Abstract
The murine γ-herpesvirus 68 has many similarities to EBV, and induces a syndrome comparable to infectious mononucleosis (IM). The frequency of activated CD8+ T cells (CD62Llo) in the peripheral blood increased greater than fourfold by 21 d after infection of C57BL/6J (H-2b) mice, and remained high for at least a further month. The spectrum of T cell receptor usage was greatly skewed, with as many as 75% of the CD8+ T cells in the blood expressing a Vβ4+ phenotype. Interestingly, the Vβ4 dominance was also seen, to varying extents, in H-2k, H-2d, H-2u, and H-2q strains of mice. In addition, although CD4 depletion from day 11 had no effect on the Vβ4 bias of the T cells, the Vβ4+CD8+ expansion was absent in H-2IAb–deficient congenic mice. However, the numbers of cycling cells in the CD4 antibody–depleted mice and mice that are CD4 deficient as a consequence of the deletion of MHC class II, were generally lower. The findings suggest that the IM-like disease is driven both by cytokines provided by CD4+ T cells and by a viral superantigen presented by MHC class II glycoproteins to Vβ4+CD8+ T cells.
The murine γ-herpesvirus 68 (MHV-68)1 is classified as a type 2 γ-herpesvirus (γHV; references 1, 2), along with Herpesvirus saimiri (3), and a novel γHV that has recently been implicated in Kaposi's sarcoma (4–6). However, the disease process induced in mice infected intranasally with MHV-68 is much more similar to the syndrome associated with prototypic human type 1 γHV, EBV in people (7), than to that caused by the T lymphotrophic H. saimiri in nonhuman primates (8). The key characteristic is that MHV-68 replicates in the epithelial cells of the respiratory tract, with subsequent infection of B cells in lymphoid tissue (9–12). The productive growth phase in the lung cells is terminated by the CD8+ T cell response within 10–13 d. Little, if any, infectious virus can be recovered directly from homogenized lymphoid tissue, although reactivation of latent MHV-68 in B cells occurs readily after cocultivation on susceptible fibroblast monolayers (9–13).
Infectious mononucleosis (IM) is a debilitating condition of adolescents resulting from primary infection with EBV. The disease is characterized by lymph node enlargement and the prolonged presence of greatly increased numbers of activated CD8+ T cells in peripheral blood, after an initial influenza-like phase reflecting the entry of EBV via the oropharyngeal/respiratory mucosa. Apart from the viral etiology, the pathogenesis of this selective lymphocytosis is not understood. Few of the circulating CD8+ T cells can be shown to be EBV-specific, while the virus persists as a latent infection in predominantly B, rather than T, lymphocytes (14–18). Analysis of the pathogenesis of MHV-68– induced IM described in this report suggests a mechanism involving both cytokines and a putative viral superantigen.
Materials and Methods
Mice.
Female C57BL/6J (B6, H-2b), B10.BR (H-2k), BALB/cJ (H-2d), B10.PL (H-2u), and B10.Q (H-2q) mice were purchased from Jackson Laboratory (Bar Harbor, ME). The CD2 mice that are functionally negative for the H-2IAb gene (19) were bred at St. Jude Children's Research Hospital (Memphis, TN), under license from GenPharm Intl. (Mountain View, CA). Mice were infected with MHV-68 at 6–10 wk of age, and then maintained under otherwise specific pathogen-free conditions in BL-3 containment. In some studies, B6 mice were thymectomized at 3 wk of age.
Virus Stocks.
The original stock of MHV-68 (clone G2.4) was obtained from Dr. A.A. Nash (Edinburgh, U.K.) as a cellfree lysate derived from infected baby hamster kidney cells. This was then propagated in owl monkey kidney fibroblasts (ATCC 1566CRL; American Type Culture Collection, Rockville, MD).
Infection and Sampling.
Anesthetized (Avertin, 2,2,2,tribromoethanol) mice were infected intranasally with 400 PFU of MHV-68 at 6–10 wk of age, and sampled at various times after infection. Blood was obtained from the axilla or retroorbital sinus of anesthetized mice.
Cell Cycle Analysis.
The cell cycle analysis of CD8+ T lymphocytes was performed as previously described (20). In brief, cells were stained with FITC-conjugated antibodies to CD8α (536.72; PharMingen, San Diego, CA), permeabilized with ethanol, and the DNA was stained with propidium iodide. The cells were analyzed in two-color mode on the FACScan® (Becton Dickinson, San Jose, CA) using Modfit/Winlist software (Verity Software Inc., Topsham, ME). The program determines the status of individual CD8+ lymphocytes as being in G0/G1, S, or G2 + M by estimating the DNA content of intact nuclei.
In Vivo T Cell Depletion and Flow Cytometry.
B6 mice were depleted of CD4+ T cells by in vivo treatment with GK1.5 mAb at 2–3–d intervals, as previously described (21, 22), beginning 11 d after infection. Depletion was assessed by flow cytometry using RM4-4, which is not blocked by GK1.5 (PharMingen). Activated CD8+ lymphocytes were assessed by two-color staining with CD62L (MEL-14-FITC), or CD44 (IM7-FITC) and CD8-α (536.72-PE) (PharMingen). Lymphocytes were phenotyped using mAb to B220, CD4 (GK1.5), and CD8-α (53-6.72) (PharMingen). TCR-Vβ usage was assessed using a panel of mAbs specific for Vβ 2–14, as previously described (23, 24).
Results
Respiratory Infection with MHV-68 Induces an IM-like Syndrome in B6 Mice.
Previous data have established that respiratory challenge of C57BL/6J (B6) mice with MHV-68 results in acute viral infection that is eliminated by CD8+ T cells by day 13. Persistent, latent infection is established in B cells, which is accompanied by a significant splenomegaly (9, 12, 13, 25). In the current studies, the profile of PBL was analyzed at various stages of infection. Interestingly, the data showed a greater than fourfold increase in the frequency of activated/memory (26–29) CD8+CD44hiCD62Llo PBL from day 21 after infection (Fig. 1 A), which is reminiscent of the IM that is frequently (∼50% of cases) a consequence of EBV infection in humans (30). This IM-like blood picture was also seen in mice that had been thymectomized as adults (Fig. 1 B), suggesting that the tendency for human IM to be more common in adolescents is not due to the provision of new thymic emigrants.
Figure 1.

Phenotypic analysis of CD8+ T cells from peripheral blood of MHV-68–infected B6 mice. CD8+ PBL (hatched bars) from intact (A) and adult thymectomized (B) mice were assessed for the activation phenotype, CD62Llo (open bars), and CD44hi (stippled bars) at various time points after infection, as indicated. Percentages are based on total PBL.
