Abstract
Autoreactive anti–MHC class II T cells are found in Brown Norway (BN) and Lewis (LEW) rats that receive either HgCl2 or gold salts. These T cells have a T helper cell 2 (Th2) phenotype in the former strain and are responsible for Th2-mediated autoimmunity. In contrast, T cells that expand in LEW rats produce IL-2 and prevent experimental autoimmune encephalomyelitis, a cell-mediated autoimmune disease. The aim of this work was to investigate, using T cell lines derived from HgCl2-injected LEW rats (LEWHg), the effect of these autoreactive T cells on the development of Th2-mediated autoimmunity. The five LEWHg T cell lines obtained protect against Th2-mediated autoimmunity induced by HgCl2 in (LEW × BN)F1 hybrids. The lines produce, in addition to IL-2, IFN-γ and TGF-β, and the protective effect is TGF-β dependent since protection is abrogated by anti-TGF-β treatment. These results identify regulatory, TGF-β–producing, autoreactive T cells that are distinct from classical Th1 or Th2 and inhibit both Th1- and Th2-mediated autoimmune diseases.
Mercuric chloride or gold salts induce in Brown Norway (BN)1, in (Lewis [LEW] × BN)F1 hybrids, and in susceptible mice, a transient Th2-dependent B cell polyclonal activation (1–4) responsible for an increase in serum IgE concentration and for the production of various autoantibodies including anti-DNA and antilaminin antibodies (for review see reference 1). These latter antibodies are associated with the occurrence of an autoimmune glomerulonephritis as observed in some patients treated with gold salts (5). T cells that recognize either self–MHC class II molecules or an ubiquitous self-peptide presented by MHC class II molecules play an important role in the induction of B cell polyclonal activation in this model (6, 7). In contrast, HgCl2 provokes in LEW rats, a nonantigen-specific suppression and protects from autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) to which this strain is otherwise highly susceptible. Autoreactive anti-self– MHC class II T cells have also been detected in HgCl2- injected LEW rats (8). Autoreactive T cell lines have been derived from gold-injected BN rats and HgCl2-injected LEW rats (LEWHg T cell lines) by repeated stimulations with syngeneic APCs. In both strains, these anti–MHC class II T cell lines are RT1.B- (mouse IA equivalent), but not RT1.D- (mouse IE equivalent) restricted (7, 8). Whereas T cell lines derived from BN rats produce IL-4 and are able to passively transfer autoimmunity into CD8-depleted naive BN rats (7), the LEWHgA T cell line, derived from HgCl2-injected LEW rats, produces IL-2 and IFN-γ and protects LEW rats against EAE, a Th1-mediated autoimmune disease, by inducing regulatory CD8+ T cells (8).
The aim of this study was to assess the effect of adoptive transfer of LEWHg T cell lines on the course of Th2mediated autoimmunity induced by HgCl2 in (LEW × BN)F1 hybrids. We show that (a) the lines produce TGF-β in addition to IL-2 and IFN-γ, (b) adoptive transfer of the lines prevents HgCl2-induced autoimmunity, and (c) this protection is abrogated after anti-TGF-β mAb administration.
Materials and Methods
Rats
BN and LEW rats were obtained from Charles River (Rouen, France) and maintained in our facilities. F1 hybrids were obtained by crossing BN male and LEW female rats in our animal house. 8–12-wk-old males or females were used in the experiments.
Culture Medium
RPMI 1640 (Biochrom KG, Berlin, Germany) was supplemented with streptomycin (100 μg/ml) and penicillin (100 U/ml), nonessential amino acids (0.1 mM), L-glutamine (2 mM; GIBCO, Paisley, U.K.), sodium pyruvate (1 mM; Biochrom KG), and 2-ME (5 × 10−5 M); Sigma Chemical Co., St. Louis, MO).
mAbs and FACS® Analysis
R73, W3/25, OX8, OX81, and B10.H2 mAbs are mouse IgG1 mAbs that recognize rat TCR-α/β chains (9), CD4 (10), CD8 (11), rat IL-4 (12), and thyroglobulin, respectively. The IgG1 1D11.16 and the IgG2a 2G.7 anti-TGF-β mAbs were provided by Dr. W. Waegell (Celtrix Pharmaceuticals, Santa Clara, CA; 13) and by Dr. C.M. Melief (University of Leiden, Leiden, Netherlands; 14), respectively. The IgG1 DB.1 and DB.12 antiIFN-γ mAbs were provided by Dr. P.H. Van der Meide (15) and B10.H2 mAb by Dr. D. Glotz (Paris, France). The W3/25 and OX8 hybridomas were obtained from the Public Health Laboratory Service (Oxford, U.K.); Dr. T. Hunig (Würzburg, Germany), and Dr. D. Mason (Medical Research Council, Oxford, U.K.) provided the R73 and OX81 hybridomas, respectively. Ascites and purified antibodies were prepared as described (16).
The phenotype of cells was determined by double staining; cells were incubated with FITC-labeled W3/25 and biotinylatedOX8 mAb, and then streptavidin-phycoerythrin (Sigma Chemical Co.) was added. The cells were analyzed with a Coulter Epics XL (Coultronics, Margency, France); a minimum of 5,000 cells were counted.
