Abstract
Human monocytes undergo spontaneous apoptosis upon culture in vitro; removal of serum from the media dramatically increases the rate of this process. Monocyte apoptosis can be significantly abrogated by the addition of growth factors or proinflammatory mediators. We have evaluated the role of the endogenous Fas–Fas ligand (FasL) interaction in the induction of this spontaneous apoptosis and found that a Fas–immunoglobulin (Ig) fusion protein, an antagonistic anti-Fas monoclonal antibody and a rabbit anti-FasL antibody all greatly reduced the onset of apoptosis. The results indicate that spontaneous death of monocytes is mediated via an autocrine or paracrine pathway. Treatment of the cells with growth factors or cytokines that prevented spontaneous apoptosis had no major effects on the expression of Fas or FasL. Additionally, monocyte-derived macrophages were found to express both Fas and FasL but did not undergo spontaneous apoptosis and were not sensitive to stimulation by an agonistic anti-Fas IgM. These results indicate that protective mechanisms in these cells exist at a site downstream of the receptor–ligand interaction.
The Fas–Fas ligand (FasL) system is recognized as a major pathway for the induction of apoptosis (programmed cell death) in cells and tissues (for reviews see references 1, 2). Fas (CD95), a type I membrane protein of ∼45 kD, is a member of the TNF-receptor (TNFR) family of proteins (3). FasL is a type II membrane protein of ∼37 kD, belonging to the TNF and CD40 ligand family of proteins (4, 5). Fas is widely expressed in many tissue types, either constitutively or following activation of the cells (6–11). In B and T cells, Fas is expressed at low levels on the surface of resting cells and expression is enhanced after lymphocyte activation (6, 11, 19). In contrast with Fas, the expression of FasL is reported to be much more restricted and often requires cell activation (7, 12–14). Cell surface expression of FasL is very low in resting lymphocytes but can be induced on both T and B cells after activation of the cells (13, 14, 20). The interaction of FasL with Fas on a target cell stimulates an intracellular cascade of events that leads to the induction of apoptosis. Because the expression of FasL appears to be regulated more strictly, the cell surface expression of FasL by the effector cells is thought to be the triggering event in the induction of programmed cell death. The Fas–FasL system has been shown to play a critical role in the development of the T and B cell repertoire (15, 16). Additionally, it has been proposed that target cell killing by CTLs is, in part, mediated through the interaction of FasL on the activated T cell with Fas on the target cell (17–19).
Human monocytes cultured in vitro undergo spontaneous apoptosis without requiring additional external stimuli (21–23). This process can be accelerated and enhanced by the removal of serum. Even in the presence of 20% serum, the majority of monocytes will undergo apoptosis over several days (23); surviving cells differentiate into macrophages. In culture, it is possible to prevent the rapid onset of apoptosis in monocytes by treatment with inflammatory mediators such as TNF, LPS, the ligand to CD40 (CD154), and growth factors and cytokines including GM-CSF and IFN-γ (21–23). Monocytes and macrophages express low but detectable levels of Fas but the role of the endogenous Fas and FasL in the spontaneous apoptosis has not been established. Recent reports have shown that monocytes in medium containing serum can rapidly undergo apoptosis following the ligation of the Fas on the surface of the cells with an agonistic mAb to Fas (mAb CH-11) (9). However, no studies have been presented on the direct role of endogenous FasL in the spontaneous apoptosis of purified monocytes. Furthermore, the regulation of expression of FasL and its role in monocyte and macrophage function has not been explored.
In this report, we show that peripheral monocytes isolated by elutriation express both Fas and FasL and that the onset of apoptosis of human peripheral monocytes in culture is prevented by the addition of a nonstimulatory antiFas mAb, an antagonistic rabbit anti-FasL Ab, or a soluble Fas–Ig fusion protein, all of which block the interaction between Fas and FasL. Therefore, monocytes are able to undergo apoptosis via an autocrine or paracrine mechanism that is dependent on the expression of both Fas and FasL but is independent of another source of FasL, such as activated T cells. In contrast, monocyte-derived macrophages cultured for 7 d in vitro developed resistance to Fas-induced apoptosis despite expressing significant levels of Fas and FasL on the cell surface.