The T cell activation seen in the peripheral blood is likely to be a consequence of events occurring in lymphoid tissue. Analysis of splenic lymphocytes showed that although the spleens were enlarged, the relative prevalence of the CD8+ subset remained fairly constant (Fig. 2 A), indicating that all categories of lymphocytes are cycling at a high rate (Fig. 2 B). However, the frequencies of T cells in S to G2 + M phase were generally greater for the CD8+ than the CD4+ subset of T cells, with the difference being most apparent at the later time points (days 18 and 25 after infection, Fig. 2 B), corresponding to the expansion of CD8+ CD62Llo T cells in the PBL, first observed at day 21 after infection (Fig. 1 A).
Figure 2.

Phenotype and activation profile of splenic lymphocytes from MHV-68–infected B6 mice. The percentage of CD4+ (closed bars), CD8+ (hatched bars), and B220+ (cross-hatched bars) splenic lymphocytes (A) and the proportion of cycling cells within those subsets (B) were determined at various time points after infection.
There are several possible mechanisms to explain virusinduced massive proliferation in lymphoid tissue that could lead to an IM-like profile in the blood. First, it is well known that viral infection results in clonal expansion of cytotoxic T lymphocyte precursors (CTLp) specific for viral peptides + class I MHC glycoproteins (31, 32). However, it has been shown that not all of the activated CD8+ T cells in the peripheral blood of EBV-induced IM patients are virus specific (30, 33). Thus, additional mechanisms must contribute to the CD8+ T cell expansion. One possibility is cytokine-induced bystander activation. For example, it has been shown that cytokines contribute to T cell proliferation during viral infection (20, 34, 35). Another possibility is superantigen-driven T cell proliferation. Superantigens are potent stimulatory molecules secreted by microbial pathogens that cause Vβ-specific T cell expansion (36). The existence of an EBV-encoded superantigen has been suggested by some groups (36–38), but remains controversial (39–41).
Vβ Profile of Activated T Cells.
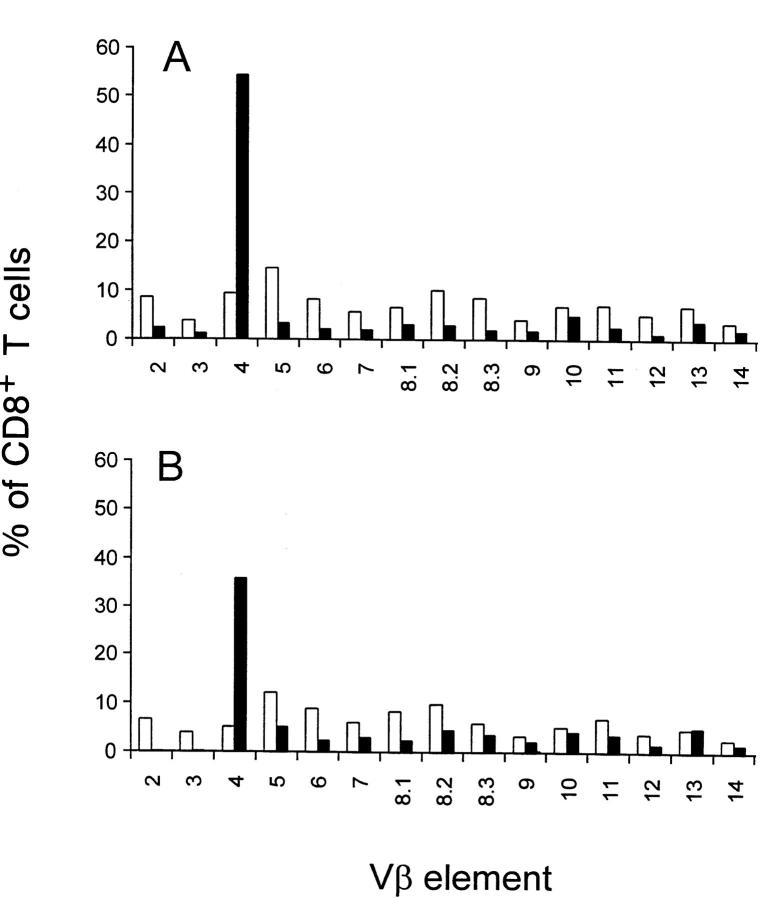
As a first step in determining the mechanism of T cell activation, the TCR-Vβ profile was examined. The results showed a striking predominance of Vβ4+ T cells among the CD8+ T cells in the peripheral blood, and a compensatory decrease in all other Vβs (Fig. 3 A). This Vβ4 expansion was also seen in splenic CD8+ T cells (Fig. 3 B), but not in peripheral lymph nodes (data not shown). The Vβ4 expansion among the CD8+ peripheral blood T cells was not evident in the first 2 wk of infection, but was consistently seen at day 21 after infection, although the magnitude of the increase varied in individual B6 mice (Fig. 4 A). A variable and much reduced Vβ4 expansion was also transiently observed in CD4+ T cells from peripheral blood (Fig. 4 B) and spleen (data not shown). Elevated levels of Vβ4+CD8+ T cells were still observed at 90 d after infection (Fig. 4 A).
Figure 3.
TCR-Vβ usage. TCR-Vβ usage in CD8+ T cells isolated from peripheral blood (A) and spleen (B) of MHV-68–infected (closed bars) and uninfected (open bars) B6 mice analyzed 21 d after infection was determined by two-color staining using a panel of Vβ-specific mAb and anti-CD8. The data are expressed as the percentage of CD8+ T cells expressing a particular TCR-Vβ among total CD8+ T cells.
Figure 4.
Kinetics of Vβ4 expression after MHV-68 infection. Percentage of Vβ4+ T cells among CD8+ T cells (A) and CD4+ T cells (B) from peripheral blood of individual B6 mice at various time points after MHV-68 infection. 3–9 mice were analyzed at each time point, but, in many cases, the values are so close as to be indistinguishable on the graph. The percent Vβ4+ T cells among total CD4+ and CD8+ T cells was determined by two-color flow cytometry using mAb specific for Vβ4, CD4, and CD8. Note that the scale for the y-axis differs in the two panels.
