Abstract
The recruitment of eosinophils into the airways after allergen exposure is dependent on interleukin (IL) 5 secreted from antigen-specific CD4+ T cells of the T helper cell (Th) 2 subset. However, while it is established that costimulation through CD28 is required for TCR-mediated activation and IL-2 production, the importance of this mechanism for the induction of a Th2 immune response is less clear. In the present study, we administered the fusion protein CTLA-4 immunoglobulin (Ig) into the lungs before allergen provocation to determine whether CD28/CTLA-4 ligands are required for allergen-induced eosinophil accumulation and the production of Th2 cytokines. Administration of CTLA-4 Ig inhibited the recruitment of eosinophils into the lungs by 75% and suppressed IgE in the bronchoalveolar lavage fluid. CTLA-4 Ig also inhibited the production of IL-4, IL-5, and IL-10 by 70–80% and enhanced interferon-γ production from CD3–T cell receptor–activated lung Thy1.2+ cells. Allergen exposure upregulated expression of B7-2, but not B7-1, on B cells from the lung within 24 h. Moreover, airway administration of an anti-B7-2 monoclonal antibody (mAb) inhibited eosinophil infiltration, IgE production, and Th2 cytokine secretion comparable in magnitude to that observed with CTLA-4 Ig. Treatment with an anti-B7-1 mAb had a small, but significant effect on eosinophil accumulation, although was less effective in inhibiting Th2 cytokine production. The anti-B7-2, but not anti-B7-1, mAb also inhibited antigen-induced airway hyperresponsiveness in vivo. In all of the parameters assessed, the combination of both the anti-B7-1 and anti-B7-2 mAb was no more effective than anti-B7-2 mAb treatment alone. We propose that strategies aimed at inhibition of CD28 interactions with B7-2 molecules may represent a novel therapeutic target for the treatment of lung mucosal allergic inflammation.
Bronchial asthma is characterized by the infiltration of eosinophils into the airway submucosa (1). Activation of eosinophils with the subsequent secretion of highly charged granular cationic proteins such as major basic protein is believed to be important in the pathogenesis of bronchial asthma (2–4). While the precise mechanisms by which eosinophils are recruited into the lungs are not fully understood, there is increasing evidence suggesting that activation of antigen-specific CD4+ T cells of the Th2 subset in the lungs resulting in IL-5 secretion plays a major role in allergic inflammation of the airways (5–7).
T cell activation requires interaction between the TCR and specific antigen, presented in the form of processed peptides in association with MHC class II molecules. However, for complete T cell activation, a second signal termed costimulation is required (8, 9). The most widely studied costimulatory molecule is CD28, which is constitutively expressed on the surface of both CD4+ and CD8+ T cells. Activation of CD28 in conjunction with the CD3–TCR complex is required for T cell proliferation and IL-2 production (10–12). In the absence of CD28-mediated signaling, a state of unresponsiveness or anergy develops (9–11). The natural ligands for CD28 have more recently been identified as B7-1 or CD80 (13–15), and B7-2 or CD86 (16, 17), and have been demonstrated to be present on the surface of antigen-pulsed B cells (13–15) and dendritic cells (18) and may account for the efficacy of these cells as APCs.
B7-1 and B7-2 also bind to CTLA-4, a homologue of CD28 (16, 19). A soluble chimeric fusion protein consisting of the extracellular domain of CTLA-4 and a human γ-1 constant region, CTLA-4 Ig (20), binds to both B7-1 and B7-2 with an affinity 20 times greater than CD28, and inhibits CD28– and CTLA-4–mediated signaling (19–22). In vitro studies using murine and human cells have demonstrated that CTLA-4 Ig inhibits T cell proliferation and IL-2 production, and induces a state of T cell unresponsiveness (19–22). However, while it is clearly established that CD28mediated signaling is required for IL-2 production, the requirement of costimulation through CD28 for the induction of a Th2 immune response is less clear. In the present series of experiments, we have administered CTLA-4 Ig into the airways before aerosol antigen challenge to determine whether costimulation through these molecules is required for the recruitment of eosinophils into the lungs and the production of Th2 cytokines. To further delineate the involvement of B7-1 and/or B7-2, the expression of these molecules on lung B cells after allergen provocation was assessed and neutralizing mAbs to either B7-1 and/or B7-2 were administered into the lung before allergen provocation. The results of this study indicate that allergen exposure selectively upregulates B7-2 expression and that costimulation through this molecule is required for the induction of a lung mucosal Th2 immune response and for allergen-induced altered airway responsiveness.
Materials and Methods
Antibodies and Cytokines
The CTLA-4 Ig used is a fusion protein between the extracellular domain of murine CTLA-4 and human γ-1 obtained from a CTLA-4 Ig–producing hybridoma (20) provided by Dr. Peter Lane (Basel Institute of Immunology, Basel, Switzerland). Supernatants were then concentrated and purified over a protein A column. PE-labeled Thy1.2 (30-H12), PE-labeled B220 (RA36B2), FITC-labeled B7-1 (16-10A1), FITC-labeled and purified B7-2 (GL-2), and CD16/CD32 (2.4G2, Fc Block™) were purchased from PharMingen, (San Diego, CA). Purified B7-1 (1610A1; reference 23) was obtained from Dr. Hans Reiser (Dana Farber Cancer Institute, Boston, MA). Human recombinant IL-2 and anti-CD3 (2C11) was obtained from Ciba-Geigy AG. (Basel, Switzerland).
In Vivo Experiments
Quantification of Eosinophil Recruitment to the Lung.