Materials and Methods
Cells.
Peripheral monocytes were prepared from healthy donors as described previously (23) in RPMI containing 2.5 mM EDTA, 10 μg/ml polymyxin B, 100 U/ml penicillin, and 100 μg/ml streptomycin. In brief, the PBMC were separated on Ficoll and the T cells were depleted from the PBMC fraction by rosetting with SRBC. The monocytes were then separated from the remaining PBMC by centrifugal elutriation. After elutriation, the monocytes were collected from the appropriate fractions by centrifugation and then resuspended in RPMI-1640 media with additions as noted in the figure legends. The monocytes isolated by this procedure were >90% pure as measured by staining the cells to determine expression of CD14, CD16, CD19, and CD3. Staining of monocytes for FACS® analysis was performed immediately after isolation. Monocyte-derived macrophages were prepared from peripheral monocytes by culturing the cells in RPMI containing 20% FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml), in Teflon beakers at 37°C for 7 d; half the media was changed after 4 d. Before analysis, dead cells were removed by centrifugation over Histopaque (Sigma Chem. Co., St. Louis, MO).
Reagents and Antibodies.
The Fas–Ig and B7–Ig fusion proteins were constructed essentially as described previously (8, 23, 24), based on published sequences of the extracellular domains (3, 25) together with the constant region of human IgG1. Modified pCDM8 vectors containing the fusion genes were transiently expressed in COS cells for 6 d. The cell supernatants were harvested, clarified by centrifugation, and the fusion proteins then purified on protein A. Soluble recombinant FasL (CD8–FasL) was expressed as a fusion protein consisting of the extracellular domain of murine CD8 fused to the entire extracellular domain of human FasL. CD8–FasL was produced by transient transfection of COS cells and purified on immobilized anti-murine CD8 as described for sCD154 (23). Human GM-CSF, IFN-γ, IL-4, TNF-α, and FITC-labeled annexin V were obtained from R & D Systems (Minneapolis, MN). LPS (Escherichia coli 055:B5) was from Whittaker Bioproducts (Walkersville, MD). The mouse monoclonal anti-Fas IgM and anti-Fas IgG1 were obtained from Immunotech (Westbrook, ME); rabbit anti-FasL polyclonal antibodies (C20 and N20) were obtained from Santa Cruz Labs (Santa Cruz, CA); anti-FasL mAb was from Transduction Laboratories (Lexington, KY); FITC-labeled anti-rabbit and anti-mouse antibodies were from Jackson ImmunoResearch Labs., Inc. (West Grove, PA) or Tago (Burlingame, CA). Human IgG and all other reagents were from Sigma.
Analysis of Cell Surface Antigen Expression.
After the appropriate incubations, the cells were harvested, washed once in 2% FBS– RPMI (staining media) at 4°C, resuspended in 200 μl 2% FBS– RPMI containing 1 mg/ml BSA, and 250 μg/ml human IgG for 20 min. The cells were pelleted and resuspended in PBS containing 2% FBS, before the addition of the primary antibodies (at 10 μg/ml). The cells were incubated for 30 min at 4°C, washed once with staining media, and then incubated with FITC-labeled antimurine IgG or anti-rabbit IgG (1:50 dilution) in staining media for an additional 30 min at 4°C. For single color analysis, the samples were washed twice in PBS and fixed in 2% formaldehyde. In most analyses, the cells were stained both for the uptake of propidium iodide and for specific antigen expression. For this two-color staining, the cells were not fixed; instead, after staining cells with Ab and washing them in PBS, propidium iodide (PI) (5 μg/ml) was added immediately before analysis on a FACScan® analyzer (Becton Dickinson and Co., Mountain View, CA). In the measurement of cell surface antigen expression, the gates of the analyzer were set to exclude PI-positive cells. The FACS® data are reported as mean fluorescence ratios. This represents the mean fluorescence determined using the Fas or FasL Ab divided by the mean fluorescence of the control Ab for each treatment of the monocytes.
