Abstract
The homing of lymphocytes from the blood is controlled by specialized processes of lymphocyte–endothelial cell interaction. Interference with these processes offers the potential to manipulate lymphocyte traffic, and thus to modulate normal and pathologic immune and inflammatory responses. We selected antilymphocyte monoclonal antibodies (mAbs) for inhibition of lymphocyte binding in vitro to lymph node high endothelial venules (HEV), specialized vessels that support lymphocyte recruitment into lymph nodes. mAb L11 blocks T cell binding to lymph node and Peyer's patch HEV and inhibits T cell extravasation from the blood into organized secondary lymphoid tissues. In contrast, L11 has no effect on lymphocyte binding to purified vascular ligands for L-selectin, α4β7, or LFA-1, suggesting that it inhibits by a novel mechanism. The L11 antigen is CD43, a sialomucin implicated in vitro in regulation of lymphocyte activation, whose expression is often dysregulated in the Wiskott-Aldrich syndrome. CD43 represents a novel target for experimental and therapeutic manipulation of lymphocyte traffic and may help regulate T cell distribution in vivo.
Regulated trafficking of lymphocytes through secondary lymphoid tissues is key to systemic immunity and is controlled by active mechanisms of lymphocyte–endothelial cell (EC) recognition (1, 2). Recruitment of lymphocytes from the blood has been separated into multiple sequential steps characterized as contact initiation (“tethering”), rolling, pertussis toxin-sensitive Gαi-mediated activation, and activation-dependent integrin triggering and arrest (1–4). Each step may be mediated by different adhesion or activation receptors allowing specificity through use of unique combinations of receptors to create specific homing pathways (1–4).
A number of adhesion molecules involved in lymphocyte homing via high endothelial venules (HEV) have been identified. In Peyer's patches (PP), the adhesion cascade for naive lymphocytes appears to involve a series of overlapping adhesion events with L-selectin, and to a lesser extent α4β7, initiating interaction, L-selectin and α4β7, both participating in rolling, and Gαi-linked activation-triggered arrest that requires both α4β7 and LFA-1 (5). L-selectin, but not α4 integrins, are also implicated in lymphocyte homing to LN; in this site, L-selectin appears critical in targeting the entry of most lymphocytes and LFA-1 participates in activation-dependent arrest as well (6, 7). However, additional molecules may be involved even in these relatively wellstudied models. For example, recent studies (Salmi, M., E.L. Berg, E.C. Butcher, and S. Jalkanen, personal communication) raise the possibility that vascular adhesion protein 1 (VAP1) may play an important role in primary (activationindependent) lymphocyte interactions with HEV in human LN, perhaps acting in sequence with or, for some lymphocyte subsets, as a substitute for L-selectin–initiated interactions. Moreover, the molecules involved in activation events during lymphocyte–HEV recognition have not been identified. In addition to its importance for understanding the physiology of lymphocyte trafficking, identification of molecules involved in or capable of modulating lymphocyte–EC recognition may reveal novel targets for the therapeutic regulation of pathological inflammatory and immune responses.
Lymphocyte–HEV recognition is readily studied in vitro in analyses of lymphocyte binding to HEV in frozen tissue sections in an assay developed by Stamper and Woodruff (8). In this assay, the molecular elements involved both in primary adhesion and activation-dependent interactions have been identified or shown to participate; thus, it represents a powerful tool for dissecting this cellular event in its molecular basis (9; see Discussion). Therefore, to identify novel molecular targets for controlling lymphocyte homing, we selected mAbs for their ability to block lymphocyte binding to HEV in frozen sections. We describe here an mAb, L11, that inhibits lymphocyte–HEV interaction in vitro and lymphocyte recruitment to LN, PP, and spleen in vivo. Inhibition is selective for T cells, suggesting an experimental and therapeutic approach and potentially a physiologic mechanism for differential control of T versus B cell homing. We demonstrate that the L11 antigen is CD43, a major membrane sialoglycoprotein of hematopoietic cells (10, 11), implicated in the regulation of T cell activation and adhesion in vitro.
