Abstract
The development of pre–T cells with productive TCR-β rearrangements can be mediated by each the pre–T cell receptor (pre-TCR), the TCR-αβ as well as the TCR-γδ, albeit by distinct mechanisms. Although the TCR-γδ affects CD4−8− precursor cells irrespective of their rearrangement status by TCR-β mechanisms not involving TCR-β selection, both the preTCR and the TCR-αβ select only cells with productive TCR-β genes for expansion and maturation. The TCR-αβ appears to be much less effective than the pre-TCR because of the paucity of TCR-α proteins in TCR-β–positive precursors since an early expressed transgenic TCR-αβ can largely substitute for the pre-TCR. Thus, the TCR-αβ can assume a role not only in the rescue from programmed cell death of CD4+8+ but also of CD4−8− thymocytes. In evolution this double function of the TCR-αβ may have been responsible for the maturation of αβ T cells before the advent of the pre–TCR-α chain.
During development of αβ T cells in the thymus most TCR genes rearrange in temporal order such that most TCR-β rearrangement occurs before TCR-α rearrangement (1, 2). Over the years, it became clear that the products of the rearranged genes, i.e., the TCR-β and TCR-α chains, have an important role in controlling T cell development: the first produced TCR-β chain covalently binds to the pre–TCR-α (pTα)1 chain (3, 4) and forms the pre-TCR that rescues from programmed cell death CD4− 8−44−25+ cells that have succeeded in TCR-β chain rearrangement. The selected cells assume the CD4−8−44−25− phenotype (5), proliferate extensively, and eventually become CD4+8+ cells that bear the TCR-αβ on the cell surface while expression of the pTα is terminated (6, 7). The CD4+8+-expressing cells are programmed to die unless the TCR-αβ binds to thymic MHC molecules and cells are rescued from cell death once more and eventually become mature T cells that leave the thymus (8, 9). Both the preTCR and the TCR-αβ associate with signal-transducing CD3 molecules and may signal through activation of src kinases like p56lck and fyn (3, 10). In fact, recent experiments have established that p56lck- and fyn-deficient, double mutant mice exhibited a developmental block at the CD4−8−44−25+ stage where the pre-TCR normally assumes its role (11). Even earlier experiments in either rearrangement-deficient RAG−/− mice (12, 13) or CD3ε−/− mice (14) had already indicated that a signaling receptor that contains at least one chain encoded by a rearranging gene was required to rescue CD4−8−44−25+ cells from apoptotic cell death (15).
Experiments in pre-TCR–deficient TCR-β−/− or pTα−/− mice had shown that the pre-TCR, while having an important function in generating large numbers of CD4+8+ cells from CD4−8− precursors, was likely not to be the only TCR able to mediate these events since both types of mutant mice still contained significant though reduced numbers of CD4+8+ thymocytes (6, 16). In fact, the origin of the CD4+8+ cells in TCR-β−/− mice was obscure and the possibility was discussed that they may belong to the γδ lineage (16). In pTα−/− mice, however, some of the CD4+8+ cells expressed TCR-αβ on the cell surface and could undergo positive selection to become mature T cells, i.e., they belonged to the αβ lineage. Therefore, it is important to define alternative rescue pathways that can avoid a total deficiency of αβ T cells in pTα-defective mice. Indeed, by defining such pathways, one may gather further information on how the pre-TCR functions in immature T cells. In this report we show that not only the pre-TCR but both the TCR-γδ as well as the TCR-αβ can mediate the differentiation of CD4−8−25+ pre–T cells albeit by distinct mechanisms.
Materials and Methods
Mice.
The pTα−/− mice, TCR-α−/− mice, and TCR-δ−/− mice have been described (6, 17, 18). TCR-α−/− pTα−/− mice were bred in the animal colony of the Basel Institute for Immunology. Breeding of TCR-δ−/− pTα−/− mice was done in the animal facilities at the Hôpital Necker (Paris, France). C57BL/6 mice were purchased from IFFA CREDO (L'Arbresle, France). The TCR-αβ transgenic mice, with a transgenic TCR specific for the male antigen (H-Y) in the context of H-2Db MHC molecules, have been described previously and were crossed on the C57BL/6 (B6) background (19). TCR-αβ transgenic pTα−/− mice were bred in the animal colony of the Basel Institute for Immunology. Animals were analyzed at 6-8 wk of age. Animal care was in accordance with institutional guidelines.