T Cell Lines
The five LEWHg T cell lines (LEWHg A, B, C, D, and E) used in this study have been previously described (8). They were derived from lymph node cells of five distinct LEW rats injected with HgCl2 for 7 d; they are CD4+CD8−TCR-α/β+, proliferate in the presence of syngeneic APCs, and are MHC class II (RT1.B)–restricted. The LEWOVA T cell line was also a CD4+ CD8− TCR-α/β+ T cell line derived from the draining lymph nodes of a LEW rat immunized 10 d before with 100 μg OVA in CFA. Except when otherwise mentioned, T cell lines were stimulated repeatedly every 2 wk as follows: 2 × 105 cells/well were cultured in 24-well flat-bottomed plates (Nunc, Kamstrup, Denmark), in a 6% CO2 incubator in the presence of normal irradiated (3,000 rads) syngeneic thymocytes (5–10 × 106/well), in a volume of 1 ml/well culture medium supplemented with 10% heatinactivated FCS and 10% Con A supernatant (8). The LEWOVA T cell line was stimulated with 50 μg/ml OVA (Sigma Chemical Co.).
Cytokine Assays
T cell lines were stimulated with irradiated thymocytes as described above, in the absence of Con A supernatant; 24-, 48-, 72-, 96-, and 120-h culture supernatants were collected and stored at −20°C. IL-2 production was assessed using proliferation of the IL-2–dependent CTLL-2 cell line (7). IFN-γ production was determined by two-site sandwich ELISA (7). Values were expressed as U/ml IFN-γ referring to a standard curve constructed using serial dilutions of recombinant purified rat IFN-γ (a gift from Dr. P. van der Meide, Vrije Universiteit, Amsterdam, Netherlands). IL-4 detection was based upon the ability of this cytokine to upregulate MHC class II molecule expression on B cells (12). The assay was performed, in the presence or in the absence of the OX81 anti–rat IL-4 mAb (50 μg/ml), by fluorocytometry. Recombinant rat IL-4, (provided by Dr. D. Mason, Medical Research Council, CIU, Oxford, U.K.) was used as a positive control.
TGF-β was measured by ELISA (R&D Sys., Inc., Abingdon, U.K.) according to the manufacturer's instructions. To demonstrate that TGF-β was produced by T cells and not by APCs, the LEWHgA T cell line was also stimulated as follows: 96 flat-bottomed well plates were precoated with rabbit anti–mouse Ig antibodies (DAKOPATTS, Copenhagen, Denmark) overnight; the LEWHgA T cell line was then added (5 × 104/well), together with mouse anti–rat TCR-α/β mAb (50 μg/ml) as described elsewhere (9). Supernatant was collected after 24, 48, and 96 h. Cells were also cultured for 72 h to assess the proliferative response. The anti–rat TCR-α/β mAb was replaced in control cultures by the B10.H2 mAb.
Experimental Design
HgCl2 Injections and Follow Up of Mercury Disease.
Rats were injected with HgCl2 (100 μg/100 g body wt, subcutaneously, 3 times/wk) during 4 to 6 wk (6). All the rats were bled once a week for determination of serum IgE concentration, antilaminin, and occasionally, anti-DNA antibody titers as previously described (17, 18). Kidneys were processed at the time of killing for immunofluorescence studies, using FITC-conjugated sheep anti– rat IgG antiserum as previously described (7). The intensity of fluorescence was scored on a scale from 0 to 4 in blind study by two independent examiners.
Adoptive Transfer of T Cell Lines.
T cell lines (LEWHgA, LEWHgB, LEWHgC, LEWHgD, and LEWHgE) were stimulated for 3 d with syngeneic irradiated thymocytes only or LEWOVA with syngeneic irradiated thymocytes plus ovalbumin. T cell lines (107 cells) were then intraperitoneally injected into naive F1 rats 2 wk before the first HgCl2 injection.
Thymectomy, Anti-CD8, and Anti-TGF-β Treatment.
Rats were thymectomized at 8 wk of age (19), allowed to recover for 3 wk, and then injected with the LEWHgA T cell line; 15 d later, rats were injected with HgCl2. In addition, some rats received the anti-CD8 (OX8) mAb or the control isotype-matched B10.H2 mAb (500 μg intraperitoneally, once a week for 1 mo) from the first HgCl2 injection (8). The effect of TGF-β neutralization was assessed using the anti-TGF-β mAbs 1D11.16 or 2G.7 (2 mg intraperitoneally on days −2, 0, 3, 6, and 9 with respect to the first HgCl2 injection).
Statistical Analysis
Serum IgE concentration, antilaminin, and anti-DNA antibody titers were compared between the different groups by using nonparametrical tests. The number of rats per group with kidneybound IgG was compared by chi-square analysis.
Results
Description of T Cell Lines.