Assay of Apoptosis and DNA Fragmentation.
Monocytes were isolated by elutriation and cultured at 1 × 106/ml in RPMI containing 500 ng/ml polymyxin B together with no further additions, or with the agents as indicated in the text. After these incubations, the cells were harvested by centrifugation and then either permeabilized with buffer containing sodium citrate (0.3%), Triton X-100 (0.01%), and PI (50 μg/ml) for FACS® analysis or assayed for cell viability by measuring the exclusion of Trypan blue. Alternatively, the binding of FITC–annexin V was used to follow the expression of phosphatidylserine on early apoptotic cells (8, 26). The staining was carried out essentially according to the manufacturer's instructions. After the appropriate incubations cells, 5 × 105/500 μl, were incubated with saturating concentrations of FITC– annexin V for 30 min at room temperature; the cells were then immediately analyzed by FACS®.
Results
Expression of Cell Surface Fas and FasL on Human Monocytes and Macrophages.
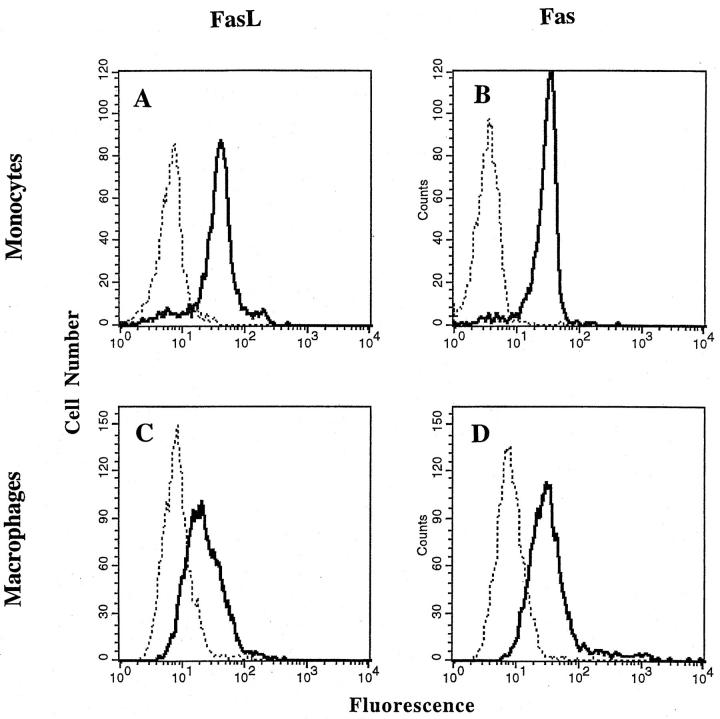
To evaluate the expression of Fas and FasL on the cell surface, monocytes were isolated by elutriation and analyzed immediately; human macrophages were derived from peripheral monocytes by culturing the cells for 7 d in Teflon culture dishes. The different cells were then analyzed, without fixation, by FACS®. The results showed that freshly isolated monocytes and cultured monocyte-derived macrophages expressed both Fas and FasL on the cell surface (Fig. 1), although the levels of both Fas and FasL were slightly lower in the macrophages (Fig. 1, C and D). In the analysis of cells from seven different donors the ratio of specific mean fluorescence of Ab to Fas relative to the control Ab was 8.4 ± 0.7 and 5.5 ± 1.7 in monocytes and macrophages, respectively. For FasL, the ratio of specific mean fluorescence to the control was 5.7 ± 0.5 and 4.7 ± 1.0 in monocytes and macrophages, respectively.
Figure 1.