Sv129 mice (20– 25 g) of either sex were immunized intraperitoneally with 10 μg of OVA (grade V; Sigma Chemical Co., St. Louis, MO) in 0.2 ml of alum (Serva, Heidelberg, Germany). 10 d later, animals were anesthetized by inhaled 2% Forene™ (isofluran; Abbott, Cham, Switzerland) and 100 μg of CTLA-4 Ig administered to the lungs (in a volume of 50 μl) by the intranasal route. Control mice were treated with 100 μg of human γ-1 as the appropriate control antibody. In preliminary experiments, we have used Evans blue dye to document that intranasal delivery results in 75% of the dye deposited in the airways, with no detectable dye in the esophagus or stomach. Moreover, studies using an anti-Fas mAb, which when given systemically were fatal due to fulminant hepatitis, did not induce mortality when given intranasally, supporting the argument that administration of agents via this route does not reach the systemic circulation (24).
In a separate series of experiments, we administered 100 μg of mAbs to B7-1 (16-10A1) and/or B7-2 (GL-2) (also in a volume of 50 μl) via the intranasal route. Control mice received 100 μg of rat IgG as an isotype control antibody. 4 h after each antibody treatment, mice were placed in a plastic box and exposed, while conscious, to an aerosol of OVA (50 mg/ml), of which 90% of the particles are <5 μm in size, for 20 min daily for a total of 5 d. Control mice were exposed to an aerosol of PBS for the same time period. 72 h after the last antigen inhalation, mice were anesthetized with urethane, the trachea cannulated, and bronchoalveolar lavage (BAL)1 performed by four repeated lavages with 0.3 ml of PBS injected into the lungs via the trachea. Total cell counts were performed, cytospins prepared (Shandon, Scientific Ltd., Cheshire, U.K.), stained with Diff-Quik® (Baxter Dade AG., Dudingen, Switzerland), and a differential count of 200 cells was performed.
In Vitro Experiments
Determination of BAL Fluid IgE.
Total IgE in the BAL fluid was determined by ELISA as described previously (25) with the following alterations. In brief, microtiter plates were coated with rat anti–murine IgE 3B-39 (04-6100; Zymed Labs., Inc., S. San Francisco, CA). After incubation with dilutions of BAL fluid, sample-bound mouse IgE was detected with biotinylated rat anti– murine IgE 3-11. Data were expressed as total IgE per ml of BAL fluid. The limit of detection of the assay was 2 ng/ml of IgE.
Purification of Lung T Cells.
To analyze the lung T cell cytokine profile, experiments were performed 72 h after the last antigen challenge. After the four repeated lavages for assessment of the inflammatory cell infiltrate, the lungs were perfused via the right ventricle with 5 ml of PBS containing 100 U/ml of heparin to remove any blood and intravascular leukocytes. The lungs were then removed and placed into DMEM containing 10% FCS, 2-mercaptoethanol (50 μM), sodium pyruvate (1 mM), Hepes (10 mM), and gentamycin (50 μg/ml). The lungs were then gently homogenized, the cell suspension was filtered through a 70-μM filter, and lymphocytes enriched over a single step Ficoll gradient. B cells were depleted from the cell suspension using magnetic sheep anti–mouse Ig beads (Dynabeads, Nycomed, Norway). Cells were then labeled with Thy1.2-PE and purified by flow cytometry (Beckton Dickinson, San Jose, CA) as described previously (5). In all experiments, purity was >99% Thy1.2+.
Cell Culture.
Lung Thy1.2+ cells were then plated at a concentration of 2 × 105 in 96-well microtiter plates coated with an anti-CD3 antibody (2C11, 50 μg/ml). Cells were cultured for 72 h in the presence of human IL-2 (200 U/ml). Supernatants were harvested and cytokine production determined by ELISA as described elsewhere (5). The limits of detection were IL-4, 0.5 U/ml; IL-5, 100 U/ml; IL-10, 10 U/ml, IL-2, 0.2 U/ml; IFN-γ, 50 U/ml.
Immunofluoresence and Flow Cytometry Analysis.
To determine the expression of CD28 ligands on lung B lymphocytes 24 h after the first, third, or fifth aeroallergen challenge, lymphocytes were enriched from the lung as described above and stained with antiB220-PE. Nonspecific binding to Fcγ receptors was then blocked by incubating cells at 4°C for 30 min with CD16/CD32 mAb (2.4G2). Cells were then washed twice and incubated for a further 30 min with FITC anti-B7-1 or FITC-B7-2. Expression of B7 molecules after allergen exposure was compared to either before challenge or after repeated challenge with PBS. Fluorescence was analyzed by flow cytometry, gated on the region of B220+ cells (FACScan®) using consort 30 software, and 10,000 events acquired.
Measurement of Airway Hyperresponsiveness In Vivo.
Airway responsiveness was measured 24 h after the last aerosol challenge by recording respiratory pressure curves by whole body plethysmography (Buxco®, EMKA Technologies, Paris, France) in response to inhaled methacholine (Aldrich-Chemie, Steinhein, Germany) at a concentration of 3 × 10−2 M for 20 s, as described previously (26, 27). This method allows measurements of spontaneous breathing in a nonrestrained mouse. Airway responsiveness was expressed in enhanced pause (Penh), a calculated value, which correlates with measurement of airway resistance, impedance, and intrapleural pressure in the same mouse. Penh = (Te/Tr − 1) × (Pef/Pif ) (Te, expiration time; Tr, relaxation time; Pef, peak expiratory flow; Pif, peak inspiratory flow × 0.67 = coefficient; reference 28). The relaxation time is the time it takes for the box pressure to change from a maximum to a user-defined percentage of the maximum. In this study, Tr measurement begins at the maximum box pressure and ends at 40%. Immunized mice were treated with either control Ig, anti-B7-1, and/or anti-B7-2 mAb and exposed to either PBS or OVA.
Results
CTLA-4 Ig Inhibits Antigen-induced Eosinophil Recruitment to the Lungs.
