Abstract
Staphylococcus aureus produces a set of proteins (e.g., staphylococcal enterotoxin A [SEA], SEB, toxic shock syndrome toxin 1 [TSST-1]) which act both as superantigens (SAgs) and toxins. Although their mode of action as SAgs is well understood, little is known about how they enter the body via the intestine and cause food poisoning. To examine this problem we used an in vitro culture system to study the capacity of class II MHC-negative human intestinal epithelial cells (Caco-2) to transcytose several staphylococcal toxins. We found that Caco-2 cells are capable of dosedependent, facilitated transcytosis of SEB and TSST-1, but not SEA. We extended these studies in vivo in mice by showing that ingested SEB appears in the blood more efficiently than SEA. Our data suggest that these toxins can cross the epithelium in an immunologically intact form. These results may have important implications for the pathogenesis of food poisoning.
S taphylococcus aureus produces a set of exotoxins including staphylococcal enterotoxins (SEs)1 and toxic shock syndrome toxin 1 (TSST-1) which cause food poisoning and toxic shock syndrome in human beings and other species (1–4). These toxins are intermediate molecular weight proteins (22–30 kD) that also act as superantigens (SAgs; 5–7), due to their ability to bind to MHC class II molecules on APCs, and stimulate all T cells bearing particular Vβs on their T cell receptors (5–11). For example, the mouse in SEA stimulates T cells bearing Vβs 1, 3, 11, 17, and the closely related SEB and SECs stimulate T cells bearing Vβ7 and members of the Vβ8 family (12). TSST-1 stimulates nearly all T cells bearing Vβ15 in the mouse or Vβ2 in human (13). Thus, an individual toxin can potentially activate a very high proportion of T cells (5–30%). The toxic shock associated with these toxins has been attributed to the flood of cytokines (TNF, IL-2, etc.) released during this massive T cell stimulation. For example, in mice SEB can cause a shock-like syndrome resulting in rapid weight loss and death (14). Nude mice or mice lacking T cells bearing the relevant Vβ elements are resistant to the toxic effects of SEB (14). A role for T cells in generation of shock is also suggested by the greatly elevated percentage of T cells bearing Vβ2 in patients with TSST-1–mediated toxic shock syndrome (13, 15).
On the other hand, in toxin-mediated food poisoning, the symptoms of which include diarrhea and vomiting within about 4–6 h of ingesting contaminated food, pathogenesis is not so well understood. It is possible that these toxins stimulate intestinal lymphocytes to release cytokines which induce the symptoms by local inflammation. Alternatively, they may have an additional functional domain which can interact directly with receptors on the cells lining the intestine to cause the symptoms. Distinguishing between these possibilities has been difficult due to the lack of a rodent model for toxin-mediated food poisoning.
To shed some light on the problem, we performed experiments to see if these toxins can cross the intestinal epithelium, since to stimulate T cells, ingested toxin must escape the intestine in an intact form that is capable of interacting with the host immune system. We performed two types of experiments. In the first we directly examined transepithelial transcytosis of toxins in vitro using a well-characterized human enterocytic cell line, Caco-2 (16, 17). In the second we used T cell stimulation/deletion assays to detect toxins in the blood of mice after they had been fed toxins. Our results show that these toxins cross epithelial membranes, in some cases by a facilitated mechanism. While our results do not rule out a direct effect of these toxins on intestinal epithelium, they show that the toxins can gain rapid access to the immune system after ingestion supporting a role for T cells in the pathogenesis of food poisoning.
Materials and Methods
Cell Lines.
The human adenocarcinoma cell line, Caco-2 (18), was obtained from American Type Culture Collection (Rockville, MD). Two T cell hybridomas were used in these studies. 2B10.D20 (Vβ8.2+) was derived from a B10.D2 mouse and recognizes SEB presented by a variety of class II MHC molecules (Kappler, J., and P. Marrack, unpublished data). 5KC (Vβ3+) was derived from a B10.BR mouse and responds to SEA, again presented by class II MHC molecules (19). The HLA-DR1 transfected fibroblast, DAP-DR1, and the B lymphoblastoid cell line, LG2, were used as APCs for SEB and SEA (20–22).
Toxins.
SEA and TSST-1 were obtained from Toxin Technology (Madison, WI). Recombinant wild-type and mutant SEB proteins were affinity purified from lysates of Escherichia coli transfected with plasmids encoding the appropriate toxins as previously described (22).
Mice.
B10. BR animals were bred in the animal care facility at the National Jewish Center (Denver, CO).
mAbs.
In this study, three anti-SEB mAbs (B327, 2B33, and B344; reference 23), two anti-SEA mAbs (A116.3 and A108.6; Kappler, J., and P. Marrack, unpublished data), and two anti– TSST-1 mAbs (TS327H1.1 and 477L; reference 24) were used. The anti–TSST-1 mAbs were a gift of M. Matsumura (NeXstar Pharmaceuticals, Lakewood, CO). Detecting mAbs were biotinylated using NHS-S-S-biotin (Sigma Chemical Co., St. Louis, MO). Biotin conjugated anti-TCR Vβ mAbs, KJ25 (anti-Vβ3), and F23.1 (Vβ8) were purchased from PharMingen (San Diego, CA). The anti-CD4 mAb GK1.5 (25) was FITC labeled using a standard protocol.
Preparation and Selection of Complete Monolayers.