Antibodies and Flow Cytometry.
The following mAbs were used for staining: anti-CD4 (H129.19, PE-conjugated; GIBCO BRL, Gaithersburg, MD; or H129.19, FITC-conjugated; GIBCO BRL), anti-CD8 (Ly-2, FITC-conjugated; PharMingen, San Diego, CA; or 53-6.7, biotinylated; GIBCO BRL; or 53-6.7, RED613conjugated; GIBCO BRL), anti-CD25 (3C7, PE-conjugated; PharMingen), anti-CD44 (biotinylated KM81; American Type Culture Collection, Rockville, MD), anti-panTCR-β (H57-597, FITC-conjugated [20]), anti-TCR-δ (GL3, FITC-conjugated; PharMingen), T3.70 (specific for the TCR-α chain of the HYreactive TCR, FITC-conjugated), and F23.1 (specific for the TCR-β chain of HY-reactive TCR, FLUOS-conjugated [21]).
Two- and three-color stainings were performed with FITC-, PE-, and biotin-labeled antibodies at optimal concentrations. Biotin-conjugated antibodies were revealed by either streptavidin-PE (Southern Biotechnology, Birmingham, AL) or streptavidin-Tricolor (Caltag Laboratories, San Francisco, CA). Thymocytes were resuspended in cold PBS supplemented with 2% FCS. All stainings were done in 96-well plates (0.5 × 106 cells per well) in 20 μl of mAb in PBS plus 2% FCS plus 0.1% sodium azide for 20 min on ice. Between first and second step reagents cells were washed in PBS plus 2% FCS plus 0.1% sodium azide as was done after the last step. Data were analyzed on a FACScan® (Beckton Dickinson, Mountain View, CA), using Lysis II software (Beckton Dickinson).
For intracellular/extracellular double staining of thymocytes cells were first incubated with culture supernatant of mAB 2.4G2 to block FCγRII/III. Cells were then stained for surface markers as described above. After washing in PBS, cells were fixed in PBS plus 1% paraformaldehyde for 15 min at room temperature, followed by two washing steps in PBS. Cells were then permeabilized in 0.5% saponin (Sigma, Heidelberg, Germany) for 10 min at room temperature and washed in PBS. Intracellular staining with FITC-conjugated antibodies diluted in PBS plus 0.5% saponin was performed for 20 min at room temperature, followed by two washing steps in PBS and 2 × 15 min on a rocking platform in PBS plus 2% FCS plus 0.5% saponin on ice. Finally, cells were washed in PBS plus 2% FCS and analyzed on a FACScan®, using Lysis II software.
Results and Discussion
In initial experiments, it was determined whether either the TCR-γδ or the TCR-αβ could be responsible for the production of CD4+8+ T cells in pTα−/− mice by analyzing the cellular composition of thymuses from either pTα−/− TCR-α−/− or pTα−/− TCR-δ−/− double mutant mice that can only produce the γδ and the TCR-αβ, respectively. As shown in Table 1 both types of mutant mice contained CD4+8+ T cells that were further analyzed by cytoplasmic staining with antibodies specific for TCR-β and TCR-δ chains. For this purpose cells were double stained for surface expression of CD4 and CD8 molecules as well as either for cytoplasmic TCR-β or TCR-δ chains by double fluorescence using CD4 and CD8 antibodies in one color (green) and TCR-β or TCR-δ antibodies in another color (red). In this analysis single positive CD4+8− and CD4−8+ cells show an intermediate fluorescence between that of CD4−8− and CD4+8+ thymocytes and cells were gated accordingly into double negative, double positive (DP), and single positive cells (Fig. 1).
Table 1.
CD4+8+ Thymocyte Subsets of Wild-type and Mutant Mice
| Genotypes | Absolute number (×106) of thymocytes (mean ± SD) | Proportion of CD4+8+ thymocytes (mean % ± SD) | ||
|---|---|---|---|---|
| Wild type (C57BL/6) | 42.3 ± 6.4 | 80.0 ± 1.6 | ||
| pTα−/− | 2.7 ± 2.7 | 57.5 ± 4.1 | ||
| pTα−/− TCR-δ−/− | 3.9 ± 1.7 | 52.6 ± 4.5 | ||
| pTα−/− TCR-α−/− | 1.9 ± 0.1 | 4.0 ± 7.1 |
Mean values were obtained of four (two for pTα−/− TCR-α−/−) different mice of each genotype from 6–8-wk-old litter. Percentages of CD4+8+ thymocytes were determined by FACScan®.