This marked TCR skewing could reflect a dominant Vβ4 usage of H-2Kb– or H-2Db–restricted MHV-68–specific CD8+ CTLp. Repertoire analysis of CTL specific for a variety of viruses shows that diversity in the recognition of a particular peptide + MHC class I glycoprotein can range from very limited to very diverse (24, 42–46). Preferential usage of a single TCR-α/β pair has been described for a long-term EBV-specific CTL response (47), and recently, oligoclonal expansion of T cells in IM patients was reported (39). There is currently no virus-specific CTL assay developed for MHV-68. However, stimulation of T cells with MHV-68, by a standard limiting dilution protocol, showed that the prevalence of microcultures containing effectors capable of CD3-ε–dependent CTL activity (48, 49) ranged from 1:500–1:2000 (Tripp, R.A., and P.C. Doherty, unpublished observations), comparable to the level described for other viruses (20). However, these CTLp frequencies may reflect a gross underestimate if highly activated CD8+ T cells are being driven to apoptosis after in vitro stimulation, as has been described for EBV (50, 51). We have observed that the activated Vβ4+CD8+ T cells from MHV-68–infected mice are short-lived ex vivo (data not shown).
Alternatively, the Vβ4 bias might be the consequence of a superantigen-driven response, as superantigens stimulate T cells in a Vβ-specific manner. Superantigens bind to MHC class II (52–58), but, in contrast to T cell recognition of conventional viral antigen, T cell responses to superantigens are generally not MHC restricted (58–60). Therefore, as a first step in examining a role for a viral superantigen in the T cell activation, we examined the MHC haplotype dependence of the elevated Vβ4 pattern seen in B6 mice. TCR profiles were determined for MHV-68–infected H-2k, H-2d, H-2u, and H-2q mice. The data show a clear Vβ4 expansion among CD8+ PBL (Table 1), but not CD4+ PBL (data not shown) in mouse strains representing each of the haplotypes. Therefore, although we are currently unable to rule out the possibility of a conventional viral peptide that promiscuously binds to MHC class I (61), the data suggest that the Vβ4 expansion seen during the IM phase of MHV-68 infection is driven by a virally-encoded superantigen.
Table 1.
Vβ4 Expression among CD8+ T Cells in MHV-68–infected Mice
| Mouse strain | MHC haplotype | % Vβ4+CD8+≳ | Days after infection | |||||
|---|---|---|---|---|---|---|---|---|
| Naive | MHV-68–infected | |||||||
| C57BL/6J | H-2b | 3.9 ± 0.2 | 46.8 ± 12.6 | 24 | ||||
| BALB/c | H-2d | 9.0 ± 1.2 | 29.2 ± 8.9 | 41 | ||||
| B10.BR | H-2k | 3.0 ± 0.8 | 11.1 ± 3.3 | 24–28 | ||||
| B10.PL | H-2u | 4.4 ± 1.6 | 22.0 ± 8.1 | 21 | ||||
| B10.Q | H-2q | 3.9 ± 0.9 | 8.4 ± 2.4 | 28 | ||||
PBL were obtained from control mice or MHV-68–infected mice at the indicated time points after infection. The percentage of Vβ4+CD8+ T cells among total CD8+ T cells was determined by two-color flow cytometry using biotinylated mAb specific for TCR-Vβ4+(KT4) and FITC-conjugated mAb specific for CD8 (53-6.72), using standard protocols.
Role of MHC Class II and CD4 in IM.
Previous studies established that the splenomegaly characteristic of MHV-68 infection is greatly diminished in mice that lack CD4+ T cells (9–12) and MHC class II glycoproteins (13). Therefore, we analyzed CD4-deficient MHC class II −/− mice for evidence of IM. The results showed that both the extent of MHV-68–induced CD8+ T cell proliferation in the spleen (Fig. 5 A) and the relative prevalence of CD8+CD62Llo T cells in the blood (Fig. 5, B and C) were much lower than in the MHC class II +/+ controls. Furthermore, the pattern of TCR-Vβ4+CD8+ dominance associated with the increase in frequency for the CD8+CD62Llo set in the PBL (Fig. 5 B) was not observed for MHC class II −/− mice (Fig. 5 C). These differences between the MHC class II +/+ and −/− mice could reflect the absence of the H-2IAb MHC class II glycoprotein, perhaps because of a requirement for MHC class II presentation of a viral superantigen (62), and/or because of a role for cytokines produced by MHV-68–immune CD4+ T cells in the antiviral CD8+ T cell response (63–65). To test this, +/+ mice were treated in vivo with the GK1.5 mAb to CD4 from day 11 after virus challenge. The data show that this treatment decreased the extent of CD8+ T cell cycling in the spleen from ∼28% in intact mice to ∼16 and 9% at days 17 and 23 after infection, respectively (Table 2). However, >80% of the blood CD8+ T cells still showed the characteristic IM-like CD62Llo profile, and there was little effect on the prevalence of TCR-Vβ4+ T cells in the CD8+ PBL at 23 d after infection (Table 2). These data raise the possibility that the high frequency of Vβ4+CD8+ T cells in the peripheral blood is a consequence of selective protection from apoptosis rather than a direct expansion. However, the data are most consistent with selective expansion of Vβ4+CD8+ T cells. First, although cycling is dramatically reduced, it is not eliminated, and is still elevated compared with naive animals, in which <5% of CD8+ T cells are cycling (Fig. 5 A and data not shown). In particular, ∼16% of CD8+ T cell in the spleen are cycling at day 17 in CD4-depleted animals, a time point just before the dramatic increase in percentage of Vβ4+CD8+ T cells (Fig. 4). Second, there is no evidence for the massive reduction in numbers of CD8+ T cells that would be necessary to account for the compensatory increase in Vβ4+CD8+ T cells (Table 2). Thus, the data suggest that eliminating >90% of the CD4+ T cells through the time that the IM-like phase of MHV-68 infection is developing did not prevent the emergence of the prominent TCR-Vβ4+ CD8+CD62Llo population. Cytokines derived from the CD4+ population are not, therefore, primarily responsible for the selective expansion of the Vβ4+ CD8+ T cells.
Figure 5.

Cell cycle analysis. The profiles of CD8+ T cell cycling in the spleen (A) and the prevalence and activation phenotypes (CD62Llo) of the CD8+ PBL populations in normal B6 mice (+/+) (B) and congenic mice that are CD4+ T cell deficient as a consequence of homozygous disruption (−/−) of the H-2IAb MHC class II gene (C). The data are from pooled (days 0, 21, and 29) or individual (days 14 and 17) spleens and individual PBL samples (groups of five, mean ± SD) from uninfected controls (day 0), or from 8-wk-old mice that had been challenged with MHV-68 14–29 d before. CD8+ PBL were examined for Vβ4 expression on days 14, 17, and 21. The data are expressed as a percentage of total PBL.
Table 2.
