Abstract
Recent evidence indicates that chronic autoimmune disease can result from breakdown of regulation and subsequent activation of self-reactive T cells. In many murine autoimmune disease systems and in the Lewis rat, antigen-specific T cells utilizing the T cell receptor (TCR) Vβ8.2 gene segment play a major role. In the myelin basic protein–induced experimental autoimmune encephalomyelitis (EAE) model in H-2u mice, we had shown that T cells recognizing a peptide determinant within the framework 3 region of the Vβ8.2 chain have a critical role in influencing the course of the disease. Here, we report experiments in another disease system, collagen II (CII)–induced arthritis (CIA) in DBA/1LacJ (H-2q) mice, indicating a remarkably parallel control circuit to that found for EAE. A critical role is played by CII-specific Vβ8.2bearing T cells in the CIA system, which we have confirmed. Animals treated with the superantigen SEB before CII administration are significantly protected from CIA. Next, we tested the ability of peptides encompassing the entire Vβ8.2 chain to induce proliferative responses. Only TCR peptide B5 (amino acids 76–101), a regulatory peptide in EAE, induced proliferation. B5 was then used to vaccinate DBA/1LacJ mice and was shown to reduce greatly the severity and incidence of CIA as measured by joint inflammation or histology. Furthermore, similar protection was found when B5 was administered after CII immunization. It was shown that there is physiological induction of a proliferative response to B5 during CIA and that the determinant within B5 is produced from a single chain TCR construct containing the entire Vβ8.2 chain. Finally, the regulation of CIA is discussed in the context of other experimental autoimmune diseases, especially EAE, with emphasis on what appear to be strikingly common mechanisms.
In recent years, a number of antigen-induced and spontaneous autoimmune diseases in susceptible strains of rodents have been extensively studied. The accumulated evidence, as well as clinical studies have led to the inference that each of these autoimmune diseases is a representative model for the human condition it most closely resembles. For example, experimental autoimmune encephalomyelitis (EAE)1 for multiple sclerosis, collagen II (CII)–induced arthritis (CIA) for rheumatoid arthritis and diabetes in nonobese diabetic (NOD) mice for insulin-dependent juvenile diabetes. A common theme found in studies with mice of the TCRVβb haplotype but of many different H-2 haplotypes and in Lewis rats is the key role of T cells using the Vβ8.2 gene segment in primary recognition of autoantigens (1; see Discussion). These results suggest a common regulatory system that controls potentially pathogenic self-reactive T cells. Experimental support for this idea comes from studies reported here with CIA in H-2q mice that appear to employ similar regulation to that found for myelin basic protein (MBP)-induced EAE in H-2u mice.
Self-reactive T cells appear to be pivotal in the development of several human autoimmune disorders, including multiple sclerosis, rheumatoid arthritis, and type 1 diabetes. The plethora of observations linking a large number of experimental autoimmune diseases in mice and rats with the use of TCR encoded by the Vβ8.2 gene segment has prompted us to make a detailed comparison of initiation of disease and regulation of potentially pathogenic self reactive T cells in two well-defined murine experimental models, namely CIA and EAE. It has previously been shown that T cells using the TCR Vβ8.2 gene segment are required for induction of EAE in H-2u mice (2, 3). Antigen-induced EAE, using MBP or peptide Ac1-9 of MBP as antigen, was strongly inhibited with pretreatment by anti-Vβ8.2. We have recently demonstrated in mice that the response to self-MBP is controlled by potent regulatory T cell circuitry, which is based on recognition of different determinants derived from the TCR Vβ8.2 chain, in the context of class I and class II MHC molecules (4–7). This TCR-based regulation appears to be involved in maintaining peripheral tolerance to MBP, thus protecting mice from autoimmune demyelination. Furthermore, TCR peptide-specific regulatory T cells appear to be responsible for natural recovery from antigen-induced EAE in B10.PL mice (4–7).
