Abstract
CD81 is a cell surface molecule expressed on many cell types and associated with the CD19/ CD21/Leu13 signal-transducing complex on B cells. A recent report implies that CD81 expression on thymic stromal cells is important in the maturation of thymocytes from CD4− CD8− to CD4+CD8+. However, we have produced CD81-null mice by gene targeting, and find that they undergo normal development of thymocytes and express normal numbers of T cells. B cells are also found in normal numbers in the spleen, blood, and peritoneal cavity of CD81-null mice, but they express a lower level of CD19 compared to heterozygous littermates. Finally, early antibody responses to the protein antigen ovalbumin are weaker in CD81null mice compared to their heterozygous littermates. This is consistent with the proposed role of the CD19/CD21/CD81-signaling complex in lowering the threshold for B cell responses. These results show that CD81 is not required for maturation of T cells, but is important for optimal expression of CD19 on B cells and optimal stimulation of antibody production.
CD81 was first identified as the target of an anti-proliferative antibody (TAPA-1) (1) to a B lymphoma cell line. It is a member of the tetraspanin, or transmembrane 4 superfamily (TM4SF), which also includes CD9, CD37, CD53, CD63, CD82, and an expanding number of other proteins. Many of these molecules are present on leukocytes and are involved in signal transduction, cell–cell adhesion, and cellular activation or development (reviewed in reference 2).
As part of a complex on B cells that includes CD19, CD21, and Leu13 (3, 4), CD81 can provide costimulatory signals that lower the threshold required for B cells to respond to antigen (5). Also, CD81 ligation on human B cells can deliver a signal that increases interleukin (IL)-4 synthesis by T cells (6). Thus, CD81 may be important for the development of Th2 immune responses.
In addition to its potential functions on B cells, a recent report implicates a role for CD81 in T cell development as well (7). Boismenu et al. (8) found that an antibody to mouse CD81 could inhibit maturation of thymic pre–T cells from CD4−CD8− to CD4+CD8+. Furthermore, CD81transfected fibroblasts were sufficient to induce maturation of double-negative into double-positive thymocytes in reaggregation cultures. Thus, it was predicted that CD81 may be necessary for the development of thymocytes beyond the double-negative stage. Another report shows that CD81 cross-linking can be costimulatory with signals through the T cell receptor complex on human thymocytes (8). Thus, CD81 might be predicted to be important for later stages of thymocyte development as well.
In this report, we have generated CD81-null mice by gene targeting. Surprisingly, these mice appear to undergo normal thymic development and produce normal numbers of mature T cells. However, CD81-null B cells express lower levels of CD19 and show decreased early antibody production in response to a protein antigen, indicating that CD81 is important for the development of humoral immune responses.
Materials and Methods
Production of Targeting Vector.
A vector for gene targeting, pNT (9), was kindly provided by Greg Barsh (Stanford University, Stanford, CA). Genomic clones containing CD81 sequences were screened and selected from a bacteriophage P1 library by Genome Systems (St. Louis, MO). From one of these P1 clones, an 8-kb segment from the 5′ region of CD81 and a 1.8-kb segment from the 3′ region of CD81 were cloned into the pNT plasmid (Fig. 1 A).
Figure 1.