Consequences of CD4+ T Cell Depletion
| Day | CD4 depletion* | % CD8+ T cells in PBL | Spleen | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total‡ | CD62Llo | Vβ4+CD8+ | % Cycling CD8+§ | Total CD8+ (× 10−7) | Total CD4+ (× 10−7) | |||||||||
| 17 | − | 34.1 ± 10.1 | 26.9 ± 7.2 | ND | 28.1 ± 2.7 | 5.3 ± 1.3 | 3.8 ± 0.9 | |||||||
| + | 33.3 ± 6.6 | 25.6 ± 6.7 | ND | 16.1 ± 4.7‖ | 3.6 ± 0.7 | 0.2 ± 0.1¶ | ||||||||
| 23 | − | 34.8 ± 5.6 | 32.9 ± 5.6 | 46.8 ± 4.2 | 28.3 ± 5.0 | 2.3 ± 0.3 | 2.1 ± 0.2 | |||||||
| + | 38.8 ± 9.2 | 35.9 ± 9.0 | 39.5 ± 9.9 | 9.4 ± 2.2‖ | 1.5 ± 0.4 | 0.1 ± 0.1¶ | ||||||||
B6 mice were injected with the GK1.5 mAb to CD4 at 2-d intervals starting from day 11 after infection with MHV-68.
The number of CD8+ T cells in the PBL was relatively constant (7–11 × 106/ml) in all experiments.
Cycling in CD8+ T cells from naive animals is ⩽5%.
Significantly different from the corresponding values in intact mice (P ⩽0.01) by Wilcoxon rank analysis.
CD4 staining on residual cells was downmodulated.
However, there does appear to be a role for CD4+ T cells in the pathogenesis of IM. The numbers of cycling CD8+ T cells in the spleen were much lower in both the MHC class II −/− (Fig. 5 A) and CD4-depleted mice (Table 1). In addition, the frequency of the CD8+ PBL was not significantly increased as a consequence of infection in either group of CD4-deficient, MHV-68–infected mice (Fig. 5 C and Table 2). It should be noted that the elevated CD8 frequency in the MHC class II −/− mice is evident before infection, and thus reflects a compensatory increase because of the lack of CD4+ T cells rather than an increase as a consequence of infection. These data suggest that although CD4+ T cells are not involved in the specific Vβ4 expansion, they play a part in the generalized proliferation and activation of the CD8+ subset. Thus, the IM phase of MHV-68 infection appears to be a consequence of at least two separate activation events: a generalized, CD4-dependent, presumably cytokine-driven expansion that precedes, but does not control, the later, perhaps superantigen-driven, expansion of Vβ4+CD8+ T cells. The relationship between cell cycling in the spleen and activated CD8+ T cells in the peripheral blood is likely to be complex, but is an important issue for understanding the pathogenesis of IM, and thus warrants further investigation.
Discussion
Initial characterization of MHV-68 has revealed striking biological similarities to EBV. For example, major aspects of the pathogenesis of viral infection are similar in humans and mice, including the initial acute respiratory infection and the establishment of viral latency in B cells (9–12).
In the current studies, we describe a syndrome of T cell activation that occurs late in infection, well after the clearance of infectious virus and the establishment of latent infection of B cells. The activated T cells in the peripheral blood are predominantly CD8+ and express a CD62Llo-, CD44hi-activated phenotype, with as many as 75% of the CD8+ T cells expressing Vβ4+ TCR. These activated cells are a reflection of a more generalized activation in the enlarged spleen, in which CD4+ and B220+ cells are also activated. The activated phenotype is sustained in vivo for >2 mo (Figs. 1 and 4). This pathology has two key features in common with EBV-induced IM, namely, activated CD8+ T cells in the peripheral blood and splenomegaly. Taken together with previously identified similarities between MHV-68 and EBV infection (10), the similarities in the IM profile strengthen the relevance of MHV-68 as an experimental mouse model for EBV.
The availability of a mouse model of a γ-herpesvirus– induced IM promises to be a valuable tool in understanding the pathogenesis of the disease. For example, these initial studies show that the T cell–activation profile is unaltered in adult thymectomized mice, suggesting that availability of newly emerging thymocytes is not necessary for IM. In another example, we have been able to establish the relationship between initial infection and the onset of IM. Although this has been difficult to ascertain in EBV-induced IM because, in most cases, IM is the first clinical evidence of infection, the mouse model clearly indicates that the onset of IM is a late event in the viral infection, beginning as early as 2–3 wk after infection, well after the clearance of infectious virus from the lung. Finally, it has been long known that activated CD8+ T cells characteristic of EBVinduced IM are not all virus-specific, and the cause of the lymphoproliferation has been extensively investigated. There are conflicting reports supporting a role for viral antigens (17, 39, 66), superantigens (37, 38), and nonspecific activation (17, 18, 67) in the generation of activated CD8+ T cells. An intriguing possibility suggested by the late kinetics of CD8+ T cell activation is that the IM is a consequence of new viral antigen expression during the establishment of latency. The MHV-68 virus model will allow us to directly address the mechanism of CD8+ T cell activation. Although we don't yet know whether these T cells are specific for virus, our current studies suggest that both a TCR-mediated event, characterized by Vβ4+CD8+ T cell expansion, and a generalized T cell activation that is probably mediated by CD4-dependent cytokines, are involved in the MHV-68– induced IM.
The selective expansion of Vβ4+CD8+ T cells described in this report has two intriguing characteristics. First, the effect is not MHC-restricted, in that Vβ4 expansion is seen among activated CD8+ T cells in the peripheral blood and spleen of MHV-68–infected mouse strains expressing five different MHC haplotypes. Second, the Vβ4+CD8+ expansion is MHC class II–dependent because it is not observed in MHC class II −/− mice, but does occur in mice that were depleted of CD4+ T cells by in vivo antibody administration. There are two possible explanations for the MHC-unrestricted, Vβ4+ T cell expansion. The first possibility is an oligoclonal T cell response to a conventional viral antigen. The MHC promiscuity could be explained by the existence of a dominant epitope capable of binding multiple class I haplotypes, as has been described (61). The second possibility is superantigen-driven T cell activation. We believe the data are most consistent with a superantigen-driven response since Vβ-specific, class II–dependent, MHC-unrestricted stimulation of T cells are hallmarks of superantigen activation. As additional support of this, the response appears to be relatively independent of the α chain of the TCR (Blackman, M.A., unpublished observations) and T cell responses to superantigens are characteristically independent of the non-Vβ components of the TCR (68).
