Abstract
Two novel human β-chemokines, Ckβ-8 or myeloid progenitor inhibitory factor 1 (MPIF-1), and Ckβ-6 or MPIF-2, were discovered as part of a large scale cDNA sequencing effort. The MPIF-1 and MPIF-2 cDNAs were isolated from aortic endothelium and activated monocyte libraries, respectively. Both of the cDNAs were cloned into a baculovirus vector and expressed in insect cells. The mature recombinant MPIF-1 protein consists of 99 amino acids and is most homologous to macrophage inflammatory protein (MIP)-1α, showing 51% identity. It displays chemotactic activity on resting T lymphocytes and monocytes, a minimal but significant activity on neutrophils, and is negative on activated T lymphocytes. MPIF-1 is also a potent suppressor of bone marrow low proliferative potential colony-forming cells, a committed progenitor that gives rise to granulocyte and monocyte lineages. The mature recombinant MPIF-2 has 93 amino acid residues and shows 39 and 42% identity with monocyte chemoattractant protein (MCP)-3 and MIP-1α, respectively. It displays chemotactic activity on resting T lymphocytes, a minimal activity on neutrophils, and is negative on monocytes and activated T lymphocytes. On eosinophils, MPIF-2 produces a transient rise of cytosolic Ca2+ and uses the receptor for eotaxin and MCP-4. In hematopoietic assays, MPIF-2 strongly suppressed the colony formation by the high proliferative potential colony-forming cell (HPP-CFC), which represents a multipotential hematopoietic progenitor.
Chemokines are cytokines that stimulate proinflammatory activity by eliciting the chemotactic migration of leukocytes and their adhesion to endothelial cells (1–8). All the chemokines have an 8–10-kD molecular mass, exhibit 20–75% homology at the amino acid level, and are characterized by four conserved cysteine residues that form two disulfide bonds. Based on the arrangement of the first two cysteines, they have been classified as α- or β-chemokines. The α-chemokines show the first two cysteines separated by one amino acid (C-X-C motif) while the β-chemokines are characterized by two contiguous cysteines (C-C motif) (9, 10).
Structural analysis shows that β-chemokines cluster in two major groups. The first group includes monocyte chemoattractant protein (MCP)-1, MCP-2, MCP-3, MCP-4, and eotaxin, the second group includes macrophage inflammatory protein (MIP)-1α, MIP-1β, and RANTES. All the MCP chemokines are NH2 terminally blocked, attract activated T lymphocytes, and are without activity on neutrophils. All of them except eotaxin are chemotactic for monocytes and all of them except MCP-1 activate eosinophils. With the exception of MCP-1, MCP chemokines are not active on freshly purified resting T lymphocytes. (9, 11–16).
The β-chemokines of the second group, MIP-1α, MIP1β, and RANTES are chemotactic for activated T lymphocytes and monocytes. However, MIP-1α is also active on eosinophils and, moderately active on neutrophils, whereas RANTES attracts eosinophils and resting and activated T lymphocytes (9, 11, 12). Finally, MIP-1α and MIP-1β exibit activity on hematopoiesis. MIP-1α inhibits the primitive bone marrow progenitors as defined by spleen colony assay (CFU-S), and MIP-1β prevents the inhibitory activity of MIP-1α (13, 14). Both MIP-1α and MIP-1β inhibit megakaryocyte development (15).
This paper describes two novel β-chemokines, Ckβ-8 or myeloid progenitor inhibitory factor (MPIF)1 1 that exhibits homology to MIP-1α and Ckβ-6 or MPIF-2 that exhibits homology to both MIP-1α and MCP-3. MPIF-1 and MPIF-2 inhibit two distinct classes of myeloid progenitors in vitro.
Materials and Methods
Materials, Reagents, and Chemicals.
HBSS, IMDM, RPMI 1640, and MEM tissue culture media, l-glutamine, penstrep, and poly (A) RNA purification kits were purchased from GIBCO BRL (Gaithersburg, MD). Heat-inactivated fetal bovine serum (FBS), histopaque 1077 and 1119, DMSO, platelet-activating factor (PAF), and PMA were from Sigma Chemical Co. (St. Louis, MO). MyeloCult™ H5100 and MethoCult™ H4535 media were purchased from Stem Cell Technologies Inc. (Vancouver, Canada). Bacto-agar was from Difco Labs. (Detroit, MI). EXCELL 400 medium was from J.R. Scientific (Woodland, CA). Tissue culture grade plasticware was from Costar Corp. (Cambridge, MA). QBEND/10 CD34 cell isolation kit, RS and CS columns, and VarioMac were purchased from Miltenyi Biotech Inc. (Sunnyvale, CA). The cDNA ZAP Express synthesis kits were from Stratagene Corp. (La Jolla, CA). Poly (A) RNA from various tissues was purchased from Clontech (Palo Alto, CA). Nylon membranes for Northern blot analysis were from Zetabind (Cuno, Inc., Meriden, CT). Restriction enzymes and protease inhibitors were from Boehringer Mannheim (Indianapolis, IN). POROS CM20 and HS50 columns were from PerSeptive Biosystems (Framingham, MA). Sephacryl S-200 was from Pharmacia BioProcess Technology ASB (Uppsala, Sweden). ProBlott™ membranes and Blot CartridgesTM were from Perkin-Elmer Corp./ Applied Biosystems, (Foster City, CA). PA-1 column was from Dionex Corp. (Sunnyvale, CA). Limulus amebocyte lysate assay was from Associates of Cape Cod, Inc. (Woods Hole, MA). The intracellular fluorescence probes were from Molecular Probes Inc. (Eugene, OR).
mAbs and Cytokines.
The mAbs were purchased from Becton Dickinson (San Jose, CA). The mAb HIT3a anti–human CD3 was from PharMingen (San Diego, CA). Human and mouse recombinant cytokines were purchased from R&D Sys., Inc. (Minneapolis, MN). MCP-4 was produced as reported (16).
Cell Lines and Viruses.
The cell lines Sf9 (CRL-1711), K562 (CCL-243), HL60 (CRL-1964), THP-1 (TIB-202), Jurkat (TIB152), Molt-3 (CRL-1552), RS4-11 (CRL-1873), CCRF-CEM (CCL-119), KG1 (CCL-246), and KG1a (CCL-246.1) were from American Type Culture Collection (Rockville, MD). Most of the cell lines were cultured in RPMI 1640 supplemented with 10% FBS, 2 mM l-glutamine, and penstrep. KG1 and KG1a cells were grown in IMDM with 10% FBS. For the chemical induction studies, ∼2 × 106 K562 or HL-60 cells were distributed in T-75 flasks with 20 ml of fresh media with or without 1% DMSO or 10 μM PMA, and processed for total RNA at various time points. Sf9 cells were grown at 27°C in EX-CELL 400 medium with 1% FBS. The Autographa californica virus was from PharMingen.
cDNA Libraries, RNA Isolation, and Northern Blot Analysis.
Poly (A) RNA was purified from a variety of tissues or cell lines using purification kits according to the manufacturer's recommendations. Total RNA was isolated using the guanidine isothiocynate–CsCl2 method (17). All the cDNA libraries were then constructed by using the ZAP-cDNA kit according to the manufacturer's instructions. Poly (A) RNA (1 μg) from tissues or total RNA from cell lines (10 μg) were fractionated on a 1% agaroseformaldehyde gel, transferred to nylon membranes, and hybridized under high stringency conditions with 32P-cDNA probes as previously described (18).
Automated Gene Sequencing.