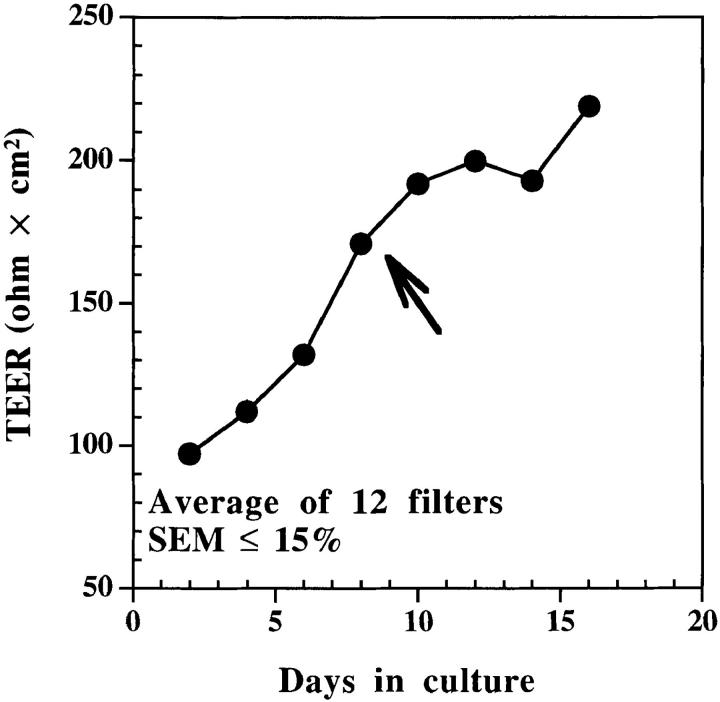
Caco-2 cells were grown to confluence on plastic flasks (225 cm2) and subcultured every week using trypsin-EDTA (GIBCO BRL, Gaithersburg, MD). After trypsinization, the cells (between passages 11–32) were washed once, separated from dead cells using ficoll-hypaque density gradient centrifugation, washed again, and then resuspended in tissue culture medium at 5 × 105 cells/ml. Cell suspensions (400 μl) were added to the chambers of 12-mm nitrocellulose filters (Millicell-HA; Millipore Corp., Bedford, MA) and placed into 24-well tissue culture plates containing 400 μl culture medium. Medium was replaced every other day until confluent monolayers with tight junctions developed as assessed by a hydrostatic pressure test and transepithelial electrical resistance (TEER) of monolayers. The hydrostatic pressure test was performed on filters 24 h before an experiment. Each monolayer was filled with medium to the rim and incubated overnight at 37°C. Any filter that showed appreciable leakage was excluded. Transepithelial electrical resistance across other membranes that passed the hydrostatic pressure test was measured with a Millicell-ERS instrument (Millipore Corp., reference 26). Only monolayers with TEER of ⩾150 ohm × cm2 after correction for intrinsic value of empty filter were used in the experiments described in this study (Fig. 1). At this point the medium in both the filter inserts (apical medium) and culture wells (basal medium) was replaced and the cultures were used to study transcytosis.
Figure 1.
Caco-2 cells grown on filters make complete monolayers within 6–8 d. 2 × 105 cells were added per filter that was placed into a 24-well plate at 37°C. Apical and basal media were replaced every other day and TEERs of monolayers were measured using Millicell-ERS (Millipore Corp.). Monolayers were considered confluent when TEER reached ⩾150 ohm × cm2 (arrow).
Measurement of Transcytosis.
The ability of Caco-2 cells to transcytose toxins was tested using confluent monolayers grown on Millicell-HA filters as described above. Media were removed from selected filters and 400 μl of fresh culture medium containing various concentrations of each toxin (SEA, SEB, TSST-1) were added together to either the same apical (upper) or basal (lower) chamber of each culture. In each case an equal quantity of the enzyme horseradish peroxidase (HRP) was added as an internal control for nonspecific transcytosis due to leakage, fluid pinocytosis, etc. Among the available fluid phase markers, HRP (40 kD) was chosen because its molecular weight is close to those of the toxins (22–28 kD) so their pinocytosis should occur at more or less the same rate. Another 400 μl of culture medium was added to the other chamber. After incubation at 37°C for the indicated period of time, apical or basal media were collected depending on whether toxins were added to the basal or apical media. Amounts of each toxin and HRP present in collected samples were assayed as described below.
ELISA Analysis.
A capture ELISA was used to determine amounts of each toxin present in different samples. For SEB, wells of Immulon® 3 plates (Dynatech Labs., Inc., Chantilly, VA) were coated overnight at 4°C with 100 μl of capture antibody B327 (10 μg/ml PBS), washed, and blocked with 25% FCS. Serial dilutions of the samples were added and the plates incubated overnight at 4°C. The captured toxin was detected using biotinylated B344 mAb followed by alkaline phosphatase–conjugated extravidin (Fisher Scientific, Pittsburgh, PA) and p-nitrophenyl phosphate as substrate. The binding sites on SEB of B327 and B344 do not overlap, nor does substitution of residue N23 or F44 significantly reduce their binding (23). The OD of the wells was read at 405 nm using an automated plate reader (Bio-Tek Instrs. Inc., Winooski, VT). Samples containing a known concentration of SEB were used to construct a standard curve. The amount of SEB present in each sample was calculated by comparison to the standard using a multiple regression computer program MKASSAY (available on request). In the experiments where all toxins (SEA, SEB, and TSST-1) and HRP were added to the same filter, specific sets of mAbs (descibed above) were used to determine the concentration of each toxin in the sample using essentially the same procedure.
An enzymatic assay was used to determine the concentrations of HRP in collected samples. In brief, samples were titrated in PBS, and then 100 μl of TMB microwell peroxidase substrate (Kirkegaard & Perry Labs., Inc., Gaithersburg, MD) was added to each well and incubated at room temperature until blue color developed. Wells were read at 630 nm using the ELISA reader. Concentration of HRP in each sample was determined using computer analysis by comparison to the HRP standard.
IL-2 Assay.
Stimulations of T cell hybridomas to produce IL-2 was done in 96-well microtiter plates as previously described (27) using 5 × 104 hybridoma cells and 5 × 104 DR1-transfected fibroblast (DAP-DR1) as APCs of toxins. IL-2 production was used as a readout of T cell activation and was measured using the MTT (3-[4-5-Dimethylthiazol-2-LX]-2,5-diphenyltetrazolium bromide) assay (28).
Flow Cytometric Analysis.
T cells purified from blood of mice treated with SEA or SEB were stained simultaneously with biotinylated anti-Vβ and FITC-conjugated anti-CD4 antibodies and analyzed on a FACScan® (Becton Dickinson, San Jose, CA) as described elsewhere (12).