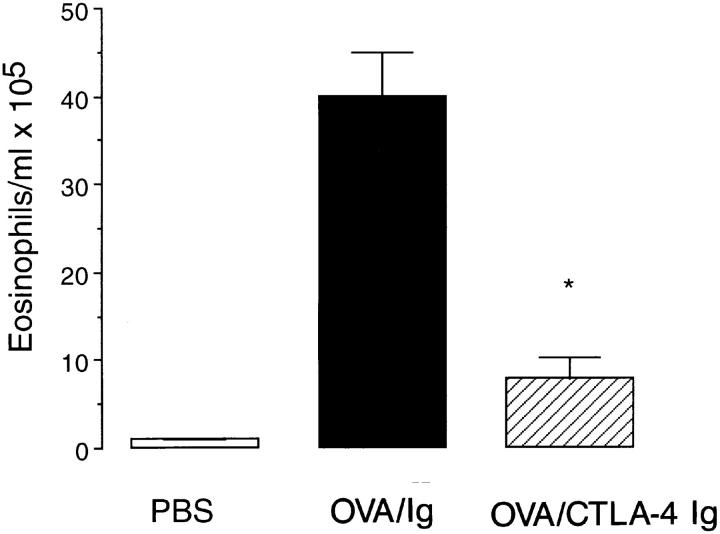
Exposure to five repeated aerosol provocations of OVA induced a selective infiltration of eosinophils into the airways as assessed by BAL (40.0 ± 11.0 eosinophils/ml × 105, n = 6). Administration of the human γ-1 as the isotype-matched control antibody 4 h before the first allergen provocation had no significant effect on antigeninduced eosinophil recruitment (38.9 ± 6 eosinophils/ml × 105, P >0.1, n = 6). Pretreatment with 100 μg of CTLA4 Ig induced a marked attenuation in the recruitment of eosinophils into the airways (7.9 ± 2.4 eosinophils/ml × 105, n = 6, P <0.05, Fig. 1). Administration of CTLA-4 Ig before PBS provocation of immunized animals failed to induce any inflammatory changes in the airways (Fig. 1).
Figure 1.
Inhibition of eosinophil infiltration by CTLA-4 Ig. Immunized mice were treated with either CTLA-4 Ig (striped bar) or human γ-1 as the isotype control Ig (filled bar) before allergen challenge. Immunized, PBS-challenged mice were also treated with CTLA-4 Ig and are shown for comparison (open bar). Data are expressed as mean ± SEM of eosinophils/ml × 105 for n = 4–7 mice. Statistical significance (*) was determined by a Student's t test and a value of P <0.05 was considered to be significant.
CTLA-4 Ig Inhibits the Local IgE Production in the Lungs.
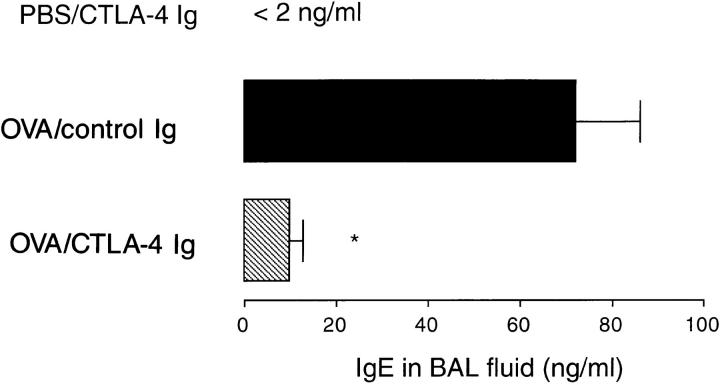
Repeated allergen provocation increased levels of IgE in the BAL fluid as compared to PBS-exposed animals (PBS challenged, <2 ng/ml, n = 3; antigen challenged, 72 ± 14 ng/ml, n = 8, P <0.01). Local airway administration of CTLA-4 Ig inhibited the production of IgE in the BAL fluid (9.7 ± 2.9 ng/ml, n = 5, P <0.001; Fig. 2). In contrast, CTLA-4 Ig failed to suppress total serum IgE (data not shown).
Figure 2.
Inhibition of IgE in the BAL fluid by CTLA-4 Ig. Immunized mice were treated with either CTLA-4 Ig (striped bar) or human γ-1 as the isotype control Ig (filled bar) before allergen challenge. Immunized, PBS-challenged mice were also treated with CTLA-4 Ig and are shown for comparison. Data are expressed as mean ± SEM of IgE in ng/ml for n = 4–7 mice. Statistical significance (*) was determined by a Student's t test and a value of P <0.05 was considered to be significant.
CTLA-4 Ig Inhibits Th2 Cytokine Production and Enhances IFN-γ Production.
Activation of FACS® purified Thy1.2+ cells from the lung via the CD3–TCR complex from immunized, PBS-challenged mice produced IL-2 and IFN-γ, with no detectable IL-4, IL-5, or IL-10. In contrast, repeated allergen provocation of immunized mice treated with human γ-1 resulted in the secretion of high amounts of IL-4, IL-5, and IL-10, elevated levels of IL-2, and reduced amounts of IFN-γ (Fig. 3). Pretreatment with CTLA-4 Ig inhibited the production of Th2 cytokines by 70–80%, enhanced the production of IFN-γ by threefold, but had no significant effect on IL-2 production compared to control Ig-treated mice.
Figure 3.
Effect of CTLA-4 Ig on cytokine production from in vitro–activated lung T cells. Immunized mice were treated with either CTLA-4 Ig (striped bars) or human γ-1 (filled bars) before allergen challenge. Immunized, PBS-challenged mice were also treated with CTLA-4 Ig and are shown for comparison (open bars). Data are expressed as mean ± SEM of triplicate cultures and shown in U/ml.
B7-2, but Not B7-1, Is Upregulated on Lung B Cells after Allergen Provocation.