As previously described (8), the five LEWHg T cell lines used in this study are CD4+ CD8−TCR-α/β+ and proliferate in the presence of normal syngeneic MHC class II+ APCs. They all produced IL-2 and IFN-γ (Table 1) with a peak at 24 h for IL-2 and 48 or 72 h for IFN-γ. The LEWHg, but not the LEWOVA, T cell lines produced TGF-β (Table 1) with a peak at 96 or 120 h of stimulation. None of these cytokines were detected in supernatants from irradiated APCs only, or in supernatants of resting T cell lines (Table 1). To eliminate a role for APCs in producing TGF-β, the LEWHgA T cell line was stimulated by plate-bound anti-TCR mAb in the absence of APCs. In such conditions, the LEWHgA T cell line proliferated (index 3 ± 0.5) and produced TGF-β (1.72 ± 0.05 ng/ml). No proliferation and no TGF-β secretion were observed when the anti-TCR mAb was replaced by the control B10.H2 mAb.
Table 1.
Cytokines Produced by the LEWHg and LEWOVA T Cell Lines
| Line (No. of exp.) | IL-2* | IFN-γ | TGF-β | |||
|---|---|---|---|---|---|---|
| cpm × 10−3 | U/ml | ng/ml | ||||
| LEWHgA (8) | 12 ± 3.5 | 15 ± 9 | 2.3 ± 0.9 | |||
| LEWHgB (2) | 8, 6 | 10, 13 | 1.7, 0.9 | |||
| LEWHgC (2) | 14, 7 | 21, 9 | 0.45, 0.39 | |||
| LEWHgD (2) | 8, 12 | 2, 4.5 | 0.15, 0.3 | |||
| LEWHgE (2) | 9.6, 15 | 13, 15 | 0.6, 0.5 | |||
| LEW OVA (5) | 10.5 ± 1.5 | 12 ± 3 | <0.1 | |||
| Unstimulated lines | 0.5 ± 0.1 | 0 | <0.1 |
Results are expressed as mean ± SD when five or eight experiments were performed; otherwise individual values were given.
The background value of CTLL-2 cells was 700 ± 150 cpm.
The supernatant from the stimulated LEWHgB T cell line increased MHC class II expression on B cells (mean fluorescence intensity of 51 versus 10 for B cells cultured alone), and this increase was abolished by addition of the anti-IL-4 OX81 mAb (mean fluorescence intensity of 14) suggesting that this line produced IL-4. None of the other LEWHg or LEWOVA T cell lines produced detectable amounts of IL-4 in this assay (not shown).
Anti–MHC Class II LEWHg T Cell Lines Prevent HgCl2induced Autoimmunity in F1 Rats
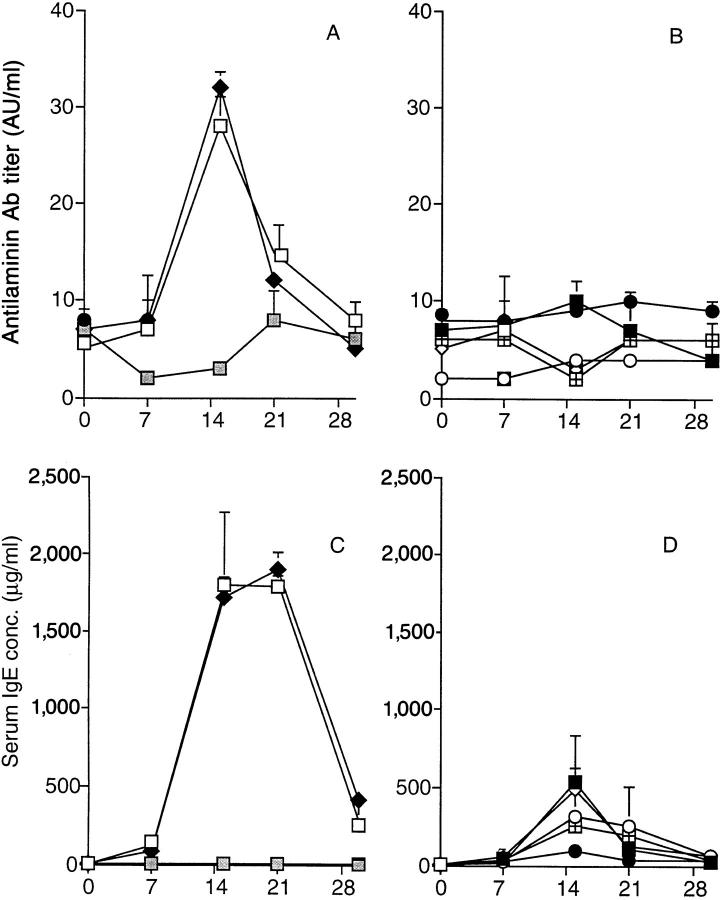
Transfer of the LEWHgA T cell line at the time of the first HgCl2 injection attenuated the autoimmune manifestations, but did not completely prevent the polyclonal activation of B cells nor renal IgG deposition (data not shown). By contrast, HgCl2-induced immunopathological manifestations were abrogated or considerably reduced in rats that received the LEWHg T cell lines 15 d before the first HgCl2 injection. In these rats, antilaminin antibody titer was much lower as compared to that of HgCl2-injected rats (P <0.005) and did not differ from that observed in normal rats (Fig. 1, A and B). Similar results were obtained concerning anti-DNA antibodies (not shown). Serum IgE concentration dramatically decreased when compared to HgCl2-injected rats (P <0.01), but remained higher than in normal rats (Fig. 1, C and D). The number of rats with glomerular IgG deposits was reduced in those who received the LEWHg T cell lines (from 66 to 19% depending upon the line, versus 100% in rats that received HgCl2 only; P <0.01); when considering rats that still exhibited IgG glomerular deposits, the intensity of immunofluorescence was greatly diminished when compared to rats injected with HgCl2 only (Fig. 2). Transfer of the LEWOVA T cell line had no effect on HgCl2-induced autoimmunity; these rats behaved as rats injected with HgCl2 only with respect to serum IgE concentration, antilaminin antibody titer (Fig. 1, A and C), and glomerular IgG deposits (Fig. 2).