Oral Administration of Toxins to Mice.
Toxins were administered to mice either orally or intraperitoneally. For oral administration of toxins, a special curved needle (Fisher Scientific) was used. Soybean trypsin inhibitor (Sigma Chemical Co.) was added to reduce gastric digestion of orally administered toxins (29). Sera were collected from the treated mice and then tested for presence of toxins as described below.
Levels of Functional Toxins in Sera.
The ELISA described above was not sensitive enough to detect the low levels of toxins present in the blood of mice a few hours after administration of the toxins. Therefore, a functional assay was used which was at least one order of magnitude more sensitive than the ELISA. Dilutions of a serum to be tested for SEA or SEB were used in vitro to stimulate a T cell hybridoma bearing the appropriate Vβ element (Vβ3 for SEA and Vβ8.2 for SEB). At 24 h, the level of IL-2 produced was assayed as described above. As controls, dilutions of a standard preparation of SEA or SEB were tested similarly. A plot of the volume of sera or standard used versus the amount of IL-2 produced was constructed and the amount of toxin in the sera calculated by comparison to the SAg standard using multiple regression. Since the highest amount of serum that could be added to a culture was 10 μl, the limit of sensitivity of this assay was ∼10 pg/ml for SEA and ∼100 pg/ml for SEB.
Results
Transcytosis of SEB through Caco-2 Monolayers.
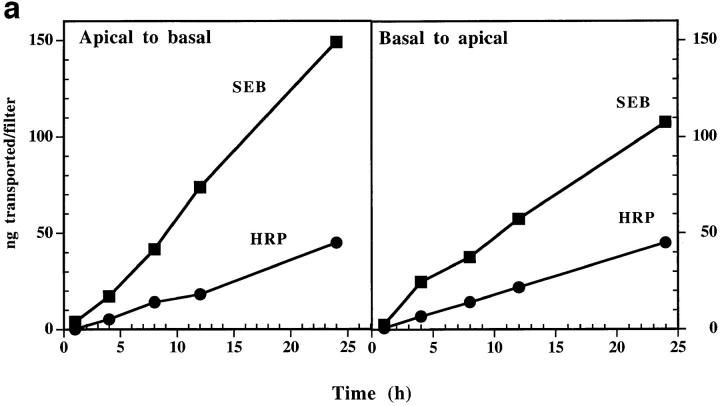
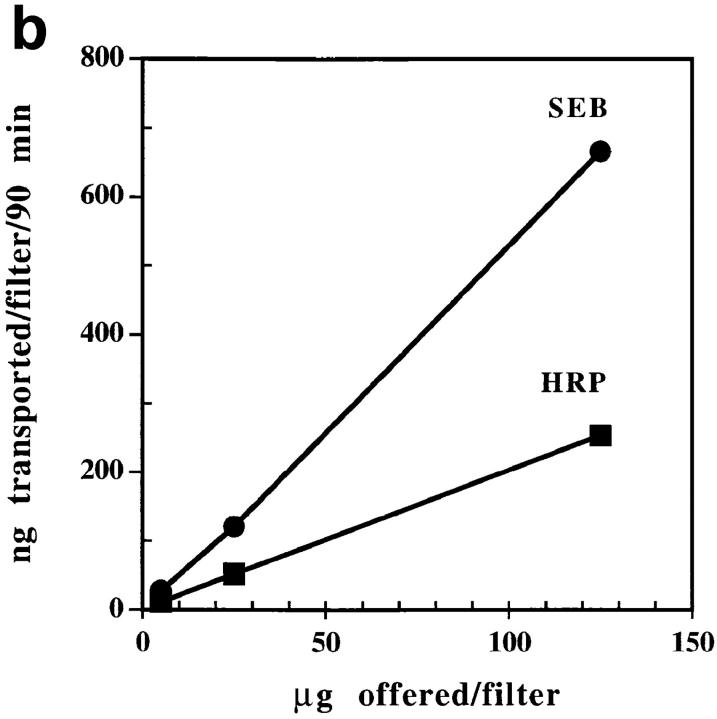
We prepared two chambered cultures separated by a monolayer of confluent Caco-2 cells as described in Materials and Methods (see Fig. 1). We initially studied SEB transcytosis to characterize this system, using HRP as an internal control to distinguish between specific transcytosis and nonspecific mechanisms such as paracellular diffusion with water (30). Two types of experiments were done. In both, SEB and HRP were added to either apical or basal media and their appearance in the opposite chamber assessed as described in the Materials and Methods. In the first experiment (Fig. 2 a), a fixed concentration of each protein (7.5 μg/ml) was used and the kinetics of transcytosis followed in either direction through the monolayer. In the second (Fig. 2 b), various concentrations of the protein were added to the apical media and the amount transcytosed to the basal medium assayed at 90 min. These results established several points. First, transcytosis of SEB was detected in both directions across the Caco-2 monolayer and the rate of SEB transcytosis was linear for the 24 h of the experiment. Second, SEB was transcytosed faster than HRP, especially in the apical to basolateral direction where the SEB transcytosis rate was usually three to six times faster than that of HRP. These results suggested that the transcytosis of SEB by Caco-2 was facilitated, perhaps by a specific surface receptor. However, we found no evidence in these experiments for saturation of the SEB transcytosis mechanism even using concentrations of SEB up to 300 μg/ml (Fig. 2 b).
Figure 2.
(a) SEB transcytosis by Caco-2 cells is bidirectional and time dependent. (Apical to basal) Toxin and HRP (3 μg each in 400 μl medium) were added to the apical chamber. Samples were collected from basal chambers at indicated time points, over a period of 24 h. Transcytosed toxin and HRP were then measured as described in Materials and Methods. (Basal to apical) The experiment was done as above except the toxin and HRP were added to the basal chamber and apical samples were collected. Each point is the mean of three determinants. SEM was usually <15%. This experiment is a representative of three similar experiments. (b) Transcytosis of SEB by Caco-2 cells is dose dependent but not saturable. Different concentrations of SEB and HRP were added to the apical medium of the same filter. Basal media were collected 90 min later and assayed for SEB and HRP transcytosed. Each point was performed in triplicate. SEM was usually <15%. This experiment is a representative of three separate experiments with similar results.