Cell surface staining of Fas and FasL on monocytes and monocyte-derived macrophages. Dotted line, control antibodies; solid line, Fas or FasL Abs as indicated. Peripheral monocytes were isolated by elutriation and then stained immediately (A and B), or cultured for 7 d in Teflon dishes (C and D) and then stained for cell surface expression of FasL (A and C, using anti-FasL polyclonal Ab, C20) and Fas (B and D, using anti-Fas, murine IgG1) as described in Material and Methods. Staining with control antibodies was carried out using mouse IgG1 or rabbit IgG as appropriate. The histograms are from a single experiment representative of seven experiments with cells from different donors.
Role of Endogenous FasL in the Spontaneous Apoptosis of Human Monocytes.
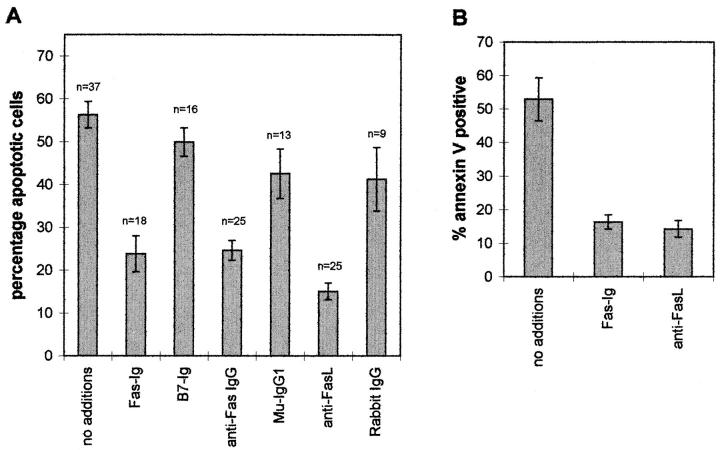
In the absence of serum, freshly isolated human monocytes rapidly undergo programmed cell death (21, 22); even in the presence of serum a significant proportion of the cells die (23). Studies were undertaken to determine the role of FasL in this spontaneous death of the cells. Peripheral monocytes were isolated and then immediately placed into culture for 18 h in serum-free RPMI alone, with the soluble fusion protein Fas–Ig, or with blocking anti-Fas or anti-FasL antibodies. The spontaneous apoptosis was markedly reduced by culturing the monocytes with Fas–Ig but not by the control fusion protein B7– Ig (Fig. 2 A). An antagonistic mouse anti-Fas mAb (IgG1) also protected the cells to a similar extent, whereas control mouse IgG1 gave rise to only marginal protection. The polyclonal anti-FasL antibody (C20) was the most effective at inhibiting the spontaneous apoptosis of the peripheral monocytes; normal rabbit IgG yielded little protection (Fig. 2 A).
Figure 2.
Effect of fusion proteins and antibodies on spontaneous apoptosis of peripheral monocytes. (A) Staining of nuclei with PI. (B) Staining of the cell surface with annexin V. Elutriated peripheral monocytes were cultured at 1 × 106/ml in polypropylene tubes in RPMI media containing 1 μg/ml polymyxin B together with no additions, 50 μg/ml Fas–Ig, 50 μg/ml B7.1–Ig, 10 μg/ml anti-Fas IgG, 10 μg/ml muIgG1, 10 μg/ml rabbit anti-FasL (C20) or 10 μg/ml rabbit IgG. For PI staining, the cells were harvested by centrifugation after 18 h at 37°C ; they were then analyzed for apoptotic nuclei with PI as described in Materials and Methods. n, the number of different donors in each treatment set. For staining with annexin V the cells were harvested after 8 h in culture, washed, and then stained for 30 min with FITC–annexin V. The bars represent the mean ± SEM of the number (n) of different donors.
