Abstract
Intranasal Herpes simplex virus type 1 (HSV-1) infection of mice caused pneumonia. Manifestations of the disease included: histological pneumonitis, pulmonary influx of lymphocytes, decreased pulmonary compliance, and decreased survival. Immunohistochemical staining demonstrated iNOS induction and the nitrotyrosine antigen in the lungs of infected, but not uninfected mice, suggesting that nitric oxide contributes to the development of pneumonia. To elucidate the role of nitric oxide in the pathogenesis of HSV-1 pneumonia, infected mice were treated either with the inhibitor of nitric oxide synthase activity, NG-monomethyl-l-arginine (l-NMMA), or, as a control, with PBS or d-NMMA. l-NMMA treatment decreased the histological evidence of pneumonia and reduced the bronchoalveolar lavage lymphocyte number to one-quarter of the total measured in control-treated mice. l-NMMA treatment significantly improved survival and pulmonary compliance of HSV-1–infected mice. Strikingly, the l-NMMA–mediated suppression of pneumonia occurred despite the presence of a 17-fold higher pulmonary viral titer. Taken together, these data demonstrated a previously unrecognized role of nitric oxide in HSV-1–induced pneumonia. Of note, suppression of pneumonia occurred despite higher pulmonary virus content; therefore, our data suggest that HSV-1 pneumonia is due to aspects of the inflammatory response rather than to direct viral cytopathic effects.
Herpes simplex virus type 1 (HSV-1)1 pneumonia is of considerable clinical significance, particularly in newborns and immunocompromised patients (1–6), but the pathogenesis is poorly understood. A broader mechanistic understanding of the disease would provide a basis for prophylactic and therapeutic strategies in HSV-1 pneumonia patients (7).
Nitric oxide (NO) produced by the inducible isoform of NO-synthase (iNOS, NOS2) plays a multifunctional role in the cytotoxicity of activated macrophages, in the pathophysiology of septic shock, and in the suppression of cardiac function (8, 9). Studies of iNOS-deficient mutant mice demonstrated that NO contributes to endotoxic shock and to the immune response to bacteria and parasites (10, 11). Experiments with NG-monomethyl-l-arginine (l-NMMA) or aminoguanidine, inhibitors of nitric oxide synthase (NOS), showed the involvement of NO in influenza virus pneumonia (12), graft versus host disease (13), experimental autoimmune encephalomyelitis (14), and alloreactivity (15).
NO production can be beneficial as an antiviral effector mechanism against HSV-1 and other viruses (16–19). On the other hand, NO may also be detrimental by contributing to pathology during viral infections (20–22); for example, NO induced by gp41 of HIV-1 contributes to the dementia seen in AIDS patients (23).
NO is crucial for the pathogenesis of influenza virus pneumonia in mice (12). Inhibition of iNOS by l-NMMA treatment improved survival of virus-infected mice without exerting an effect on the pulmonary viral titer. Thus, NO is important for the development of influenza virus pneumonia but not as an antiviral effector mechanism (12). In contrast, HSV-1 is susceptible to NO (18, 19). Furthermore, NO is produced during HSV-1 infection (24). Therefore, we wanted to determine whether iNOS inhibition in HSV-1–induced pneumonia would lead to a higher pulmonary viral burden and exacerbation of pneumonitis, or alternatively, to a reduction in proinflammatory effects of NO with suppression of pneumonitis. Therefore, inhibition of NO production could affect the development of viral pneumonitis in opposite ways: it could worsen the disease by diminishing the antiviral effect of NO, or it could improve the pneumonitis by reducing a proinflammatory mechanism.
Our data suggest that NO is an important mediator of HSV-1 pneumonia. Inhibition of NO production by l-NMMA treatment led to a significantly improved survival rate and to a strong suppression of HSV-1–induced pneumonitis despite the presence of a higher pulmonary virus content. These results suggest that iNOS inhibition is beneficial; the proinflammatory effects of NO are more important for the pathology of HSV-1 pneumonia than the antiviral effects of NO.
Materials and Methods
Mice.
Female CBA/J (H-2k) mice, purchased from The Jackson Laboratory (Bar Harbor, ME), were used at 12–26 wk of age.
Infection of Mice.
Mice were infected intranasally with 1 × 106 PFU HSV-1, strain KOS 1.1 (25), kindly provided by Drs. R. Finberg and J. Brubaker (Dana-Farber Cancer Institute, Boston, MA), given in a final volume of 0.03 ml sterile 0.9% sodium chloride (Fujisawa USA, Inc., Deerfield, IL) per mouse. For survival experiments, mice were infected with 2 LD50 (5 × 107 PFU per mouse in this system). The virus stock used for this study was clarified supernatant derived from infected Vero cell cultures (Advanced Biotechnologies, Inc., Columbia, MD). The Vero cells and culture media were screened and shown to be free of mycoplasma and endotoxin contamination.
Treatment of Mice with NMMA.
l-NMMA (Alexis Corp., San Diego, CA) dissolved in PBS (pH 7.4), was given at a dose of 2 mg/day i.p. in a volume of 0.2 ml from the day of virus infection until analysis of mice as described (12). Infected control mice were treated with the same amount of NG-monomethyl-d-arginine (d-NMMA) (Sigma Chem. Co., St. Louis, MO) or with PBS. Treatment of uninfected mice with l-NMMA did not affect any of the tested parameters, when compared to uninfected, untreated mice (data not shown).
Tissue Sampling.
Mice were euthanized by CO2 asphyxiation 7 d after infection, blood was collected by cardiac puncture, and the trachea was cannulated using a 19-gauge tubing adapter (Becton Dickinson, Rutherford, NJ) inserted through an incision immediately distal to the larynx. Bronchoalveolar lavage (BAL) was performed with sterile 0.9% sodium chloride, using three separate 1.0-ml aliquots, each of which was infused and withdrawn five times. The cells were centrifuged and resuspended in RPMI medium containing 10% FCS for further analysis. When indicated, the left lung was processed for virus titration, and the right lower lobe of the lung was used for histological examination. Previous studies demonstrated that the virus titration and histology were not affected by the lung lavage procedure (Adler, H., L. Kobzik, and I.J. Rimm, unpublished results).