Bacterial colonies containing plasmids with inserts were selected, lysed, and the cDNA insert was amplified by PCR. Expressed sequence tag (EST) clones were discovered as reported (19, 20). The EST clones used in this study were the result of a collaboration between The Institute for Genomic Research (Gaithersburg, MD) and Human Genome Sciences, Inc. (Rockville, MD). For sequence analysis and assembly, Sequencher Software (version 2.1.1; Gene Codes Corp., Ann Arbor, MI) was used.
Expression and Purification of Recombinant Proteins.
The coding sequences of MPIF-1 and MPIF-2 were amplified by PCR during which unique restriction sites (BglII and Asp 718) were introduced and then cloned into the baculovirus transfer vector A2. This vector contains the β-galactosidase gene of Escherichia coli to facilitate the identification of recombinant viruses that form blue plaques. The Sf9 cells were infected at a multiplicity of infection of two and the supernatant of the infected culture was collected after 72 h. The recombinant MPIF-1 and MPIF-2 were purified in the presence of protease inhibitors (20 μg/ml pefabloc SC, 1 μg/ ml leupeptin, 1μg/ml E-64, and 1 mM EDTA) on a POROS HS 50 cation exchange column. The column was eluted with 500 mM NaCl in 40 mM Na acetate buffer at pH 5.5; the chemokine positive fractions were pooled and diluted with a 40 mM Na acetate buffer, pH 5.5, to adjust NaCl concentration to 100 mM. This mixture was applied to a POROS CM20 cation exchange column and eluted with a linear salt gradient (0.1–1 M NaCl). The positive fractions were pooled and applied to a Sephacryl S-200 column. The resulting preparations were >95% pure as determined by SDS-PAGE. Endotoxin was monitored by the limulus amebocyte lysate assay.
The NH2-terminal amino acid sequence of purified MPIF-1 and MPIF-2 was analyzed by an ABI-494 sequencer (Perkin-Elmer). The glycosylation of each chemokine was determined by a Dionex carbohydrate analyzer (21).
Human Blood Cells.
Peripheral blood mononuclear neutrophils, eosinophils, T lymphocytes, monocytes, and PBMC were purified from single donor leukopacks (American Red Cross, Rockville, MD) as previously described (22–24).
Resting T lymphocytes were enriched by negative selection by passing PBMC on T cell columns (R&D Sys.) that retained monocytes and B lymphocytes. Resting CD4+ and CD8+ T lymphocytes were 90% pure. Enriched T lymphocytes (CD4+ and CD8+) were activated by 16 h incubation in RPMI medium in the presence of 10 U/ml IL-2 in 100-mm dishes coated with HIT3a mAb anti–CD3 (1 μg/ml). Elutriated monocytes were 70–80% pure as assessed by CD14 positivity. Eosinophils were >95% pure as assessed on stained cytospun preparations. CD34+ cells were isolated from 45 ml of cord blood collected with 5 mM EDTA and 20 U/ml heparin. After 10 min centrifugation at 200 g the buffycoat was resuspended in PBS supplemented with 5 mM EDTA (PBS/EDTA) and centrifuged on histopaque 1077. The cells were collected, washed, resuspended in cold PBS/EDTA with 0.5% BSA, and subjected to the QBEND/10 kit and magnetic cell sorting according to the manufacturer's instructions. This procedure yielded a 95% CD34+ population of cells.
Mouse Bone Marrow Cells.
Bone marrow cells were isolated from 6–8-wk-old C57Bl/6 female mice (Jackson Labs., Bar Harbor, ME) according to Metcalf (25). In brief, cells were obtained from the femur and tibia by flushing with IMDM 5% FBS and centrifuged at 750 g for 30 min over a cushion of histopaque 1119. Cells sedimenting at the interface were washed with IMDM medium, plated in 10-cm dishes, and incubated for 1 h at 37°C to remove plastic adherent cells. The nonadherent cell population was used in clonogenic assays.
Chemotaxis.
Cells were washed in HBSS with 0.1% BSA (HBSS/BSA) and resuspended in the same medium at 2 × 106/ml with 1 μM calcein-AM. After 30 min at 37°C, the cells were washed in HBSS/BSA, resuspended in the same medium at 8 × 106/ml, and 25 μl of this suspension was dispensed into each upper chamber of a 96-well chemotaxis plate filter (Neuro Probe, Cabin John, MD). Different concentrations of the chemotactic agents were distributed in the bottom chamber of each well. Cells were allowed to migrate for 45–90 min through the polycarbonate filter between upper and bottom chambers (5–8 μm pores; polyvinylpyrrolidone-free), and the number of migrated cells (both attached to the bottom surface of the filter as well as in the bottom chamber) were quantitated using a fluorescence plate reader (Cytofluor PerSeptive Biosystems). The ratio between the number of cells migrated in the presence of chemokines and the number of cells migrated in the presence of buffer control is defined as the chemotactic index.
Ca2+ Mobilization.
Monocytes were loaded for 30 min at 37°C with 4 μM Indo-1/acetoxymethylester per 5 × 106 cells/ml in prewarmed buffer (1 mM CaCl2, 2 mM MgSO4, 5 mM glucose, 10 mM Hepes buffer, pH 7.4). Cells were then washed, resuspended in the same buffer at 5 × 105 cells/ml, and stimulated with the indicated chemokines at 37°C. Eosinophils were loaded with 2.5 μM fura-2/5 × 106 cells/ml in prewarmed buffer (1 mM CaCl2, 1 mM MgCl2, 125 mM NaCl, 5 mM KCl, 0.5 mM glucose, 20 mM Hepes, pH 7.4, 0.025% BSA). Cells were washed in loading buffer and resuspended at 106 cells/ml. The fluorescence signal induced by the changes in the intracellular Ca2+ was measured on a fluorescence spectrophotometer (F-2000; Hitachi Instruments Inc., San Jose, CA) by monitoring Indo-1 excitation at 330 nm, emission at 405 and 485 nm, and fura-2 excitation at 340 and 380 nm, emission at 510 nm.
Clonogenic Assays on Mouse Bone Marrow Cells.
High proliferative potential colony-forming cells (HPP-CFC) and low proliferative potential CFC (LPP-CFC) colony-formation assays were performed in a two-layered agar culture system as described by Bradley and Hodgson (26). In brief, the bottom layer was prepared in 3.5-cm diam dishes with 1 ml of MEM supplemented with 20% FBS, 0.5% Difco agar, 7.5 ng/ml mouse (m)IL-3, 150 ng/ml mouse stem cell factor (mSCF), 7.5 ng/ml human monocyte (hM)-CSF, and 15 ng/ml mIL-1α. Chemokines were incorporated into the bottom agar where indicated. This layer was then overlayed with 0.5 ml of murine bone marrow cell suspension to have 1,500 cells/dish in MEM with 20% FBS and 0.3% agar. The dishes were then incubated for 14 d in a tissue culture incubator (37°C, 88% N2, 5% CO2, and 7% O2) and colonies were scored under an inverted microscope. In these conditions HPP-CFC and LPP-CFC gives rise to colonies that are >5 mm and <1 mm diam, respectively (27, 28). In some experiments, impact of MPIF-1 and MPIF-2 on the growth of burst-forming unit–erythroid (BFU-E), CFU-E, and CFU–granulocyte and monocyte (GM) was also determined. In brief, 5 × 104 bone marrow cells were suspended in 1 ml of MethoCult™ semisolid medium and plated in 3.5-cm dishes. For CFU-E assay, the medium was supplemented with 3 U/ml h erythropoietin (Epo) and the dishes were incubated for 2 d. The hemoglobinized colonies (4–16 cells/ colony) were identified by acid benzidine stain and scored under an inverted microscope. For BFU-E and CFU-GM assay, the medium was supplemented with 5 ng/ml mIL-3, 10 ng/ml mGMCSF, and 3 U/ml hEpo. The dishes were incubated for 11 d and the colonies were scored under an inverted microscope.