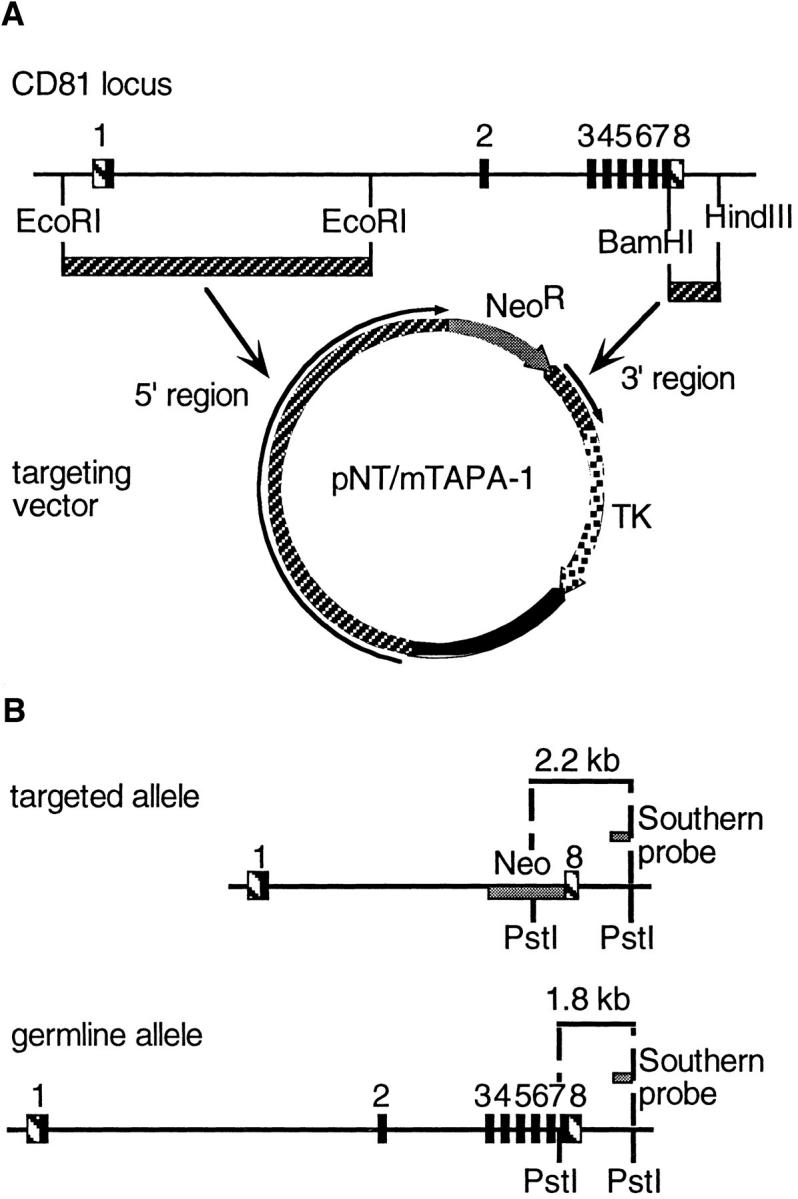
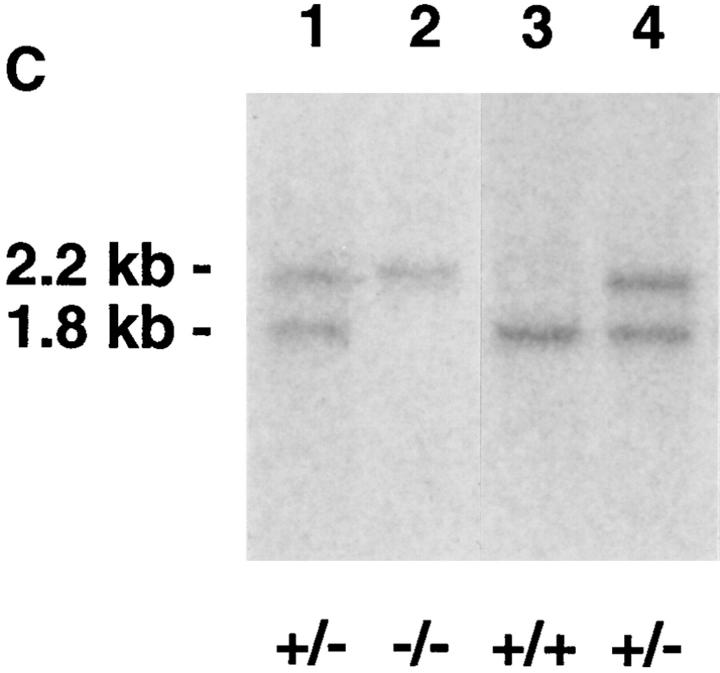
(A) Strategy for production of a CD81-targeting vector. 5′ and 3′ sequences of CD81 were cloned into pNT, such that the NeoR gene would replace exons 2–8 of CD81. The thymidine kinase (TK) gene provided negative selection by conferring gancyclovir sensitivity to those cells that took up the plasmid but did not undergo homologous recombination with the CD81 locus to remove the TK gene. (B) A probe for Southern blotting was designed to detect the difference, in a PstI digest, of DNA from a targeted allele of CD81 (2.2-kb band) versus a germline allele (1.8-kb band). (C) Southern blot of tail DNA from pups derived by crossing two CD81+/− mice. Two pups were heterozygous (+/−), one was wild type (+/+), and one was CD81-null (−/−).