Flow Cytometry.
BAL lymphocytes were stained for flow cytometry and analyzed using CellQuest on a FACScan® (Becton Dickinson). The following monoclonal antibodies, conjugated either with fluorescein isothiocyanate or phycoerythrin were used for staining: anti-B220, anti-CD4, anti-CD8, anti-CD3, anti-α/β T cell receptor, and anti-γ/δ T cell receptor (PharMingen, San Diego, CA). Fluorescein isothiocyanate- or phycoerythrin-conjugated isotype-matched antibodies were used as negative controls.
Virus Titration and Histology.
The left lung was homogenized by grinding in 1 ml of sterile 0.9% sodium chloride and stored frozen at −80°C. Viral titers were determined from thawed aliquots by plaque assay on Vero cells, and the number of plaques was determined after staining the cells with crystal violet.
For histology, the lower lobe of the right lung was inflated with O.C.T. compound (Miles Inc., Elkhart, IN) and fixed in 10% formalin in PBS (Fisher Scientific, Fair Lawn, NJ). After fixation, the samples were dehydrated and embedded in paraffin. 5-μm-thick sections were cut and stained with hematoxylin/ eosin (H/E). The samples were analyzed and scored microscopically in a blind study on coded slides as described (26). Two types of pathological changes were scored separately. Histopathologic changes around airways and vessels were termed periluminal, and histopathologic changes within the alveolar parenchyma were scored as parenchymal abnormalities. A final score of pneumonitis from 0 to 9 was assigned for each pathologic process (periluminal and parenchymal) as the product of severity grade by the extent. The score that is presented reflects the sum of both scores.
Staining for iNOS and Nitrotyrosine.
For immunohistochemistry, mouse lungs were inflated by intratracheal instillation of O.C.T. compound and immediately frozen in a dry ice/2-methylbutane bath. Immunostaining was performed on 8-μM cryostat sections using a standard peroxidase anti-peroxidase protocol (27). Primary antibodies included rabbit anti-NOS2 or anti-nitrotyrosine (Upstate Biotechnology Inc., Lake Placid, NY) or normal rabbit IgG (Sigma) used at 1 μg/ml. Diaminobenzidine was used as a chromogen, and slides were counterstained with hematoxylin before microscopic evaluation.
Pulmonary Function Test.
As parameter of lung function, dynamic compliance (Cdyn, defined as the increase in lung volume obtained from a given increase in distending pressure at points of zero flow) was determined in vivo 7 d after infection. Cdyn is a parameter that generally reflects the condition of the lung parenchyma, and is decreased by pulmonary interstitial edema, or inflammation, or by any condition that reduces alveolar patency. We used a previously described plethysmographic technique for making measurements of lung function (28). Anesthetized mice (70–90 mg/kg Na pentobarbital i.p.) underwent placement of a tracheostomy tube for mechanical ventilation (7 ml/kg/breath, 150 breaths/min) and thoracotomy to eliminate any effect that the chest wall may have on pulmonary function.
Determination of HSV-1–specific Antibodies.
HSV-1–specific IgG2a antibodies in serum samples of mice 7 d after infection were determined using a specific ELISA (29). In brief, microtiter plates coated with HSV-1 Macintyre Strain Viral Lysate (Advanced Biotechnologies Inc., Columbia, MD) were incubated with serial dilutions of a standard serum or test samples, and after extensive washing, alkaline phosphatase conjugated goat anti–mouse IgG2a (Southern Biotechnology, Birmingham, AL) was added. The plates were developed using p-nitrophenyl phosphate (Sigma) in DEA buffer. The reaction was stopped with 3 N NaOH, and absorbance was read at 405 nm using an ELISA reader. In all assays, samples and standards were run at least in duplicates. The calculation of the HSV-1–specific antibodies was performed by the method of Zollinger and Boslego (30). As expected, no HSV-1–specific IgG2a antibodies were detected in sera of uninfected mice. In contrast, in all sera of infected mice, regardless of their treatment, HSV-1–specific IgG2a antibodies were readily detected (data not shown). Thus, all infected animals seroconverted in response to infection.
Statistical Analyses.
The Student's t test (unpaired) and the Wilcoxon rank sum test were used for data analysis where appropriate. Treatment-group differences in the viral titer per lung were analyzed by one-way analysis of variance (ANOVA).
Results
Intranasal Infection of Mice with HSV-1 Led to the Development of Pneumonia.
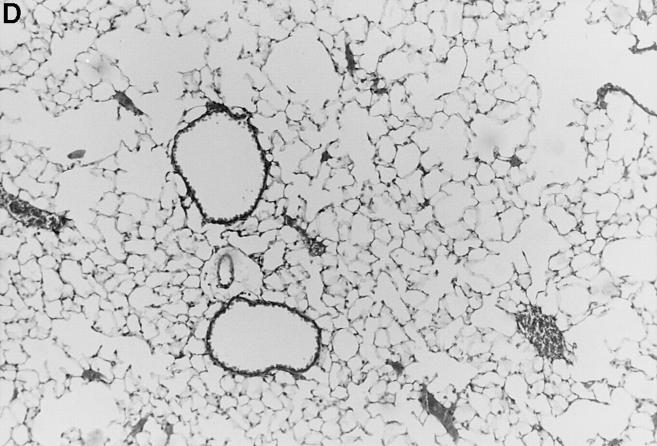
Intranasal infection of mice with HSV-1 led to the development of pneumonia. Histopathological changes in infected animals were most evident at day 7 after infection. The pneumonia was characterized histologically by mononuclear infiltrates (Fig. 1, compare A with D), an increase in the histology score (Fig. 2, compare uninfected/ l-NMMA with infected/PBS), an increase in the number of cells recovered by BAL (Fig. 3, A and B, compare uninfected/l-NMMA with infected/PBS), a change in the CD4/ CD8 ratio (Table 1, compare uninfected/untreated with infected/PBS), and by a decrease in lung compliance (see below).