Figure 1.
Intracytoplasmic staining for TCR-β (TCR-βIC) and TCR-δ (TCR-δIC) within thymocyte subsets from C57BL/6 (WT), pTα−/− mice (A), and pTα−/− TCR-α−/−, pTα−/− TCR-δ−/− mice (B). Total thymocytes were surface stained with PE-conjugated CD4 antibodies, biotinylated CD8 antibodies followed by PE-streptavidin; cytoplasmic staining was performed with anti-panTCR-β or anti–TCR-δ antibodies. The cells were gated as indicated at the top of each histogram. The percentages of cells and absolute numbers (in brackets) are indicated.
Fig. 1 shows that 64% of CD4−8− cells in wild-type mice expressed TCR-β chains, and that due to TCR-β selection by the pre-TCR (22) the vast majority of CD4+8+ cells contained TCR-β chains in their cytoplasm. On the other hand, the expression of cytoplasmic TCR-δ chains was mostly restricted to CD4−8− cells. The picture was different in pTα−/− mice where, due to the diminution of rapidly cycling TCR-β–selected CD4−8−44−25− cells (6), only 21% of the CD4−8− cells were TCR-β positive. In addition, only 39% of the CD4+8+ cells contained TCR-β chains in their cytoplasm indicating that in the pTα−/− mice the majority of the CD4+8+ cells were generated by a mechanism that did not involve TCR-β selection. The fact that not all single positive cells in these mice were TCR-β+ is due to the fact that these cells are in part immature TCR-β− single positive cells, on their way from CD4−8− to CD4+8+ cells. Such cells constituted a higher proportion of all cells in pTα−/− mice. The TCR-δ+ single positive cells had a mature CD4+8− phenotype as confirmed by independent three-color stainings indicating also that these cells expressed TCR-γδ receptors on the cell surface. These cells were present in a higher number in pTα−/− mice consistent with the notion that the pre-TCR may have a role in regulating γδ rearrangement and/or expression (23 and unpublished observations).
In pTα−/− TCR-α−/− mice the proportion of TCR-β+ CD4+8+ and TCR-β single positive cells was even further reduced. When looking at the absolute numbers of various cell subsets (Table 1 and Fig. 1) it is clear that there was a very marked reduction in cell numbers of CD4+8+ thymocytes and more mature cells in pTα−/− and pTα−/− TCR-α−/− mice, whereas the numbers of CD4−8− cells were within the same range.
pTα−/− TCR-δ−/− mice also had reduced numbers of DP cells but here the picture differed from that in pTα−/− and pTα−/− TCR-α−/− mice in that all of the CD4+8+ cells were TCR-β positive, i.e., were exclusively generated through a mechanism that involved TCR-β selection. The single positive TCR-β+ cells in pTα−/− TCR-δ−/− mice were exported from the thymi and CD4+8− as well as CD4−8+ cells could be detected in lymphnodes of these mice (not shown). This excludes the possibility that these cells belong exclusively to the NK1.1+CD4+ subset that exhibits an unusual phenotype (24).
The above results were reproducible in the different mice with marginal deviations in either the percentage of cells or absolute cell numbers and are schematically presented in Fig. 2. The main message from this analysis is that the TCR-αβ can generate CD4+8+ cells through TCR-β selection, i.e., by intracellular or cell-autonomous signaling only. In contrast, the TCR-γδ can generate CD4+8+ cells that are either TCR-β+ or TCR-β− but all TCR-δ− through a mechanism that may involve intercellular communication of unknown nature. If the TCR-γδ would generate a significant number of DP cells by cell-autonomous signaling one might expect to find some TCR-δ expression in these cells. However, the fact that the CD4+8+ cells are TCR-δ negative suggests that these cells are not selected by cell-autonomous signaling by the TCR-γδ even though it can not be entirely excluded that TCR-γδ expression is abruptly switched off in CD4+8+ cells. The notion of intercellular communication is in line with experiments that involved transfer of γδ T cells into thymuses of rearrangement-deficient mice that resulted in generation of CD4+8+ cells of host origin (25) and also with earlier data by Shores et al. (26). Our experiments suggest that in the latter experiments γδ but not αβ T cells promoted the development of CD4+8+ thymocytes and make the additional point that the generation of DP cells was not due to an artefact caused by adoptive transfer of cells.