CIA in rodents is an acute and severe experimental model of autoimmune polyarthritis that develops following immunization with heterologous type II collagen in adjuvant (8–10); homologous CII appear to induce chronic and progressive arthritis (7). Both MHC class II and non-MHC genes play important roles in the susceptibility to disease induction, with H-2q and H-2r being the most susceptible MHC haplotypes (12). Although the relative contribution of antibody and cellular mechanisms in disease is not yet clear, there is evidence that T cells are critically involved in the pathogenesis of CIA (13–16).
It has clearly been shown that the depletion of CD4+ T cells in mice attenuates the incidence and delays the onset of CIA (13). Similarly, CIA has been induced in rats by adoptive transfer of CD4+ T cells specific for CII (14, 15). It has been suggested that in susceptible mouse strains expressing either the complete TCR Vβ gene repertoire (TCRVβb) or lacking part of the Vβ gene segments (TCRVβa), CIA is mediated by CII-specific T cells using a limited set of TCR V gene segments (17–21). These findings are in agreement with the hypothesis that pathogenic T cells use a restricted repertoire of V gene segments, as has previously been demonstrated in the EAE system. It has been shown that T cells specific for CII in DBA/1 mice as well as T cells infiltrating the joints in B10.Q mice use a limited number of TCR V genes (20, 21). The significant reduction in the incidence of CIA in both DBA/1 as well as in B10.RIII mice treated with anti-Vβ8 antibodies indicates that T cells expressing Vβ8 TCR are important in the development of CIA (20– 23). Selective depletion of Vβ8-expressing T cells has been shown to be as effective as the depletion of all αβ T cells in B10.R III mice (23). Here, using superantigenic stimulation of T cells in vivo, we show that CII-specific T cells expressing Vβ8.2 genes are crucial for the induction of CIA in DBA/1LacJ mice. Our studies indicate that TCR Vβ8.2 chain–derived determinants are physiological targets for the regulation of CIA and TCR–peptide-based regulation may be critical for the maintenance of peripheral tolerance to CII. These data also show that the same framework 3 region of the Vβ8.2 chain appears to be crucially involved in regulation of both CIA and EAE.
Materials and Methods
Mice.
DBA/1LacJ mice (H-2q) were obtained from The Jackson Laboratory (Bar Harbor, ME), and were bred at UCLA under specific pathogen-free conditions. 8 to 12 wk-old male mice were used for induction of arthritis.
Induction of CIA in DBA/1LacJ Mice.
Arthritis was induced in DBA/1LacJ male mice after injection of 100 μg of heterologous bovine CII. The bovine CII (Institut Jacques Bois, Reims, France) was dissolved in 0.01 M acetic acid at 4°C overnight and emulsified in an equal volume of CFA (DIFCO, Detroit, MI). 3 wk after the primary injection, mice were again immunized with 100 μg CII emulsified in IFA, intraperitoneally. A second injection with collagen was necessary to induce severe and reproducible disease.
Peptide Synthesis.
Peptides were synthesized as described earlier (5). The following are amino acid sequences of the TCR peptides and are shown in the single letter amino acid code. B1 (1–30L), EAAVTQSPRNKVAVTGGKVTLSCNQTNNHNL; B2 (21– 50), LSCNQTNNHNNMYWYRQDTGHGLRLIHYSY; B3 (41–70), HGLRLIHYSYGAGSTEKGDIPDGYKASRPS; B4 (61– 90), PDGYKASRPSQENFSLILELATPSQTSVYF; B5 (76–101), LILELATPSQTSVYFCASGDAGGGYE.
T Cell Proliferation Assay.
Mice were immunized as above with CII, or subcutaneously with 3–7 nmol of TCR peptide emulsified in CFA (DIFCO, Detroit, MI). Draining (para-aortic and inguinal) lymph node cells were obtained 9–10 d later, and used in antigen-induced proliferation assays. 5 × 105 lymph node cells were cultured in 0.2 ml of serum-free medium (HL-1; Ventrex, Portland, ME or X-Vivo 10; BioWhittaker, Walkersville, MD) medium alone, containing 2 mM glutamine, or with varying concentrations of collagen or specific TCR peptide in 96-well culture dishes for 4 d. Antigen-induced proliferation was assessed by the incorporation of 1 μCi of [3H]thymidine during the last 18 h of culture. For splenic proliferation assays, erythrocytes were lysed and the residual cells were plated as above in HL-1 or X-Vivo 10 medium at a density of 1 × 106 cells in a total volume of 200 μl per well in a 96-well plate with varying concentrations of antigens.