Transfection of Embryonic Stem Cells.
Early passage (P10) embryonic stem (ES) cells of the R1 cell line (10) were kindly provided by Andras Nagy (Mount Sinai Hospital, Toronto, Canada). ES cell growth and gene targeting was done using standard methods as outlined in Joyner et al. (11). In brief, R1 cells were transfected with the modified pNT plasmid (pNT.mTAPA-1) by electroporation, then plated on feeder layers of mouse embryonic fibroblasts (MEF; Genome Systems) and grown under positive/ negative selection (200 μg/ml G418, 2 μM gancyclovir in DMEM medium containing 15% fetal bovine serum (Intergen, Purchase, NY), 100 μM nonessential amino acids, 1 mM sodium pyruvate, 100 μg/ml gentamycin, and 1,000 U/ml leukemia inhibitory factor (GIBCO BRL, Gaithersburg, MD). After 10 d, visible colonies were picked with a pipette tip, trypsinized, and plated in 24well plates with fresh MEF feeders. After growth in 24-well plates, an aliquot of each clone was frozen, while a second aliquot was expanded and used for purification of DNA using DNAStat 60 (Tel-Test “B”, Inc., Friendswood, TX).
Selection of ES Clones and Blastocyst Injection.
A probe for Southern blotting, external to the region of CD81 included in pNT.mTAPA-1, was chosen as shown in Fig. 1 B. The BamHI– HindIII fragment was cloned into pUC19, and re-isolated by restriction digestion and gel extraction (Qiagen Gel Extraction Kit; Qiagen Corp., Chatsworth, CA). The probe was labeled using the Random Primers DNA Labeling kit from GIBCO BRL. Southern blotting was carried out with ZetaProbe nylon membrane (BioRad Corp., Hercules, CA), using alkaline blotting and standard hybridization protocols recommended by the supplier. Out of 90 clones screened, 4 clones were chosen which had undergone homologous recombination. 3 of these were used for microinjection into C57Bl/6 blastocysts and reimplantation into pseudopregnant hosts, done by the ES Cell Gene Altered Mouse Service (University of Cincinnati, Cincinnati, OH). One line yielded two germline-transmitting chimeras. Agouti offspring of these chimeras were bred and screened for homozygosity by Southern blot, performed as above on tail clips prepared using the QiaAmp Tissue Kit (Qiagen). Homozygous (CD81−/−), and heterozygous (CD81+/−) littermates (see Fig. 1 C) were chosen for experiments comparing cell development and immune responses.
Flow Cytometry of Lymphocytes.
Thymus and spleen were harvested from four to five week old mice and cells extracted with a tissue homogenizer. Peripheral blood was obtained by tail bleeding and peritoneal cavity cells by peritoneal lavage (12). All cells were subjected to hypotonic lysis of red cells by 15 min incubation in 0.144 M NH4Cl + 0.017 M Tris, pH 7.2, followed by washing in PBS/BSA and staining for flow cytometry using standard conditions (12). Labeled antibodies were as follows: anti–CD4-FITC, anti–CD3-FITC, B220-FITC (Caltag Corp., South San Francisco, CA); anti–CD4-PE, anti–CD8-PE (Biosource International, Camarillo, CA); anti–CD19-PE (PharMingen Corp., San Diego, CA); and anti-mouse CD81-biotin (2F7; a gift of Wendy Havran, University of California, San Diego). Streptavidin-PE (Becton Dickinson & Co., Mountain View, CA) was used as a second step for the 2F7-biotin. Irrelevant mouse IgG1-FITC and IgG1-PE (Becton Dickinson) were used as negative controls.
Immunization and Serum ELISA.