Figure 1.
Ability of the LEWHg T cell lines to protect F1 rats against HgCl2-induced immune disorders. Antilaminin antibody titer (A and B) and serum IgE concentration (C and D) in HgCl2-injected rats (□, n = 25); normal rats ( , n = 5); rats injected with HgCl2 and, 2 wk before, with the LEWOVA (♦, n = 5), LEWHgA (▪, n = 21), LEWHgB (○, n = 3), LEWHgC (•, n = 3), LEWHgD (◫̶ , n = 3) or LEWHgE (⋄, n = 6) T cell line. AU, arbitrary units.
Figure 2.
Incidence of F1 rats with glomerular IgG deposits (number in brackets) and immunofluorescence score in HgCl2-injected rats, control rats, and rats injected with HgCl2 and, 2 wk before, with one of the T cell lines. The immunofluorescence score concerns only rats that exhibited glomerular IgG deposits.
CD8+ Cells and Recent Thymic Emigrants Are Not Involved in the Protection.
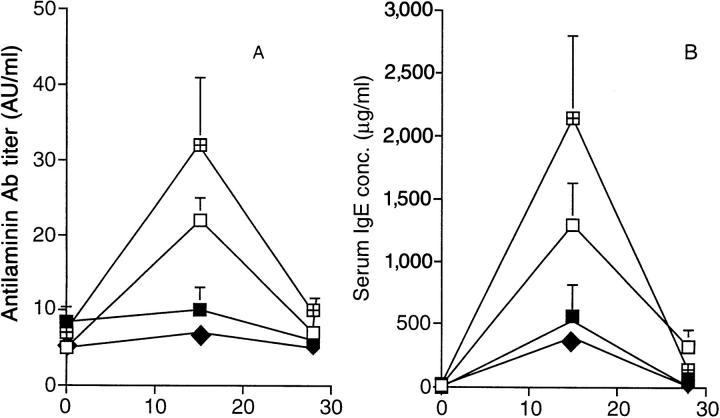
Since we had previously observed that the LEWHgA T cell line protects from EAE in a CD8-dependent manner (8), the potential role of CD8+ cells was addressed in the present model. To achieve CD8+ cell depletion, rats were thymectomized and injected with the anti-CD8 mAb. These rats displayed <1.5% CD8+ cells in spleen and lymph nodes at the time of killing, compared to 15 ± 3% in control rats. In these rats, HgCl2 injections induced similar, or even more severe, manifestations than in control rats injected with HgCl2 (Fig. 3, A and B). Transfer of the LEWHgA T cell line inhibited HgCl2-induced antilaminin antibody production (Fig. 3 A), increase in serum IgE concentration (Fig. 3 B), and glomerular IgG deposits (not shown) whether rats were thymectomized and treated with the anti-CD8 mAb or not. These results show that neither CD8+ cells nor recent thymic emigrants were involved in the protection.
Figure 3.
Effect of thymectomy and CD8+ cell depletion on the protective effect of the LEWHgA T cell line on HgCl2-induced disease. Antilaminin antibody titer (A) and serum IgE concentration (B) in HgCl2injected rats (□, n = 5), HgCl2-injected rats that received the LEWHgA T cell line two weeks before (▪, n = 9), thymectomized rats injected with HgCl2 and anti-CD8 mAb (◫̶ , n = 4), and thymectomized rats injected with the LEWHgA T cell line 2 wk before HgCl2 and anti-CD8 mAb administration (♦, n = 4).
Protection of Mercury-induced Autoimmunity by LEWHg CD4+ T Cell Lines Is TGF-β–dependent.
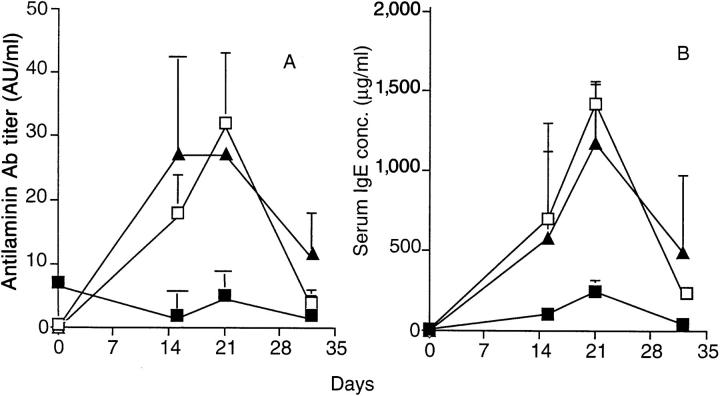
We next examined whether the inhibition of mercury disease induced by transfer of LEWHg T cell lines depends on the production of TGF-β in vivo. Interestingly, blocking TGF-β by administration of 1D11-16 mAb at the time of mercury disease induction completely abrogated the LEWHgA-mediated protective effect (Fig. 4). This is exemplified by the restoration of autoantibody production (Fig. 4 A), IgE synthesis (Fig. 4 B), and the reappearance of kidney Ig deposits to levels comparable to those observed in HgCl2-injected control rats (not shown). Administration of a different TGF-β–specific mAb (2G.7) also prevented the protection induced by the transfer of LEWHgA T cells (Table 2). Treatment with anti-TGF-β mAb alone did not influence HgCl2-induced autoimmune syndrome.