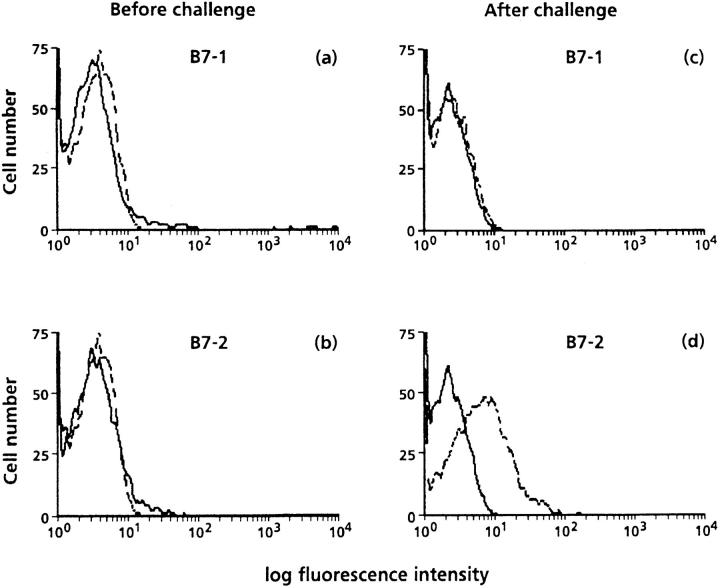
We next investigated whether antigen provocation leads to the expression of the CD28 counterligands B7-1 and B7-2. B cells obtained from the lungs of immunized mice before challenge expressed low levels of B7-1 and B7-2 (Fig. 4, a and b). However, B7-2 expression was markedly upregulated within 24 h after the first allergen exposure and was maintained after three or five allergen provocations (data not shown). In contrast, B7-1 was not upregulated on lung B cells at any time point after antigen challenge (Fig. 4, c and d).
Figure 4.
Representative FACS® analysis of expression of B7-1 and B7-2 on lung B220+ cells before (a and b) and 24 h after antigen challenge in immunized mice. Cells were stained with PE-B220 alone (solid lines) or PE-B220 and FITC-B7-1 or B7-2 (dotted lines). Data are expressed as histograms of B7 expression after gating on B220 positive cells. Data are representative of three separate experiments.
B7-2 Is the Primary CD28 Counterligand Required for the Induction of a Th2 Immune Response.
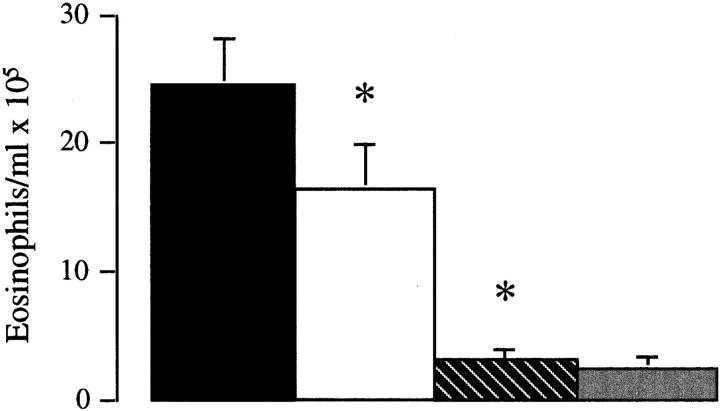
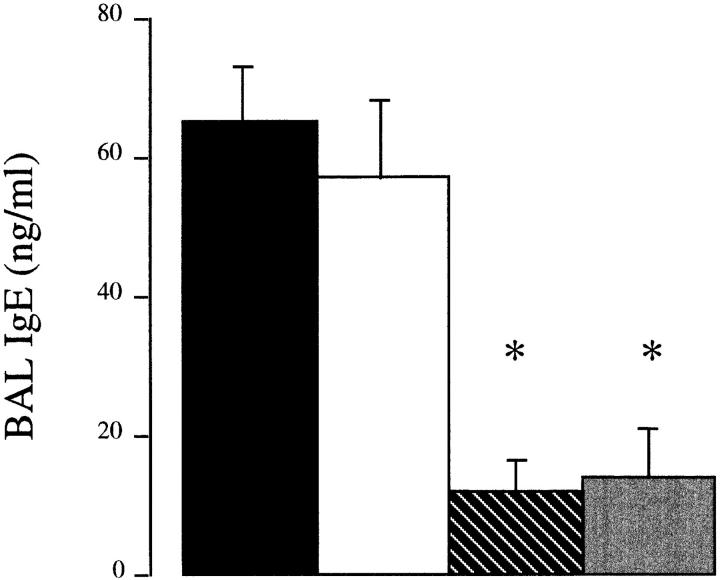
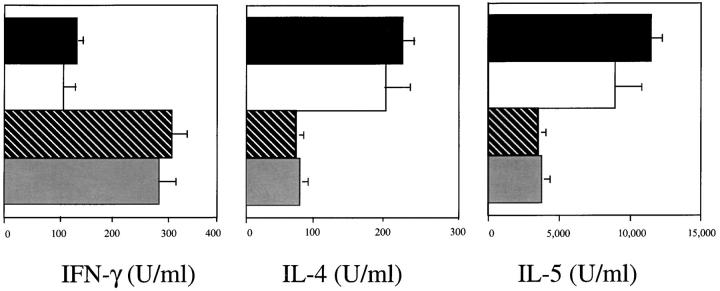
It then became important to determine whether B7-1, B7-2, or both molecules are required for the induction of a lung Th2 immune response. Anti-B7-2 mAb treatment inhibited the recruitment of eosinophils in the lung by >85%, compared to control Ig-treated mice (control Ig, 24.6 ± 3.6 eosinophils/ml × 105, n = 10; anti-B7-2 mAb, 3.07 ± 1.1 eosinophils/ml × 105, n = 5, P <0.005; Fig. 5). In contrast, the anti-B7-1 mAb had a minor, although significant, inhibitory effect on eosinophil recruitment into the lung (anti-B7-1 mAb, 16.8 ± 3.8 eosinophils/ml × 105, n = 9, P <0.05). However, the combination of both mAbs was no more effective than anti-B7-2 mAb alone (2.4 ± 0.80 eosinophils/ ml × 105, n = 10, P >0.05, compared to anti-B7-2 mAb alone). In addition, in vivo treatment with anti-B7-2 mAb inhibited the production of IL-4 and IL-5 and enhanced production of IFN-γ from purified lung T cells activated through the CD3–TCR complex, whereas treatment with anti-B7-1 mAb had a minor effect on Th2 cytokine secretion (Fig. 6). The combination of both mAbs was no more effective than anti-B7-2 mAb in inhibiting Th2 cytokine production. Finally, measurement of IgE levels in the BAL supported the principle role for costimulation through B7-2, as IgE production was suppressed by the anti-B7-2 mAb (control Ig, 65 ± 9 ng/ml; anti-B7-2 mAb, 12 ± 5 ng/ml, P <0.05), but not by the anti-B7-1 mAb (57 ± 12 ng/ml, P >0.05). The combination of both mAbs was no more effective than anti-B7-2 mAb in IgE production (14 ± 8 ng/ ml; Fig. 7).