Analysis of pre-immune serum isotypes was done by ELISA, using anti-mouse Ig (5 μg/ml; Southern Biotechnology Associates, Birmingham, AL) to coat microtiter plates, followed by serial dilutions of whole serum, and then isotype-specific detector antibodies (all at 1:5,000 dilution; Southern Biotech.). CD81-null mice and heterozygous littermates were injected intraperitoneally with 100 μg ovalbumin precipitated in alum at ∼4 wk of age. They were bled and serum analyzed for anti-ovalbumin antibodies using ELISA as described (12a) at 4, 8, and 12 wk post-immunization, and again 18 d after a second injection as above. Concentrations were determined by comparison to mouse anti-ovalbumin serum of known concentration, or monoclonal anti-ovalbumin antibodies of IgG1 or IgG2a isotype (for isotype-specific ELISA).
Results and Discussion
Generation of CD81-null Mice.
CD81−/− mice were generated by standard gene-targeting techniques, as described in Materials and Methods (see Fig. 1 A). Homozygous (CD81−/−) and heterozygous (CD81+/−) littermates were determined by Southern blotting (Fig. 1, B and C). CD81null mice were born in the expected Mendelian ratios, and developed without gross anatomic abnormalities, morbidity, or mortality. Thus, although CD81 is expressed in most tissues (1, 13, 14) and is present even on pre-implantation embryos (15), it is not necessary for embryonic development or viability of mice.
T Cell Development in CD81-null Mice.
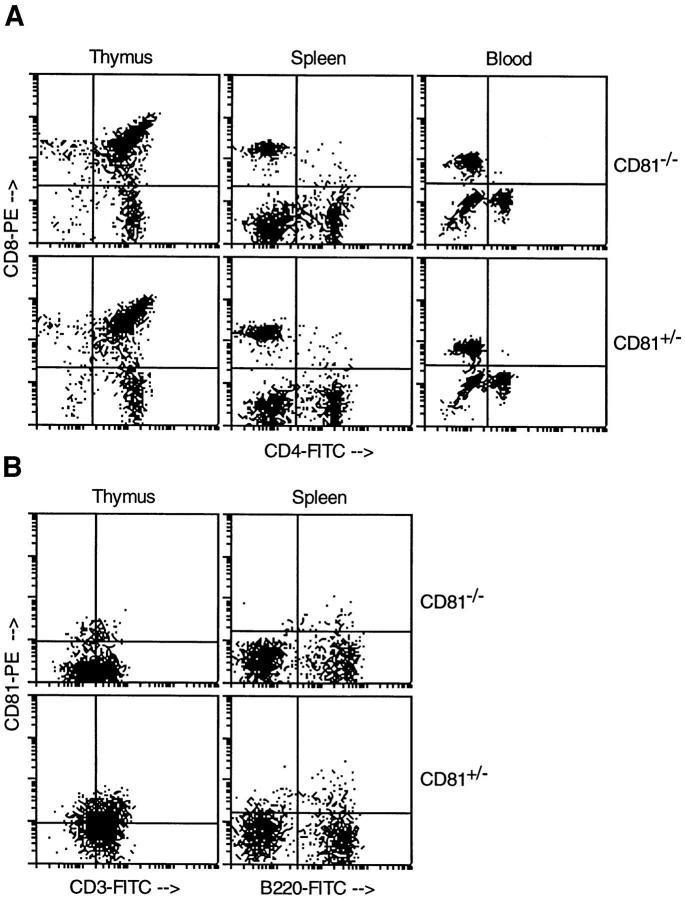
Several CD81−/− and CD81+/− littermates were killed at 4–5 wk of age and their thymus, spleen, blood, and peritoneal cavity cells were analyzed by flow cytometry. Heterozygous animals were used as controls since their expression of CD81 was comparable to wild-type mice (data not shown). The thymus and spleen of CD81-null animals were not significantly different in size from those of heterozygotes, and lymphocyte counts were not significantly different from normal (not shown). As shown in Fig. 2 A and Table 1, normal ratios of CD4−CD8−, CD4+CD8+, and single positive thymocytes were observed in the thymuses of knockout animals. T cells also migrated to spleen and peripheral blood in normal numbers, indicating no block in T cell development due to lack of CD81 (Table 1).
Figure 2.