One of the early changes in cells undergoing apoptosis is the exposure of phosphatidyl serine on the surface of the cells, which can be detected by the ability of the phospholipid to bind annexin V (8, 26). FACS® analysis of changes in the phospholipids in the plasma membrane of the monocytes using FITC–annexin V confirmed the onset of apoptotic responses observed in Fig. 2 A. After 8 h in culture, 50–60% of the peripheral monocytes bound annexin V, whereas culture of the cells in Fas–Ig or anti-FasL reduced the number of positive staining cells to less than 20% (Fig. 2 B). These results indicate that the autocrine or paracrine interaction of Fas and FasL is largely responsible for the spontaneous induction of programmed cell death that occurs upon culture of peripheral monocytes in serum-free media.
Effect of Cytokines on Apoptosis and Fas and FasL Expression.
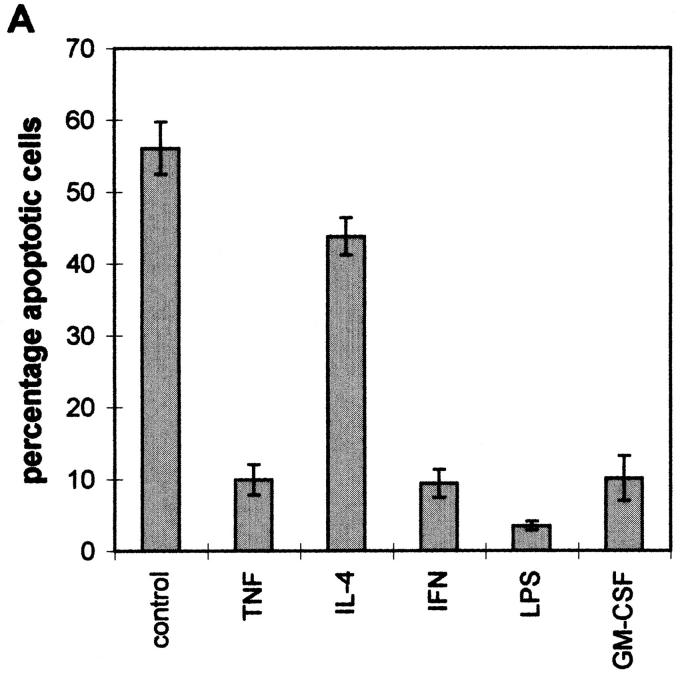
It has previously been shown that different cytokines, growth factors, and LPS can prevent the onset of apoptosis in human monocytes (9, 21, 22). In agreement with these earlier findings, we found that IFN-γ, TNF-α, GM-CSF, and LPS all effectively reduced the degree of apoptosis of monocytes when added at the initiation of the cell culture (Fig. 3 A). In addition, IL-4 alone appeared to provide slight protection from apoptosis (Fig. 3 A), but, as reported previously (21, 22), it was able to abrogate most of the rescue of the cells mediated by TNF (data not shown). Over the time course of these incubations (18 h), no major changes in the level of cell surface expression of Fas or FasL (Fig. 3, B and C) were observed. However, TNF and LPS did appear to induce a small decrease in the level of expression of FasL, and IL-4 induced a small increase in the expression of FasL. These results suggest that while the Fas– FasL interaction is essential for the spontaneous induction of programmed cell death in monocytes, the effect of cytokines, growth factors, and LPS on the apoptotic process is at a site downstream of the cell surface receptors.
Figure 3.
Effect of IFN-γ, IL-4, GM-CSF, TNF-α, and LPS on the Fas apoptotic pathway in peripheral monocytes. (A) Apoptosis. Elutriated peripheral monocytes were cultured at 1 × 106/ml in polypropylene tubes in RPMI media containing 1 μg/ml polymyxin B together with no additions, 10 ng/ ml TNF-α, 500 U/ml IL-4, 500 U/ml IFN-γ, 5 μg/ml LPS or 10 ng/ml GM-CSF. After 18 h at 37°C, the cells were harvested by centrifugation and analyzed for apoptotic nuclei as described in Materials and Methods. (B and C) Cell surface expression of Fas (B) and FasL (C). Elutriated peripheral monocytes were cultured at 1 × 106/ml in polypropylene tubes in RPMI media containing 1 μg/ml polymyxin B together with no additions, 500 U/ml IFN-γ, 10 ng/ml GM-CSF, 500 U/ml IL-4, 5 μg/ml LPS or 10 ng/ml TNF-α. After 18 h, the cells were harvested and stained for cell surface expression of Fas (anti-Fas, murine IgG1) and FasL (anti-FasL, polyclonal Ab, C20) as described in Materials and Methods. Staining with control antibodies was carried out using mouse IgG1 or rabbit IgG as appropriate. The data are expressed as ratio of the mean fluorescence intensity (mfi) of Ab against the specific antigen, relative to the mfi of control Abs for each treatment. The data represent mean ± SEM of four independent experiments.