Despite the fact that the Vβ4-specific T cell activation fits several basic criteria for superantigen-driven responses, it is unusual that CD8+ T cells are preferentially activated. Superantigens characteristically stimulate both CD4+ and CD8+ T cells (69, 70), or in the case of weak superantigens, CD4+ T cells are preferentially activated (71). It is possible that the putative MHV-68 superantigen preferentially activates CD8+ T cells. In support of this possibility, a superantigen-like activity in Toxoplasma gondii was shown to selectively activate CD8+ T cells under some experimental conditions (72, 73). It is also possible that MHV-68 activates both CD4+ and CD8+ T cells, but the CD4+ T cells are preferentially driven to apoptosis, resulting in the selective retention of the CD8+ T cells. Selective apoptosis of CD4+ T cells after staphylococcal enterotoxin B stimulation has been described (74). This possibility is consistent with the modest elevation of Vβ4+CD4+ T cells seen in some experiments (Fig. 4 B). Thus, although we cannot eliminate the possibility that the Vβ4+CD8+ expansion represents a restricted response to a conventional viral peptide with promiscuous MHC class I binding, the Vβ-specific, MHC class II–dependent, non–MHC-restricted, TCR-α chain–independent Vβ4+CD8+ activation during the IM phase of MHV-68 infection is most readily explained by the expression of a viral superantigen.
One trivial explanation for the Vβ4 expansion is that the effect is mediated by a retroviral superantigen. Perhaps the MHV-68 infection is activating an endogenous superantigen or the virus stock is contaminated with superantigen-expressing murine retroviruses. Although we cannot formally rule out these possibilities, we think that they are unlikely. First, no endogenous retrovirus specific for Vβ4+ T cells has been identified. Also, if the mice were harboring an unknown Vβ4-specific endogenous retrovirus, it would be expected that Vβ4+ T cells would be deleted in these mouse strains, which was not observed (Table 1). Recently, an exogenous mouse mammary tumor virus (MMTV) that activates Vβ4+ T cells was described in the swiss IBM moro mouse strain (75). However, this MMTV was shown to require the presence of MHC class II I-E molecules, which are absent in B6 mice, for efficient superantigen presentation. Second, with regard to the possibility that the MHV-68 virus stock is contaminated with murine retroviruses, plaquepurified virus has been cultivated in owl monkey kidney fibroblast cells, which would not be permissive for MMTV replication and would not be a source for introduction of MMTV into the MHV-68 virus stock. In addition, the delayed and sustained expansion of Vβ4+ T cells seen after infection with MHV-68 are not consistent with the kinetics of superantigen expression during a typical MMTV infection (76).
Earlier studies have suggested the presence of a viral superantigen expressed by EBV. Smith et al. described a Vβ-specific expansion in the peripheral blood of patients with IM, which was absent in two cases examined after resolution of the acute phase of the disease (37). Other studies, however, have not shown a Vβ-specific expansion either in vivo during IM nor after in vitro stimulation with EBV-infected cells (40, 41). More recently, Sutkowski et al. reported an MHC class II–dependent, but MHC-unrestricted proliferation of naive T cells in response to EBVtransformed B cells in which the virus was reactivated (38). Analysis of early activation markers indicated a selective Vβ13 activation. In contrast, in another recent study, Callan et al. showed clonal or oligoclonal populations of activated T cells in the peripheral blood of IM patients, in which the Vβ-specific expansion varied among individuals, suggesting viral antigen-driven proliferation (39). It should be noted that variation in the Vβ profile from individual patients does not in itself rule out a role for a viral superantigen. It is possible that there is sequence variation in different isolates, analogous to MMTV, resulting in expansion of different Vβ populations of T cells. However, in support of a conventional virus-specific response, Callan et al. showed that, in some cases, the specificity of the β chain was identical to that identified in EBV-reactive T cell clones by an independent group (39).
There are also reports of viral superantigens in other herpesviruses. For example, evidence for a CMV-encoded superantigen has been reported (77). In addition, an open reading frame of the H. saimiri genome, ORF 14, which has significant homology to a mouse mammary tumor virus-encoded superantigen (78), encodes a protein that binds to MHC class II molecules and stimulates T cell proliferation, although the Vβ profile of the proliferating T cells has not been reported (79).
It is interesting to speculate on the role of a putative superantigen in MHV-68 infection. In the case of MMTV superantigens, expression of the viral superantigen is essential for productive infection (62, 80–82). MHC class II molecules on virally infected B cells present a superantigen that activates a subset of T cells expressing the appropriate Vβ element. The activated T cells subsequently promote the proliferation and differentiation of infected B cells, resulting in clonal expansion and the establishment of memory cells that serve as a stable reservoir for the virus (81). It is clear from our studies that expression of the putative superantigen is not essential for infection or the establishment of latency of MHV-68 because MHC class II −/− mice still became infected and established latency (13), although they did not exhibit IM (Fig. 5). It is possible that superantigen-driven T cell activation is required for expansion of the pool of latently infected B cells. A role for a putative EBV superantigen in establishing an equilibrium between latency and activation, resulting in the maintenance of a constant EBV burden, has been postulated (38). In this light, more detailed studies of latency and malignancy in MHV-68–infected B6 and MHC class II −/− mice should be revealing (13). An intriguing feature of the putative MHV-68 viral superantigen is that the effect on the TCRVβ4+CD8+ T cells is not apparent by day 14, when all lytic virus has been cleared from epithelial sites, and only becomes obvious from ∼2 wk after evidence of latent MHV-68 infection is first detected in B lymphocytes. It is likely that the putative superantigen is a viral gene product that binds to MHC class II glycoproteins on the surface of persistently infected B cells but, if this is so, the kinetics of IM development suggest either that the protein is not expressed during the acute stage of the disease, or that the emergence of the TCR-Vβ4+CD8+ T cells is in some way inhibited during the time that the effectors that deal with the lytic phase of the infection are operating. One possible scenario is that the superantigen is not expressed on infected epithelial cells during acute infection, but can be expressed on B cells during reactivation of latent virus.
In conclusion, we have described an IM phase of MHV-68 infection of mice characterized by at least two activation events: a CD4-dependent expansion that is presumably cytokine dependent, and a preferential expansion of Vβ4+ CD8+ T cells, perhaps driven by a viral superantigen. This MHV thus provides a valuable experimental model for understanding the pathogenesis of IM and the equilibrium between the lytic and latent stages of herpesvirus infection.
Acknowledgments
We thank Anthony McMickle for help with the flow cytometry.
Footnotes
These experiments were supported by National Institutes of Health grants CA21765, AI38349, P30 CA21765, and by the American Lebanese Syrian Associated Charities.