Figure 4.
Ability of the anti-TGF-β (1D11.16) mAb to reverse LEWHgAmediated protection from mercury disease. Antilaminin antibody titer (A) and serum IgE concentration (B) in HgCl2-injected rats (□, n = 5), and HgCl2-injected rats 2 wk after adoptive transfer of the LEWHgA T cell line treated (▴, n = 4) or not (▪, n = 4) with the 1D11.16 anti-TGF-β mAb (2 mg at day −2, 0, 3, 6, and 9 with respect to the first HgCl2 injection). The result is a pool of two independent experiments.
Table 2.
Effect of the Anti-TGF-β mAb (2G.7) on the Protection Mediated by the LEWHg T Cell Lines on HgCl2-mediated Autoimmunity in F 1 Rats
| T cell line | No. of rats | Anti-TGF-β mAb (2G.7) | Serum IgE conc.* | Antilaminin antibody titer* | Glomerularbound IgG‡ | Loss of body weight | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μg/ml | AU/ml | % initial weight | ||||||||||
| − | 5 | − | 2,010 ± 640 | 24 ± 9 | 3.4 ± 0.4 | 22 ± 5 | ||||||
| − | 3 | + | 3; 315 ± 550 | 33 ± 5 | 2.7 ± 0.6 | 25 ± 3 | ||||||
| LEWHgA | 5 | − | 370 ± 150 | 9 ± 6 | 0.6 ± 0.9 | 8 ± 5 | ||||||
| LEWHgA | 2 | + | 3,800; 2,850 | 31; 66 | 4; 3 | 25; 24 | ||||||
| LEWHgB | 3 | − | 270 ± 300 | 4 ± 6 | 0.4 ± 0.6 | 5 ± 1 | ||||||
| LEWHgB | 2 | + | 5,040; 4,860 | 40; 37 | 4; 1 | 26; 27 | ||||||
| LEWHgC | 3 | − | 315 ± 260 | 2 ± 4 | 0.7 ± 0.6 | 5 ± 3 | ||||||
| LEWHgC | 2 | + | 5,040; 4,990 | 29; 37 | 3; 3 | 23; 21 |
Results are expressed as mean ± SD when the number of rats was ≥3; otherwise individual values are given.
Values for serum IgE concentration and antilaminin antibody titer represent values at the peak of the disease (day 14 or 21 after the first injection of HgCl2).
Kidneys were processed at time of killing for immunofluorescence studies and scored on a scale from 0 to 4.
To demonstrate that TGF-β–dependent inhibition of Th2-mediated autoimmunity is not a unique property of LEWHgA T cell line, we tested the effect of anti-TGF-β mAb administration on the protection induced by the LEWHgA and two other self–MHC class II reactive T cell lines. LEWHgA, LEWHgB, and LEWHgC T cell lines have a comparable regulatory potential when transferred to F1 rats before induction of HgCl2-induced disease (Table 2). Administration of the anti-TGF-β 2G.7 mAb at the time of disease induction completely reverted the protective effect induced by the transfer of LEWHg T cell lines as shown by the complete restoration of the disease criteria including loss of body weight (Table 2).
Taken together our data show that three autoreactive anti-self–MHC class II T cell lines independently derived from HgCl2-injected LEW rats prevent Th2-mediated autoimmune disorders in (LEW × BN)F1 rats, and that their strong immunoregulatory potential is dependent on the production of TGF-β.
Discussion
This study shows that five autoreactive anti-self–MHC class II T cell lines derived from distinct HgCl2-injected LEW rats protect susceptible (LEW × BN)F1 rats from HgCl2-induced, Th2-mediated autoimmunity. These lines produce TGF-β that is responsible for the protection observed.
An increase in serum IgE concentration, although considerably less than in controls, persisted in HgCl2-injected rats transferred with the LEWHg T cell lines. This was possibly due to the recently described direct effect of HgCl2 on IL-4 gene transcription (20).
Autoreactive anti–MHC class II T cells that may enhance or suppress immune responses have been described in normal situations and are responsible for the autologous mixed lymphocyte reaction (see review in reference 21). Similar cells may also participate in the peripheral control of pathogenic autoreactive T cells. Thus, autoreactive anti–MHC class II T cell lines derived either from diabetic biobreeding rats, or from nondiabetic NOD mice prevent autoimmune diabetes when transferred to diabetes-prone biobreeding rats (22) or NOD mice (23), respectively. HgCl2 has been recently shown to induce a polyclonal activation of T cells in both BN and LEW rats (24), and thus, probably allows, among other T cells, the expansion of autoreactive antiself–MHC class II T cells, from precursors present in normal animals (25).