Materials and Methods
Antibodies.
mAb L11 was produced by immunizing Fisher 344 rats four times at 3-wk intervals with the monocytoid cell line WEHI78/24 (12; gift of R. Coffman, DNAX, Palo Alto, CA). Spleen cells were fused with SP2/0 myeloma cells (American Type Culture Collection; Rockville, MD) using traditional polyethylene glycol fusion methods. Hybridoma supernatants were screened for their ability to block binding of peripheral lymph node (PLN) and mesenteric lymph node (MLN) lymphocytes to PLN HEV in Stamper-Woodruff frozen section assays (described below). L11 hybridoma was cloned three times by limiting dilution. The isotype (IgG2a) was determined by Ouchterloney analysis (ICN Biomedicals, Inc., Costa Mesa, CA). FITC-labeled Thy1.2, anti-CD43 mAb S7, and FITC-labeled S7 were purchased from PharMingen (San Diego, CA) and PE-conjugated goat anti–rat IgG was purchased from Jackson ImmunoResearch Labs. (West Grove, PA). RA3-6B2 (anti-B220; gift of R. Coffman; 13) was produced, purified, and FITC-conjugated. MECA367 (anti–mouse mucosal addressin [MAdCAM-1]; 14), MJ64, anti– mouse CD44 (15), and rat IgG negative control mAbs 9B5 (16) and 30G12 (anti-T200) were prepared in the laboratory.
Stamper-Woodruff Frozen Section Assays.
Modified Stamper-Woodruff frozen section assays were performed (8, 17) using isolated BALB/c PLN and MLN lymphocytes (2 × 107/ml) mixed 1:1 with tretramethylrhodamine B isothiocyanate (TRITC)-labeled rat MLN lymphocytes in DMEM (BioWhittaker, Walkersville, MD) without bicarbonate containing 20 mM Hepes, pH 7.0, and 0.1% (vol/vol) fetal bovine serum (assay buffer). TRITC labeling of rat internal standard cells (not recognized by L11) was performed as previously described using 1.5 μg TRITC/ml of assay buffer for 20 min at 37°C (18). Cells were preincubated with isotypematched control mAb (30G12) or with L11 (0.25 μg/106 cells) for 20 min on ice and added to freshly cut PLN frozen sections (100 ml/section) and allowed to bind for 30 min with constant rotation (76 rpm) at 4°C. Slides were gently placed in 1.5% gluteraldehyde in PBS containing 2 mM Ca2+ and 2 mM Mg2+. The number of mouse and rat lymphocytes bound to >30 HEV on each of the quadruplicate sections was determined by fluorescence microscopy. The ratio of the number of L11-treated cells bound/rat internal standard to the number of control antibody–treated cells bound/rat internal standard was calculated and multiplied by 100 to express sample cell binding as percent of control cell binding. T cells were isolated by negative selection on anti–B cell and antimacrophage columns (R & D Sys., Inc., Minneapolis, MN) and were >97% Thy1+. B cells were identified in mixed populations by prestaining cell suspensions with FITC-conjugated anti-B220. Data represents the mean and standard error of four experiments.
Adhesion Assays.
The ability of L11 to inhibit lymphocyte binding to purified vascular ligands was assessed as previously described (19, 20).
Flow Cytometry.
BALB/c MLN and PLN lymphocytes were immunostained with L11 (1 μg/106 cells) or isotype-matched control mAb, PE-conjugated mouse anti–rat IgG followed by FITCconjugated anti–Thy-1. Cells were analyzed on a FACScan® (Becton Dickinson, San Jose, CA). Cross-blocking experiments were performed by preincubating lymphocytes with 9B5, MJ64, S7, or L11 (5 μg/106 cells) for 20 min and then adding FITC-labeled S7, MJ64, or 30G12. After an additional 20 min, incubation cells were washed and analyzed as above.
In Vivo Homing.
Lymphocytes from BALB/c MLN, PLN, and spleens were labeled with 10 mM Cell-Tracker Orange (Molecular Probes Inc., Eugene, OR) according to the manufacturer's instructions or with TRITC, washed, and treated with L11 or isotype-matched negative control mAb (1 μg/106 cells) for 20 min. Cell suspensions were diluted and centrifuged. The supernatant was then removed and 5 × 107 cells were resuspended in 0.5 ml of HBSS (BioWhittaker) and injected via the tail vein. In other experiments, excess antibody (50 μg) was coinjected with cells. At the appropriate time point, peripheral blood was collected by heart puncture and lymphocyte suspensions prepared from lymphoid organs. Cells were stained with FITC-conjugated anti–Thy-1 and analyzed by two-color flow cytometry.
Transfection and Immunofluorescence.
Chinese hamster ovary P (CHO-P) cells were transfected with mouse CD43 or mouse MAdCAM-1 cDNA as described (21) and stained with L11 mAb or control mAb MECA367 (anti-MAdCAM-1) followed by PEconjugated goat anti–rat IgG and visualized by fluorescence microscopy.
Results
mAb L11 Inhibits T Cell Binding to HEV In Vitro and In Vivo.