Figure 5.
Effect of mAbs to B7-1 (open bar), B7-2 (striped bar), or B7-1 and B7-2 (shaded bar) on antigen-induced eosinophil infiltration. Control mice were treated with rat IgG ( filled bar). Data are expressed as mean ± SEM of eosinophils/ml × 105 for n = 9–10 mice. Statistical significance (*) was determined by a Student's t test and a value of P <0.05 was considered to be significant.
Figure 6.
Inhibition of IgE in the BAL fluid by anti-B7-2 mAb. Immunized mice were treated with either anti-B7-1 (open bar), anti-B7-2 mAb (striped bar), anti-B7-1 and anti-B7-2 (shaded bar), or rat IgG ( filled bar) as the isotype control Ig before allergen challenge. Data are expressed as mean ± SEM of IgE in ng/ml for n = 6–8 mice. Statistical significance (*) was determined by a Student's t test and a value of P <0.05 was considered to be significant.
Figure 7.
Inhibition of Th2 cytokine production by anti-B72 mAb from in vitro–activated lung T cells. Immunized mice were treated with anti-B7-1 and/or anti-B7-2 mAb or rat IgG as the isotype control Ig before allergen challenge. Data are expressed as mean ± SEM of triplicate cultures and shown in U/ml.
B7-2, but Not B7-1, Is Required for the Induction of Airway Hyperresponsiveness In Vivo.
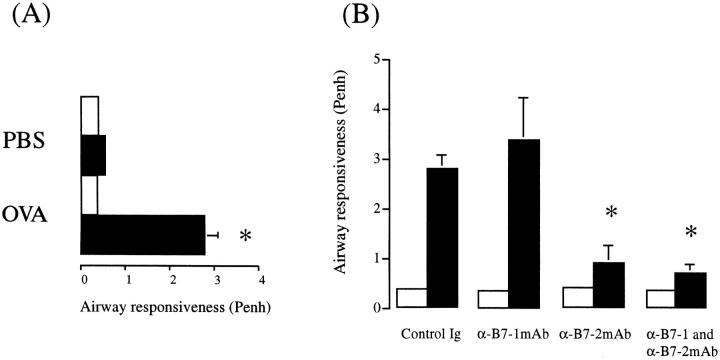
We next investigated whether B7-1 and/or B7-2 contribute to the development of airway hyperresponsiveness (AHR) to methacholine (MCh). Allergen provocation of immunized mice treated with control rat Ig resulted in the development of AHS (baseline Penh, 0.37 ± 0.04; after MCh, 2.8 ± 0.27, n = 5), as compared to immunized mice treated with control rat Ig and exposed to PBS (baseline Penh, 0.39 ± 0.01; after MCh 0.56 ± 0.03, n = 4, P >0.05; Fig. 8 A). There was no change in AHR in PBS-exposed mice that were treated with either B7-1 and/or B7-2 mAb (data not shown). Pretreatment with anti-B7-2 mAb abrogated the development of allergen-induced AHR (baseline Penh, 0.39 ± 0.04; after MCh, 0.90 ± 0.33, P <0.05). In contrast, anti-B7-1 mAb had no significant effect, and the MCh response was comparable to that observed in Ig-treated, antigen-exposed mice. (baseline Penh, 0.35 ± 0.03; after MCh, 3.38 ± 0.85, P = 0.48). Pretreatment with the combination of anti-B7-1 and B7-2 mAbs was no more effective than antiB7-2 mAb treatment alone AHR (baseline Penh, 0.36 ± 0.02; after MCh, 0.70 ± 0.16, P >0.05), compared to anti-B7-2 mAb treatment alone (Fig. 8 B).
Figure 8.
Inhibition of airway hyperresponsiveness to methacholine by an anti-B7-2 mAb. (A) Mice were immunized and exposed to either an aerosol of PBS or OVA for 5 consecutive days. (B) Effect of anti-B7-1 and/or B7-1 mAb on antigeninduced AHR. Control mice were treated with rat IgG. Results are expressed as the mean ± SEM of the Penh before (open bar) and after ( filled bar). MCh provocation for n = 4–6 animals. Statistical significance (*) was determined by a Student's t test and a value of P <0.05 was considered to be significant.