Figure 1.
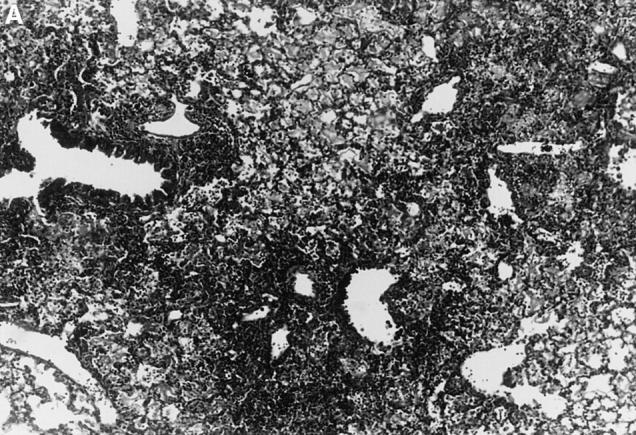
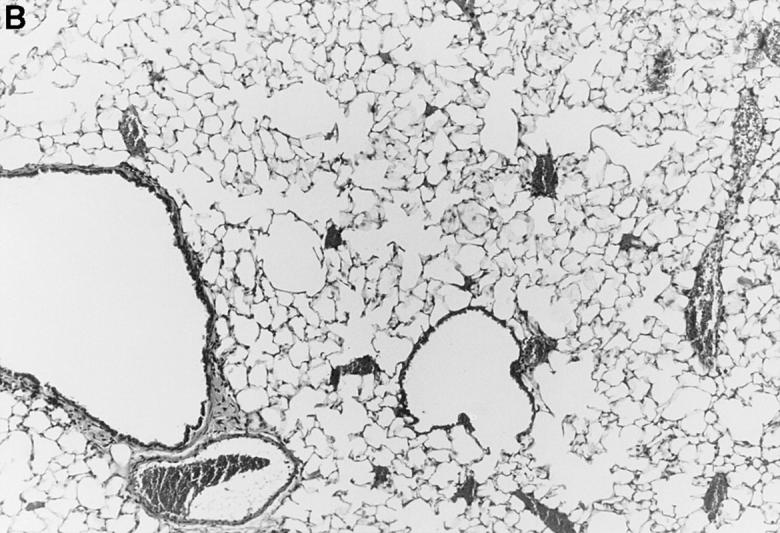
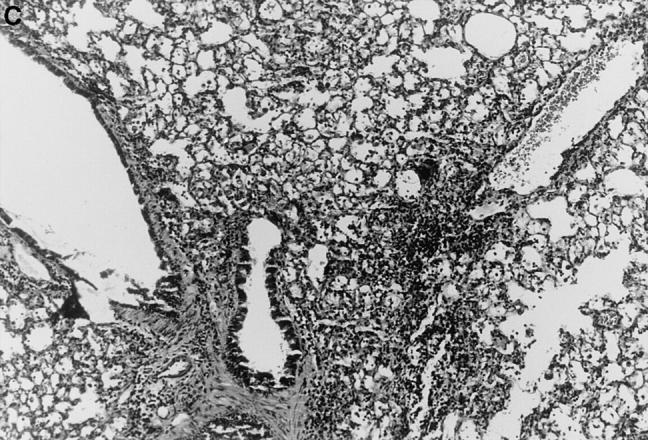
l-NMMA treatment decreased histological evidence of pneumonia. Mice were infected with HSV-1 and treated with l-NMMA, d-NMMA, or PBS. Photomicrographs of lung sections stained with H/E show the histopathological changes at day 7 after infection. (A) infected, treated with PBS; (B) infected, treated with l-NMMA; (C) infected, treated with d-NMMA; (D) uninfected, treated with l-NMMA. Original magnification: (A and C) ×100; (B and D) ×40.
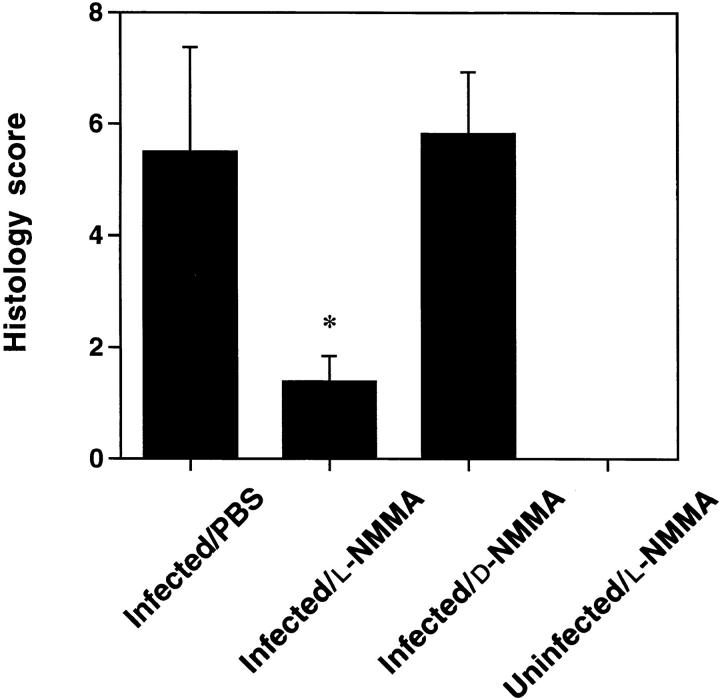
Figure 2.
l-NMMA treatment decreased pneumonitis scores. HSV1–infected mice were treated with l-NMMA, d-NMMA or PBS daily from the day of virus infection. Pathology scores were determined 7 d after infection. Data represent means ± SE of 6–13 mice in each group, pooled from 3–5 separate experiments. The asterisk indicates a significant difference from both infected/d-NMMA and infected/PBS (P <0.01; Student's t test).