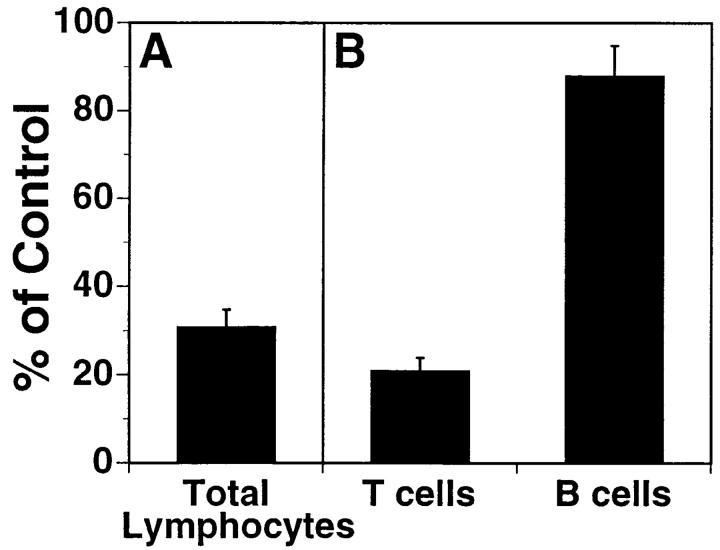
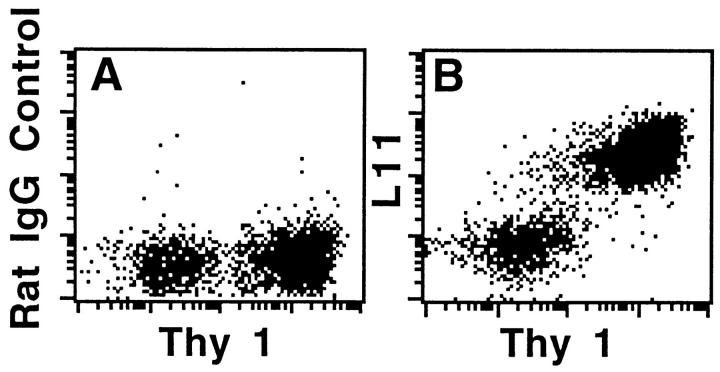
To identify antibodies capable of inhibiting lymphocyte trafficking, mAbs were generated and screened for their ability to block lymphocyte binding to HEV in LN frozen sections, using a modified Stamper-Woodruff assay (8, 17). One antibody isolated in this screen, L11 (isotype IgG2a), blocks T cell binding to normal and inflamed PLN HEV by >80% (Fig. 1). Interestingly, it has much less effect on LN B cell binding. Two-color flow cytometric analysis of LN lymphocytes reveals that L11 antigen is highly expressed by T cells but also weakly by B lymphocytes (Fig. 2), correlating with the predominant inhibitory effect of L11 on T cells.
Figure 1.
mAb L11 inhibits T cell binding to HEV in vitro. (A) L11 blocks binding of total LN lymphocytes to PLN HEV in modified Stamper-Woodruff frozen section assays performed using isolated BALB/c PLN and MLN lymphocytes mixed 1:1 with TRITC-labeled rat MLN cells (internal standard cells not recognized by L11). Cells were preincubated with isotype-matched control mAb or with L11, added to freshly cut PLN frozen sections, and incubated for 30 min with constant rotation (76 rpm) at 4°C. The number of mouse and rat lymphocytes bound to >30 HEV on each of quadruplicate sections was determined by fluorescence microscopy. The ratio of the number of L11-treated cells bound/rat internal standard to the number of control antibody treated cells bound/rat internal standard was calculated and sample cell binding expressed as percent of control cell binding. (B) Blocking of T versus B cells was assessed as described for total lymphocytes using T cells isolated by negative selection (>97% Thy 1+). B cells were identified in mixed populations by prestaining cell suspensions with FITC-conjugated antiB220.
Figure 2.
Two-color flow cytometric analyses of expression of L11 antigen by LN T cells (Thy 1+) and weak expression by Thy 1− cells (predominantly B cells). MLN and PLN lymphocytes were immunostained with isotype-matched control mAb (A) or L11 (B), PE-mouse anti–rat IgG and finally FITC-conjugated anti–Thy-1. Cells were analyzed using a FACScan® and CellQuest\xa9 software; x- and y-axis are log10 fluorescence.