1 Abbreviations used in this paper: γHV, γ-herpesvirus; CTLp, CTL precursor; IM, infectious mononucleosis; MHV-68, murine γ-herpesvirus 68; MMTV, mouse mammary tumor virus.
Peter C. Doherty and Marcia A. Blackman made equal contributions to this manuscript.
References
- 1.Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol. 1990;71:1365–1372. doi: 10.1099/0022-1317-71-6-1365. [DOI] [PubMed] [Google Scholar]
- 2.Efstathiou S, Ho YM, Minson AC. Cloning and molecular characterization of the murine herpesvirus 68 genome. J Gen Virol. 1990;71:1355–1364. doi: 10.1099/0022-1317-71-6-1355. [DOI] [PubMed] [Google Scholar]
- 3.Honess RW, Craxton MA, Williams L, Gompels UA. A comparative analysis of the sequence of the thymidine kinase gene of a gammaherpesvirus, herpesvirus saimiri. J Gen Virol. 1989;70:3003–3013. doi: 10.1099/0022-1317-70-11-3003. [DOI] [PubMed] [Google Scholar]
- 4.Cesarman E, Moore PS, Rao PH, Inghirami G, Knowles DM, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 5.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, Moore PS. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science (Wash DC) 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 6.Renne R, Zhong W, Herndier B, McGrath M, Abbey N, Kedes D, Ganem D. Lytic growth of Kaposi's sarcoma–associated herpesvirus (human herpesvirus 8) in culture. Nat Med. 1996;2:342–346. doi: 10.1038/nm0396-342. [DOI] [PubMed] [Google Scholar]
- 7.Moss DJ, Burrows SR, Khanna R, Misko IS, Sculley TB. Immune surveillance against Epstein-Barr virus. Semin Immunol. 1992;4:97–104. [PubMed] [Google Scholar]
- 8.Neubauer RH, Dunn FE, Rabin H. Infection of multiple T-cell subsets and changes in lymphocyte functions associated with Herpesvirus saimiri infection of owl monkeys. Infect Immun. 1981;32:698–706. doi: 10.1128/iai.32.2.698-706.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehtisham S, Sunil-Chandra NP, Nash AA. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J Virol. 1993;67:5247–5252. doi: 10.1128/jvi.67.9.5247-5252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash AA, Sunil-Chandra NP. Interactions of the murine gammaherpesvirus with the immune system. Curr Opin Immunol. 1994;6:560–563. doi: 10.1016/0952-7915(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 11.Sunil-Chandra NP, Efstathiou S, Nash AA. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J Gen Virol. 1992;73:3275–3279. doi: 10.1099/0022-1317-73-12-3275. [DOI] [PubMed] [Google Scholar]
- 12.Usherwood EJ, Ross AJ, Allen DJ, Nash AA. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol. 1996;77:627–630. doi: 10.1099/0022-1317-77-4-627. [DOI] [PubMed] [Google Scholar]
- 13.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell–mediated control of a γ-herpesvirus in the absence of CD4+T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henle G, Henle W, Diehl V. Relation of Burkitt's tumor–associated herpes-type virus to infectious mononucleosis. Proc Natl Acad Sci USA. 1968;59:94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyawaki T, Kasahara Y, Kanegane H, Ohta K, Yokoi T, Yachie A, Taniguchi N. Expression of CD45R0 (UCHL1) by CD4+ and CD8+T cells as a sign of in vivo activation in infectious mononucleosis. Clin Exp Immunol. 1991;83:447–451. doi: 10.1111/j.1365-2249.1991.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds DJ, Banks PM, Gulley ML. New characterization of infectious mononucleosis and a phenotypic comparison with Hodgkin's disease. Am J Pathol. 1995;146:379–388. [PMC free article] [PubMed] [Google Scholar]
- 17.Strang G, Rickinson AB. In vitro expansion of Epstein-Barr virus-specific HLA-restricted cytotoxic T cells direct from the blood of infectious mononucleosis patients. Immunology. 1987;62:647–654. [PMC free article] [PubMed] [Google Scholar]
- 18.Tomkinson BE, Maziarz R, Sullivan JL. Characterization of the T cell-mediated cellular cytotoxicity during acute infectious mononucleosis. J Immunol. 1989;143:660–670. [PubMed] [Google Scholar]
- 19.Grusby MJ, Johnson RS, Papaioannou VE, Glimcher LH. Depletion of CD4+T cells in major histocompatibility complex class II–deficient mice. Science (Wash DC) 1991;253:1417–1420. doi: 10.1126/science.1910207. [DOI] [PubMed] [Google Scholar]
- 20.Tripp RA, Hou S, McMickle A, Houston J, Doherty PC. Recruitment and proliferation of CD8+T cells in respiratory virus infections. J Immunol. 1995;154:6013–6021. [PubMed] [Google Scholar]
- 21.Allan W, Tabi Z, Cleary A, Doherty PC. Cellular events in the lymph node and lung of mice with influenza. Consequences of depleting CD4+T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 22.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+T cells. J Immunol. 1992;149:1319–1325. [PubMed] [Google Scholar]
- 23.Cole GA, Katz JM, Hogg TL, Ryan KW, Portner A, Woodland DL. Analysis of the primary T-cell response to Sendai virus infection in C57BL/6 mice: CD4+T-cell recognition is directed predominantly to the hemagglutinin-neuraminidase glycoprotein. J Virol. 1994;68:6863–6870. doi: 10.1128/jvi.68.11.6863-6870.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deckhut AM, Allan W, McMickle A, Eichelberger M, Blackman MA, Doherty PC, Woodland DL. Prominent usage of Vβ8.3 T cells in the H-2Db-restricted response to an influenza A virus nucleoprotein epitope. J Immunol. 1993;151:2658–2666. [PubMed] [Google Scholar]
- 25.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73:2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 26.Ceredig R, Allan JE, Tabi Z, Lynch F, Doherty PC. Phenotypic analysis of the inflammatory exudate in murine lymphocytic choriomeningitis. J Exp Med. 1987;165:1539–1551. doi: 10.1084/jem.165.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou S, Doherty PC. Partitioning of responder CD8+T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J Immunol. 1993;150:5494–5500. [PubMed] [Google Scholar]
- 28.Tabi Z, Lynch F, Ceredig R, Allan JE, Doherty PC. Virus-specific memory T cells are Pgp-1+and can be selectively activated with phorbol ester and calcium ionophore. Cell Immunol. 1988;113:268–277. doi: 10.1016/0008-8749(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 29.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin–low phenotype for virus-specific CD8+memory T cells. J Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 30.Rickinson, A.B., and E. Kieff. 1996. Epstein-Barr virus. In Fields Virology. B.N. Fields, D.M. Knipe, and P.M. Howley, editors. Lippincott-Raven Publishers, Philadelphia. 2397–2446.