Figure 3.
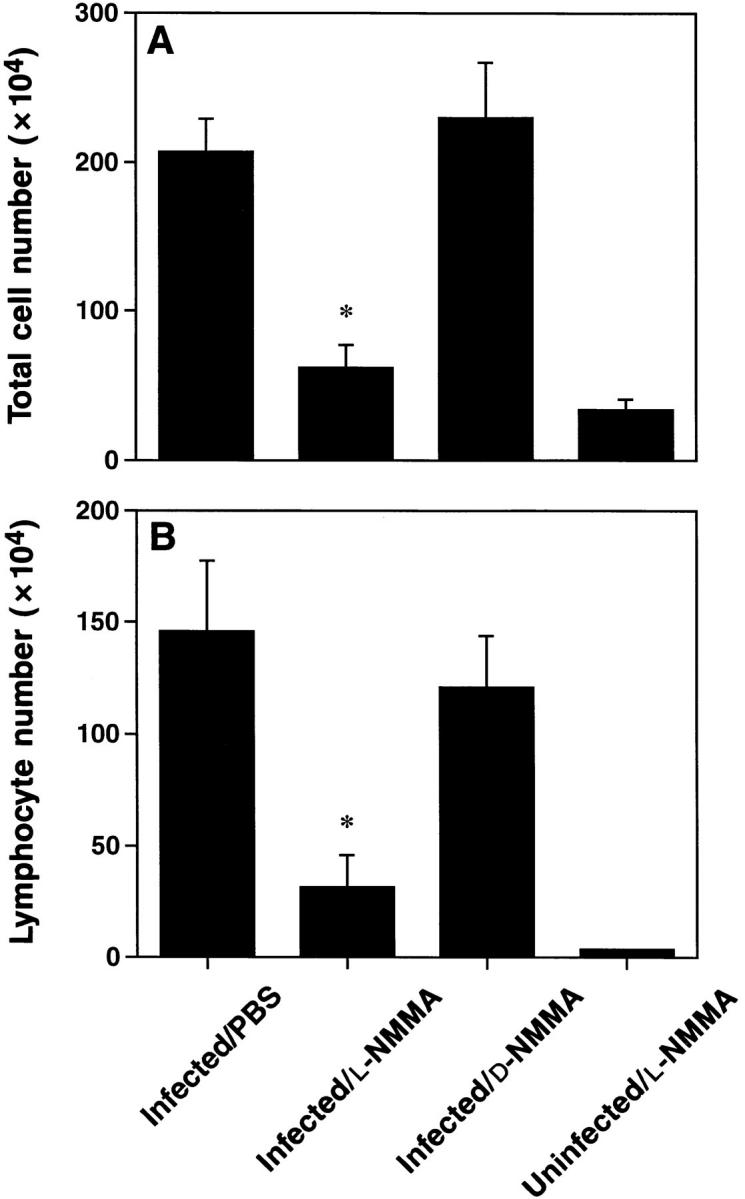
Treatment with l-NMMA suppressed the influx of cells recovered by BAL. Mice were treated with l-NMMA, d-NMMA or PBS. BAL cell numbers were determined 7 d after infection. (A) Total cell number recovered by BAL. (B) Lymphocyte numbers were calculated using total cell number in BAL and percent lymphocytes gated during FACS®-analysis. Values presented are means ± SE of 6–13 mice per group, pooled from 3–5 separate experiments. The asterisks indicate a significant difference from both infected/d-NMMA and infected/PBS (P <0.003; Student's t test).
Table 1.
Treatment with l-NMMA Did Not Affect the Lymphocyte Subset Distribution at Day 7 after Intranasal HSV-1 Infection
| Lymphocyte subsets (%) | Uninfected/untreated or l-NMMA (n = 13) | Infected/ l-NMMA (n = 13) | Infected/ d-NMMA (n = 7) | Infected- PBS (n = 6) | ||||
|---|---|---|---|---|---|---|---|---|
| Ratio CD4/CD8 | 2:1 | 1:4 | 1:4 | 1:5 | ||||
| B220+ | 1.8 ± 0.4 | 2.4 ± 0.6 | 3.4 ± 0.4 | 2.5 ± 0.6 | ||||
| CD3+ | 40.2 ± 8.5 | 73.2 ± 6.2 | 82.4 ± 1.4 | 82.7 ± 1.6 | ||||
| CD4+ | 20.4 ± 5.7 | 15.0 ± 0.9 | 14.9 ± 1.7 | 13.1 ± 1.6 | ||||
| CD8+ | 11.0 ± 1.8 | 56.6 ± 7.5 | 65.7 ± 3.2 | 68.7 ± 4.0 |
Animals were treated from day 0 to 6 with l-NMMA, d-NMMA, or PBS. BAL was performed at day 7 after infection, and the recovered cells were analyzed by FACS® analysis. Values presented are means ± SEM of the number of mice shown in brackets. Data are pooled from 3–5 separate experiments.
Immunolocalization of iNOS and Nitrotyrosine Antigens.
To demonstrate the induction of iNOS and the production of NO during HSV-1–induced pneumonia, we performed immunohistochemistry on lung tissue of uninfected and infected mice at days 3 and 7 after infection, using antibodies against murine iNOS and nitrotyrosine. iNOS was detected within inflammatory cells in infected mice, but not in uninfected mice. Similarly, nitrotyrosine staining was observed in infected mice, but not in uninfected mice (data not shown). Thus, these results demonstrated that NO is indeed produced during the course of HSV-1 infection, confirming previous data (24). NO reacts with O2 − to generate peroxynitrite, which is involved in microbial killing as well as toxicity (9, 31, 32). Nitrotyrosine is a marker of peroxynitrite generation, since nitrotyrosine is a product resulting from peroxynitrite action (12).
l-NMMA Treatment Decreased Histological Evidence of Pneumonia.