Clonogenic Assay of hCD34+ Progenitors.
CD34+ cord blood cells (5 × 104 cells/ml) were cultured in myelocultTM medium supplemented with 10 ng/ml hIL-3 and 50 ng/ml hSCF for 4 d at 37°C in a tissue culture incubator (5% CO2, 7% O2, and 88% N2). The resulting populations of progenitors were suspended (103 cells/ml) in MethocultTM semisolid medium supplemented with a cocktail of cytokines (10 ng/ml hIL-3, 10 ng/ml hGM-CSF, 50 ng/ml hSCF, 3U/ml hEpo, and 10 ng/ml h thrombopoietin), and plated in the presence or absence of chemokines. Colonies were allowed to grow by incubating the dishes at 37°C in a humidified incubator (5% CO2, 7% O2, and 88% N2) for 14 d, and colonies were scored under an inverted microscope.
Results
Characterization of the MPIF-1 and MPIF-2 cDNA Clones.
The ESTs representing MPIF-1 and MPIF-2 cDNAs were identified in the Human Genome Sciences, Inc. data base on the basis of the C-C motif and the homology with known β-chemokines. The MPIF-1 cDNA clone was isolated from a human aortic endothelial library. Sequencing of both strands of the insert revealed a full coding region for a 120–amino acid protein, of which the 21 NH2-terminal amino acids represent the signal peptide (Fig. 1 A). In comparison with the known human β-chemokines, MPIF-1 shows six cysteine residues and is most closely related to hMIP-1α, exhibiting 51% identity and 67% similarity over a stretch of 99 amino acids (Fig. 2). The amino acid sequence of MPIF-1 revealed no N-linked glycosylation sites. The MPIF-2 cDNA clone was isolated from an activated monocyte library and encodes for a 119–amino acid protein, of which the 26 NH2-terminal amino acids represent the signal peptide (Fig. 1 B). MPIF-2 is a β-chemokine with four cysteine residues that exhibits 39% identity and 59% similarity to MCP-3, and 42% identity and 65% similarity to MIP-1α over a stretch of 93 amino acids. The overall comparison of MPIF-1 with MPIF-2 revealed 30% identity and 50% similarity to one another (not shown).
Figure 1.
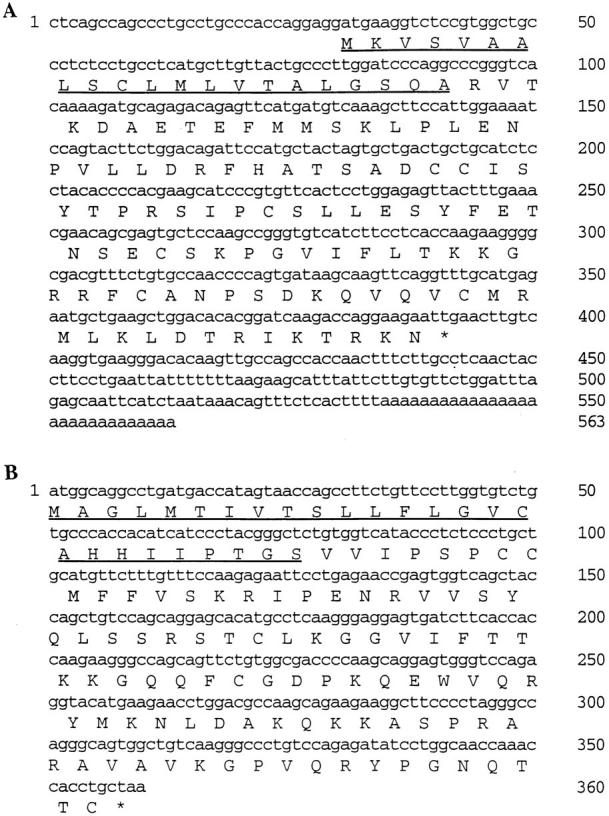
The nucleotide sequences of human MPIF-1 (A) and MPIF-2 (B) cDNAs are shown along with the deduced amino acid sequence using a single letter amino acid code. The underlined NH2-terminal amino acids represent the leader sequences that were experimentally determined of proteins expressed in baculovirus expression system. These sequence data are available from EMBL/GenBank/DDBJ under accession number U85767 for MPIF-1 and U85768 for MPIF-2. (See note added in proof.)
Figure 2.

Comparison of the amino acid sequences of human MPIF-1 (A) and MPIF-2 (B) with the human β-chemokines MIP-1α, MCP-2, MCP-3, and MCP-4. The regions of amino acid sequence homology were determined according to MegAlign Program (version 1.05; DNASTAR Inc., Madison, WI).
Tissue Distribution of MPIF-1 and MPIF-2.
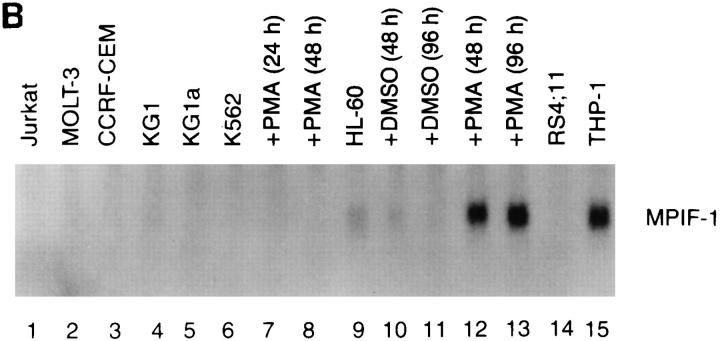
MPIF-1 ESTs were most frequently found in the liver, lung, and bone marrow cDNA libraries, but MPIF-2 ESTs were restricted to the activated monocyte and T lymphocyte cDNA libraries. To confirm this observation, Northern blot analysis was performed. As shown in Fig. 3 A, MPIF-1 cDNA hybridized to a 0.8-kb mRNA species that is most abundant in the adult lung and liver (Fig. 3 A, lanes 7 and 8), and is present at moderate levels in fetal liver (Fig. 3 A, lane 4), adult bone marrow and placenta (Fig. 3 A, lanes 11 and 13). Analysis of various cell lines showed that MPIF-1 mRNA is readily detectable in myelomonocytic cell lines (Fig. 3 B). Similar analysis with MPIF-2 cDNA turned out negative. The MPIF-2 mRNA was only detectable by reverse transcriptase-PCR in monocytes activated with GM-CSF for 96 h and in T lymphocytes activated by mAb anti–CD3 (HIT3a) in the presence of IL-2 (not shown).
Figure 3.
(A) Northern blot analysis of MPIF-1 mRNA steady-state levels in various human tissues (A) and cell lines (B). (A) Upper panel was probed with MPIF-1 cDNA, whereas lower panel with actin cDNA.
MPIF-1 and MPIF-2 Expression, Purification, and Analysis.