Figure 2.
A schematic overview of various gene-deficient mice and the corresponding defects in T cell development. Percentages indicate the proportion of cells with cytoplasmic TCR-β. The thickness of the bars is meant to correlate with the numbers of cells within the various subsets.
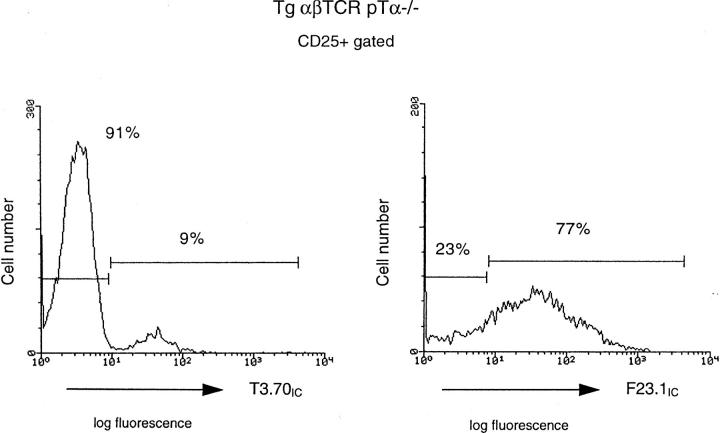
The fact that in the absence of the pre-TCR the generation of CD4+8+ cells by the TCR-αβ is rather inefficient, i.e., 240 × 104 versus 2,880 × 104 in pTα−/− TCR-δ−/− versus wild-type mice, could depend on the fact that the TCR-αβ is inefficiently formed in CD4−8− cells due to the late TCR-α rearrangement and/or the fact that TCR-αβ can only inefficiently replace the pre-TCR. To analyze this question in some more detail we studied mice that express a transgenic TCR-αβ early in development on CD4−8− cells, i.e., TCR-αβ transgenic pTα−/− mice. The transgenic TCR-αβ could indeed overcome the cellular deficiency in the CD4+8+ compartment as TCR-αβ transgenic pTα−/− mice contained approximately one-half the number of thymocytes found in TCR-αβ transgenic pTα+ mice and many more than the number found in nontransgenic pTα−/− mice (Fig. 3). However, there was a subtle difference between TCR-αβ transgenic pTα+ and TCR-αβ transgenic pTα−/− mice in that the latter, but not the former, contained a discrete subset of CD25+ cells, indicating that in spite of the presence of the transgenic TCR-αβ, the preTCR had its role in the exit from this compartment. This could be due to the lack of expression of the transgenic TCR-αβ in a fraction of cells in the CD25+ compartment of the TCR-αβ transgenic, pTα−/− mice. This was in fact confirmed by cytoplasmic staining: while only nine percent of CD25+ cells in TCR-αβ transgenic pTα−/− mice expressed the transgenic TCR-α chain the majority of these cells expressed the transgenic TCR-β chain suggesting that expression of the two transgenes is differentially regulated (Fig. 4). Thus, in TCR-αβ transgenic pTα+ mice it is the combined action of the pre-TCR and the TCR-αβ (mice that have only a TCR-β transgene still exhibit a significantly larger CD25+ compartment than TCR-αβ transgenic mice, not shown) that reduce the number of CD25+ cells while in TCR-αβ transgenic pTα−/− mice this compartment is bigger in size because of the absence of the preTCR. From these data it would appear that the TCR-αβ can at least partially mimic the function of the pre-TCR and that in normal mice the contribution of the TCR-αβ to the generation of the CD4+8+ compartment is limited due to relatively late expression of most TCR-α chains (1, 2).
Figure 3.

Comparision of surface phenotype of thymocytes from TCR-αβ pTα−/− vs. TCR-αβ transgenic mice. (Top) total thymocytes were double stained for CD4 (FITC-conjugated anti-CD4) and CD8 (RED613-conjugated anti-CD8) surface antigens as described. Percentages and absolute numbers of thymocytes (in brackets) are given. TCR-αβ pTα−/− transgenic mice contained approximately one-half of the number of thymocytes found in TCR-αβ transgenic mice (2,870 × 104 vs. 4,720 × 104 cells). (Bottom) cells were stained with FITC-conjugated CD4 and CD8 antibodies in combination with biotinylated CD44 and PEconjugated anti-CD25 antibodies. Biotin was detected with a streptavidin–PE conjugate. The expression of CD25 and CD44 was analyzed by three-color flow cytometry, using electronic gating to exclude FITC-positive cells. The percentages of cells in each quadrant are indicated.