Facilitated Transcytosis of SEB and TSST-1 but Not SEA by Caco-2 Cells.
To see if this accelerated transcytosis was specific to SEB, we tested two other staphylococcal exotoxins in this system, SEA and TSST-1. All three toxins were added to either the basal or apical medium of Caco-2 cultures and their appearance in the opposite chamber followed. Again, HRP was included in each culture as internal control. The results (Fig. 3) showed that TSST-1 was transcytosed by Caco-2 cells similarly to SEB. In each case, transcytosis was bidirectional and severalfold faster than the rate of HRP transcytosis. Also in both cases we again saw no evidence for saturation of the transcytosis mechanism with the concentration of toxins used here (up to 4 μg/ml). The results with SEA were very different. Although SEA was transcytosed in both directions, the rate was very similar to that of HRP, suggesting that SEA crossed the monolayer nonspecifically with water.
Figure 3.
Transcytosis of SEB and TSST-1, but not SEA, by Caco-2 cells. (A, Apical to basal). Equal concentrations of each toxin and HRP were added to the apical media of the same filter. Basal media were collected and assayed for toxins and HRP as indicated in Materials and Methods. (B, Basal to apical) The same experiment done in the opposite direction. Each point was performed in triplicate. SEM was usually <15%. This experiment is a representative of at least four separate experiments with similar results.
SEB Transcytosis by Caco-2 Cells Is Affected by Mutations of Residues N23 and F44.
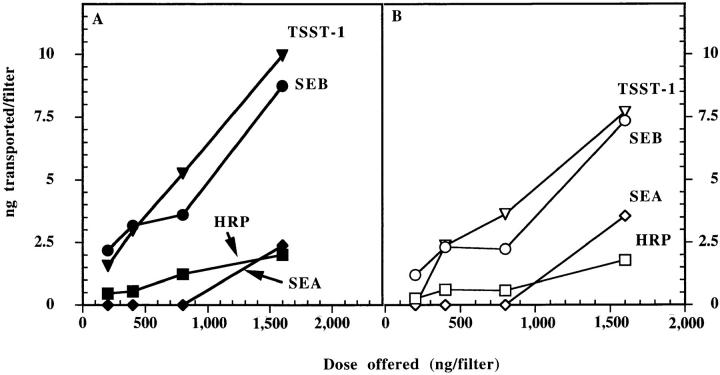
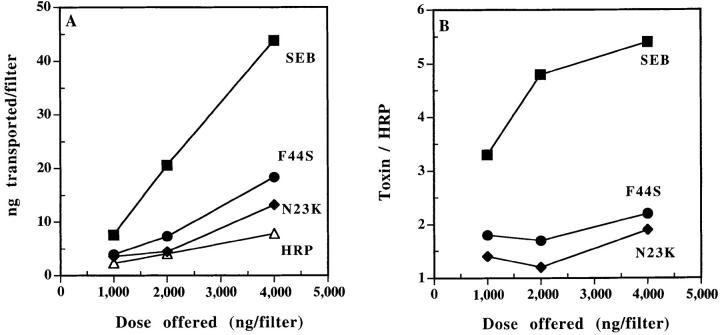
Residues N23 and F44 of SEB play critical roles in its SAg activity. Previously, we have shown that residue N23 is essential for interaction with the TCR (22), whereas the stretch of six amino acids located in the loop between SEB β strands 1 and 2 (including residue F44) are involved in binding to MHC class II molecules (22, 31, 32). In an attempt to determine if these domains of SEB also may play a role in transcytosis, we compared the rate of transcytosis of SEB through Caco-2 monolayers to that of two SEB mutants: N23 >K and F44 >S. Although, in our experience, these mutations do not affect the sensitivity of SEB detection by the pair of the mAbs used (23), standard curves of mutants were used to calibrate their concentrations in the tested supernatants as an added precaution. These mutant SEBs are reduced in their capacity to stimulate T cells by at least 1,000-fold (22). Either mutation reduced the rate of SEB transcytosis by 50–70% (Fig. 4). These results suggest that these regions of SEB have a function in the transcytosis and provide additional evidence for the specificity of SEB transcytosis by Caco-2 cells.
Figure 4.
Mutation of residues N23 and F44 affects SEB transcytosis by Caco-2 cells. (A) The amount of wild type and mutants SEB transcytosed (apical to basal) per filter in 4 h. The experiment was done as in Fig. 3. Wild type and mutant SEB were used to construct standard curves. (B) The amounts of wild type and mutants transcytosed relative to that of HRP. Each point is the mean of three determinations. SEM was usually <15%. This experiment is representative of three separate experiments with similar results.
Presentation of SEB but Not SEA to T Cells by Caco-2.
Our results suggested that transcytosis by Caco-2 cells of SEB, but not SEA, may be facilitated by a specific receptor. To examine this idea further, we tested the ability of Caco-2 cells to present SEA and SEB to T cell hybridomas bearing the appropriate Vβ regions in their T cell receptors. This experiment was possible because, unlike normal T cells, T cell hybridomas do not require professional APC-specific coreceptors for activation. Also, recent experiments have established that MHC class II is not an essential part of the SAg ligand for T cells, provided that the SAg is presented in the proper orientation for T cell receptor interaction (23, 33–35).