- 31.Sprent J. T and B memory cells. Cell. 1994;76:315–322. doi: 10.1016/0092-8674(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 32.Tripp RA, Lahti JM, Doherty PC. Laser light suicide of proliferating virus-specific CD8+T cells in an in vivo response. J Immunol. 1995;155:3719–3721. [PubMed] [Google Scholar]
- 33.Strang G, Rickinson AB. Multiple HLA class I–dependent cytotoxicities constitute the “non-HLA-restricted” response in infectious mononucleosis. Eur J Immunol. 1987;17:1007–1013. doi: 10.1002/eji.1830170717. [DOI] [PubMed] [Google Scholar]
- 34.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science (Wash DC) 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 35.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- 36.Kotzin BL, Leung DY, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 37.Smith TJ, Terada N, Robinson CC, Gelfand EW. Acute infectious mononucleosis stimulates the selective expression/expansion of Vβ6.1-3 and Vβ7 T cells. Blood. 1993;81:1521–1526. [PubMed] [Google Scholar]
- 38.Sutkowski N, Palkama T, Ciurli C, Sekaly RP, Thorley-Lawson DA, Huber BT. An Epstein-Barr virus–associated superantigen. J Exp Med. 1996;184:971–980. doi: 10.1084/jem.184.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Callan MFC, Steven N, Krausa P, Wilson JDK, Moss PAH, Gillespie GM, Bell JI, Rickinson AB, McMichael AJ. Large clonal expansions of CD8+T cells in acute infectious mononucleosis. Nat Med. 1996;2:906–911. doi: 10.1038/nm0896-906. [DOI] [PubMed] [Google Scholar]
- 40.Slobod KS, Leggiadro RJ, Presbury G, Smith FS, Hurwitz JL. Peripheral T cell receptor repertoire among CD4+ and CD8+subsets during acute infectious mononucleosis. Virol Immunol. 1994;7:151–153. doi: 10.1089/vim.1994.7.151. [DOI] [PubMed] [Google Scholar]
- 41.Slobod KS, Freiberg AS, Allan JE, Rencher SD, Hurwitz JL. T-cell receptor heterogeneity among Epstein-Barr virus–stimulated T-cell populations (erratum published, 196:914) Virology. 1993;196:179–189. doi: 10.1006/viro.1993.1466. [DOI] [PubMed] [Google Scholar]
- 42.Aebischer T, Oehen S, Hengartner H. Preferential usage of Vα4 and Vβ10 T cell receptor genes by lymphocytic choriomeningitis virus glycoprotein-specific H-2Db–restricted cytotoxic T cells. Eur J Immunol. 1990;20:523–531. doi: 10.1002/eji.1830200310. [DOI] [PubMed] [Google Scholar]
- 43.Yanagi Y, Tishon A, Lewicki H, Cubitt BA, Oldstone MB. Diversity of T-cell receptors in virus-specific cytotoxic T lymphocytes recognizing three distinct viral epitopes restricted by a single major histocompatibility complex molecule. J Virol. 1992;66:2527–2531. doi: 10.1128/jvi.66.4.2527-2531.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanagi Y, Maekawa R, Cook T, Kanagawa O, Oldstone MB. Restricted V-segment usage in T-cell receptors from cytotoxic T lymphocytes specific for a major epitope of lymphocytic choriomeningitis virus. J Virol. 1990;64:5919–5926. doi: 10.1128/jvi.64.12.5919-5926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cole GA, Hogg TL, Woodland DL. The MHC class I–restricted T cell response to Sendai virus infection in C57BL/6 mice: a single immunodominant epitope elicits an extremely diverse repertoire of T cells. Int Immunol. 1994;6:1767–1775. doi: 10.1093/intimm/6.11.1767. [DOI] [PubMed] [Google Scholar]
- 46.Casanova JL, Romero P, Widmann C, Kourilsky P, Maryanski JL. T cell receptor genes in a series of class I major histocompatibility complex–restricted cytotoxic T lymphocyte clones specific for a Plasmodium bergheinonapeptide: implications for T cell allelic exclusion and antigen-specific repertoire. J Exp Med. 1991;174:1371–1383. doi: 10.1084/jem.174.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argaet VP, Schmidt CW, Burrows SR, Silins SL, Kurilla MG, Doolan DL, Suhrbier A, Moss DJ, Kieff E, Suclley TB. Dominant selection of an invariant T cell antigen receptor in response to persistent infection by Epstein-Barr virus. J Exp Med. 1994;180:2335–2340. doi: 10.1084/jem.180.6.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mentzer SJ, Barbosa JA, Burakoff SJ. T3 monoclonal antibody activation of nonspecific cytolysis: a mechanism of CTL inhibition. J Immunol. 1985;135:34–38. [PubMed] [Google Scholar]
- 49.Spits H, Yssel H, Leeuwenberg J, De Vries JE. Antigen-specific cytotoxic T cell and antigen-specific proliferating T cell clones can be induced to cytolytic activity by monoclonal antibodies against T3. Eur J Immunol. 1985;15:88–91. doi: 10.1002/eji.1830150117. [DOI] [PubMed] [Google Scholar]
- 50.Moss DJ, Bishop CJ, Burrows SR, Ryan JM. T lymphocytes in infectious mononucleosis. I. T cell death in vitro. Clin Exp Immunol. 1985;60:61–69. [PMC free article] [PubMed] [Google Scholar]
- 51.Uehara T, Miyawaki T, Ohta K, Tamaru Y, Yokoi T, Nakamura S, Taniguchi N. Apoptotic cell death of primed CD45RO+T lymphocytes in Epstein-Barr virus– induced infectious mononucleosis. Blood. 1992;80:452–458. [PubMed] [Google Scholar]
- 52.Fischer H, Dohlsten M, Lindvall M, Sjogren HO, Carlsson R. Binding of staphylococcal enterotoxin A to HLA-DR on B cell lines. J Immunol. 1989;142:3151–3157. [PubMed] [Google Scholar]
- 53.Fleischer B, Schrezenmeier H. T cell stimulation by staphylococcal enterotoxins. Clonally variable response and requirement for major histocompatibility complex class II molecules on accessory or target cells. J Exp Med. 1988;167:1697–1707. doi: 10.1084/jem.167.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carlsson R, Fischer H, Sjogren HO. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988;140:2484–2488. [PubMed] [Google Scholar]
- 55.Fraser JD. High-affinity binding of staphylococcal enterotoxins A and B to HLA– DR. Nature (Lond) 1989;339:221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- 56.Mollick JA, Cook RG, Rich RR. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science (Wash DC) 1989;244:817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- 57.Scholl PR, Diez A, Geha RS. Staphylococcal enterotoxin B and toxic shock syndrome toxin-1 bind to distinct sites on HLA-DR and HLA-DQ molecules. J Immunol. 1989;143:2583–2588. [PubMed] [Google Scholar]
- 58.White J, Herman A, Pullen AM, Kubo R, Kappler JW, Marrack P. The Vβ–specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989;56:27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]
- 59.Janeway CA, Jr, Yagi J, Conrad PJ, Katz ME, Jones B, Vroegop S, Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 60.Scholl PR, Diez A, Karr R, Sekaly RP, Trowsdale J, Geha RS. Effect of isotypes and allelic polymorphism on the binding of staphylococcal exotoxins to MHC class II molecules. J Immunol. 1990;144:226–230. [PubMed] [Google Scholar]
- 61.Sidney, J., R.T. Kubo, P.A. Wentworth, J. Alexander, R.W. Chesnut, H.M. Grey, and A. Sette. 1996. Broadly reactive HLA restricted T cell epitopes and their implications for vaccine design. In Concepts in Vaccine Design. S.H.E. Kaufman, editor. Walter de Gruyer, Berlin. 169–186.