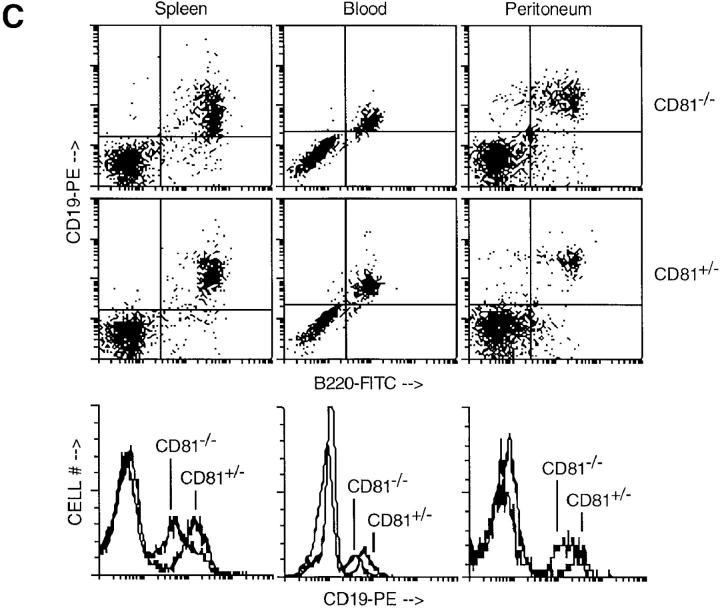
Flow cytometry analysis of lymphocytes from CD81−/− and CD81+/− mice. (A and B) T cell development was normal in CD81−/− mice, as shown by normal CD4/CD8 and CD3 staining. CD81 staining revealed low-level expression on CD81+/− thymocytes and a small population of B220+ splenocytes. (C) B cell development was normal in CD81−/− mice, except that CD19 staining intensity was decreased compared to heterozygous animals (see histogram overlays). These data are representative of at least three independent experiments.
Table 1.
Percentages of CD4/CD8 Subsets in Thymus, Spleen, and Blood of CD81−/− Versus CD81+/− Mice
| Compartment | Cell phenotytpe | CD81−/−≳ | CD81+/−≳ | |||
|---|---|---|---|---|---|---|
| Thymus | CD4−CD8− | 1.1 ± 0.4 | 1.2 ± 0.2 | |||
| CD4+CD8+ | 79.4 ± 15.8 | 79.3 ± 17.7 | ||||
| CD4+CD8− | 16.0 ± 12.6 | 16.5 ± 14.9 | ||||
| CD4−CD8+ | 3.5 ± 2.8 | 3.0 ± 2.6 | ||||
| Spleen | CD4−CD8− | 75.5 ± 3.5 | 75.2 ± 11.2 | |||
| CD4+CD8− | 12.8 ± 4.7 | 13.6 ± 4.0 | ||||
| CD4−CD8+ | 11.2 ± 1.0 | 10.8 ± 6.9 | ||||
| Blood | CD4−CD8− | 40.8 ± 18.5 | 30.7 ± 16.6 | |||
| CD4+CD8− | 25.7 ± 15.3 | 19.1 ± 10.6 | ||||
| CD4−CD8+ | 34.5 ± 9.7 | 49.6 ± 13.9 |
Mean ± SD is given for groups of 3–6 animals of each type, analyzed in two or more independent experiments.
In Fig. 2 B, the expression of CD3 in thymus was analyzed and no defect in the number of CD3+ cells or the level of CD3 expression was seen. This is surprising in light of the recent findings of Boismenu et al. (7), which implied a role for CD81 in the development of CD4+CD8+ thymocytes from CD4−CD8− precursors. Thus, although CD81 expression may induce the maturation of CD4−CD8− thymocytes, our data clearly show that CD81 is not necessary for this process to occur. Also, Todd et al. (8) have shown that CD81 may function as a costimulatory molecule on human CD3+ thymocytes. However, our data imply that this function is not essential for maturation of normal numbers of T cells in mice.
Expression of CD81 on Lymphoid Cells.
Using an antibody to mouse CD81, we also showed that a large percentage of CD81+/− thymocytes express CD81 (Fig. 2 B), as do wild-type thymocytes (not shown). The low-level staining using the 2F7 antibody to mouse CD81 can be judged to be specific by comparison to CD81−/− cells, where only a small amount of background staining was seen (Fig. 2 B). The level of CD81 staining in wild-type and heterozygous animals was comparable (data not shown). Expression of CD81 on mouse thymocytes is analogous to the findings of others in human thymocytes (8, 16).