Since iNOS and nitrotyrosine staining demonstrated the involvement of NO in HSV-1 pneumonia, experiments were designed to test the effect of l-NMMA treatment on HSV-1 pneumonia. Mice were infected intranasally with 1 × 106 PFU of HSV-1 per mouse, and histological examination of lungs was performed at day 7 after infection. In HSV-1–infected, PBS-treated control mice, pneumonitis was characterized by mononuclear infiltrates around vessels and bronchioles (periluminal), and a diffuse intraalveolar infiltrate consisting mainly of mononuclear cells (parenchymal) (Fig. 1 A). In contrast, the development of pneumonia was strongly suppressed in mice treated with l-NMMA (Fig. 1 B). The suppression of pneumonia was specific, since the biologically inactive stereoisomer d-NMMA did not show this effect (Fig. 1 C). Normal lungs were observed in uninfected mice either treated with l-NMMA (Fig. 1 D) or left untreated (data not shown). Histological scores reflecting the periluminal and parenchymal changes in the lungs of infected mice were significantly lower in infected animals treated with l-NMMA, compared to infected mice treated with d-NMMA or PBS. In fact, treatment with l-NMMA suppressed the pneumonitis scores nearly to the level observed in uninfected mice (Fig. 2).
l-NMMA Treatment Reduced the Number of Cells Recovered by BAL.
Intranasal infection with HSV-1 led to a pronounced increase in the total cell number as well as in the lymphocyte number recovered by BAL at day 7 after infection, when compared to uninfected animals (Fig. 3, A and B). l-NMMA treatment, however, markedly inhibited the influx of inflammatory cells. In contrast, treatment with d-NMMA showed no effect. Thus, the BAL data were consistent with the results obtained by histology.
Since lymphocytes were the predominant cells recruited to the lung after HSV-1 infection, we analyzed whether treatment with l-NMMA changed the composition of the lymphocyte population. Neither treatment with l-NMMA nor d-NMMA altered the lymphocyte subpopulations in infected mice, when compared with infected, PBS-treated control mice (Table 1). All groups of infected mice showed a dominance of CD3+ T cells. The CD4/CD8 ratio changed from 2:1 in uninfected mice to ∼1:4 in infected mice. The vast majority of the cells were α/β TCR+; very low numbers of γ/δ TCR+ cells were detected (data not shown).
l-NMMA Treatment Improved Lung Compliance in Infected Mice.
To assess the effect of l-NMMA treatment on the development of pneumonia, we also measured lung compliance as a parameter of in vivo global lung function. Pulmonary compliance is a quantitative measure of bronchopulmonary injury; additionally these results estimate the magnitude of the physiological derangement resulting from the infection. A reduction in lung compliance, or the most closely related human pulmonary function test parameter, total lung capacity, to 30% of a previously normal level would be considered a life-threatening change of pulmonary status. Lung compliance was 0.032 ± 0.002 (mean ± SE; n = 3) in uninfected mice treated with l-NMMA, which was similar to values measured in uninfected, untreated mice (data not shown). In infected, d-NMMA– treated mice, compliance was reduced to 0.010 ± 0.003 (n = 3), i.e., to 30% of the level in uninfected mice. In contrast, treatment of infected mice with l-NMMA significantly improved lung compliance to 0.029 ± 0.001 (n = 4), when compared to infected, but d-NMMA–treated mice (P <0.004; Student's t test). Thus, l-NMMA treatment improved lung compliance of infected animals nearly to the levels measured in uninfected mice.
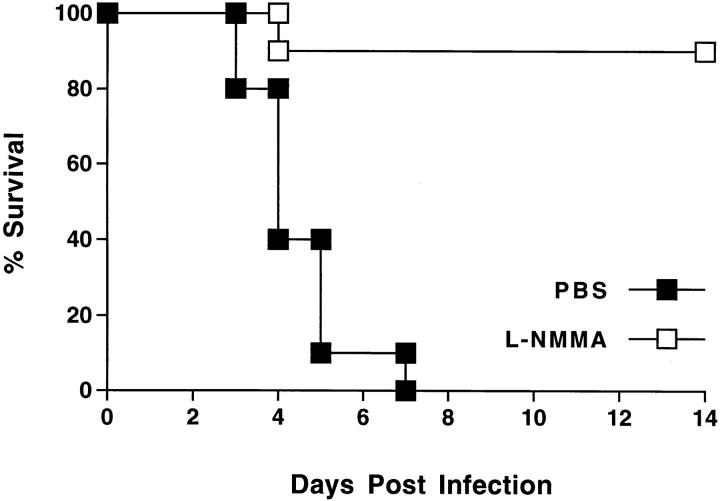
l-NMMA Treatment Increased Survival after HSV-1 Infection.
Mice were infected intranasally with 5 × 107 PFU of HSV-1 per mouse (∼2 LD50), and treated with l-NMMA or PBS daily for 7 d after infection. Survival of mice was monitored over a period of 14 d after infection. l-NMMA treatment led to a significant improvement in the survival rate (Fig. 4).
Figure 4.
l-NMMA treatment increased survival of mice after intranasal infection with HSV-1. 10 mice per group were infected with 2 LD50 of HSV-1, and treated with l-NMMA or PBS daily from the day of virus infection until day 7. l-NMMA treatment significantly improved the survival rate (P = 0.0001; Wilcoxon rank sum test).