The mechanism of action of these autoreactive, regulatory T cells is not yet completely understood. We will first discuss the role of cytokines and then the nature of cells involved in protection. Our results show that protection is provided by TGF-β. We cannot rule out that other cytokines produced by these lines also play a role in the regulation observed, but neutralization of TGF-β alone abrogated the regulatory effect of the line indicating that this cytokine was of major importance. The regulatory T cell lines described in other systems (26) were not tested for their ability to produce TGF-β, except for those derived from animals orally tolerized with myelin basic protein that were able to prevent EAE in a TGF-β–dependent manner (27–29). However, there is now large evidence that TGF-β plays a major role in controlling the emergence of several autoimmune or inflammatory diseases (30–39).
A second point to be discussed concerns the nature of the cells involved in the protection. One possibility is that the T cell lines are responsible by themselves for the effect through TGF-β production. Powrie and co-workers have shown that autoaggressive and regulatory T cells coexist in normal rats and mice (40, 41). The autoaggressive Th1-like subset induces severe immunopathological manifestations, such as colitis in mice, when transferred into immunocompromised recipients. The regulatory Th2-like subset, by contrast, has no pathogenic effect and, in addition, prevents manifestations induced by the former subset. Powrie et al. have shown in mice that this protection was due to TGF-β, and not to IL-4 (42). The LEWHg T cell lines might belong to this regulatory subset, but interestingly, do not have a Th2-like phenotype since they produce IL-2 and IFN-γ but no detectable IL-4 with a biological assay except for one T cell line. Taken together, these results identify selfreactive T cells producing, in addition to TGF-β, either Th1- or Th2-associated cytokines, with a strong regulatory potential in both Th1- and Th2-mediated autoimmune diseases. Another nonexclusive possibility is that the T cell line recruits other T cells that participate in protection. In this respect, CD8+ T cells are crucial in the protective effect of the LEWHgA CD4+ T cell line towards EAE in LEW rats (8). CD8+ cells play no role in the protection of F1 rats from HgCl2-induced autoimmunity since protection was still observed in rats profoundly depleted of CD8+ cells after thymectomy and anti-CD8 mAb treatment. The difference in CD8+ cell requirement in both situations could be due to the different genetic make up of the recipients or to differences in experimental models. That the LEWHgA T cell line had to be injected 2 wk before the first HgCl2 injection is compatible with a recruitment of CD4+ cells by the T cell line. If recruited cells play a role in protection against HgCl2-induced autoimmunity, they could resemble three previously described regulatory T cell populations. (a) The first possiblity is recent thymic emigrants, CD4+, or CD8+ T cells that have been converted into regulatory cells by the autoreactive T cells selected on thymic epithelium in an IL-4–independent manner (43). It is unlikely that recruited recent thymic emigrant cells were at play in our model since thymectomized rats injected 3 wk later with the LEWHgA T cell line and injected with HgCl2 were still protected. (b) The recruited T cells could be similar to the antiidiotypic or antiergotypic T cells described by Lohse et al. (44). (c) They could also be related to regulatory cells guided to tolerance in the so-called infectious tolerance model (45). These hypotheses are currently being tested.
To conclude, the fact that HgCl2 can induce the expansion, among other T cells, of a subset of regulatory T cells that may recognize ubiquitous peptides in the context of MHC class II molecules is probably of importance for our understanding of the regulatory circuits involved in autoimmunity. Depending upon the strain tested, those T cells could produce different cytokines: self MHC class II–reactive T cells developing in BN rats produce IL-4 and induce Th2-mediated disorders, whereas T cells expanding in LEW rats preferentially produce TGF-β responsible for downregulation of autoimmunity.
Acknowledgments
We acknowledge Drs. H. Groux and J.C. Guéry for helpful discussion, Celtrix Pharmaceuticals Inc. and Dr. C.M. Melief for providing anti-TGF-β mAbs, M. Calise for her excellent technical assistance, and Dr. L. Chatenoud for providing purified 2G.7 antibody.
This work was supported in part by a grant from the European Community Biotech program BIO-CT920316. A. Badou is supported by the Association pour la Recherche sur le Cancer. A. Saoudi is supported by the Centre National de la Recherche Scientifique.
Footnotes
1 Abbreviations used in this paper: AU, arbitrary units; BN, Brown Norway; EAE, experimental autoimmune encephalomyelitis; LEW, Lewis; LEWHg, HgCl2-injected LEW rats.