Figure 4.
Assessment of transgenic TCR-α and TCR-β expression by intracytoplasmic staining. For intracellular/extracellular double staining, thymocytes isolated from transgenic TCR-αβ mice and transgenic TCR-αβ pTα−/− mice were stained with PE-conjugated CD25 antibodies and then with FITC-conjugated T3.70 antibodies specific for the transgenic TCR-α chain of the HY-reactive TCR or FLUOS-conjugated F23.1 antibodies, specific for the transgenic TCR-β chain of the HY-reactive TCR.
Thus, all of the three known TCRs can have a role in promoting the development of pre–T cells: the TCR-γδ most likely by intercellular communication that furthers the development of CD4+8+ cells irrespective of whether or not they have succeeded in TCR-β rearrangement, the TCR-αβ that depends strictly on intracellular, cell-autonomous signals generated by the TCR-αβ chains and the pre-TCR that operates by a similar mechanism as the TCR-αβ but is much more efficient because of the early and abundant expression of the pTα gene during the phase of TCR-β rearrangement. Therefore, only mice that cannot produce any of these receptors will exhibit complete arrest at the CD4−8− stage of development as evident in RAG−/− mice or mice that are deficient in both TCR-β and TCR-δ chains and therefore, can make neither TCR-γδ, pre-TCR, nor TCR-αβ (14). In normal mice, the contribution of the TCR-γδ in development of cells of the αβ lineage appears to be limited based on the fact that the vast majority of CD4+8+ cells are TCR-β+ and thus are TCR-β selected. Likewise, in normal mice, the contribution of the TCR-αβ to the transition of DN to DP cells may be limited because of the small number of DP cells in pTα−/− TCR-δ−/− mice. However, in the absence of pTα these receptors avoid a severe immunodeficiency by enabling the formation of a significant number of mature αβ T cells. It would appear that both the preTCR and the TCR-αβ do not only mediate maturation but also proliferation since in wild-type mice and pTα−/− TCR-δ−/− mice the proportion of large CD4+8+ blasts that are derived from dividing CD4−8− precursors (15) is very similar (Table 2). There are only slightly fewer blasts in pTα−/− TCR-α−/− mice indicating that also the TCR-γδ generates dividing CD4+8+ cells.
Table 2.
Proportion of CD4+8+ Lymphoblasts in Wild type and Mutant Mice
| Genotypes | Proportion of CD4+8+ blasts (%) | |
|---|---|---|
| Wild type (C57BL/6) | 8.8 | |
| pTα−/− | 6.4 | |
| pTα−/− TCR-δ−/− | 8.5 | |
| pTα−/− TCR-α−/− | 5.4 |
Percentages of CD4+8+ blasts were determined by FACScan® using forward scatter as an index of size. The various mice were analyzed on the same day in the same experiment.
With regard to the role of the src kinases in early development, our data is consistent with the notion that signaling through the pre-TCR involves both lck and fyn kinases but is equally consistent with the idea that the fyn kinase is involved only in signaling through the TCR-γδ or -αβ, and thereby responsible for the incomplete developmental arrest observed in lck−/− mice. The fact that the TCR-αβ promotes development much in the same way as the preTCR, i.e., by cell-autonomous signaling and thereby TCR-β selection, suggests that T cell development may have proceeded in this way before the advent of the pre–TCR-α chain in evolution and that the pre-TCR had simply the advantage of making the pairing of a single TCR-β chain with different TCR-α chains more effective.
Acknowledgments
We thank Diane Mathis for critical review of the manuscript.
This work was supported in part by the Institut National de la Santé et Recherche Médicale, (Paris), and by the Faculté Necker Enfants Malades, Déscartes Université (Paris). J. Buer is supported by a grant from the Deutsche Forschungsgemeinschaft. I. Aifantis is a recipient of a Biotechnology grant from the European Commission. J.P. DiSanto is supported by a grant from the Association Pour La Recherche Contre Le Cancer. H. von Boehmer is supported by the Institut Universitaire de France. The Basel Institute for Immunology is supported by Hoffman-La Roche (Basel).