A small proportion of splenic B220+ cells may also express CD81 in heterozygous (Fig. 2 B) and wild-type mice (not shown). Human B cells all express CD81 as detected by antibodies, and germinal center B cells express the highest levels of CD81. Murine splenocytes also express mRNA for CD81 (13,14), but most do not stain detectably with the 2F7 antibody. However, by analogy to humans, the CD81 antibody-positive cells observed in mouse spleen (Fig. 2 B) may be germinal center B cells.
B Cell Development in CD81-null Mice.
Fig. 2 C shows analyses of mouse spleen, peripheral blood, and peritoneal cavity cells from CD81−/− and CD81+/− mice using antibodies to B cell–specific markers, B220 and CD19. Normal numbers of splenic and peripheral blood B cells expressing B220 and CD19 were seen in the CD81-null mice. However, the intensity of CD19 staining was slightly higher in heterozygous compared to CD81-null animals in each compartment (see histogram overlays, Fig. 2 C). Since CD81 is part of a B cell–specific complex which includes CD19 (17, 18), these results suggest that lack of CD81 impairs the expression of this complex on the B cell surface.
CD19 is known to be important for the development and self-renewal of B-1 cells in the peritoneal cavity (19– 21). We examined B-1 cells (CD5+B220+ or CD5+IgM+) in the peritoneal cavity of CD81−/− versus CD81+/− mice, and found these cells to be expressed in normal numbers (data not shown). Thus, the lower level CD19 expression in CD81−/− animals does not preclude the development of B-1 cells.
Humoral Immune Response in CD81-null Mice.
Because CD19 increases the efficiency of B cell responses, and because of the lowered expression of CD19 on CD81-null B cells, one might predict an impairment in humoral responses in CD81-null mice. This might be particularly true in the case of weak or limiting amounts of antigen, since the CD81-null mice might have a higher threshold for B cell responses. Also, a recent study found that CD81 triggering on human B cells from allergic donors increased IL-4 synthesis by allergen-specific T cells (6). Thus, another prediction for CD81-null mice would be that they might produce less IL-4 during immune responses, and thus have impaired Th2 responses.
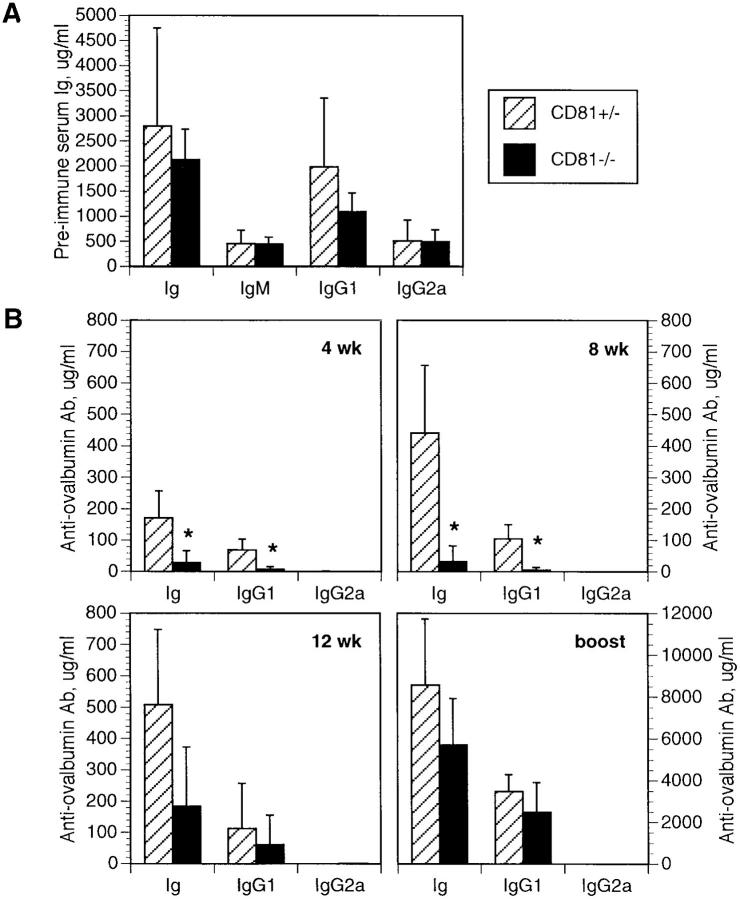
Because of these two predictions, we tested the immune response of CD81-null versus heterozygous mice to a weak, Th2-inducing antigen, ovalbumin precipitated in alum. CD81- null mice did not differ significantly in their pre-immune serum IgM, IgG1, and IgG2a, or total Ig levels (Fig. 3 A). However, they did produce significantly (P ⩽0.0005) less total Ig and IgG1 antibodies specific to ovalbumin at 4 and 8 wk after immunization with 100 μg ovalbumin in alum (Fig. 3 B). This difference was less apparent at 12 wk postimmunization, and was insignificant after boosting with a second injection of ovalbumin in alum. Thus, there is a transient defect in humoral immunity in the CD81-null mice, consistent with a defect in signaling through the CD19/CD21/ Leu13 complex. This defect appears to be overcome with prolonged or repeated exposure to the antigen.