Discussion
Activation of antigen-naive CD4+ T cells produce predominately IL-2. However, under the influence of IL-4, these cells differentiate into a phenotype that produces high levels of Th2 cytokines (29–31). In recent years, evidence has increased to suggest that activation of antigen-specific CD4+ Th2 cells in the lungs of allergic individuals plays a central role in the pathogenesis of bronchial asthma by providing IL-4, which is essential for B cell switching to IgE to occur (32), and IL-5, which is required for eosinophil mobilization and/or accumulation (5–7). It is now clearly established that in addition to interactions between peptides bound to MHC class II molecules and the CD3–TCR-α/β complex, a second signal is required. CD28-mediated signaling via its natural ligands, B7-1 and B7-2, provides the signal necessary for T cell proliferation and IL-2 gene transcription (11–18). However, the costimulatory signals that are required for the induction of a Th2 immune response are less clear. In the present study, we use the fusion protein CTLA-4 Ig to demonstrate that costimulation through CD28 ligands is required for the local production of IgE and the recruitment of eosinophil into the lungs. These observations are supported by the demonstration that CTLA-4 Ig treatment suppresses the production of IL-4, IL-5, and IL-10 from lung T cells. In contrast, pretreatment with CTLA-4 Ig enhanced the production of IFN-γ from lung T cells by threefold. Thus, in the absence of costimulation through CD28 ligands, cells can default to a phenotype that produces less Th2 cytokines and more IFN-γ. As IFN-γ has been shown to inhibit the proliferation of Th2 cells (33), reciprocally regulate IL-4-dependent IgE production (34), and can inhibit the accumulation of eosinophils into the lung in vivo (35), it remains to be clarified, using antibodies to IFN-γ or mice with a disrupted IFN-γ system, whether CTLA-4 Ig suppresses eosinophil infiltration and IgE production at least in part by the upregulation of IFN-γ production.
The requirement of CD28 as a costimulatory molecule for activation of Th2 cells is controversial at present. In vitro studies have demonstrated that Th2 cells can use other costimulatory signals besides CD28, and attention has focused on an important role for IL-1 (36). More recently, IL-4 production has been shown to be independent of CD28mediated stimulation, although CD28 signaling was required for inducing responsiveness to IL-4 (37). CTLA-4 Ig has also been demonstrated to inhibit alloantigen-specific responses, and IL-2 and IFN-γ, but not IL-4 secretion (38). In addition, antigen-specific stimulation of CD4+ T cells from TCR transgenic mice have shown that IL-4 secretion from Th2 cells is relatively independent of costimulation through CD28 (38). These in vitro observations are also supported by in vivo experiments showing that CTLA-4 Ig specifically inhibits the production of Th1 cytokines, but spares the production of Th2 cytokines in a rat renal allograft model (39). In contrast, after infection with Heligmosomoides polygyrus, CD28-mediated stimulation is required for IL-4 production and B cell switch to IgE production, although the development of a peripheral blood eosinophilia was largely independent of CD28-mediated costimulation (40). Likewise, after infection with Leishmania major (41) or in a primary immune response induced by anti-IgD (42), CTLA-4 Ig inhibits the appearance of IL-4–producing cells. However, in these two models, the production of IL-5 and IL-10 was independent of costimulation through CD28. Interestingly, administration of CTLA-4 Ig treatment several days after treatment with either anti-IgD or H. polygyrus failed to inhibit the development of IgE-producing cells (40, 42). These results suggest that in vivo priming of naive T cells generally requires B7-CD28–mediated costimulation, whereas activation of differentiated Th2 cells is largely B7 independent.
If CD28 is required for differentiation towards a Th2 immune response, it still remains unclear whether activation of CD28 is mediated by either B7-1 and/or B7-2. B7 molecules have been shown to be expressed on activated B cells (14–17), functional mature dendritic cells (18), and more recently, on activated CD4+ and CD8+ T cells (43). However, the expression of these molecules is differentially controlled in that B7-2 expression is upregulated within 6 h of B cell activation after LPS, Con A, or antigen-specific stimulation, whereas B7-1 was not increased until 72 h later. Moreover, while LPS and Con A induced B7-1 expression, cross-linking of the Ig receptor failed to upregulate B7-1 expression (44, 45). These in vitro studies are supported by the present in vivo observations that 24 h after aeroantigen exposure, B7-2, but not B7-1, expression was increased on lung B cells and expression was maintained after the repeated antigen provocations (data not shown). However, it is important to note that despite the lower level of expression of B7-1, the slower off-rate of B7-1, compared to B7-2, may allow this molecule to exert potent costimulatory functions (46). In addition, the ability of B7-1 and B7-2 to bind to distinct CD28 determinants with different affinities (47) raises the possibility that these molecules play distinct functions (48). Freeman et al. have suggested that repeated alloantigen stimulation by B7-2–, but not B7-1–transfected cells selectively primes naive cells for IL-4 production (49). Likewise, in a recent study using 1-Ad–restricted, antigen-specific CD4+ T cells obtained from transgenic mice, it was shown that anti-B7-2 mAb markedly suppressed the development of IL-4– and IL-10–producing cells, whereas blockade of B7-1 facilitated differentiation towards IL-4 production and inhibited IFN-γ secretion (50).
To investigate the role of these two molecules in the induction of the allergic inflammatory response, we administered neutralizing mAbs to B7-1 and/or B7-2 directly into the lungs. Our observations suggest that costimulation through B7-2 is the primary CD28 counterreceptor involved in the induction of a lung mucosal Th2 immune response, since administration of the anti-B7-2 mAb inhibited the recruitment of eosinophils into the lungs and Th2 cytokine production. However, we can not rule out a small contribution of B7-1–mediated costimulation, since eosinophil accumulation was reduced by 25%. However, coadministration of both mAbs was no more effective than treatment with antiB7-2 alone, supporting the evidence that B7-2 is the principal CD28 ligand required in this response. Distinct roles for B7 ligands have also been suggested in another in vivo study where neutralization of B7-2 inhibited the production of Th2 cytokines, enhanced the production of IFN-γ, and was associated with enhanced disease severity in a model of experimental allergic encephalomylitis (51). Likewise, differential functions for B7-1 and B7-2 have also been suggested in the development of diabetes in nonobese diabetic mice whereby anti-B7-2 mAb inhibited the development of diabetes, compared to anti-B7-1 mAb, which accelerated the disease development (52). It therefore became important to determine whether B7-2–mediated costimulation was not only associated with the inflammatory response in the lungs, but was also implicated in a pathophysiological-relevant response. Our results show that the induction of airway hyperresponsiveness, which is a characteristic feature of bronchial asthma, was abrogated by treatment with the anti-B7-2 mAb. In contrast, administration of the anti-B7-1 mAb provided no protection, despite the small reduction in the number of eosinophils in the lungs after allergen provocation. Taken together, we suggest that interaction of B7-2 with CD28 is important in the development of a lung inflammatory response and is essential for the induction of airway hyperresponsiveness. Nevertheless, further studies are required to elucidate whether B7-1 and B7-2 also provide distinct costimulatory functions during different immune responses (soluble protein antigens compared to intact pathogens), or whether both molecules can elicit similar responses with the appropriate cytokine secretion.