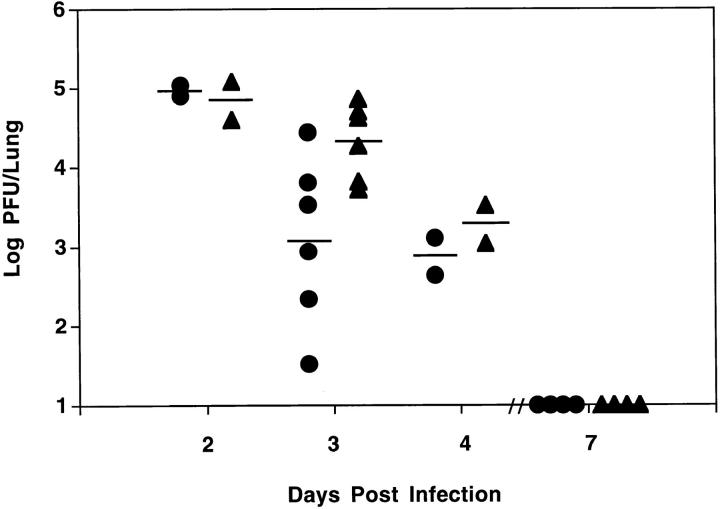
l-NMMA Treatment Increased Viral Titers in the Lung.
To assess the role of NO in the antiviral defense, pulmonary viral titers were determined at days 2, 3, 4, and 7 after infection. Similar viral titers were observed at day 2 after infection between mice treated either with l-NMMA or PBS (Fig. 5). However, a 17-fold higher pulmonary viral titer was observed at day 3 in the l-NMMA–treated mice, when compared to mice treated with PBS. At day 4 after infection, a 2.5-fold higher viral titer was measured in the l-NMMA–treated mice. Virus was cleared at day 7 after infection in all groups of mice. The difference in pulmonary viral titer between the l-NMMA and PBS group was significant.
Figure 5.
Treatment with l-NMMA led to a significant higher pulmonary viral titer. Animals were treated from day 0 until analysis with l-NMMA (triangles) or PBS (circles). Each data point represents the pulmonary viral titer (Log PFU/Lung) obtained from an individual animal. The means for the groups are shown by the solid lines. The viral titer in the l-NMMA– treated group at day 3 post infection was significantly higher than in PBStreated animals. The difference in viral titer per lung between the l-NMMA and PBS group was significant (P = 0.0199 using ANOVA).
The significantly higher pulmonary viral titer observed at day 3 after infection in the l-NMMA–treated mice was not accompanied by an increase in the extent of pneumonitis, as demonstrated by a comparable histopathology score and a similar number of BAL inflammatory cells for both the l-NMMA– and PBS-treated mice (Table 2).
Table 2.
The Higher Pulmonary Viral Titer at Day 3 after Intranasal HSV-1 Infection Was Not Associated with Increased Pulmonary Pathology
| Infected/ l-NMMA | Infected/ PBS | |||
|---|---|---|---|---|
| Histology score | 0.3 ± 0.2 | 0.8 ± 0.4 | ||
| Total cell number in BAL (×104) | 69.0 ± 7.0 | 72.0 ± 17.0 | ||
| Lymphocyte number in BAL (×104) | 8.3 ± 1.4 | 6.5 ± 3.6 | ||
| Pulmonary virus content (Log PFU/lung) | 3.7 ± 0.2* | 2.7 ± 0.4 |
Animals were treated from day 0 until analysis with l-NMMA or PBS. Histopathology scores, BAL cell numbers and pulmonary viral titers were determined in parallel 3 d after infection. Values presented are means ± SE of five individual mice per group.
The difference in viral titer per lung between the l-NMMA and PBS group was significant (P <0.05; Student's t test).
Discussion
Intranasal infection with HSV-1 led to the development of pneumonia. We demonstrated that inhibition of NO production led to a significantly improved survival rate as well as to a strong suppression of the pneumonia despite the presence of a 17-fold higher viral titer in the lung at 3 d after infection. These results suggested that the proinflammatory effects of NO are more important than its antiviral effector functions for the development of HSV-1–induced pneumonia. Furthermore, these results also support the notion that the inflammatory response to HSV-1 infection, rather than direct viral cytopathic effects, is critical for the development of pneumonia. Immunopathology is involved in other HSV-1 infections, for example, an immune-mediated pathogenesis that involves T cells is the basis for herpetic stromal keratitis (33, 34). A T cell–mediated immunopathology is also believed to be involved in the pathogenesis of CMV-induced pneumonia (35). Consequently, reduction of virus growth did not prevent, but did moderate, murine CMV pneumonitis (36, 37). Therefore, treatment of HSV-1–induced pneumonia with antiviral drugs alone might not be sufficient, and a modulation of components of the immune response may also be required. Administration of selective inhibitors of NOS activity in the treatment of inflammatory airway diseases has been suggested (38, 39).
In our study, inhibition of NO production led to a 17-fold increase in pulmonary viral titer at 3 d after infection, confirming earlier reports on an antiviral activity of NO against HSV (18, 19). Therefore, it was remarkable that inhibition of NO production strongly suppressed the development of pneumonia. A recent study of the pathogenesis of influenza virus-induced pneumonia in mice demonstrated that inhibition of NO production by l-NMMA administration resulted in a significant improvement of the survival rate of virus-infected mice (12). However, in this case, l-NMMA treatment did not significantly affect the propagation of influenza virus in vivo.
Inhibition of NO production might lead to suppression of pneumonia by preventing the influx of inflammatory cells to the lung. NO can cause vasodilation, leading to alterations in blood flow, which may promote leukocyte infiltration. NO can also modulate leukocyte adhesion to endothelial cells and alter microvascular permeability during inflammation (9, 31). In vivo neutralization of IFN-γ reduced the magnitude of the pulmonary cellular infiltrate after pulmonary influenza virus infection (40). Thus, IFN-γ may recruit leukocytes into inflamed lung parenchyma. IFN-γ is a strong iNOS inducer, able to induce NO production as a single stimulus (41). Thus, the mechanism of IFN-γ action in this system may be to increase NO production. IFN-γ is also induced during HSV-1 infection of the lung (Adler, H., and I.J. Rimm, unpublished results).
NO or its reaction products might directly cause tissue damage and the release of cytokines and chemokines, which then attract inflammatory cells to the site of virus infection. Influenza A virus infection selectively induced chemokines responsible for the predominant mononuclear leukocyte infiltration in virally infected tissue (42). NO induces NF-kB binding activity and may lead to secretion of proinflammatory lymphokines and cytokines from activated lymphocytes (8, 43).