Figure 3.
(A) Preimmune levels of serum Ig, IgM, IgG1, and IgG2a were not significantly different between CD81+/− and CD81−/− mice. Error bars represent the standard deviation of 4–6 animals per group. (B) The humoral response to a single injection with ovalbumin in alum was measured at 4, 8, and 12 wk post-immunization, and 18 d post-boost. Levels of total Ig and IgG1 were significantly lower in CD81−/− mice at 4 and 8 wk (*P ⩽0.0005), but high levels of IgG2a were not produced, indicating there was not a significant shift to a Th1-dominated immune response. The differences between CD81+/− and CD81−/− antibody levels were not significant at 12 wk, and disappeared completely after boosting, indicating that the defect in antibody production was transient, and could be overcome by repeated exposure to the antigen. Note the different scale after boosting. Error bars represent the standard deviation of 9–10 animals per group. The data shown are representative of three independent experiments.
After boosting and in vitro stimulation with ovalbumin protein, IL-4 and IFN-γ synthesis from splenocytes was not different in CD81-null mice compared to controls (not shown). There was also no significant difference in IgG2a levels between groups at any time point (Fig. 3 B), suggesting that these mice did not substitute a Th1 response for a Th2 response. Thus, although CD81 signaling may lead to IL-4 production, our data argues that a Th2-dominated immune response can still be mounted in the absence of CD81. It is of course still possible that early levels of IL-4 synthesis (which we were unable to measure by ELISA) may be lower in CD81-null mice, or that other immunization schemes may show a difference in cytokine secretion between CD81-null and control animals.
CD19-deficient mice (19, 20) and CD21-deficient mice (22, 23) both have severely defective antibody responses. Lack of CD81, which is part of the same signal transduction complex on B cells, confers a similar but more subtle phenotype, since CD81-null mice recover antibody production with time and with repeated antigen exposure.
In summary, we have shown that CD81-null animals undergo normal T cell maturation, despite the suspected role(s) for CD81 expression in thymocyte development (7, 8). CD81-null mice also have normal B cell development, but express lower CD19 levels. This latter defect probably accounts for their impaired antibody production in response to immunization with ovalbumin in alum, since the defect is transient and is not reflected in noticeably impaired IL-4 production or a significant Th1 bias. In conclusion, CD81 is likely to have a greater role in the control of immune responses than in the development of immune cells.
Acknowledgments
The authors thank John Duffy (University of Cincinnati) for his expertise in blastocyst injection, and Greg Barsh, Lutz Hein, Andrzej Chruscinski, and Scott Todd (Stanford University) for providing invaluable advice and reagents at various stages in the project. We especially thank Ronald Levy (Stanford University) for continual support and helpful discussions.
This work was supported by Public Health Service grant CA 34233 from the National Institutes of Health. H.T. Maecker is supported by a National Research Service Award from the National Institutes of Health.
Footnotes
Note added in proof. After analysis of anti-ovalbumin responses in more animals, we have found that many CD81-null mice do not recover normal autibody titers, even after boosting. Thus, the humoral response in CD81-null mice may be even more impaired than suggested by Fig. 3 B.