Inhibition of binding to HEV suggests involvement of the L11 antigen in T cell homing. We therefore assessed the effect of L11 on short-term localization of intravenously injected lymphocytes in vivo. Pretreatment of syngeneic LN cells with L11 results in significant inhibition of 1-h homing of T cells, but not B cells, to PLN, MLN, PP, and spleen (Fig. 3 A). Although similar inhibition of T cell trafficking is observed whether L11-pretreated lymphocytes are washed before injection or are co-injected with excess mAb, if lymphocytes are injected with excess antibody, significant reduction (⩽30% inhibition) of B lymphocyte homing is also observed (data not shown). Importantly, L11-treated cells were significantly enriched in the blood of recipients, consistent with mAb inhibition of EC interaction and recruitment rather than nonspecific or toxicity-induced clearance of injected cells from the circulation. Fluorescence microscopic examination of frozen sections of LN following in vivo homing reveals decreased accumulation of L11-treated cells in HEV compared to control cells, in spite of enhanced blood levels, suggesting that the antibody inhibits events involved in lymphocyte–HEV recognition in vivo.
Figure 3.
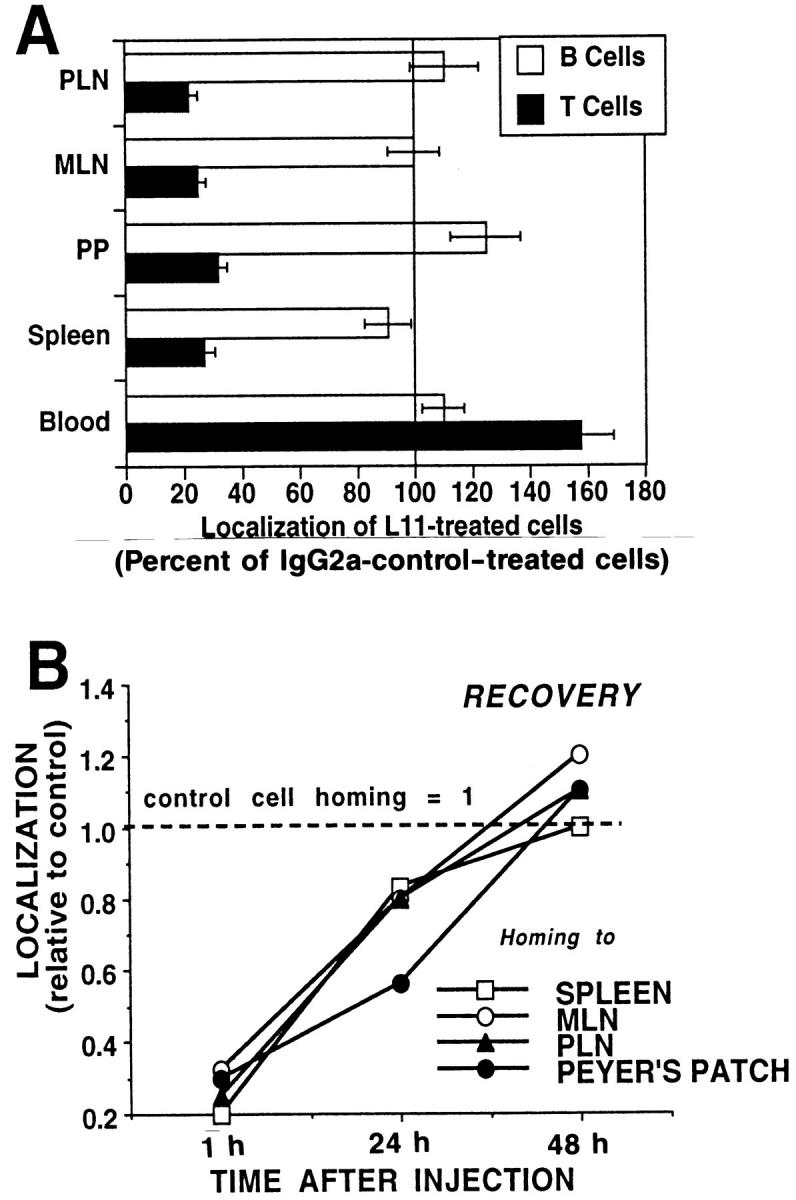
mAb L11 inhibits T cell binding to HEV in vivo. (A) L11 pretreatment inhibits T (closed bars) but not B (open bars) cell localization to PLN, MLN, PP, and spleen 1 h after intravenous injection. TRITClabeled lymphocytes were treated with L11 or isotype-matched negative control mAb for 20 min, washed, and 5 × 107 cells injected via the tail vein. After 1 h, peripheral blood was collected by heart puncture and lymphocyte suspensions prepared from lymphoid organs were stained with FITC-conjugated anti–Thy-1, spiked with internal standard fluorescent beads for enumeration, and analyzed by two-color flow cytometry. (B) Homing of L11-pretreated T cells returns to that of control cells over 48 h. Redistribution studies were performed as described for A using cells labeled with 10 mM Cell-Tracker Orange.