Recombinant MPIF-1 and MPIF-2 proteins were expressed in Sf9 insect cells infected with a recombinant baculovirus. SDS-PAGE analysis of a purified MPIF-1 preparation revealed a single Coomassie blue–stained band with a molecular mass of 11–12 kD (Fig. 4 A). Edman degradation analysis showed that the MPIF-1 protein was processed to yield a protein with RVTKDAE as the NH2-terminus and an expected molecular mass of ∼11.2 kD, thus confirming that the observed sequence is identical to that predicted from the cDNA sequence (Fig. 4 C). Consistent with the lack of N-glycosylation sites no mannose or N-acetylglucosamine were detected in the MPIF-1 protein.
Figure 4.
Analysis of MPIF-1 (A) and MPIF-2 (B) proteins. SDSPAGE was performed on 5 μg of purified protein under reducing conditions. The gel was stained with Coomassie blue. Lane 1 of each panel shows the molecular weight standards. C shows the NH2-terminal sequences of MPIF-1 and MPIF-2 proteins as determined by Edman degradation.
Amino acid sequence analysis indicated that the NH2 terminus of the MPIF-2 protein is not blocked and begins with VVIPSP (Fig. 4 C). This predicts the calculated molecular mass of the mature MPIF-2 protein to be 10.5 kD, which is in agreement with the SDS-PAGE analysis of Coomassie blue–stained protein (Fig. 4 B). MPIF-2 contains up to 0.9 moles of N-acetylglucosamine or mannose per mole of the protein. No galactose or N-acetylgalactosamine were detected, suggesting the absence of O-linked oligosaccharides.
Chemotactic Activity of MPIF-1 and MPIF-2.
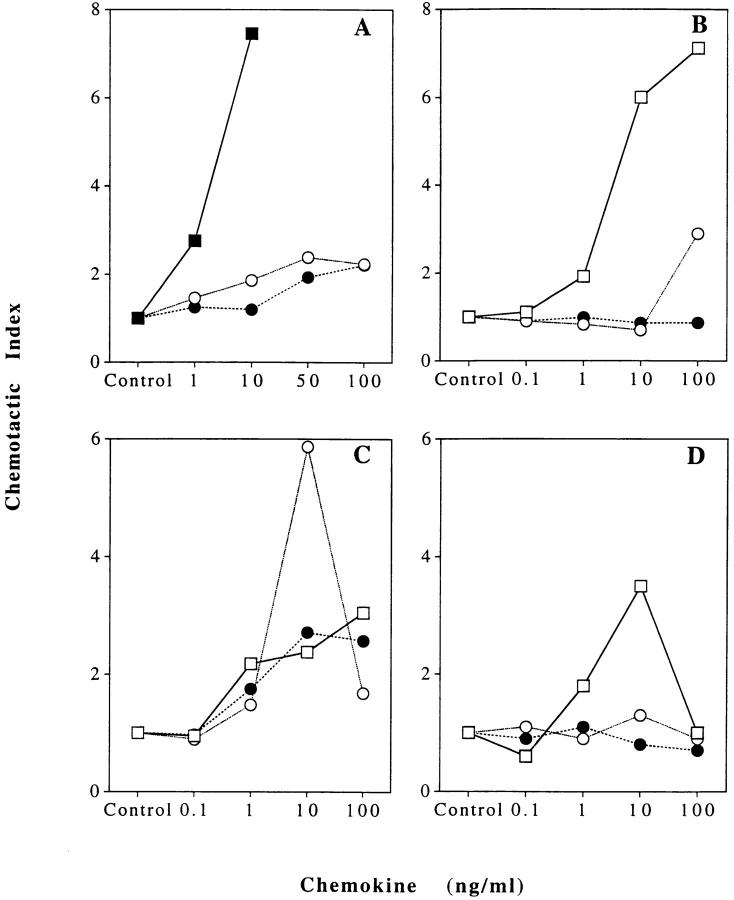
The chemotactic properties of MPIF-1 and MPIF-2 were studied on purified resting and anti-CD3–activated T lymphocytes, neutrophils, and monocytes. The activity on T lymphocytes and monocytes was tested in comparison with MCP-1, which is a potent attractant for these cells. The activity on neutrophils was tested, and compared with IL-8. Several experiments were performed using cells obtained from different donors, and representative experiments are summarized in Fig. 5.
Figure 5.
Chemotactic activity of MPIF-1 and MPIF-2 on peripheral blood leukocytes: neutrophils (A), monocytes (B), resting T lymphocytes (C), and activated T lymphocytes (D). The data for MPIF-1 (open circles), MPIF-2 (closed circles), IL-8 (closed squares), and MCP-1 (open squares) are presented as chemotactic index (ratio between the number of cells migrated in the presence of chemokines and the number of cells migrated in the presence of buffer control). One of three to five experiments performed is shown.
On resting T lymphocytes, MPIF-1 reached its maximum activity at 10 ng/ml, and was more potent and three times more effective than MCP-1. In contrast, MPIF-2 and MCP-1 showed comparable potency and efficacy (Fig. 5 C). Both MPIF-1 and MPIF-2 were negative on anti-CD3– activated T lymphocytes (Fig. 5 D). MPIF-1 induced migration of monocytes at concentrations ranging from 100 ng/ml to 1 μg/ml, but this activity was not observed with all the monocyte preparations tested. MPIF-2 was negative on monocytes (Fig. 5 B). Both MPIF-1 and MPIF-2 induced some migration of neutrophils. Although minimal, this activity was consistently evident at 50 ng/ml, whereas IL-8 was already four times more potent at 10 ng/ml (Fig. 5 A).
Cytosolic Calcium Changes and Desensitization Experiments.
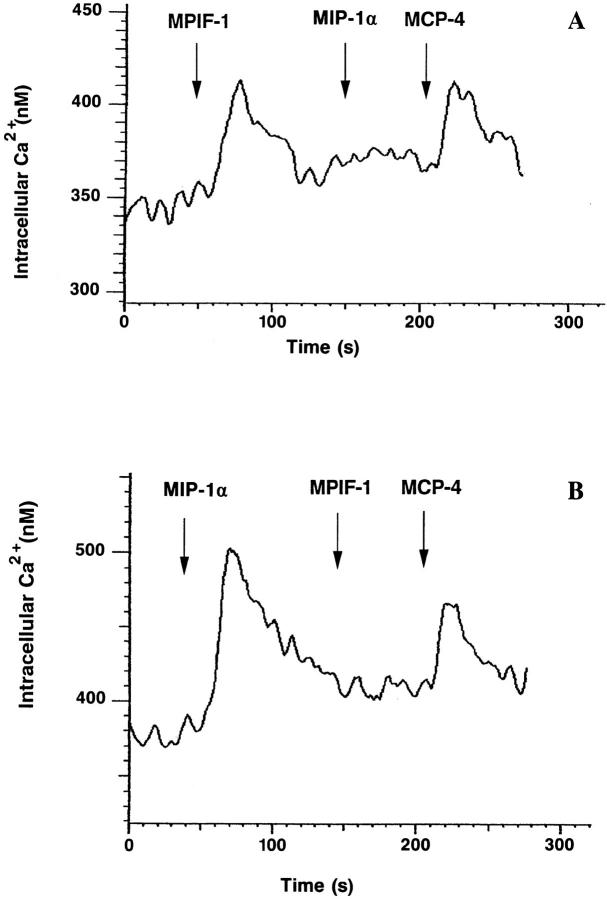
The capacity of MPIF-1 and MPIF-2 to induce a rapid and transient rise of cytosolic Ca2+ concentration [Ca2+]i was assessed on monocytes, and eosinophils. MPIF-1 was active on monocytes, and MPIF-2 produced changes of [Ca2+]i on eosinophils. On monocytes, a significant rise of the [Ca2+]i was observed using 10 ng/ml MIP-1α, and the maximum value was recorded at 100 ng/ml MIP-1α. In similar conditions, 1 μg/ml MPIF-1 induced only 50% of the change of [Ca2+]i that was observed with 100 ng/ml MIP-1α. In the desensitization experiments, the [Ca2+]i changes after repeated stimulation were monitored in monocytes to assess receptor usage by MPIF-1. The stimulation of monocytes with 100 ng/ml MIP-1α abolished responsiveness to 1 μg/ ml MPIF-1, but not to 100 ng/ml MCP-4 (Fig. 6 B). When monocytes were first exposed to 1 μg/ml MPIF-1 and then to 100 ng/ml MIP-1α followed by 100 ng/ml MCP-4, MPIF-1 was able to abolish MIP-1α response without affecting the ability of the same cells to respond to MCP-4 (Fig. 6 A).