References
- 1.Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maecker, H.T., S.C. Todd, and S. Levy. 1997. The tetraspanin superfamily: molecular facilitators. FASEB J. In press. [PubMed]
- 3.Matsumoto AK, Martin DR, Carter RH, Klickstein LB, Ahearn JM, Fearon DT. Functional dissection of the CD21/CD19/TAPA-1/Leu-13 complex of B lymphocytes. J Exp Med. 1993;178:1407–1417. doi: 10.1084/jem.178.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- 5.Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science (Wash DC) 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 6.Secrist H, Levy S, DeKruyff RH, Umetsu DT. Ligation of TAPA-1 (CD81) or major histocompatibility complex class II in co-cultures of human B and T lymphocytes enhances interleukin-4 synthesis by antigen-specific CD4+ T cells. Eur J Immunol. 1996;26:1435–1442. doi: 10.1002/eji.1830260706. [DOI] [PubMed] [Google Scholar]
- 7.Boismenu R, Rhein M, Fischer WH, Havran WL. A role for CD81 in early T cell development. Science (Wash DC) 1996;271:198–200. doi: 10.1126/science.271.5246.198. [DOI] [PubMed] [Google Scholar]
- 8.Todd SC, Lipps SG, Crisa L, Salomon DR, Tsoukas CD. CD81 expressed on human thymocytes mediates integrin activation and IL-2–dependent proliferation. J Exp Med. 1996;184:2055–2060. doi: 10.1084/jem.184.5.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tybulewicz VL, Crawford CE, Jackson PK, Bronson RT, Mulligan RC. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991;65:1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- 10.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyner, A.L. 1993. Gene targeting: a practical approach. In The Practical Approach Series. D. Rickwood and B.D. Hames, eidtors. Oxford University Press, New York.
- 12.Coligan, J.E., A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober. 1995. Curr. Prot. Immunol. Wiley, London. 3.1.3–3.1.5, 3.5.10–3.5.11.
- 12a.Maecker, H.T., D.T. Umetsu, R.H. DeKruyff, and S. Levy. 1997. DNA vaccination with cytokine fusion constructs biases the immune response to ovalbumin. Vaccine. In press. [DOI] [PubMed]
- 13.Nagira M, Imai T, Ishikawa I, Uwabe KI, Yoshie O. Mouse homologue of C33 antigen (CD82), a member of the transmembrane 4 superfamily: complementary DNA, genomic structure, and expression. Cell Immunol. 1994;157:144–157. doi: 10.1006/cimm.1994.1212. [DOI] [PubMed] [Google Scholar]
- 14.Tomlinson MG, Wright MD. Characterisation of mouse CD37: cDNA and genomic cloning. Mol Immunol. 1996;33:867–872. doi: 10.1016/0161-5890(96)84612-8. [DOI] [PubMed] [Google Scholar]
- 15.Andria ML, Barsh GS, Levy S. Expression of TAPA-1 in preimplantation mouse embryos. Biochem Biophys Res Commun. 1992;186:1201–1206. doi: 10.1016/s0006-291x(05)81533-5. [DOI] [PubMed] [Google Scholar]
- 16.Engel, P., and T.F. Tedder. 1994. New CD from the B cell section of the Fifth International Workshop on Human Leukocyte Differentiation Antigens. Leuk. Lymphoma. 13(Suppl.): 61–64. [DOI] [PubMed]
- 17.Tedder TF, Zhou LJ, Engel P. The CD19/ CD21 signal transduction complex of B lymphocytes. Immunol Today. 1994;15:437–442. doi: 10.1016/0167-5699(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 18.Fearon DT, Carter RH. The CD19/CR2/ TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–149. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 19.Engel, P., L.J. Zhou, D.C. Ord, S. Sato, B. Koller, and T.F. Tedder. 1995. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 3:39–50. [DOI] [PubMed]
- 20.Rickert RC, Rajewsky K, Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature (Lond) 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 21.Krop I, de Fougerolles AR, Hardy RR, Allison M, Schlissel MS, Fearon DT. Self-renewal of B-1 lymphocytes is dependent on CD19. Eur J Immunol. 1996;26:238–242. doi: 10.1002/eji.1830260137. [DOI] [PubMed] [Google Scholar]
- 22.Ahearn JM, Fischer MB, Croix D, Goerg S, Ma M, Xia J, Zhou X, Howard RG, Rothstein TL, Carroll MC. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- 23.Molina H, Holers VM, Li B, Fung Y, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]