In conclusion, repeated allergen provocation results in the commitment of cells to the Th2 phenotype, the selective recruitment of eosinophils to the lung, and the production of IgE, which is dependent on the activation of CD28 ligands. In particular, we suggest an important role primarily for B7-2, based on the selective upregulation of this molecule, the marked suppression of lung Th2 mucosal immune responses, and abrogation of airway hyperresponsiveness after allergen exposure by the anti-B7-2 mAb. These observations open up the attractive therapeutic potential that inhibiting B7-2–CD28 interactions in the lungs of allergic individuals may lead to the development of novel therapies for the treatment of allergic lung disease.
Acknowledgments
We are grateful to Drs. M.A. Bray, C. Heusser, C. Bertrand, Y. Chvatchko, and F. Erard for their critical advice and help with this work. Mr. J. Bews and M.D. Tyers for technical help, and Mr. Wesp for performing the FACS® analysis and sorting.
Footnotes
1 Abbreviations used in this paper: AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; MCh, methacholine.
References
- 1.Gleich G. The eosinophil and bronchial asthma. Current understanding. J Allergy Clin Immunol. 1990;85:422–436. doi: 10.1016/0091-6749(90)90151-s. [DOI] [PubMed] [Google Scholar]
- 2.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig derived major basic protein on tracheal epithelium. Lab Invest. 1980;42:35–42. [PubMed] [Google Scholar]
- 3.Coyle AJ, Perretti F, Manzini S, Irvin CG. Cationic protein induced sensory nerve activation. Role of substance P in airway hyperresponsiveness and plasma protein extravasation. J Clin Invest. 1994;94:2301–2306. doi: 10.1172/JCI117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coyle AJ, Ackerman S, Burch R, Proud D, Irvin CG. Human eosinophil granule major basic protein and synthetic polycations induce airway hyperresponsiveness in vivo dependent on bradykinin generation. J Clin Invest. 1995;95:1735–1740. doi: 10.1172/JCI117850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coyle AJ, Le Gros G, Bertrand C, Tsukuki S, Heusser C, Kopf M, Anderson GP. IL-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima H, Iwamoto I, Tomoe S, Matsumara R, Tomioka H, Takatsu K, Yoshida S. CD4+ lymphocytes and interleukin-5 mediate antigen induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992;144:374–379. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 7.Van Oosterhout AJM, Rudof A, Ladenius C, Savelkoul H, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti–IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz R, Mueller DL, Jenkins MK. T cell clonal anergy. Cold Spring Harbor Symp Quant Biol. 1989;54:605–617. doi: 10.1101/sqb.1989.054.01.072. [DOI] [PubMed] [Google Scholar]
- 9.Schartz RH. A cell culture model for T lymphocyte clonal anergy. Science (Wash DC) 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 10.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen specific IL-2 production by human T cells. J Immunol. 1991;147:2461–2466. [PubMed] [Google Scholar]
- 11.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28 mediated signaling costimulates murine T cells and prevents induction of anergy in T cell clones. Nature (Lond) 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 12.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science (Wash DC) 1991;251:313–315. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 13.Linsley PS, Brady W, Grosmaire L, Aruffo A, Damle NK, Ledbetter JA. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin-2 mRNA accumulation. J Exp Med. 1991;173:721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linsley PS, Clark EA, Ledbetter JA. T cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimmi, C.D., G.J. Freeman, J.D. Gribben, K. Sugita, A.S. Freedman, C. Morimoto, and L.M. Nadler. B cell surface antigen B7 provides a costimulatory signal that induces T cells to proliferate and secrete IL-2. Proc. Natl. Acad. Sci. USA. 88: 6575–6579. [DOI] [PMC free article] [PubMed]
- 16.Freeman GJ, Gribben JG, Boussiotis VA, Hg JW, Restivo VA, Lombard LA, Gray GS, Nadler LM. Cloning of B7-2: a CTLA-4 counter receptor that costimulates human T cell proliferation. Science (Wash DC) 1993;262:909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- 17.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science (Wash DC) 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Ritchie SC, Pearson TC, Linsley PS, Lowry RP. Functional expression of the costimulatory molecule B7/BB1, on murine dendritic cell populations. J Exp Med. 1992;176:1215–1220. doi: 10.1084/jem.176.4.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linsley PS, Brady W, Urnes M, Grosmaire L, Damsle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane P, Gerhard W, Hubele S, Lanzavecchia A, McConnell F. Expression and functional properties of mouse B7/BB1 using a fusion protein between mouse CTLA-4 and human γ-1. Immunology. 1993;80:56–61. [PMC free article] [PubMed] [Google Scholar]
- 21.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, Singh C, Tepper MA. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science (Wash DC) 1992;257:792–795. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 22.Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of allospecific hyporesponsiveness in human T lymphocytes by blocking interaction between CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Razi-Wolf Z, Galvon F, Gray G, Reiser H. Evidence for an additional ligand, distinct from B7, for the CTLA-4 receptor. Proc Natl Acad Sci USA. 1993;90:11182–11186. doi: 10.1073/pnas.90.23.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuyuki S, Bertrand C, Erard F, Trifilieff A, Tsuyuki J, Wesp M, Anderson GP, Coyle AJ. Murine lung eosinophils express the Fas receptor. Induction of apoptosis and resolution of lung eosinophilic inflammation. J Clin Invest. 1995;96:2924–2931. doi: 10.1172/JCI118364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ledermann F, Schlienger C, Wagner K, Heusser C. A sensitive and efficient induction system for murine IgE: single cell analyses at the clonal level. J Immunol Methods. 1991;141:263–275. doi: 10.1016/0022-1759(91)90153-7. [DOI] [PubMed] [Google Scholar]