To further assess the fate of antibody-treated cells and the possibility of cell damage or toxicity caused by L11, longer-term redistribution studies were performed. Lymphocytes were labeled with 10 μM Cell-Tracker Orange, preincubated with saturating levels of L11 or control antibody, washed, and injected into syngeneic recipients. Animals were killed at 1, 24, and 48 h. As above, 1-h homing was blocked by L11, but by 48 h, the distribution and number of L11-treated cells in lymphoid tissues was similar to that of control cells (Fig. 3 B).
mAb L11 Does Not Inhibit Binding of T Cells to Purified Vascular Ligands.
In situ lymphocyte interactions with HEV involve sequential overlapping engagement of L-selectin, α4β7, and LFA-1 (5). To determine if L11 was directly inhibiting engagement of these adhesion receptors, the ability of L11 to inhibit lymphocyte binding to purified vascular ligands was assessed. L11 had no effect on T cell rolling on the L-selectin ligand peripheral node addressin (PNAd; data not shown) or on binding to the α4β7 ligand MAdCAM-1 (Fig. 4 A). As described (22), lymphocytes require activation to bind the LFA-1 ligand intracellular adhesion molecule 1 (ICAM-1); L11 had no effect on this interaction when integrins were triggered by replacement of Mg2+ with Mn2+ in the binding buffer (Fig. 4 B). These results suggest that the L11 antigen is not acting as a receptor for known vascular adhesion ligands, and that L11 does not directly inhibit engagement of these receptors.
Figure 4.
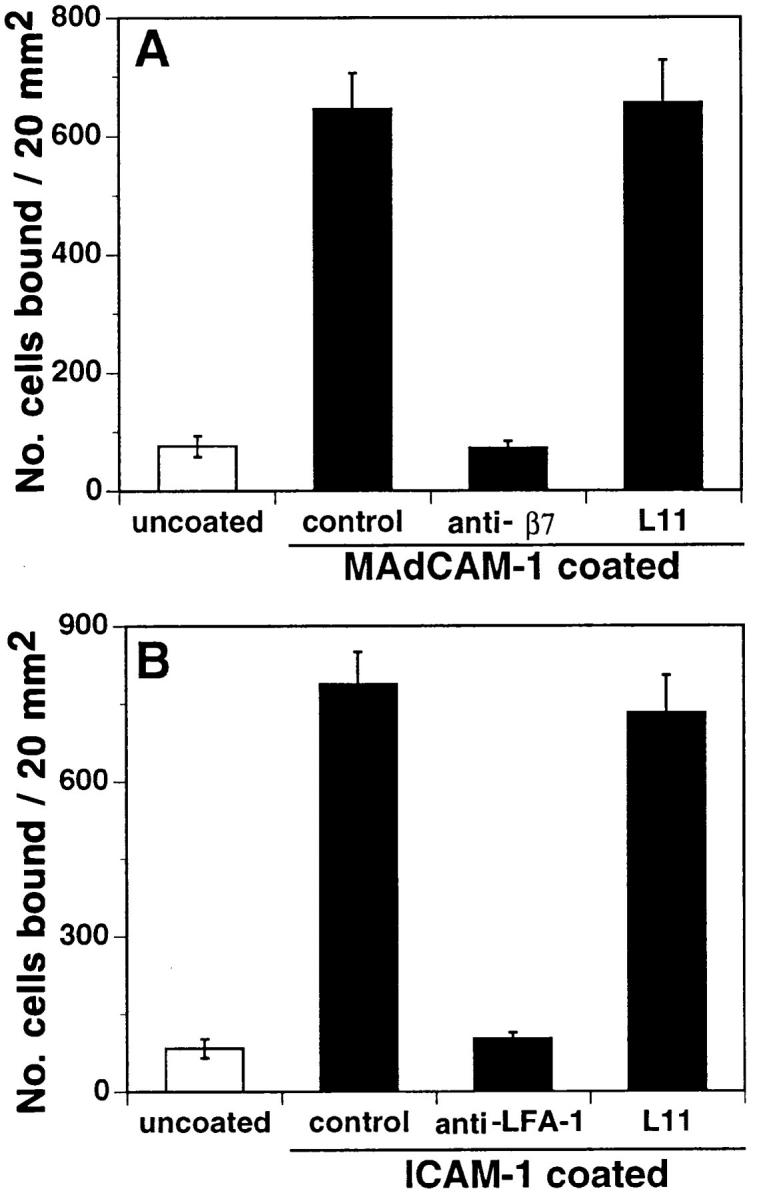
L11 fails to block binding of lymphocytes to MAdCAM-1 or ICAM-1. (A) antiβ7 mAb cocktail containing FIB504, FIB30, and DATK32 (26) blocks binding of MLN lymphocytes to purified MAdCAM-1 and (B) anti–LFA-1 mAb blocks binding of Mn2+treated MLN lymphocytes to purified ICAM-1; however, L11 fails to block binding to either ligand.