Treatment with l-NMMA clearly did not prevent the development of pneumonia by interfering with the viral infection of the lung for the following reasons: (a) higher viral titers were detected in l-NMMA treated animals (Fig. 5). (b) The change in the CD4/CD8 ratio of the BAL lymphocytes, which is characteristic for a viral infection of the lung (44), was not affected by the treatment (Table 1). (c) All infected animals seroconverted in response to the intranasal infection (data not shown).
Although there is currently controversy regarding the production of NO by human macrophages (45), increasing numbers of reports demonstrate iNOS induction or NO production in human diseases (46–50). Moreover, NO can be produced by iNOS of a number of other cells types including for example pulmonary epithelial cells (31, 39, 51). In conclusion, we demonstrated an important role of NO in HSV-1–induced pneumonia in mice that may have substantial implications for the therapy of this disease.
Acknowledgments
The authors thank Dr. Steven Burakoff for helpful discussions and Dr. Paul Martin for critical reading of the manuscript.
This study was supported by grants from the National Institutes of Health, the Mallinckrodt Foundation and the Aid for Cancer Research. I.J. Rimm is a Claudia Adams Barr Investigator. H. Adler is supported by a grant from the Deutsche Forschungsgemeinschaft.
Footnotes
1 Abbreviations used in this paper: BAL, bronchoalveolar lavage; Cdyn, lung compliance; H/E, hematoxylin/eosin; HSV-1, Herpes simplex virus-type 1; iNOS, inducible nitric oxide synthase; d-NMMA, NG-monomethyl-d-arginine; l-NMMA, NG-monomethyl-l-arginine; NO, nitric oxide; NOS, nitric oxide synthase.
Presented in part at the AAAAI/AAI/CIS Joint Meeting, San Francisco, CA, February 21–26, 1997.
References
- 1.Whitley, R.J., and J.W. Gnann, Jr. 1993. The epidemiology and clinical manifestations of Herpes simplex virus infections. In The Human Herpesviruses. B. Roizman, R.J. Whitley, and C. Lopez, editors. Raven Press, Ltd., New York. 69–97.
- 2.Wingard JR. Infections in allogeneic bone marrow transplant recipients. Semin Oncology. 1993;20:80–87. [PubMed] [Google Scholar]
- 3.Ruben FL, Nguyen MLT. Viral pneumonitis. Clin Chest Med. 1991;12:223–235. [PubMed] [Google Scholar]
- 4.Hull HF, Blumhagen JD, Benjamin D, Corey L. Herpes simplex viral pneumonitis in childhood. J Pediatr. 1984;104:211–215. doi: 10.1016/s0022-3476(84)80994-4. [DOI] [PubMed] [Google Scholar]
- 5.Hubbell C, Dominguez R, Kohl S. Neonatal Herpes simplex pneumonitis. Rev Infect Dis. 1988;10:431–438. doi: 10.1093/clinids/10.2.431. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey PG, Fife KH, Hackman RC, Meyers JD, Corey L. Herpes simplex virus pneumonia. Clinical, virologic, and pathologic features in 20 patients. Ann Intern Med. 1982;97:813–820. doi: 10.7326/0003-4819-97-6-813. [DOI] [PubMed] [Google Scholar]
- 7.Quabeck K. The lung as a critical organ in marrow transplantation. Bone Marrow Transplant. 1994;14:S19–S28. [PubMed] [Google Scholar]
- 8.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 9.Schmidt HHHW, Walter U. NO at work. Cell. 1994;78:919–925. doi: 10.1016/0092-8674(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 10.Wei X-Q, Charles IG, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Moncada S, Liew FY. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature (Lond) 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 11.MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie Q-W, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 12.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng YM, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garside P, Hutton AK, Severn A, Liew FY, Mowat AM. Nitric oxide mediates intestinal pathology in graft-versus-host disease. Eur J Immunol. 1992;22:2141–2145. doi: 10.1002/eji.1830220827. [DOI] [PubMed] [Google Scholar]
- 14.Cross AH, Misko TP, Lin RF, Hickey WF, Trotter JL, Tilton RG. Aminoguanidine, an inhibitor of inducible nitric oxide synthase, ameliorates experimental autoimmune encephalomyelitis in SJL mice. J Clin Invest. 1994;93:2684–2690. doi: 10.1172/JCI117282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worral NK, Lazenby WD, Misko TP, Lin T-S, Rodi CP, Manning PT, Tilton RG, Williamson JR, Ferguson TB., Jr Modulation of in vivo alloreactivity by inhibition of inducible nitric oxide synthase. J Exp Med. 1995;181:63–70. doi: 10.1084/jem.181.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris N, Buller RML, Karupiah G. Gamma interferon-induced, nitric oxide-mediated inhibition of vaccinia virus replication. J Virol. 1995;69:910–915. doi: 10.1128/jvi.69.2.910-915.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi Z, Reiss CS. Inhibition of vesicular stomatitis virus infection by nitric oxide. J Virol. 1995;69:2208–2213. doi: 10.1128/jvi.69.4.2208-2213.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croen KD. Evidence for an antiviral effect of nitric oxide. J Clin Invest. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karupiah G, Xie Q-W, Buller RML, Nathan C, Duarte C, MacMicking JD. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science (Wash DC) 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 20.Zheng YM, Schäfer MK-H, Weihe E, Sheng H, Corisdeo S, Fu ZF, Koprowski H, Dietzschold B. Severity of neurological signs and degree of inflammatory lesions in the brains of rats with borna disease correlate with the induction of nitric oxide synthase. J Virol. 1993;67:5786–5791. doi: 10.1128/jvi.67.10.5786-5791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dawson VL, Dawson TM, Uhl GR, Snyder SH. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci USA. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koprowsky H, Zheng YM, Heber-Katz E, Fraser N, Rorke L, Fu ZF, Hanlon C, Dietzschold B. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc Natl Acad Sci USA. 1993;90:3024–3027. doi: 10.1073/pnas.90.7.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamson DC, Wildemann B, Sasaki M, Glass JD, McArthur JC, Christov VI, Dawson TM, Dawson VL. Immunologic NO synthase: elevation in severe AIDS dementia and induction by HIV-1 gp41. Science (Wash DC) 1996;274:1917–1921. doi: 10.1126/science.274.5294.1917. [DOI] [PubMed] [Google Scholar]
- 24.Rolph MS, Ramshaw IA, Rockett KA, Ruby J, Cowden WB. Nitric oxide production is increased during murine vaccinia virus infection, but may not be essential for virus clearance. Virol. 1996;217:470–477. doi: 10.1006/viro.1996.0141. [DOI] [PubMed] [Google Scholar]
- 25.Hughes RG, Munyon WH. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975;16:275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Jr, Crawford JM, Ferrara JLM. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;8:3230–3239. [PubMed] [Google Scholar]
- 27.Kobzik, L., and H.H.H.W. Schmidt. 1996. Immunohistochemistry of nitric oxide synthase and nitric oxide-related products. In Methods in Nitric Oxide Research. M. Feelisch and J.S. Stamler, editors. John Wiley & Sons Ltd., New York. 229–236.