Figure 6.
Cross-desensitization of human blood monocytes. Indo1–loaded cells were stimulated sequentially with 1 μg/ml MPIF-1 and other β-chemokines (100 ng/ml), and [Ca2+]i-dependent fluorescence changes were recorded. (A) Stimulation with MPIF-1, MIP-1α, and MCP-4; (B) Stimulation with MIP-1α, MPIF-1, and MCP-4.
On eosinophils, the magnitude of the [Ca2+]i rise increased with the concentration of chemokines tested. A maximum response was recorded using 500 ng/ml eotaxin or 1 μg/ml MPIF-2 (data not shown). At the maximal concentration of 1 μg/ml, the response to MPIF-2 was ∼85% of the eotaxin response, while MCP-1 at 1 μg/ml was negative. In desensitization experiments, the stimulation of eosinophils with 1 μg/ml eotaxin or MCP-4 abolished responsiveness to 1 μg/ml MPIF-2, but not to 10−6 M PAF (Fig. 7, A and D). When eosinophils were first exposed to 1 μg/ml MPIF-2 and then to 1 μg/ml eotaxin or MCP-4, MPIF-2 was found to be able to abolish eotaxin or MCP-4 response without affecting the ability of the same cells to respond to PAF (Fig. 7, B and C).
Figure 7.
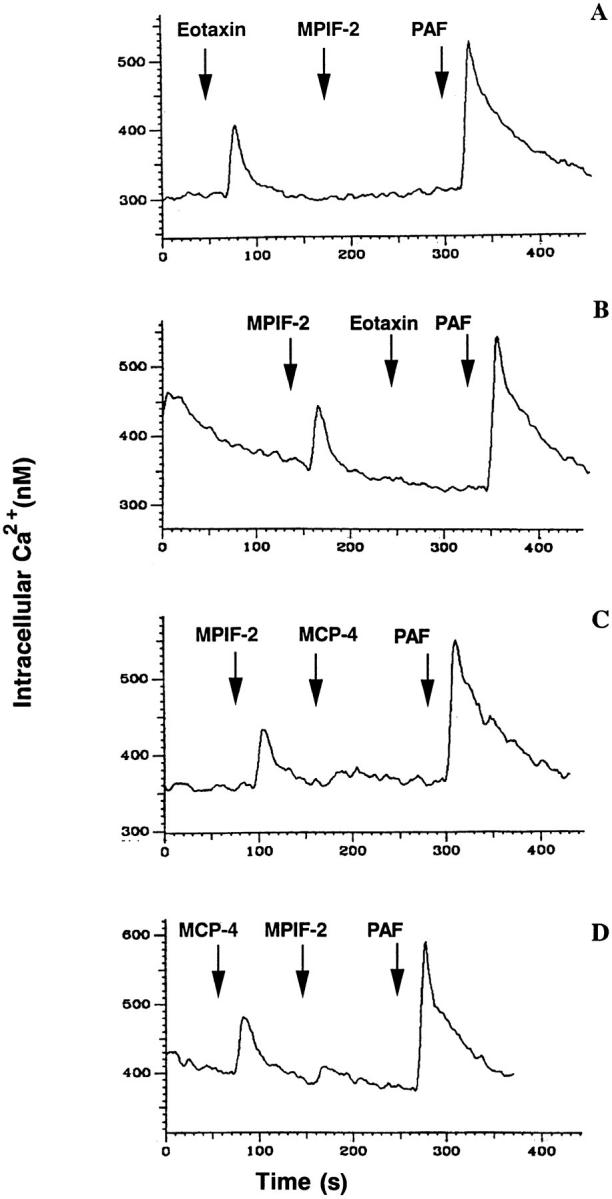
Cross-desensitization of human blood eosinophils. Fura-2–loaded cells were stimulated sequentially with 1 μg/ml MPIF-2 and other β-chemokines or 10−6 M PAF, and [Ca2+]i-dependent fluorescence changes were recorded. (A) Stimulation with eotaxin, MPIF-2, and PAF; (B) Stimulation with MPIF-2, eotaxin, and PAF; (C) stimulation with MPIF-2, MCP-4, and PAF; (D) Stimulation with MCP-4, MPIF-2, and MCP-4.
Inhibition of Mouse Myeloid Progenitors.
The effects MPIF-1 and MPIF-2 on hematopoietic cells was studied on mouse low density bone marrow cells plated in methylcellulose- or agar-containing growth medium supplemented with appropriate combinations of cytokines. No colonies were detected either in the absence of cytokines or in the presence of either MPIF-1 or MPIF-2 alone. A concentration of 50 ng/ml MPIF-1 reduced the frequency of CFU-GM and LPP-CFC colonies to 30% of that found in the control cultures (Table 1). This inhibitory effect of MPIF-1 appeared to be specific, as MPIF-1 had no effect on colony formation by CFU-E, BFU-E, and HPP-CFC (Table 1). In contrast, 50 ng/ml MPIF-2 produced 80% reduction in the numbers of HPP-CFC colonies compared to those found in the control cultures without effects on colony formation by CFU-E, BFU-E, CFU-GM, and LPP-CFC (Table 1). In the same conditions, both MCP-4 (50 ng/ml) and MIP-1α (100 ng/ml) had no effect on colony formation (Table 1 and Fig. 8). The inhibitory effect of MPIF-1 and MPIF-2 was dose dependent (Fig. 9). MPIF-2 reduced HPP-CFC colonies to 50% of the control at 10 ng/ml (Fig. 9 A), while MPIF-1 reduced LPP-CFC colonies to 50% of the control at 20 ng/ml (Fig. 9 B). This experiment confirmed the specificity of MPIF-1 and MPIF-2 inhibitory effect on LPP-CFC and HPP-CFC, respectively.
Table 1.
Effect of MPIF-1 and MPIF-2 on the Murine Hematopoietic Progenitor Cell Colony Formation In Vitro in Response to Known Cytokines
| Additions | CFU-E | Progenitor colonies | LPP-CFC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BFU-E | CFU-GM | HPP-CFC | ||||||||
| CC | 94 ± 10 | 13 ± 2.5 | 29 ± 3.0 | 35 ± 4.0 | 114 ± 12 | |||||
| +MPIF-1 | 89 ± 11 | 12 ± 2.0 | 11 ± 2.0 | 29 ± 2.0 | 27 ± 6.0 | |||||
| +MPIF-2 | 91 ± 4.0 | 12 ± 3.0 | 29 ± 2.0 | 8 ± 2.0 | 109 ± 14 | |||||
| +MCP-4 | 91 ± 11 | 12 ± 3.0 | 31 ± 4.0 | 34 ± 2.0 | 116 ± 17 | |||||
CC, appropriate cocktail of cytokines were used as described in Materials and Methods. Chemokines were added at 50 ng/ml final concentration. Data shown are pooled from two separate experiments and are expressed as mean ± SD.