- 62.Beutner U, McLellan B, Kraus E, Huber BT. Lack of MMTV superantigen presentation in MHC class II– deficient mice. Cell Immunol. 1996;168:141–147. doi: 10.1006/cimm.1996.0060. [DOI] [PubMed] [Google Scholar]
- 63.Hou S, Mo XY, Hyland L, Doherty PC. Host response to Sendai virus in mice lacking class II major histocompatibility complex glycoproteins. J Virol. 1995;69:1429–1434. doi: 10.1128/jvi.69.3.1429-1434.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarawar SR, Cardin RD, Brooks JW, Mehrpooya M, Tripp RA, Doherty PC. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol. 1996;70:3264–3268. doi: 10.1128/jvi.70.5.3264-3268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tripp RA, Sarawar SR, Doherty PC. Characteristics of the influenza virus-specific CD8+ T cell response in mice homozygous for disruption of the H-2IAbgene. J Immunol. 1995;155:2955–2959. [PubMed] [Google Scholar]
- 66.Golovkina TV, Chervonsky A, Prescott JA, Janeway CA, Jr, Ross SR. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J Exp Med. 1994;179:439–446. doi: 10.1084/jem.179.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svedmyr E, Jondal M. Cytotoxic effector cells specific for B cell lines transformed by Epstein-Barr virus are present in patients with infectious mononucleosis. Proc Natl Acad Sci USA. 1975;72:1622–1626. doi: 10.1073/pnas.72.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blackman MA, Woodland DL. Role of the T cell receptor α-chain in superantigen recognition. Immunol Res. 1996;15:98–113. doi: 10.1007/BF02918500. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald HR, Lees RK, Chvatchko Y. CD8+ T cells respond clonally to Mls-1a-encoded determinants. J Exp Med. 1990;171:1381–1386. doi: 10.1084/jem.171.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Webb SR, Sprent J. Response of mature unprimed CD8+ T cells to Mlsadeterminants. J Exp Med. 1990;171:953–958. doi: 10.1084/jem.171.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wallace VA, Rahemtulla A, Timms E, Penninger J, Mak TW. CD4 expression is differentially required for deletion of MLS-1a-reactive T cells. J Exp Med. 1992;176:1459–1463. doi: 10.1084/jem.176.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Denkers EY, Caspar P, Hieny S, Sher A. Toxoplasma gondiiinfection induces specific nonresponsiveness in lymphocytes bearing the Vβ5 chain of the mouse T cell receptor. J Immunol. 1996;156:1089–1094. [PubMed] [Google Scholar]
- 73.Denkers EY, Caspar P, Sher A. Toxoplasma gondii possesses a superantigen activity that selectively expands murine T cell receptor Vβ5-bearing CD8+lymphocytes. J Exp Med. 1994;180:985–994. doi: 10.1084/jem.180.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawabe Y, Ochi A. Programmed cell death and extrathymic reduction of Vβ8+CD4+ T cells in mice tolerant to Staphylococcus aureusenterotoxin B. Nature (Lond) 1991;349:245–248. doi: 10.1038/349245a0. [DOI] [PubMed] [Google Scholar]
- 75.Maillard I, Erny K, Acha-Orbea H, Diggelmann H. A Vβ4–specific superantigen encoded by a new exogenous mouse mammary tumor virus. Eur J Immunol. 1996;26:1000–1006. doi: 10.1002/eji.1830260507. [DOI] [PubMed] [Google Scholar]
- 76.Held W, Waanders GA, Shakhov AN, Scarpellino L, Acha-Orbea H, MacDonald HR. Superantigeninduced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 77.Dobrescu D, Ursea B, Pope M, Asch AS, Posnett DN. Enhanced HIV-1 replication in Vβ12 T cells due to human cytomegalovirus in monocytes: evidence for a putative herpesvirus superantigen. Cell. 1995;82:753–763. doi: 10.1016/0092-8674(95)90472-7. [DOI] [PubMed] [Google Scholar]
- 78.Thomson BJ, Nicholas J. Superantigen function. Nature (Lond) 1991;351:530. doi: 10.1038/351530a0. [DOI] [PubMed] [Google Scholar]
- 79.Yao Z, Maraskovsky E, Spriggs MK, Cohen JI, Armitage RJ, Alderson MR. Herpesvirus saimiriopen reading frame 14, a protein encoded by T lymphotropic herpesvirus, binds to MHC class II molecules and stimulates T cell proliferation. J Immunol. 1996;156:3260–3266. [PubMed] [Google Scholar]
- 80.Golovkina TV, Chervonsky A, Dudley JP, Ross SR. Transgenic mouse mammary tumor virus superantigen expression prevents viral infection. Cell. 1992;69:637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- 81.Held W, Acha-Orbea H, MacDonald HR, Waanders GA. Superantigens and retroviral infection: insights from mouse mammary tumor virus. Immunol Today. 1994;15:184–190. doi: 10.1016/0167-5699(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 82.Huber BT. The role of superantigens in virus infection. J Clin Immunol. 1995;15:22s–25s. doi: 10.1007/BF01540890. [DOI] [PubMed] [Google Scholar]