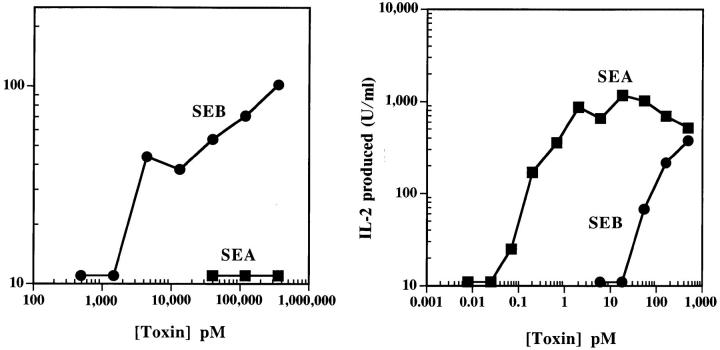
We cultured a Vβ3 and Vβ8.2 T cell hybridoma with various concentrations of SEA or SEB in the presence of either Caco-2 or DR1+ fibroblasts as the APCs. The results are shown in Fig. 5. The DR1-bearing fibroblast presented both SEA and SEB efficiently to the appropriate T cell hybridoma. On the other hand, Caco-2 presented SEB, but not SEA. However, Caco-2 was about 100-fold less efficient than the DR1+ fibroblast in presenting SEB, possibly indicating a very low affinity receptor.
Figure 5.
Presentation of SEB, but not SEA, by Caco-2 cells. (A) Monolayers of Caco-2 cells (8 d) were used to present various concentrations of each toxin to specific T cell hybrids. SEA reactive (Vβ3+) and SEB reactive (Vβ8.2+) T cell hybrids were used. (B) As a positive control, DR1+ LG2 cells were used as APCs. IL-2 produced by activated T cells was measured as described in the Materials and Methods. This experiment is representative of four similar experiments.
Transcytosis of SEA and SEB in Mice.
Transcytosis of SEB and TSST-1 by Caco-2 cells shows that intestinal epithelia have the potential to deliver toxins across the intestine. To study transcytosis under more physiological conditions, we tested the ability of orally administered toxins to cross the intestinal barrier of mice and enter systemic circulation without altering their functional capacity. Since TSST-1 is labile to digestion by proteolytic enzymes of the gastrointestinal tract especially pepsin (36), we limited the in vivo experiments to SEA and SEB which are fairly resistant to digestion by gastrointestinal secretions (37). SEA or SEB were introduced into the stomachs of mice and the appearance of the functional toxin in the circulation was followed either by using serum from these mice to stimulate in vitro T cell hybridomas bearing the appropriate Vβ elements or by following the in vivo deletion of T cells bearing these Vβs over the course of several weeks.
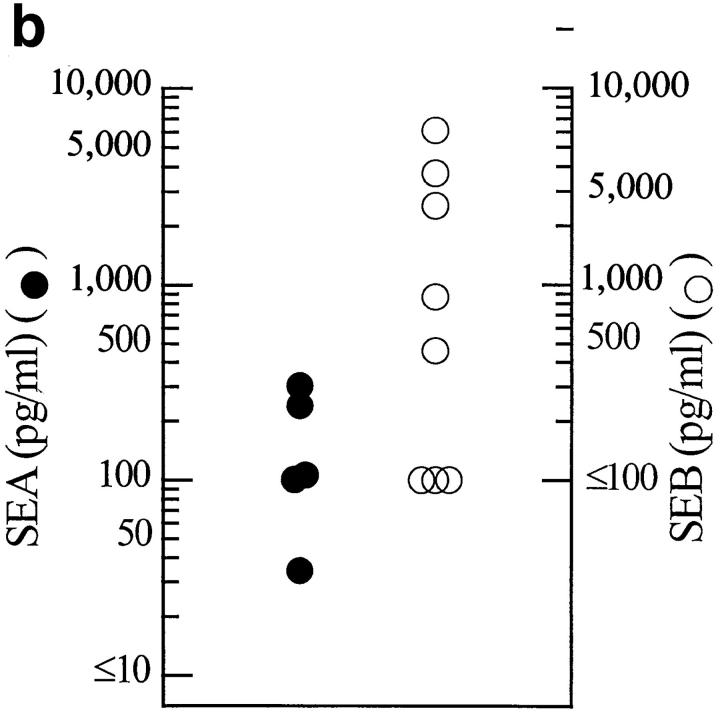
Results from the in vitro stimulation experiments are shown in Fig. 6. Various quantities of sera taken from mice after feeding either SEA or SEB were assessed for their ability to stimulate IL-2 production by Vβ3- or Vβ8.2-bearing T cell hybridomas, respectively, using DR1+ APCs. Preliminary experiments established that serum levels of toxins peaked at about 2 h (data not shown). Typical titrations are shown in Fig. 6 a. These titration data were used to calculate the levels of functional toxins in the sera as described in Materials and Methods. These data are summarized for all mice in Fig. 6 b. Functional SEB and SEA were easily detected in the sera of most of the mice. There was considerable variation in the levels achieved, particularly of SEB, but on average SEB reached levels (2,750 pg/ml) more than 20 times those of SEA (100 pg/ml). This difference may be related to the facilitated transcytosis of SEB versus the apparently passive transcytosis of SEA seen in vitro with human Caco-2 cells. However, other explanations such as differential degradation or clearance of the two toxins in vivo cannot be excluded by our data.
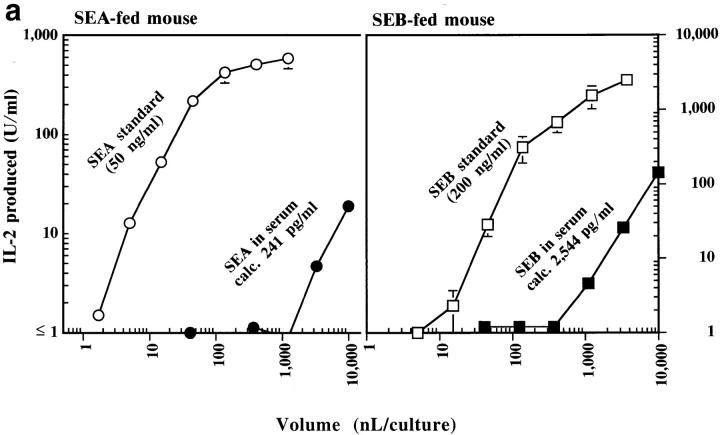
Figure 6.