References
- 1.Goldman M, Druet P, Gleichmann E. TH2 cells in systemic autoimmunity: insights from allogeneic diseases and chemically-induced autoimmunity. Immunol Today. 1991;12:223–227. doi: 10.1016/0167-5699(91)90034-Q. [DOI] [PubMed] [Google Scholar]
- 2.Mathieson PW, Thiru S, Oliveira DBG. Regulatory role of OX22highT cells in mercury-induced autoimmunity in the Brown Norway rat. J Exp Med. 1993;177:1309–1316. doi: 10.1084/jem.177.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillespie KM, Qasim FJ, Tibbats LM, Thiru S, Oliveira DBG, Mathieson PW. Interleukin-4 gene expression in mercury-induced autoimmunity. Scand J Immunol. 1995;41:268–272. doi: 10.1111/j.1365-3083.1995.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 4.Biancone L, Andres G, Ahn H, Lim A, Dai C, Noelle R, Yagita H, De Martino C, Stamenkovic I. Distinct regulatory roles of lymphocyte costimulatory pathways on T helper type 2–mediated autoimmune disease. J Exp Med. 1996;183:1473–1482. doi: 10.1084/jem.183.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fillastre, J.P., P. Druet, and J.P. Mery. 1988. Proteinuric nephropathies associated with drugs and substances of abuse. In The Nephrotic Syndrome. J.S. Cameron and R.J. Glassock, editors. Marcel Dekker, New York. 697–744.
- 6.Rossert J, Pelletier L, Pasquier R, Druet P. Autoreactive T cells in mercury-induced autoimmunity. Demonstration by limiting dilution analysis. Eur J Immunol. 1988;18:1761–1766. doi: 10.1002/eji.1830181116. [DOI] [PubMed] [Google Scholar]
- 7.Saoudi A, Castedo M, Nochy D, Mandet C, Pasquier R, Druet P, Pelletier L. Self reactive anti–class II Th2 cell lines derived from gold salt-injected rats trigger B cell polyclonal activation and transfer autoimmunity in CD8depleted normal syngeneic recipients. Eur J Immunol. 1995;25:1972–1979. doi: 10.1002/eji.1830250726. [DOI] [PubMed] [Google Scholar]
- 8.Castedo M, Pelletier L, Rossert J, Pasquier R, Villarroya H, Druet P. Mercury-induced autoreactive anti–class II T cell line protects from experimental autoimmune encephalomyelitis by the bias of CD8+antiergotypic cells in Lewis rats. J Exp Med. 1993;177:881–889. doi: 10.1084/jem.177.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunig T, Wallny H-J, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams AF, Galfré G, Milstein C. Analysis of cell surface by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977;12:663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- 11.Brideau RJ, Carter PB, Mc WR, Master, Mason DW, Williams AF. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+T cells in vitro. J Immunol. 1996;156:2406–2412. [PubMed] [Google Scholar]
- 13.Dasch JR, Pace DR, Waegell W, Inenaga D, Ellingsworth L. Monoclonal antibodies recognizing transforming growth factor-β. Bioactivity neutralization and transforming growth factor β2 affinity purification. J Immunol. 1989;142:1536–1541. [PubMed] [Google Scholar]
- 14.Lucas C, Bald LN, Fendly BM, Mora-Worms M, Figari IS, Patzer EJ, Palladino MA. The autocrine production of transforming growth factor-β1 during lymphocyte activation. A study with a monoclonal antibody– based ELISA. J Immunol. 1990;145:1415–1422. [PubMed] [Google Scholar]
- 15.van der Meide PH, Borman AH, Beljaars HG, Dubbeld MA, Botman CAD, Schellekens H. Isolation and characterization of monoclonal antibodies directed to rat interferon-gamma. Lymphokine Res. 1989;8:439–449. [PubMed] [Google Scholar]
- 16.Pelletier L, Rossert J, Pasquier R, Vial MC, Druet P. Role of CD8+T cells in mercury-induced autoimmunity or immunosuppression in the rat. Scand J Immunol. 1990;31:65–74. doi: 10.1111/j.1365-3083.1990.tb02744.x. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier L, Pasquier R, Rossert J, Vial M-C, Mandet C, Druet P. Autoreactive T cells in mercury-induced autoimmunity. Ability to induce the autoimmune disease. J Immunol. 1988;140:750–754. [PubMed] [Google Scholar]
- 18.Dubey C, Kuhn J, Vial MC, Druet P, Bellon B. Anti-interleukin-2 receptor monoclonal antibody therapy supports a role for Th1-like cells in HgCl2-induced autoimmunity in rats. Scand J Immunol. 1993;37:406–412. doi: 10.1111/j.1365-3083.1993.tb03311.x. [DOI] [PubMed] [Google Scholar]
- 19.Sedgwick JD. Long-term depletion of CD8+ T cells in vivo in the rat: no observed role for CD8+ (cytotoxic/ suppressor) cells in the immunoregulation of experimental allergic encephalomyelitis. Eur J Immunol. 1988;18:495–502. doi: 10.1002/eji.1830180402. [DOI] [PubMed] [Google Scholar]
- 20.Prigent P, Saoudi A, Pannetier C, Graber P, Bonnefoy Y, Druet P, Hirsch F. Mercuric chloride, a chemical responsible for Th2-mediated autoimmunity in Brown-Norway rats, directly triggers T cells to produce IL-4. J Clin Invest. 1995;96:1484–1489. doi: 10.1172/JCI118185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zauderer M. Origin and significance of autoreactive T cells. Adv Immunol. 1989;45:417–437. doi: 10.1016/s0065-2776(08)60698-7. [DOI] [PubMed] [Google Scholar]