Figure 8.
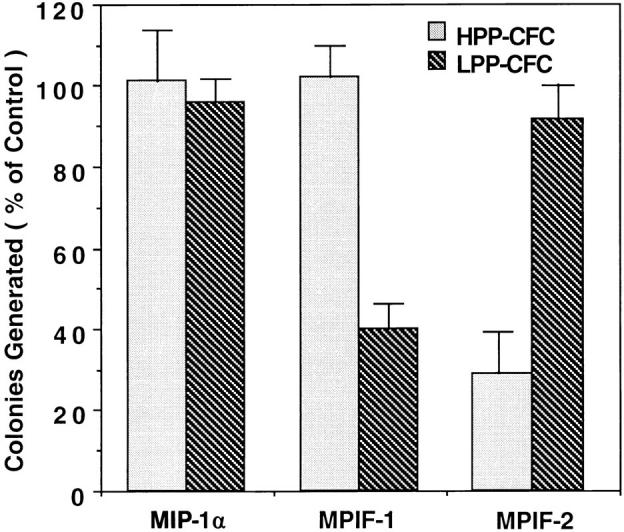
Effect of MPIF-1 and MPIF-2 on the colony formation by murine HPP-CFC and LPP-CFC in vitro. A population of 1,500 low density mouse bone marrow cells in semisolid medium with 5 ng/ml mIL-3, 100 ng/ml mSCF, 10 ng/ml mIL-1α, and 5 ng/ml hM-CSF were distributed in each plate and cultured with or without 100 ng/ml of the indicated chemokine for 14 d. The results from two experiments that were performed in duplicates are presented. The number of colonies generated in the presence of chemokines is expressed as percentage of those produced in the absence of any added chemokines ± SD.
Figure 9.
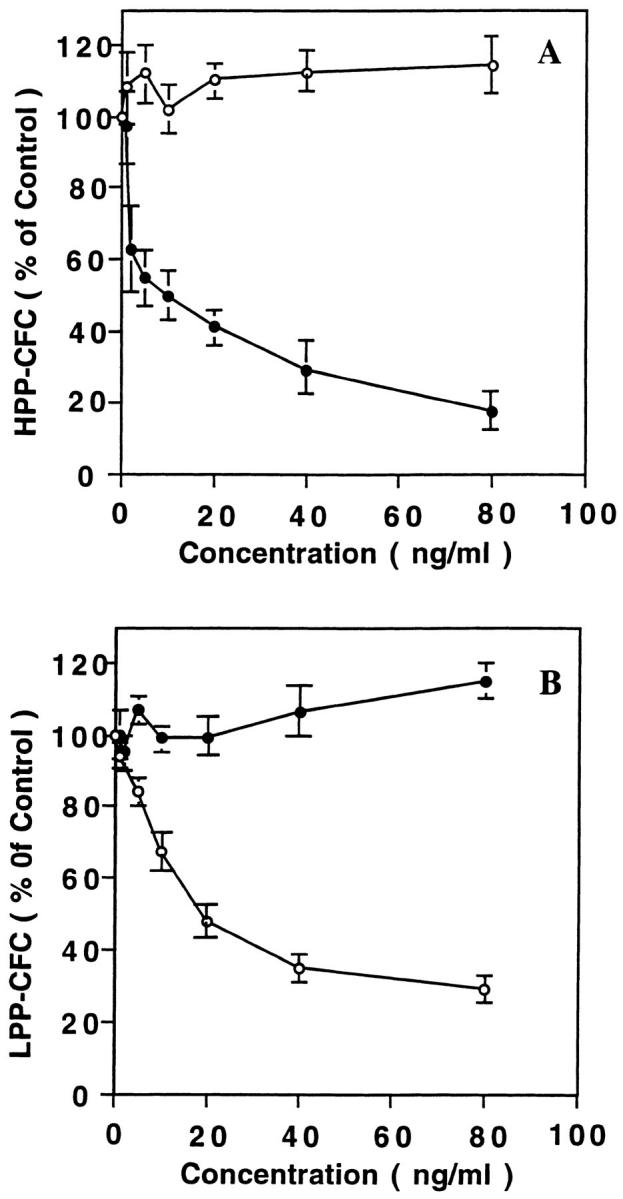
Effect of various concentrations of MPIF-1 (open circles) and MPIF-2 (closed circles) on the growth and differentiation of HPP-CFC (A) and LPP-CFC (B). The low density population of mouse bone marrow cells was plated (1,500 cells/dish) in agar-containing medium with or without the indicated concentrations of chemokines, but in the presence of a cytokine cocktail as described in the legend to Fig. 8. The dose-response curve includes data from three independent experiments that are expressed as mean ± SEM.
Effect on Human Hematopoietic Progenitors.
To determine the impact of MPIF-1 and MPIF-2 on human hematopoietic cells, CD34+ cells were isolated from cord blood and cultured for 4 d in the presence of IL-3 and SCF to generate hematopoietic progenitors. A limiting dilution of this cell population was then cultured in semisolid medium with multiple cytokines with or without test chemokines. Data from two representative experiment are shown in Table 2. Colony formation by CFU-GM and CFU-Mix in the presence of either 50 ng/ml MPIF-1 or 50 ng/ml MPIF-2 was reduced by 30–40% compared to those found in the control cultures (Table 2). Neither 50 ng/ml of MIP-1α nor MCP-4 affected CFU-GM and CFU-Mix (Table 2).
Table 2.
Effect of MPIF-1 and MPIF-2 on Human Hematopoietic Progenitor Colony Formation In Vitro
| Experiment | Additions | BFU-E | CFU-G | CFU-M | CFU-GM | CFU-Meg | CFU-Mix | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colonies/103 cells | ||||||||||||||
| 1 | CC | 13 ± 2 | 15 ± 2 | 14 ± 3 | 16 ± 3 | 11 ± 3 | 11 ± 2 | |||||||
| + MPIF-1 | 19 ± 2 | 18 ± 5 | 12 ± 2 | 8 ± 2 | 12 ± 2 | 5 ± 1 | ||||||||
| + MPIF-2 | 16 ± 4 | 12 ± 2 | 13 ± 1 | 9 ± 3 | 14 ± 3 | 3 ± 1 | ||||||||
| + MIP-1α | 17 ± 3 | 19 ± 5 | 14 ± 2 | 14 ± 4 | 12 ± 3 | 12 ± 2 | ||||||||
| 2 | CC | 14 ± 3 | 13 ± 3 | 13 ± 1 | 12 ± 3 | 13 ± 1 | 11 ± 2 | |||||||
| + MPIF-1 | 14 ± 2 | 11 ± 1 | 12 ± 2 | 5 ± 1 | 12 ± 2 | 4 ± 1 | ||||||||
| + MPIF-2 | 13 ± 2 | 12 ± 1 | 13 ± 1 | 8 ± 2 | 12 ± 3 | 6 ± 2 | ||||||||
| + MIP-1α | 12 ± 2 | 12 ± 3 | 13 ± 2 | 13 ± 2 | 14 ± 2 | 12 ± 1 | ||||||||
| + MCP-4 | 12 ± 1 | 14 ± 2 | 12 ± 1 | 12 ± 2 | 11 ± 1 | 12 ± 2 | ||||||||
CC, a cocktail of cytokines was added as described in Materials and Methods. Chemokines were added at 50 ng/ml final concentration. Data from two representative experiments performed in triplicates are shown and are expressed as mean ± SD.