Anti-Fas–induced Apoptosis in Monocytes and Monocytederived Macrophages.
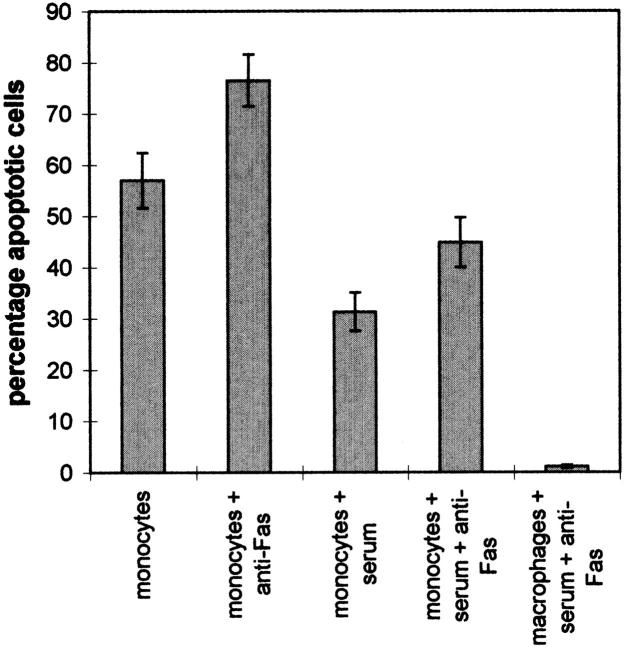
Spontaneous apoptosis was evident in peripheral monocytes, even when the cells were cultured in the presence of 20% FBS (Fig. 4). As shown here (Fig. 4) and reported previously (9), monocyte apoptosis in either the absence or presence of serum significantly increased after the addition of a stimulatory anti-Fas mAb to the cells. In contrast, human monocyte-derived macrophages were completely resistant to anti-Fas (Fig. 4) or sFasL-induced (data not shown) apoptosis. Thus, despite expressing significant levels of Fas and FasL on the cell surface (see Fig. 1), monocyte-derived macrophages were resistant to both spontaneous and anti-Fas–induced apoptosis.
Figure 4.
Induction of apoptosis in peripheral monocytes and monocyte-derived macrophages. Elutriated peripheral monocytes were cultured for 18 h alone or with 20% FBS, in the absence or presence of 200 ng/ml anti-Fas mAb (CH-11). Monocyte-derived macrophages were cultured for 7 d in RPMI containing 20% FBS and then harvested, washed once, and then resuspended in RPMI containing 20% FBS. The cells were then cultured for an additional 18 h in the presence of 200 ng/ml anti-Fas mAb. No spontaneous apoptosis of these cells was observed over the final 18 h. Cells were harvested by centrifugation and analyzed for apoptotic nuclei with PI. The bars represent the mean ± SEM of eight (monocytes) or six (macrophages) experiments with different donors.