L11 Antigen is CD43.
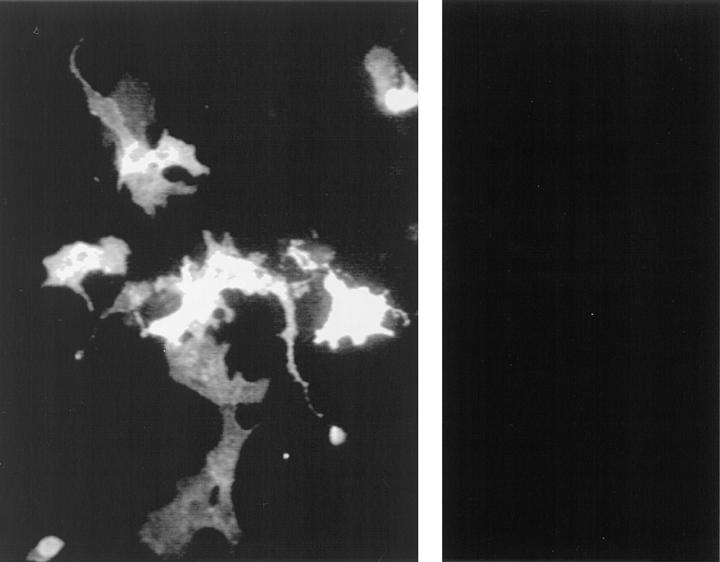
Immunofluorescence flow cytometry reveals that L11 antigen is highly expressed by bone marrow neutrophils as well as T cells (not shown). This expression pattern is similar to that of CD43 (10). In addition, the relative molecular mass of the L11 antigen, 100 kD in Western blots (not shown), is also similar to that reported for mouse CD43 (11). Therefore, we next assessed reactivity of L11 with CHO-P cell transfectants expressing mouse CD43 (11). L11 (Fig. 5, left) but not control mAb (Fig. 5, right), stained CD43-transfected CHO-P cells, as did antiCD43 mAb S7 (not shown). L11 failed to stain mocktransfected CHO-P cells or CHO-P cells expressing the irrelevant antigen MAdCAM-1. Thus, L11 can recognize CD43.
Figure 5.
L11 antigen is CD43. L11 (left), but not control mAb (right), stained CD43-transfected CHO-P cells. CHO-P cells were transfected with mouse CD43 or mouse MAdCAM-1 cDNA and stained with L11 mAb or control mAb MECA367 (anti–mouse MAdCAM-1). MECA367 recognized MAdCAM-1 transfectants (not shown) but failed to stain CD43 transfectants (right). L11 stained CD43 transfectants (left), but not MAdCAM-1 transfectants (not shown).
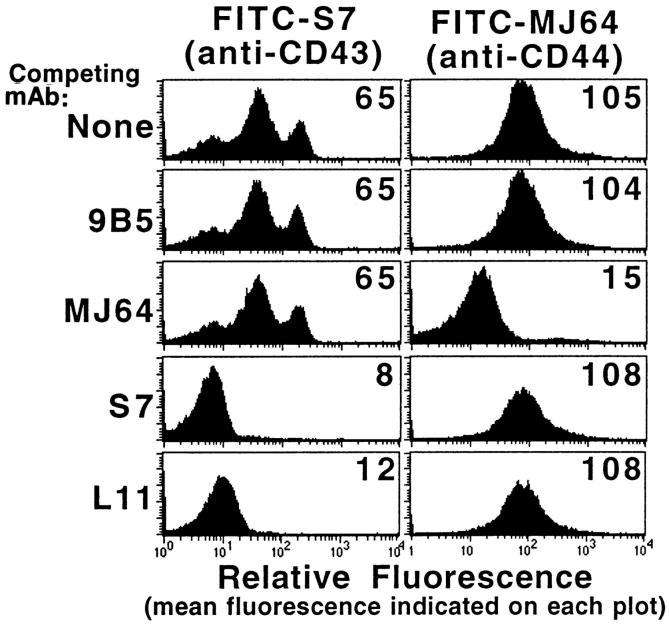
To determine if the T cell antigen recognized by L11 is CD43, LN lymphocytes were stained (at subsaturating concentrations) with L11 or S7 (detected with PE-conjugated anti–mouse IgG) and FITC-labeled S7 or FITC-labeled Thy-1. As shown above, double staining with FITC–Thy-1 and L11 (Fig. 2 B) yielded double-positive cells, indicating that these molecules are both expressed by T cells. In contrast, double staining with L11 and FITC-S7 yielded a linear relationship (not shown) indicating that the molecule recognized by these mAbs are expressed equivalently on individual T cells, a pattern suggesting that the mAbs recognize a common antigen. Finally, the epitopes recognized by L11 and S7 appear to be close or overlapping as L11 (and S7) blocks binding of FITC-labeled S7 (Fig. 6) but not binding of FITC-MJ64 (anti-CD44), whereas MJ64 blocks binding of FITC-MJ64 but not FITC-labeled S7. Together these data indicate that the antigen defined by L11 on T cells is CD43.