Discussion
In a large-scale DNA sequencing effort, two novel human β-chemokines were discovered and named Ckβ-8 or MPIF-1 and Ckβ-6 or MPIF-2 for their activity on human and mouse myeloid progenitors. The cDNA encoding MPIF-1 has been cloned from a human aortic endothelial library, and the message for this chemokine is mainly expressed by macrophages and is detectable in lung, liver, bone marrow, placenta, and fetal liver. MPIF-1 is not NH2 terminally blocked, and shares 51% identity and 67% similarity with MIP-1α. The chemotactic activity of MPIF-1 is very potent on resting T lymphocytes, significant on monocytes, and weak but consistent on neutrophils. Finally, MPIF-1 has a notable in vitro inhibitory effect on LPP-CFC, the committed progenitors that give rise to granulocyte and monocyte lineages and reduces CFU-GM, but has no significant effects on HPP-CFC multipotent hematopoietic progenitors. The experiments performed with human cord blood CD34+-derived cells show that MPIF-1 inhibits both CFU-Mix and CFU-GM, and confirm that our novel β-chemokine acts on similar targets of both murine and human origins. MPIF-1 does not show inhibitory activity on BFU-E or megakaryocyte precursors. Although the desensitization experiments suggest that MPIF-1 and MIP-1α may share the same receptor on monocytes, they are functionally unrelated. MPIF-1 and MIP-1α are active on different functional stages of T lymphocytes and hematopoietic progenitors where they may use distinct receptors. Finally, in comparison with MIP-1α, MPIF-1 is a more potent inhibitor of hematopoietic progenitors. In our study, MPIF-1 at 10–50 ng/ml produces 50–80% inhibition of mLPP-CFC and 50% inhibition of hCFU-GM and hCFU-Mix (CFU-GEMM), whereas MIP-1α at 100 ng/ ml is ineffective. These data are in agreement with previously published results showing inhibitory effect of MIP1α for BFU-E, CFU-GM, CFU-GEMM, and CFU-Megs at concentrations ranging from 100 to 500 ng/ml (15, 29, 30). Other studies show that a single addition of MIP-1α up to 1 μg/ml to the liquid or semisolid system has no effect on GM-CFU, BFU-E, and multilineage precurors. Only the daily addition of 100 ng/ml MIP-1α for 6 d produces inhibition (31, 32).
The recently cloned MRP-2 is a mouse β-chemokine that shares some characteristics with MIP-1α and shows interesting similarities to MPIF-1. It suppresses in vitro colony formation by mouse and human BFU-E, CFU-GM, and CFU-GEMM progenitors (33). MPIF-1 and MRP-2 are 42% identical at the amino acid level and show six cysteine residues that are conserved with respect to their location within both proteins. Due to the fact that the MRP-2 and MPIF-1 cDNAs exhibit only 67% identity and MPIF-1 does not inhibit BFU-E, it is unclear if MPIF-1 represents the human counterpart of MRP-2. The structural and functional properties we described for MPIF-1 suggest that this novel β-chemokine may be grouped with MIP-1α, MIP-1β, RANTES, and mMRP-2.
The novel β-chemokine MPIF-2 is weakly expressed by activated T lymphocytes and GM-CSF–treated macrophages, does not have the NH2 terminus blocked, and exhibits 40% identity and 59% similarity to MCP-3, and 42% identity and 65% similarity to MIP-1α. MPIF-2 attracts resting T lymphocytes, activates eosinophils, has minimal activity on neutrophils, and is negative on monocytes and activated T lymphocytes. On eosinophils, MPIF-2, eotaxin, and MCP-4 induce comparable transient rise of [Ca2+]i. Finally, MPIF-2 has a notable in vitro inhibitory effect on mHPP-CFC multipotent hematopoietic precursors without affecting the LPP-CFC–committed progenitors that give rise to granulocyte and monocyte lineages. Although the desensitization experiments suggest that MPIF-2, eotaxin, and MCP-4 may share the same receptor on eosinophils, MPIF-2 may use a different receptor on multipotent hematopoietic precursors. In fact, MCP-4 does not affect HPP-CFC multipotent hematopoietic precursors. The structural and functional properties we described for MPIF-2 show that this novel β-chemokine shares characteristics with both the MCP and MIP chemokines.
Presently, it is difficult to assess the physiological relevance of the novel β-chemokines we described in this paper. During early stages of inflammation, wound healing, and delayed-type hypersensitivity reactions, locally activated macrophages, granulocytes, lymphocytes, and endothelial cells release β-chemokines (i.e., MCP-1, MCP-3, MIP-1α, MIP-1β, and RANTES), cytokines (i.e., IL-1, IL-3, IL-5, IL-6), and growth factors (i.e., G-CSF, GM-CSF) to recruit mature leukocytes and to stimulate the bone marrow production of new leukocytes (34–36). It is tempting to speculate that MPIF-1 and MPIF-2 may play an important role promoting and then limiting these processes. Once released by activated T lymphocytes, monocytes, and tissue macrophages, MPIF-1 and MPIF-2 could induce the migration of these same cell types to the local microenvironment. More intriguing, the potential of MPIF-1 and MPIF-2 to reduce the capacity of the hematopoietic progenitor pool to produce mature elements could limit the local accumulation of proinflammatory cells. The inhibitory activity of MPIF-1 and MPIF-2 suggests that these novel chemokines may have the potential to protect the hematopoietic progenitors from the cytotoxic effects of the antiblastic drugs used in cancer therapy.
Acknowledgments
We wish to thank Vina Patil-Koota, Radhika Uppaluri, and Jeffrey Carrell for their excellent technical assistance. We would also like to thank the Shady Grove Hospital (Rockville, MD) for their provision of human cord blood. The contributions of the Human Genome Sciences, Inc. and The Institute of Genomic Research (Rockville, MD). Sequencing and Bioinformatics departments are appreciated. The human tissues in this study were supplied by the Cooperative Human Tissue Network (Fredrick, MD), which is funded by the National Cancer Institute.
Note added in proof. The cDNA sequence for MPIF-2 shown in Fig. 1 B has a typographical error at nucleotide 182, which is printed c rather than g. This correction results in a change of the amino acid at position 61 from a glycine (G) to an alanine (A). In addition, the MPIF-2 cDNA sequence predicts a phenylalanine at position 73. However, data from mass spectrophotometry and sequencing studies indicates that there is a serine at position 73 instead of a phenylalanine in the protein preparation used in this study.
Footnotes
1 Abbreviations used in this paper: CFC, colony-forming cells; Epo, erythropoietin; EST, expressed sequence tag; FBS, fetal bovine serum; GM, granulocyte and monocyte; h, human; HPP, high proliferative potential; LPP, low proliferative potential; m, mouse; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; MPIF, myeloid progenitor inhibitory factors; PAF, platelet-activating factor; SCF, stem cell factor.