Discussion
While the function of the Fas–FasL system in the regulation of apoptosis in lymphocytes is well established (7, 11, 14), its role in other leukocytes is less well established, though recent reports suggest that here too it may play a crucial function (8, 27, 28). Fas is constitutively expressed on other phagocytes (8–10, 28) and stimulation of Fas with an agonistic mAb induces apoptosis in neutrophils and eosinophils (8, 28). Additionally, it has been shown that the neutrophils, like activated T cells, can release biologically active soluble FasL (7, 8). Furthermore, upon isolation and culture in vitro, neutrophils undergo programmed cell death (8) mediated by interactions of Fas and FasL on the cells. It has been proposed that this apoptotic response of leukocytes in vitro reflects one of the normal mechanisms for the elimination of the cells in vivo. During an inflammatory response the eosinophils or neutrophils are recruited into the tissue. Later, the subsequent resolution of the inflammation requires that the leukocytes are removed from the site and this may be mediated by apoptosis.
Monocytes undergo spontaneous apoptosis (9, 21–23). This cell death can be inhibited by treatment of the monocytes with LPS, TNF-α, GM-CSF, IFN-γ, and sCD154 (21–23). Monocytes express Fas, but the reports vary considerably on the extent to which the cells are sensitive to apoptosis induced by the anti-Fas mAb (9, 10, 29). In addition, treatment of monocytes with inflammatory mediators such as TNF-α and IL-1β partially protects the cells from anti-Fas–induced apoptosis whereas LPS pretreatment fully protects the cells (9, 21, 22). These treatments are reported not to alter the level of Fas (9). Thus, both growth and proinflammatory factors enhance the survival of monocytes. The role of FasL in the spontaneous apoptosis of monocytes has not been explored. Furthermore, the effect of inflammatory mediators and growth factors on FasL expression is not known.
In the work reported here, we show that resting monocytes constitutively express FasL on the cell surface. This expression was not readily detected in an earlier study using the fusion protein Fas–Ig to detect FasL (9) due to the lower affinity of the reagent and due to high background binding of the Ig protein to the Fc receptors. In addition, our results indicate that spontaneous apoptosis of monocytes in vitro is mediated by the interaction of Fas and FasL on the cell surface, because apoptosis could be suppressed by reagents that block the interaction of Fas and FasL. These agents included a Fas–Ig soluble fusion protein and antagonistic antibodies to Fas or FasL. Because of the inability of any of these agents to provide complete protection from death, we cannot rule out that other mechanisms may also contribute to the induction of spontaneous apoptosis.
However, it is clear that other factors beyond the expression of Fas and FasL on the cell surface also ultimately contribute to the sensitivity of monocytes to the apoptotic pathway (27–29). We found that treatment of peripheral monocytes with TNF-α, IFN-γ, GM-CSF, or LPS, agents that protect the cells from spontaneous apoptosis, did not markedly alter the expression of either Fas or FasL. In addition, despite the expression of both Fas and FasL on the surface, monocyte-derived macrophages were able to survive 7 d in culture and were also resistant to direct stimulation of Fas with the agonistic anti-Fas mAb. This is in contrast with a report where differentiation of monocytes by adherence to plastic enhanced their sensitivity to the mAb (29). The pathways that give rise to protection of these cells are currently being explored.
The presence of FasL on the cell surface of both monocytes and macrophages suggests that the ligand may play a role, other than regulating self-apoptosis, in the effector functions of the cell. This has also recently been proposed for neutrophils that were found to both express FasL on the cell surface and also release biologically active sFasL in the cell supernatants (8). In preliminary experiments, we have found that it is possible to stimulate monocytic cells to release biologically active sFasL (data not shown). Together, these results indicate that it is very likely that FasL plays a very important role in mediating some of the physiological functions of monocytes and macrophages.
Footnotes
W.C. Liles is a Pfizer Postdoctoral fellow.