- 28.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen L, Knipe DM, Finberg RB. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- 30.Zollinger WD, Boslego JW. A general approach to standardization of the solid-phase radioimmunoassay for quantitation of class-specific antibodies. J Immunol Methods. 1981;46:129–140. doi: 10.1016/0022-1759(81)90130-7. [DOI] [PubMed] [Google Scholar]
- 31.Laskin JD, Heck DE, Laskin DL. Multifunctional role of nitric oxide in inflammation. Trends Endocrinol Metab. 1994;5:377–382. doi: 10.1016/1043-2760(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 32.Nussler AK, Billiar TR. Inflammation, immunoregulation, and inducible nitric oxide synthase. J Leuk Biol. 1993;54:171–178. [PubMed] [Google Scholar]
- 33.Doymaz MZ, Rouse BT. Immunopathology of Herpes simplex virus infections. Curr Top Microbiol Immunol. 1992;179:121–136. doi: 10.1007/978-3-642-77247-4_8. [DOI] [PubMed] [Google Scholar]
- 34.Mercadal CM, Bouley DM, DeStephano D, Rouse BT. Herpetic stromal keratitis in the reconstituted scid mouse model. J Virol. 1993;67:3404–3408. doi: 10.1128/jvi.67.6.3404-3408.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka K, Koga Y, Lu Y-Y, Zhang X-Y, Wang Y, Kimura G, Nomoto K. Murine cytomegalovirusassociated pneumonitis in the lungs free of the virus. J Clin Invest. 1994;94:1019–1025. doi: 10.1172/JCI117415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanley JD, Pesanti EL. The relation of viral replication to interstitial pneumonitis in murine cytomegalovirus lung infection. J Infect Dis. 1985;151:454–458. doi: 10.1093/infdis/151.3.454. [DOI] [PubMed] [Google Scholar]
- 37.Shanley JD, Morningstar J, Jordan MC. Inhibition of murine cytomegalovirus lung infection and interstitial pneumonitis by acyclovir and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother. 1985;28:172–175. doi: 10.1128/aac.28.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barnes PJ. Nitric oxide and airway disease. Ann Med. 1995;27:389–393. doi: 10.3109/07853899509002592. [DOI] [PubMed] [Google Scholar]
- 39.Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995;16:128–130. doi: 10.1016/0167-5699(95)80128-6. [DOI] [PubMed] [Google Scholar]
- 40.Baumgarth N, Kelso A. In vivo blockade of gamma interferon affects the influenza virus-induced humoral and the local cellular immune response in lung tissue. J Virol. 1996;70:4411–4418. doi: 10.1128/jvi.70.7.4411-4418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 42.Sprenger H, Meyer RG, Kaufmann A, Bussfeld D, Rischkowsky E, Gemsa D. Selective induction of monocyte and not neutrophil-attracting chemokines after influenza A virus infection. J Exp Med. 1996;184:1191–1196. doi: 10.1084/jem.184.3.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lander HM, Sehajpal P, Levine DM, Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993;150:1509–1516. [PubMed] [Google Scholar]
- 44.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 45.Denis M. Human monocytes/macrophages: NO or no NO? . J Leuk Biol. 1994;55:682–684. doi: 10.1002/jlb.55.5.682. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson S, da Gloria M, Bonecini-Almeida, Lapa e Silva JR, Nathan C, Xie Q-W, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, Arnelle DR, Hollis D, McDonald MI, Granger DL. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type II expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clair EWS, Wilkinson WE, Lang T, Sanders L, Misukonis MA, Gilkeson GS, Pisetsky DS, Granger DL, Weinberg JB. Increased expression of blood mononuclear cell nitric oxide synthase type 2 in rheumatoid arthritis patients. J Exp Med. 1996;184:1173–1178. doi: 10.1084/jem.184.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vodovotz Y, Lucia MS, Flanders KC, Chesler L, Xie Q-W, Smith TW, Weidner J, Mumford R, Webber R, Nathan C, et al. Inducible nitric oxide synthase in tanglebearing neurons of patients with Alzheimer's disease. J Exp Med. 1996;184:1425–1433. doi: 10.1084/jem.184.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McInnes IB, Leung BP, Field M, Wei XQ, Huang F-P, Sturrock RD, Kinninmonth A, Weidner J, Mumford R, Liew FY. Production of nitric oxide in the synovial membrane of rheumatoid and osteoarthritis patients. J Exp Med. 1996;184:1519–1524. doi: 10.1084/jem.184.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asano K, Chee CBE, Gaston B, Lilly CM, Gerard C, Drazen JM, Stamler JS. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci USA. 1994;91:10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]