(a) Transcytosis of SEA and SEB by B10.BR mice. A representative experiment is shown in which various amounts of serum from a mouse fed SEA or SEB (200 μg each), or of standard SEA (50 ng/ml) or SEB (200 ng/ml) were used to activate 5KC or 2B10 T cell hybrids, respectively, in the presence of DAP-DR1 as APCs. IL-2 produced by the activated T cells was measured as described in Materials and Methods. The figure shows a plot of the volume of serum or standard used versus the amount of IL-2 produced. (b) Oral SEB is absorbed more efficiently than SEA by B10. BR mice. The figure shows a summary of levels of SEA or SEB in the sera of individual mice (five for SEA and eight for SEB) determined 2 h after ingestion of the toxin calculated as in Fig. 6 a and Materials and Methods.
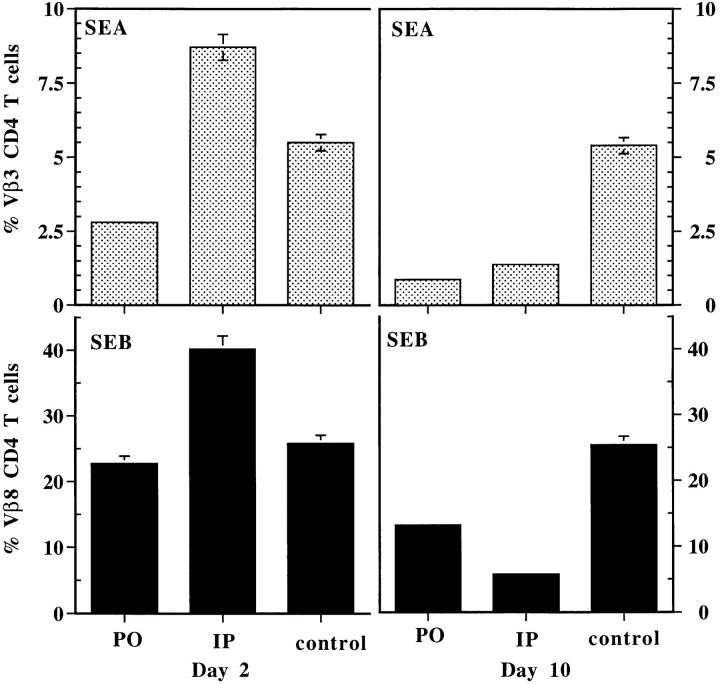
As a second indication of the transcytosis of functional SEA and SEB in these mice, we followed in vivo the fate of Vβ3- and Vβ8-bearing T cells. Exposure of mice to systemic injections of SAgs usually leads to expansion of T cells (maximum around day 2) bearing the appropriate Vβs followed by clonal deletion that becomes evident between 7 and 10 d after injection (38). Therefore, we examined the blood of the mice for the levels of T cells bearing these Vβs on day 2 and 10 after feeding SEA or SEB, and compared these results to those obtained with an intraperitoneal injection of the same dose of toxins. The results are shown in Fig. 7. In both cases, as expected, intraperitoneal injection led to expansion of the appropriate T cell pool on day 2 followed by substantial deletion by day 10. Feeding the same amount of toxin, however, led to substantial deletion on day 10 that was not preceded by an expansion on day 2. The most likely explanation for this difference lies in the lower effective level of toxin achieved with feeding over direct injection, since direct injection of very low doses of toxin has been shown to lead to in vivo T cell deletion that is not preceded by detectable T cell expansion (38). Taken together, these results show that SEB and SEA readily cross the intestinal epithelium and enter the blood stream in a fully functional form.
Figure 7.
Oral SEA and SEB cause in vivo deletion of reactive CD4+ T cells. Blood samples were collected on days 2 and 10 from mice that received oral or intraperitoneal toxins and from control (untreated) mice as well. T cells were purified and stained simultaneously using CD4 and Vβ3+ or Vβ8+ mAbs, and analyzed on FACScan®. This data is the mean from three mice in each group ± SEM. This experiment is representative of two experiments with similar results.
Discussion
The main conclusion of these studies is that the staphylococcal exotoxins, SEB, SEA, and TSST-1, can rapidly cross an epithelial membrane in intact, fully functional forms. These findings have implications for the role of SEB, SEA, and similar SAg enterotoxins in food poisoning since, if the SAg activity of these toxins is important in this pathology, they must have the ability to cross the intestinal epithelium intact to gain access to T cells.
Our experiments with the human polarized enterocytic cell line Caco-2, suggest that these toxins may cross intestinal epithelium by more than one mechanism. The rate of SEA transcytosis was similar to that of a marker for water transport, HRP, suggesting a nonspecific mechanism. SEB, on the other hand, was transcytosed at three to six times the rate of HRP, suggesting a receptor-facilitated process. Correlating with this idea was the ability of Caco-2 to act as an APC for SEB, but not SEA, in stimulating T cell hybridomas bearing the appropriate Vβ element. Other evidence in support of this conclusion was the inhibition of SEB transcytosis by mutations in N23 or F44. These mutations also interfere with the SAg activity of SEB by disrupting T cell receptor and MHC class II binding, respectively. Previously, Harris et al. (37) has shown that these mutations also interfere with SEB-induced food poisoning in monkeys. While interpreted at the time in terms of the SAg activity of SEB, our current findings suggest that the reduction in SEB transcytosis caused by these mutations may have contributed as well to the results.
Despite the appearance of a receptor-mediated mechanism for SEB transcytosis, several features usually associated with such a mechanism were not observed. The rate of transcytosis was only slightly favored in the apical to basal direction through the Caco-2 monolayer. Usually, transcytosis is biased in one direction because the distribution of the receptor involved is polarized. For example, in intestinal and mammary epithelium, transcytosis of secretory IgA from the basal to apical surface occurs via an Fc receptor (pIgR) whose distribution is polarized in favor of the basolateral surface (39–43). Reciprocally, absorption of IgG from ingested milk in neonatal rats and mice is mediated by a special Fc receptor (FcRn), and polarized to the apical surface of the intestinal epithelium (44, 45). However, there are, in fact, examples of bidirectional transcytosis of ligands such as glutathione (46) and glycylsarcosine (47). Also, there is a recent description by Ellis and Luzio (48) of a novel receptor on Caco-2 cells that can transcytose in both directions.