Footnotes
1 Abbreviations used in this paper: DP, double positive; pTα, pre-TCR-α.
References
- 1.Raulet DH, Garman RD, Saito H, Tonegawa S. Developmental regulation of T cell receptor gene expression. Nature (Lond) 1985;314:103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- 2.Snodgrass HR, Dembic Z, Steinmetz M, von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature (Lond) 1985;315:232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- 3.Groettrup M, Ungewiss K, Azogui O, Palacios R, Owen MJ, Hayday AC, von Boehmer H. A novel disulfide-linked heterodimer on pre-T cells consists of the T cell receptor beta chain and a 33 kd glycoprotein. Cell. 1993;75:283–294. doi: 10.1016/0092-8674(93)80070-u. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Ruf C, Ungewiss K, Groettrup M, Bruno L, Fehling HJ, von Boehmer H. Analysis and expression of a pre-T cell receptor gene. Science (Wash DC) 1994;266:1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- 5.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4−CD8−triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 6.Fehling HJ, Krotkova A, Saint-Ruf C, von Boehmer H. Crucial role of the pre-T-cell receptor alpha in development of alpha/beta but not gamma/delta T cells. Nature (Lond) 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 7.von Boehmer, H., and H.J. Fehling. 1997. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. In press. [DOI] [PubMed]
- 8.von Boehmer H. Developmental biology of T cells in T-cell receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 9.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 10.Anderson SJ, Levin SD, Perlmutter RM. Involvement of the protein tyrosine kinase p56lckin T cell signaling and thymocyte development. Adv Immunol. 1994;56:151–178. doi: 10.1016/s0065-2776(08)60451-4. [DOI] [PubMed] [Google Scholar]
- 11.van Oers NSC, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. Alpha/beta T cell development is abolished in mice lacking both lck and fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 12.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Steward V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt F. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 13.Mombaerts P, Iacomoni J, Johnson RS, Herrup K, Tonegawa S, Papioannou VE. RAG-1 deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 14.Malissen M, Gillet A, Ardouin L, Bouvier G, Trucy J, Ferrier P, Vivier E, Malissen B. Altered T cell development in mice with a targeted mutation of the CD3 epsilon gene. EMBO (Eur Mol Biol Organ) J. 1995;14:4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penit C, Lucas B, Vasseur F. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J Immunol. 1995;154:5103–5105. [PubMed] [Google Scholar]
- 16.Mombaerts P, Clark AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, Tonegawa S. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature (Lond) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 17.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice lacking T cell receptor alpha/beta-expressing cells. Science (Wash DC) 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 18.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor delta gene mutant mice: independent generation of alpha/beta T cells and programmed rearrangements of gamma/delta TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 19.Kisielow P, Bluethmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T cell receptor transgenic mice involves deletion of non-mature CD4+8+thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 20.Kubo R, Born W, Kappler J, Marrack P, Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha/beta T cell receptors. J Immunol. 1989;142:2736–2742. [PubMed] [Google Scholar]
- 21.Teh HS, Kishi H, Scott B, von Boehmer H. Deletion of autospecific T cells in T cell receptor transgenic mice spare cells with normal TCR levels and low levels of CD8 molecules. J Exp Med. 1989;169:795–806. doi: 10.1084/jem.169.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayday AC. Not in the thymus. Curr Biol. 1993;3:525–528. doi: 10.1016/0960-9822(93)90047-r. [DOI] [PubMed] [Google Scholar]
- 23.Bruno L, Fehling HJ, von Boehmer H. The alpha/beta T cell receptor can replace the gamma/delta receptor in the development of the gamma/delta lineage cells. Immunity. 1996;5:343–352. doi: 10.1016/s1074-7613(00)80260-5. [DOI] [PubMed] [Google Scholar]
- 24.Bendelac A. Mouse NK1+T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch F, Shevach EM. Gamma/delta T cells promote CD4 and CD8 expression of SCID thymocytes. Int Immunol. 1993;8:991–995. doi: 10.1093/intimm/5.8.991. [DOI] [PubMed] [Google Scholar]
- 26.Shores EW, Sharrow SO, Uppenkamp I, Singer A. T cell receptor negative thymocytes from SCID mice can be induced to enter the CD4/CD8 differentiation pathway. Eur J Immunol. 1990;20:69–77. doi: 10.1002/eji.1830200111. [DOI] [PubMed] [Google Scholar]