References
- 1.Nagata S. Fas and Fas ligand: a death factor and its receptor. Adv Immunol. 1996;57:129–144. doi: 10.1016/s0065-2776(08)60672-0. [DOI] [PubMed] [Google Scholar]
- 2.Nagata S, Golstein P. The Fas death factor. Science (Wash DC) 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 3.Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Human Fas ligand: gene structure, chromosomal location and species specificity. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 5.Suda T, Nagata S. Purification and characterization of the Fas-ligand that induces apoptosis. J Exp Med. 1994;179:873–879. doi: 10.1084/jem.179.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki T, Uehara T, Nibu R, Tsuji T, Yachie A, Yonehara S, Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992;149:3753–3758. [PubMed] [Google Scholar]
- 7.Tanaka M, Suda T, Takahashi T, Nagata S. Expression of the functional soluble form of human Fas ligand in activated lymphocytes. EMBO (Eur Mol Biol Organ) J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Um H-D, Orenstein JM, Wahl SM. Fas mediates apoptosis in human monocytes by a reactive oxygen intermediate dependent pathway. J Immunol. 1996;156:3469–3477. [PubMed] [Google Scholar]
- 10.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas–mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 11.Schattner E, Friedman SM. Fas expression and apoptosis in human B cells. Immunol Res. 1996;15:246–257. doi: 10.1007/BF02918252. [DOI] [PubMed] [Google Scholar]
- 12.Suda T, Takahashi T, Golstein P, Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 13.Hahne M, Renno T, Schroeter M, Irmler M, French L, Bornand T, MacDonald HR, Tschopp J. Activated B cells express functional Fas ligand. Eur J Immunol. 1996;26:721–724. doi: 10.1002/eji.1830260332. [DOI] [PubMed] [Google Scholar]
- 14.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 15.Ramsdell F, Seaman MS, Miller RE, Tough TW, Alderson MR, Lynch DH. gld/gldmice are unable to express a functional ligand for Fas. Eur J Immunol. 1994;24:928–933. doi: 10.1002/eji.1830240422. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 17.Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T-cell mediated cytotoxicity. Science (Wash DC) 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 18.Lowin B, Hahne M, Mattmann C, Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (Lond) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 19.Anel A, Simon AK, Auphan N, Buferne M, Boyer C, Golstein P, Schmitt-Verhulst AM. Two signaling pathways can lead to Fas ligand expression in CD8+cytotoxic T lymphocyte clones. Eur J Immunol. 1995;25:3381–3387. doi: 10.1002/eji.1830251227. [DOI] [PubMed] [Google Scholar]
- 20.Nishimura Y, Ishii A, Kobayashi Y, Yamasaki Y, Yonehara S. Expression and function of mouse Fas antigen on immature and mature T cells. J Immunol. 1995;154:4395–4403. [PubMed] [Google Scholar]
- 21.Managan DF, Welch GR, Wahl SM. Lipopolysaccharide, tumor necrosis factor-α, and IL-1β prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991;146:1541–1546. [PubMed] [Google Scholar]
- 22.Managan DF, Wahl SM. Differential regulation of human monocyte programmed cell death (apoptosis) by chemotactic factors and pro-inflammatory cytokines. J Immunol. 1991;147:3408–3412. [PubMed] [Google Scholar]
- 23.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 24.Hollenbaugh D, Aruffo A. Construction of immunoglobulin fusion proteins. Curr Prot Immunol. 1994;2:10–19. doi: 10.1002/0471142735.im1019as48. [DOI] [PubMed] [Google Scholar]
- 25.Freeman GJ, Freedman AS, Segil JM, Lee G, Whitman JF, Nadler LM. B7, a new member of the Ig superfamily with unique expression on activated and neoplastic B cells. J Immunol. 1989;143:2714–2722. [PubMed] [Google Scholar]
- 26.Martin SJ, Reutelingsperger CPM, McGahon AJ, Rader JA, van Schie RCAA, LaFace DM, Green DR. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J Exp Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Squier MKT, Sehnert AJ, Cohen JJ. Apoptosis in leukocytes. J Leukoc Biol. 1995;57:2–10. doi: 10.1002/jlb.57.1.2. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Schleimer RP, Saito H, Likura Y, Bochner BS. Induction of apoptosis in human eosinophils by anti-Fas antibody treatment in vitro. Blood. 1995;86:1437–1443. [PubMed] [Google Scholar]
- 29.Richardson BC, Lalwani ND, Johnson KJ, Marks RM. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]