More importantly, in our experiments we saw no evidence for saturation of SEB transcytosis, even using SEB concentrations up to 300 μg/ml. If this were a receptormediated process, we would have expected the rate to maximize as the receptor became saturated (at concentrations of ∼10 times the k D). These results suggest that the putative receptor must be of low affinity (∼k D >5 × 10−5 M), a result consistent with the finding that SEB presentation to T cells by Caco-2 is much less efficient than by MHC class II+ fibroblast. (The MHC class II affinity for SEB is ∼10−6 M.)
Surprisingly, we observed facilitated transcytosis of TSST-1 as well as SEB by Caco-2 cells. Again, this transcytosis occurred at a rate about fivefold greater than that of HRP and was bidirectional and could not be saturated. TSST-1 is not an enterotoxin, most likely because it is destroyed by digestive enzymes in the stomach and intestine (49). However, TSST-1 is a major cause of toxic shock syndrome. In women, TSST-1 produced in the vagina can find its way into the blood stream, where the subsequent toxic shock is accompanied by massive stimulation of peripheral Vβ2-bearing T cells (13, 15). Since these cases are often associated with use of a particular type of tampon during menses, the toxin may enter the body via direct contact with blood. However, if the putative receptor mediating TSST-1 transcytosis through Caco-2 is common to other types of epithelium, then transcytosis may contribute as well to the uptake of the toxin. This notion is supported by the ability of conjuctival epithelia and endothelia to endocytose and transcytose TSST-1 (50).
Previous studies have shown that there are non-MHC receptors for some of the staphylococcal toxins on intestinal epithelia, B cells, and mast cells (33–35). These studies showed that human intestinal epithelial cells and mouse B cells or mast cells could present SEB, but not SEA. In contrast, lymph node cells from class II–deficient mice could present SEC1-3 and SEE, but not SEB or SEA. Some of these results are similar to those described in this paper. Perhaps, the receptors which participate in toxins binding and transcytosis are related to some of these reported molecules.
In an attempt to relate our results to a more physiological situation we documented the appearance of ingested SEA and SEB in the blood of mice. Functional toxin was detected both by the ability of serum from these animal to stimulate the appropriate T cell hybridomas in vitro and by the in vivo partial disappearance of T cells bearing the appropriate Vβ elements. These results extend those of Migita and Ochi (29) who used Western blotting to show the appearance of intact SEB in the blood of mice fed the toxin. As in our in vitro transcytosis studies, we found that the extent of SEA uptake was less than that of SEB, although both rapidly reached functional levels in the serum. This could again reflect a passive versus active mechanism of transcytosis, but other mechanisms, such as differential degradation, are not excluded by our results.
Acknowledgments
We would like to thank Dr. Kathryn Howell (University of Colorado Health Sciences Center, Denver, CO) for helpful suggestions and Dr. Masazumi Matsumura (Nextar Pharmaceutical, Boulder, CO) for the gift of anti-TSST-1 mAbs before publication. Thanks are also due to Janice White, Janice Clement, Ella Kushnir and Frances Crawford for their help and support. The critical proofreading of the manuscript by Janice White and Jeremy Bender is greatly appreciated.
This work was supported by U.S. Public Health grants AI-47134, AI-18785, and AI-22295.
Footnotes
1 Abbreviations used in this paper: HRP, horseradish peroxidase; SAg, superantigen; SE, staphylococcal enterotoxins; TEER, transepithelial electrical resistance; TSST-1, toxic shock syndrome 1.
References
- 1.Bergdoll, M.S. 1970. Enterotoxins. In Microbial Toxins III. Bacterial protein toxins. T.C. Moutie, S. Kadis, and S.J. Ajil, editors. Academic Press, New York. 265–326.
- 2.Bergdoll, M.S. 1979. Staphylococcal Intoxications. In Food Bourne Infections and Intoxications. H.R.A.F.L. Bryan, editor. Acadamic Press, New York. 443–494.
- 3.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureusassociated with toxic shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 4.Todd JK. Toxic shock syndrome. Clin Microbiol Rev. 1988;1:432. doi: 10.1128/cmr.1.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P. The Vβ-specific superantigen Staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- 6.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, Carrel S, Posnett DN, Choi Y, Marrack P. Vβ-specific stimulation of human T cells by staphylococcal toxins. Science (Wash DC) 1989;244:811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 7.Yagi J, Baron J, Buxser S, Janeway CAJ. Bacterial proteins that mediate the association of a defined subset of T cell receptor: CD4 complexes with class II MHC. J Immunol. 1990;144:892–901. [PubMed] [Google Scholar]
- 8.Janeway CAJ, Yagi J, Conrad PJ, Katz ME, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 9.Scherer MT, Ignatowicz L, Winslow G, Kappler JW, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–128. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 10.Woodland DL, Blackman M. How do T cell receptors, MHC molecules and superantigens get together? . Immunol Today. 1993;14:208–212. doi: 10.1016/0167-5699(93)90164-G. [DOI] [PubMed] [Google Scholar]
- 11.Herman A, Kappler JW, Marrack P, Pullen AM. Superantigens-mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 12.Callahan JE, Herman A, Kappler JW, Marrack P. Stimulation of B10.BR T cells with superantigenic staphylococcal toxins. J Immunol. 1990;144:2473–2479. [PubMed] [Google Scholar]
- 13.Choi Y, Lafferty JA, Clements JR, Todd JK, Gelfand EW, Kappler J, Marrack P, Kotzin BL. Selective expansion of T cells expressing Vβ2 in toxic shock syndrome. J Exp Med. 1990;172:981–984. doi: 10.1084/jem.172.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrack P, Blackman M, Kushnir E, Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990;171:455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotzin BL, Yeung DYM, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–165. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 16.Pinto M, Robine-Leon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 17.Dantzig AH, Bergin L. Uptake of the cephalosporin cephalexin, by a dipeptide transport carrier in the human cell line, Caco-2. Biochem Biophys Acta. 1990;1027:211–217. doi: 10.1016/0005-2736(90)90309-c. [DOI] [PubMed] [Google Scholar]