Figure 6.
L11 antigen on T cells is CD43. LN lymphocytes preincubated with L11, anti-CD43 mAb S7, anti-CD44 mAb MJ64, control mAb 9B5, or buffer were stained with FITC-labeled S7 or MJ64 and their fluorescence measured by FACS® analysis. The x-axis is log10 fluorescence; mean fluorescence is indicated on each histogram. L11 blocked FITC-S7 binding but not control mAb (FITC-MJ64) binding to T cells.
Discussion
We have shown that anti-CD43 mAb L11 is a potent inhibitor of lymphocyte homing from the blood into lymphoid organs, including the LN, PP, and spleen. Consistent with the preferential expression of CD43 on T cells, the effect is selective for T cell homing, although when the mAb is co-injected in excess some redistribution in B cell trafficking can also be observed. Inhibition is not due to toxicity since there is a concurrent increase in blood levels of T cells when homing is blocked and the distribution of L11treated cells returns to normal after 48 h.
The selective effect on T cells is unusual since most previously identified lymphocyte receptors involved in homing are shared by subsets of B cells as well, reflecting the common mechanisms of B and T cell traffic at least to organized lymph tissues. Moreover, molecules previously implicated in homing generally influence traffic in a tissue- selective fashion (2), whereas the effect of anti-CD43 shown here influences trafficking to LN, PP, and even spleen to a significant extent. This suggests an effect on an event common to T cell trafficking to diverse sites.
CD43 is an abundant leukocyte surface sialomucin that has been implicated both in antiadhesive roles, acting as a passive barrier to engagement of surface adhesion receptors, and in proadhesive activities, mediated, for example, through intracellular signaling and activation of leukocyte integrins (e.g., LFA-1) (23–25). In this context, the failure of L11 to inhibit lymphocyte rolling on PNAd or lymphocyte binding to MAdCAM-1 or ICAM-1 in vitro renders it unlikely that it is inducing a passive barrier activity of CD43. On the other hand, L11 might inhibit a normal proadhesive signaling activity in the context of lymphocyte homing. However, our results are also consistent with involvement of a previously unidentified vascular ligand for CD43, with CD43–endothelial interaction representing an additional step in the multistep processes of lymphocyte–HEV (and spleen marginal sinus lining cell) interactions in vivo. In this context it is relevant that VAP-1 has recently been implicated in lymphocyte–HEV interactions in the PLN and its role in the multistep process of HEV interaction appears to precede that of LFA-1.
In conclusion, anti-CD43 mAb L11 inhibits lymphocyte recruitment to lymphoid organs, acting at the level of lymphocyte–EC recognition. Inhibition is selective for T cells, suggesting an experimental and therapeutic approach, and potentially a physiologic mechanism, for differential control of T versus B cell homing.
Acknowledgments
The authors thank Evelyn Resurrecion, Jamie Lopez, Jean Jang, and June Twelves for technical assistance and Drs. Ellen Berg, Eddie Bowman, Carlo Laudanna, and Sherry Haugejordan-Brown for comments on the manuscript.
Footnotes
L.M. McEvoy was a Senior Fellow of the American Heart Association, California Division, and the National Multiple Sclerosis Society during part of this work. H. Sun is supported by a predoctoral award by the National Cancer Institute. This work was supported by grant AI37319 from the National Institutes of Health and the Core Facilities of the Stanford Digestive Disease Center under DK38707.