References
- 1.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. CRC Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 2.Mukaida N, Harada A, Yasumoto K, Matsushima K. Properties of proinflammatory cell type–specific leukocyte chemotactic cytokines, interleukin 8 and monocyte chemotactic and activating factor (MCAF) Microbiol Immunol. 1992;36:773–789. doi: 10.1111/j.1348-0421.1992.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 3.Kuna P, Reddihari SR, Rucinski D, Oppenheim JJ, Kaplan AP. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for human basophils. J Exp Med. 1992;175:489–493. doi: 10.1084/jem.175.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff SC, Kreiger M, Brunner T, Dahinden CA. Monocyte chemotactic protein 1 is a potent activator of human basophils. J Exp Med. 1992;175:1271–1275. doi: 10.1084/jem.175.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahinden CA, Geiser T, Brunner T, Tscharner VV, Caput D, Ferraa P, Minty A, Baggiolini M. Monocyte chemotactic protein 3 is a most effective basophil- and eosinophil-activating chemokine. J Exp Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo J, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human IP-10 is a chemoattractant for T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1815. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Pozo MA, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 10.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 11.Rot A, Kreiger M, Brunner T, Bischoff SC, Schall TJ, Dahinden CA. RANTES and macrophage inflammatory protein 1α induce the migration and activation of normal human eosinophil granulocytes. J Exp Med. 1992;176:1489–1495. doi: 10.1084/jem.176.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B. Monocyte chemotactic proteins MCP-1, MCO-2, and MCP-3 are major attractants for human CD4+ and CD8+T lymphocytes. FASEB J. 1994;8:1055–1060. doi: 10.1096/fasebj.8.13.7926371. [DOI] [PubMed] [Google Scholar]
- 13.Pragnell IB, Wright EG, Lorimore SA, Adam J, Rosendaal M, Delamarter JF, Freshney M, Eckmann I, Sproul A, Wilkie N. The effect of stem cell proliferation regulators demonstrated with an in vitro assay. Blood. 1988;72:96–99. [PubMed] [Google Scholar]
- 14.Graham GJ, Wright EC, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB. Identification and characterization of an inhibitor of hematopoietic stem cell proliferation. Nature (Lond) 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz AM, Zhang J, Ratajczak M, Park KS, Li C, Yan Z, Poncz M. Chemokine regulation of human megakaryocytopoiesis. Blood. 1995;86:2559–2567. [PubMed] [Google Scholar]
- 16.Uguccioni M, Loetscher P, Forssmann U, Dewald B, Li H, Lima SH, Li Y, Kreider B, Garotta G, Thelen M, Baggiolini M. Monocyte chemotactic protein 4 (MCP-4), a novel structural and functional analogue of MCP-3 and eotaxin. J Exp Med. 1996;183:2379–2384. doi: 10.1084/jem.183.5.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 18.Kreider BL, Pillips PD, Prystowsky MD, Shirsat N, Pierce JH, Tushinski R, Rovera G. Induction of granulocyte–macrophage colony-stimulating factor (CSF) receptor by granulocyte CSF increases the differentiative options of a murine hematopoietic progenitor cell. Mol Cell Biol. 1990;10:4846–4851. doi: 10.1128/mcb.10.9.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams MD, Kelly JM, Gocayne JD, Dubnick M, Polymeropoulos MH, Hong X, Merril CR, Wu A, Olde B, Moreno RF, et al. Complementory DNA sequencing: expressed sequence tags and human genome project. Science (Wash DC) 1991;252:1651–1656. doi: 10.1126/science.2047873. [DOI] [PubMed] [Google Scholar]
- 20.Adams MD, Dubnick M, Kerlavage AR, Moreno R, Kelly JM, Utterback TR, Nagle JW, Fields C, Ventor JC. Sequence identification of 2,375 human brain genes. Nature (Lond) 1992;255:632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- 21.Hardy MR, Townsend RR. High-pH anionexchange chromatography of glycoprotein-derived carbohydrates. Methods Enzymol. 1994;230:208–225. doi: 10.1016/0076-6879(94)30014-3. [DOI] [PubMed] [Google Scholar]
- 22.Boyum, A. 1976. Isolation of lymphocytes, granulocytes and macrophages. Scand. J. Immunol. 5(Suppl.):9–15. [PubMed]
- 23.Talmadge KW, Gallati H, Sinigaglia F, Walz A, Garotta G. Identitiy between human interferon-gamma and “macrophage-activating factor” produced by human T lymphocytes. Eur J Immunol. 1986;16:1471–1477. doi: 10.1002/eji.1830161202. [DOI] [PubMed] [Google Scholar]
- 24.Hansel TT, De Vries JM, Iff T, Rihs S, Wandzilak M, Betz S, Blaser K, Walker C. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J Immunol Methods. 1991;145:105–110. doi: 10.1016/0022-1759(91)90315-7. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf, D. 1984. The Hemopoietic Colony Stimulating Factors. Elsevier Science Publishers B. V., Amsterdam. 97– 105.
- 26.Bradley TR, Hodgson GS. Detection of primitive macrophage progenitor cells in bone marrow. Blood. 1979;54:1446–1451. [PubMed] [Google Scholar]
- 27.Bertoncello I, Bradley TR, Hodgson GS, Dunlop JM. The resolution, enrichment and organization of normal bone marrow high proliferative potential colony-forming subsets on the basis of rhodamine 123 fluorescence. Exp Hematol. 1991;19:174–182. [PubMed] [Google Scholar]
- 28.Kriegler AB, Verschoor SM, Ber D, Bertoncello I. The relationship between different high proliferative potential colony-forming cells in mouse bone marrow. Exp Hematol. 1994;22:432–440. [PubMed] [Google Scholar]
- 29.Dunlop DJ, Wright EG, Lorimore S, Graham GJ, Holyoake T, Kerr DJ, Wolpe SD, Pragnell IB. Demonstration of stem cell inhibition and myeloprotective effects of SCI/rhMIP-1α in vivo. Blood. 1992;79:2221–2228. [PubMed] [Google Scholar]
- 30.Broxmeyer HE, Sherry B, Cooper S, Lu L, Maze R, Beckmann MP, Cerami A, Ralph P. Comparitive analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. J Immunol. 1993;150:3448–3458. [PubMed] [Google Scholar]
- 31.Quesniaux VFJ, Graham GJ, Pragnell IB, Donaldson D, Wolpe SD, Iscove NN, Fagg B. Use of 5-fluorouracil to analyze the effect of macrophage inflammatory protein-1α on long-term reconstituting stem cells in vivo. Blood. 1993;81:1497–1504. [PubMed] [Google Scholar]
- 32.Bonnet D, Lemoine FM, Najman A, Giugon M. Comparison of the inhibitory effect of AcSDKP, TNF-α, TGF-β, and MIP-1α on marrow-purified CD34+progenitors. Exp Hematol. 1995;23:551–556. [PubMed] [Google Scholar]
- 33.Youn B-S, Jang I-K, Broxmeyer HE, Cooper S, Jenkins NA, Gilbert DJ, Copeland NG, Elick TA, Fraser MJ, Kwon BS. A novel chemokine, macrophage inflammatory protein-related protein-2, inhibits colony formation of bone marrow myeloid progenitors. J Immunol. 1995;155:2661–2667. [PubMed] [Google Scholar]
- 34.Sherri B, Cerami A. Small cytokine superfamily. Curr Opin Immunol. 1991;3:56–60. doi: 10.1016/0952-7915(91)90077-e. [DOI] [PubMed] [Google Scholar]
- 35.Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformansinfection. J Immunol. 1995;155:4790–4797. [PubMed] [Google Scholar]
- 36.Schrum S, Probst P, Fleischer B, Zipfel PF. Synthesis of the CC-chemokines MIP-1α, MIP-1β, and RANTES is associated with a type 1 immune response. J Immunol. 1996;157:3598–3604. [PubMed] [Google Scholar]