- 18.Fogh J, Wright WC, Loveless JD. Absence of Hela cell contamination in 169 cell lines derived from human tumors. J Natl Cancer Inst. 1977;58:209–214. doi: 10.1093/jnci/58.2.209. [DOI] [PubMed] [Google Scholar]
- 19.White J, Pullen A, Choi K, Marrack P, Kappler JW. Antigen recognition properties of mutant Vb3+T cell recptors are consistent with an immunoglobulin-like structure for the receptor. J Exp Med. 1993;177:119–125. doi: 10.1084/jem.177.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gatti R, Leibold W. HLA-D typing with lymphoblastoid cell lines. IV. Allelic relationships. Tissue Antigens. 1979;13:35–44. doi: 10.1111/j.1399-0039.1979.tb01134.x. [DOI] [PubMed] [Google Scholar]
- 21.Herman A, Croteau G, Sekaly R, Kappler J, Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990;172:709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappler JW, Herman A, Clements J, Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992;175:387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamad ARA, Herman A, Marrack P, Kappler JW. Monoclonal antibodies defining functional sites on the toxin superantigen SEB. J Exp Med. 1994;180:615–622. doi: 10.1084/jem.180.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimonkevitz R, Boen E, Malmstrom S, Brown E, Hurley JM, Kotzin BL, Matsumura M. Delineation by use of specific monoclonal antibodies of the T cell receptor and major histocompatibility complex interaction sites on the superantigen toxic shock syndrome toxin 1. Infect Immun. 1996;64:1133–1139. doi: 10.1128/iai.64.4.1133-1139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dailynas D, Wilde D, Marrack P, Pierres A, Wall K, Havran W, Otten G, Loken M, Pierres M, Kappler J, Fitch F. Characterization of the murine antigenic determinant designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appear to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 26.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:C260–C267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 27.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2–restricted, interleukin-2–producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Migita K, Ochi A. Induction of clonal anergy by oral administration of staphylococcal enterotoxin B. Eur J Immunol. 1994;24:2081–2086. doi: 10.1002/eji.1830240922. [DOI] [PubMed] [Google Scholar]
- 30.Bonsdorff CV, Fuller SD, Simons K. Apical and basolateral endocytosis in Madin-Darby Kidney (MDCK) cells grown on nitrocellulose filters. EMBO (Eur Mol Biol Organ) J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swaminathan S, Furey W, Pletcher J, Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature (Lond) 1992;359:801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- 32.Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, Chi YI, Stauffacher C, Strominger JL, Wiley DC. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature (Lond) 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 33.Dohlsten MG, Hedlund G, Segren S, Lando P, Herrmann T, Kelly A, Kalland T. Human major histocompatiblity complex class II–negative colon carcinoma cells present staphylococcal superantigens to cytotoxic T lymphocytes: evidence for a novel enterotoxin receptor. Eur J Immunol. 1991;21:1229–1233. doi: 10.1002/eji.1830210520. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann T, Romero P, Sartoris S, Pailoa F, Accola RS, Maryanski JL, MacDonald HR. Staphylococcal enterotoxin-dependent lysis of MHC class II negative target cells by cytolytic T lymphocytes. J Immunol. 1991;146:2504–2512. [PubMed] [Google Scholar]
- 35.Avery AC, Markowitz JS, Grusby MJ, Glimcher LH, Cantor H. Activation of T cells by superantigen in class II–negative mice. J Immunol. 1994;153:4853–4861. [PubMed] [Google Scholar]
- 36.Spero, L., A. Johnson-Winger, and J.J. Schmidt. 1988. Enterotoxins of Staphylococci. Handbook of Natural Toxins. C. Hardegree and A. Tu, editors. Marcel Dekker Inc., New York. 131–163.
- 37.Harris TO, Grossman D, Kappler J, Marrack P, Rich RR, Betley MJ. Lack of complete correlation between emetic and T cell–stimulatory activities of staphylococcal enterotoxins. Infect Immun. 1993;61:3175–3183. doi: 10.1128/iai.61.8.3175-3183.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormack JE, Callahan JE, Kappler J, Marrack P. Profound deletion of mature T cells in vivo by chronic exposure to exogenous superantigen. J Immunol. 1993;150:3785–3792. [PubMed] [Google Scholar]
- 39.Mostov K, Friedlander M, Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature (Lond) 1984;308:37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- 40.Mostov K, Simister NE. Transcytosis. Cell. 1985;43:389–390. doi: 10.1016/0092-8674(85)90166-7. [DOI] [PubMed] [Google Scholar]
- 41.Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- 42.Underdown BJ, Schiff JM. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 43.Gruenberg J, Howell KE. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- 44.Jones EA, Waldman TA. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J Clin Invest. 1972;51:2916–2927. doi: 10.1172/JCI107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jakoi ER, Cambier J, Saslow S. Transepithelial transport of maternal antibody: purification of IgG receptor from newborn rat intestine. J Immunol. 1985;135:3360–3364. [PubMed] [Google Scholar]
- 46.Elfernik RPJ, Bakker CTM, Jansen PLM. Glutathione-conjugate transport by human colon adenocarcinoma cells (Caco-2 cells) Biochem J. 1993;290:759–764. doi: 10.1042/bj2900759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thwaites DT, McEwan GTA, Hirst BH, Simmons NL. Transepithelial dipeptide (glycylsarcosine) transport across epithelial monolayers of human Caco-2 cells is rheogenic. Eur J Physiol. 1993;425:178–180. doi: 10.1007/BF00374520. [DOI] [PubMed] [Google Scholar]
- 48.Ellis JA, Luzio JP. Identification and characterization of a novel protein (p137) which transcytoses bidirectionally in Caco-2 cells. J Biol Chem. 1995;270:20717–207123. doi: 10.1074/jbc.270.35.20717. [DOI] [PubMed] [Google Scholar]
- 49.Spero L, Morlock AB. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978;253:8787–8791. [PubMed] [Google Scholar]
- 50.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]