References
- 1.Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (Wash DC) 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu Y, Newman W, Tanaka Y, Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992;13:106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- 4.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 5.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 6.Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature (Lond) 1983;303:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 7.Steen PD, Ashwood ER, Huang K, Daynes RA, Chung HT, Samlowski WE. Mechanisms of pertussis toxin inhibition of lymphocyte–HEV interactions. I. Analysis of lymphocyte homing receptor–mediated binding mechanisms. Cell Immunol. 1990;131:67–85. doi: 10.1016/0008-8749(90)90235-j. [DOI] [PubMed] [Google Scholar]
- 8.Stamper HB, Jr, Woodruff JJ. An in vitro model of lymphocyte homing. I. Characterization of the interaction between thoracic duct lymphocytes and specialized high-endothelial venules of lymph nodes. J Immunol. 1977;119:772–780. [PubMed] [Google Scholar]
- 9.Butcher EC. Specificity of leukocyte–endothelial interactions and diapedesis: physiologic and therapeutic implications of an active decision process. Res Immunol. 1993;144:695–698. doi: 10.1016/s0923-2494(93)80053-2. [DOI] [PubMed] [Google Scholar]
- 10.Remold-O'Donnell E, Zimmerman C, Kenney D, Rosen F. Expression on blood cells of sialophorin, the surface glycoprotein that is defective in Wiskott-Aldrich syndrome. Blood. 1987;70:104–109. [PubMed] [Google Scholar]
- 11.Baecher C, Infante A, Semcheski K, Frelinger J. Identification and characterization of a mouse cell surface antigen with alternative molecular forms. Immunogenetics. 1988;28:295–302. doi: 10.1007/BF00364226. [DOI] [PubMed] [Google Scholar]
- 12.Walker EB, Lanier LL, Warner NL. Characterization and functional properties of tumor cell lines in accessory cell replacement assays. J Immunol. 1982;128:852–859. [PubMed] [Google Scholar]
- 13.Holmes, K., and B. Fowlkes. 1991. Preparation of cells and reagents for flow cytometry. In Current Protocols in Immunology. J.E. Coligan, A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober, editors. Greene Publishing Assoc. and Wiley-Interscience, New York. 5.3.1–5.3.11. [DOI] [PubMed]
- 14.Streeter PR, Berg EL, Rouse BTN, Bargatze F, Butcher C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature (Lond) 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 15.Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalkanen S, Bargatze R, Herron L, Butcher E. A lymphoid cell surface protein involved in endothelial cell recognition and lymphocyte homing in man. Eur J Immunol. 1986;16:1195–1202. doi: 10.1002/eji.1830161003. [DOI] [PubMed] [Google Scholar]
- 17.Butcher EC, Scollay RG, Weissman IL. Lymphocyte adherence to high endothelial venules: characterization of a modified in vitro assay, and examination of the binding of syngeneic and allogeneic lymphocyte populations. J Immunol. 1979;123:1996–2003. [PubMed] [Google Scholar]
- 18.Butcher, E.C., and W.L. Ford. 1986. Following cellular traffic: methods of labeling lymphocytes and other cells to trace their migration in vivo. 4th ed. In Handbook of Experimental Immunology. D. Weir and L. Herzenber, editors. Blackwell Scientific Publishers, Palo Alto. 57.1–57.23.
- 19.Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin–mediated lymphocyte rolling on MAdCAM-1. Nature (Lond) 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- 20.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 21.Seed B, Aruffo A. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu Y, Mobley J. Distinct divalent cation requirements for integrin-mediated CD4+ T lymphocyte adhesion to ICAM-1, fibronectin, VCAM-1, and Invasin. J Immunol. 1993;151:4106–4115. [PubMed] [Google Scholar]
- 23.Sperling AI, Green JM, Mosley RL, Smith PL, DiPaolo RJ, Klein JR, Bluestone JA, Thompson CB. CD43 is a murine T cell costimulatory receptor that functions independently of CD28. J Exp Med. 1995;182:139–146. doi: 10.1084/jem.182.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeSmet W, Walter H, Van Hove L. A new CD43 monoclonal antibody induces homotypic aggregation of human leucocytes through a CD11a/CD18-dependent and -independent mechanism. Immunology. 1993;79:46–54. [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Mateos P, Campanero MR, del Pozo MA, Sanchez-Madrid F. Regulatory role of CD43 leukosialin on integrin-mediated T-cell adhesion to endothelial and extracellular matrix ligands and its polar redistribution to a cellular uropod. Blood. 1995;86:2228–2239. [PubMed] [Google Scholar]
- 26.Andrew DP, Berlin C, Honda S, Yoshino T, Hamann A, Holzmann B, Kilshaw PJ, Butcher EC. Distinct but overlapping epitopes are involved in alpha 4 beta 7–mediated adhesion to vascular cell adhesion molecule-1, mucosal addressin-1, fibronectin, and lymphocyte aggregation. J Immunol. 1994;153:3847–3861. [PubMed] [Google Scholar]