- 22.Nagata M, Yoon J-W. Prevention of autoimmune type I diabetes in biobreeding (BB) rats by a newly established, autoreactive T cell line from acutely diabetic BB rats. J Immunol. 1994;153:3775–3783. [PubMed] [Google Scholar]
- 23.Reich E-P, Scaringe D, Yagi J, Sherwin S, Janeway CA. Prevention of diabetes in NOD mice by injection of autoreactive T-lymphocytes. Diabetes. 1989;38:1647–1651. doi: 10.2337/diab.38.12.1647. [DOI] [PubMed] [Google Scholar]
- 24.Fillon J, Baccala R, Kuhn J, Druet P, Bellon B. Evidence for heterogeneous TCRVβ expression in mercury- induced autoimmune disorders in rats. Int Immunol. 1997;9:263–271. doi: 10.1093/intimm/9.2.263. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal B, Manickasundari M, Fraga E, Singh B. T cells that recognize peptide sequences of self MHC class II molecules exist in syngeneic mice. J Immunol. 1991;147:383–390. [PubMed] [Google Scholar]
- 26.Saoudi A, Seddon B, Heath V, Fowell D, Mason D. The physiological role of regulatory T cells in the prevention of autoimmunity: the function of the thymus in the generation of the regulatory T cell subset. Immunol Rev. 1996;149:196–216. doi: 10.1111/j.1600-065x.1996.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (Wash DC) 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 28.Santos LMB, Al-Sabbagh A, Londono A, Weiner H. Oral tolerance to myelin basic protein induces regulatory TGF-β secreting T cells in Peyer's patches of SJL mice. Cell Immunol. 1994;157:439–447. doi: 10.1006/cimm.1994.1240. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Inobe J-I, Weiner HL. Induction of oral tolerance to myelin basic protein in CD8-depleted mice: both CD4+ and CD8+cells mediate active suppression. J Immunol. 1995;155:910–916. [PubMed] [Google Scholar]
- 30.Wahl SM. Transforming growth factor β: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuruvilla AP, Shah R, Hochwald GM, Liggitt HD, Palladino MA, Thorbecke GJ. Protective effect of transforming growth factor β1 on experimental autoimmune diseases in mice. Proc Natl Acad Sci USA. 1991;88:2918–2921. doi: 10.1073/pnas.88.7.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santambrogio L, Hochwald GM, Saxena B, Leu C-H, Martz JE, Carlino JA, Ruddle NH, Palladino MA, Gold LI, Thorbecke J. Studies on the mechanisms by which transforming growth factor-β (TGF-β) protects against allergic encephalomyelitis. J Immunol. 1993;151:1116–1127. [PubMed] [Google Scholar]
- 33.Jung S, Schluesener HJ, Schmidt B, Fontana A, Toyka KV, Hartung H-P. Therapeutic effect of transforming growth factor-β2 on actively induced EAN but not adoptive transfer EAN. Immunology. 1994;83:545–551. [PMC free article] [PubMed] [Google Scholar]
- 34.Wahl SM, Allen JB, Costa GL, Wong HL, Dasch JR. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor β. J Exp Med. 1993;177:225–230. doi: 10.1084/jem.177.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyers CM, Kelly CJ. Immunoregulation and TGF-β1. Suppression of a nephritogenic murine T cell clone. Kidney Int. 1994;46:1295–1301. doi: 10.1038/ki.1994.397. [DOI] [PubMed] [Google Scholar]
- 36.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, Strober S. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β–mediated oral tolerance. J Exp Med. 1996;183:2605–2616. doi: 10.1084/jem.183.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature (Lond) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, Gress RE, Hines KL, Tian H, Karlsson S, et al. Immune dysregulation in TGFβ1–deficient mice. J Immunol. 1994;153:1936–1946. [PubMed] [Google Scholar]
- 39.Dang H, Geiser AG, Letterio JJ, Nakabayashi T, Kong L, Fernandes G, Talal N. SLE-like autoantibodies and Sjögren's syndrome–like lymphoproliferation in TGF-β knockout mice. J Immunol. 1995;155:3205–3212. [PubMed] [Google Scholar]
- 40.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22lowsubset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhiCD4+ T cells. Immunity. 1994;1:553–582. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 42.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-β but not interleukin 4 in the suppression of T helper type 1–mediated colitis by CD45RBlow CD4+T cells. J Exp Med. 1996;183:2669–2674. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modigliani Y, Bandeira A, Coutinho A. A model for developmentally acquired thymus-dependent tolerance to central and peripheral antigens. Immunol Rev. 1996;149:156–174. doi: 10.1111/j.1600-065x.1996.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 44.Lohse A, Cohen IR. Immunoregulation: studies of physiological and therapeutic autoreactivity by T cell vaccination. Springer Semin Immunopathol. 1992;14:179–186. doi: 10.1007/BF00195293. [DOI] [PubMed] [Google Scholar]
- 45.Cobbold SP, Adams E, Marshall SE, Davies JD, Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev. 1996;149:5–33. doi: 10.1111/j.1600-065x.1996.tb00897.x. [DOI] [PubMed] [Google Scholar]