- 26.Eum SY, Haile S, Lefort J, Huerre M, Vargaftig BB. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5–dependent bronchial hyperresponsiveness. Proc Natl Acad Sci USA. 1995;92:12290–12294. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chvatchko Y, Kosco-Vilbois M, Herren S, Lefort J, Bonnefoy J-Y. Germinal centers in the lungs of mice following allergen provocation. J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.6.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelfand EW, Irvin CG. T lymphocyte: setting the tone of the airways. Nat Med. 1997;3:382–383. doi: 10.1038/nm0497-382. [DOI] [PubMed] [Google Scholar]
- 29.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (Lond) 1993;362:245–247. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 30.Swain SL, Weinberg AD, English M, Hutson G. IL-4 directs the development of Th2 effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 31.Seder RA, Paul WE, Davis MM, de St BF, Groth The presence of IL-4 during in vitro priming determines the lymphokine producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berstedt-Lindqvist S, Moon H-B, Rersson U, Möller G, Heusser CH, Severinson E. IL-4 instructs uncommitted B lymphocytes to switch to IgG1 and IgE. Eur J Immunol. 1988;18:1073–1077. doi: 10.1002/eji.1830180716. [DOI] [PubMed] [Google Scholar]
- 33.Gajewski TF, Fitch FW. Anti-proliferative effects of IFN-γ in immune regulation. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocytes clones. J Immunol. 1988;140:4245–4253. [PubMed] [Google Scholar]
- 34.Finkelman FD, Katona IM, Mosmann TR, Coffman RL. Interferon-γ regulates the isotypes of Ig secreted during in vivohumoral immune responses. J Immunol. 1988;140:1022–1029. [PubMed] [Google Scholar]
- 35.Iwamoto I, Nakajima H, Endo H, Yoshisa S. Interferon-γ regulates antigen induced eosinophil recruitment into mouse airways by inhibiting the infiltration of CD4+T cells. J Exp Med. 1993;177:5789–5804. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver CT, Hawrylowicz CM, Unanue ER. T helper subsets require the expression of distinct costimulatory signals by antigen presenting cells. Proc Natl Acad Sci USA. 1988;95:8181–8184. doi: 10.1073/pnas.85.21.8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McArthur JG, Raulet DH. CD28 induced costimulation of T helper type 2 cells mediated by induction of responsiveness to IL-4. J Exp Med. 1993;178:1645–1653. doi: 10.1084/jem.178.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKnight AJ, Perez VL, Shea CM, Gray GS, Abbas AK. Costimulator dependence of lymphokine secretion by naive and activated CD4+ T lymphocytes from TCR transgenic mice. J Immunol. 1994;152:5220–5225. [PubMed] [Google Scholar]
- 39.Sayegh MH, Akalin E, Hancock WW, Russell ME, Carpenter CB, Linsley PS, Turka LA. CD28-B7 blockade after alloantigenic challenge in vivo inhibits Th1 cytokines but spares Th2. J Exp Med. 1995;181:1869–1874. doi: 10.1084/jem.181.5.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu P, Chen S-J, Moorman M, Morris SC, Finkelman FD, Linsley P, Urban JF, Gause WC. CTLA-4 ligands are required to induce an in vivo interleukin 4 response to a gastrointestinal nematode parasite. J Exp Med. 1994;180:693–698. doi: 10.1084/jem.180.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corry DB, Reiner SL, Linsley PS, Lockley R. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4184. [PubMed] [Google Scholar]
- 42.Lu P, Zhou XD, Chen S-J, Moorman M, Schoneveld A, Morris S, Finkelman FD, Linsley PS, Claassen E, Gause WC. Requirement of CTLA-4 counter receptors for IL-4 but not IL-10 elevations during a primary systemic in vivo immune response. J Immunol. 1995;154:1078–1087. [PubMed] [Google Scholar]
- 43.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response on T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenscow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodsnow CC, Bluestone JA. Differential upregulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- 45.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 47.Kariv I, Truneh A, Sweet RW. Analysis of the site of interaction of CD28 with its counterreceptor CD80 and CD86 and correlation with function. J Immunol. 1996;157:29–38. [PubMed] [Google Scholar]
- 48.Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? . Cell. 1995;81:979–982. doi: 10.1016/s0092-8674(05)80001-7. [DOI] [PubMed] [Google Scholar]
- 49.Freeman GJ, Boussiotis VA, Anumanthan A, Bernstein GM, Ke X-Y, Rennert PD, Gray GS, Gribben JG, Nadler LM. B7-1 and B7-2 do not deliver identical costimulatory signals, since B7-2 but not B7-1 preferentially costimulates the initial production of IL-4. Immunity. 1995;2:523–532. doi: 10.1016/1074-7613(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 50.Ranger AM, Das MP, Kuchroo VK, Glimcher LH. B7-2 (CD86) is essential for the development of IL-4 producing T cells. Int Immunol. 1996;8:1549–1560. doi: 10.1093/intimm/8.10.1549. [DOI] [PubMed] [Google Scholar]
- 51.Kucheroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Gilmcher LH. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 52.Lenscow D, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of an anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J Exp Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]