Flow Cytometry Analysis.
Antibodies were purified from hybridoma supernatants by protein A chromatography. Anti-CD4– PE (GK1.5) was acquired from Becton-Dickinson (Mountain View, CA). Anti-Vβ8.2 (F23.2) (24) was used for TCR staining after SEB treatment; The F23.2-producing hybridoma was provided by Dr. M. Bevan (University of Washington, Seattle, WA). Antibodies were used in PBS containing 1% fetal bovine serum. 1 × 106 cells were stained with 0.5 μg of antibody or biotinylated Ab in a total volume of 50 μl at 4°C for 30 min. Cells were washed twice with PBS and then resuspended in 50 μl of a 1:50 dilution of either FITC-conjugated streptavidin or goat anti–mouse Ig– FITC (Southern Biotechnology, Birmingham, AL). After 20 min at 4°C, cells were washed, fixed with 1% paraformaldehyde in PBS, and analyzed using a Becton-Dickinson cytofluorograph.
Anti-CII Antibody Response.
Mice were bled retro-orbitally and serum samples were collected from individual mice and stored at −70°C until assay. A standard ELISA assay was used to quantify the antibody response to CII. In brief, microtiter plates were coated overnight with CII at a concentration of 10 μg/ml in a total volume of 50 μl/well in PBS. After blocking remaining protein-binding sites with BSA, diluted serum samples were incubated and the amount of bound antibody determined by incubation with goat anti–mouse IgG covalently coupled to alkaline phosphatase (Southern Biotechnology, Birmingham, AL). A serum pool collected from hyperimmune mice was used as a standard.
Results
Superantigen-induced Tolerance and Susceptibility to CIA.
As mentioned earlier, treatment of mice with antibodies against the TCR Vβ8.2 gene segment has been shown to reduce significantly the incidence of arthritis (21–23). Also, the majority of T cells recognizing bovine CII (bCII) utilize Vβ8.2 and Vα11 gene segments in the DBA/1LacJ mouse (21). In several experimental models, it has been demonstrated that injection of mice with the superantigen, SEB, results in an initial expansion of Vβ8.2 T cells followed by clonal exhaustion and nonresponsiveness (25). We have asked whether the disease-mediating response to bCII predominantly uses T cells expressing the Vβ8.2 gene segment; if so, clonal exhaustion or tolerance induction among Vβ8.2 T cells after SEB challenge might result in protection from CIA. As shown in Table 1, DBA/1LacJ mice given a single injection of SEB are significantly protected from disease. In the SEB-treated group, 3 of 10 mice displayed mild arthritic symptoms (arthritic index, 1.1). In contrast, within control groups, including the SEA-injected group, 8 of 10 mice developed severe arthritis (arthritic index, 6.0). These data implicate Vβ8 T cells in mediating CIA. Interestingly, mice injected with SEB 10 d after CII/ CFA injection were not protected (8 of 8) but rather developed more severe arthritis (arthritic index, 9.9) than those in the control group (arthritic index, 5.3).
Table 1.
Administration of SEB 10 d Before CII Challenge Protects Mice from CIA
| Treatment group | Incidence of disease | Arthritis severity | ||
|---|---|---|---|---|
| PBS | 8/10 | 6.0 | ||
| SEB | 3/10 | 1.1 | ||
| SEA | 8/9 | 5.7 |
Mice were injected with 50–100 μg of superantigen per animal 10 d before the injection of CII/CFA for induction of arthritis. The percentage of CD4+, Vβ8.2+ T cells was analyzed by flow cytometry and found to decrease significantly 48 h after SEB injection (7.9 ± 1.5% in controls versus 1.8 ± 0.3% in SEB-treated group). Arthritis severity was determined as in Fig. 2.
Immunogenicity of TCR–Peptides from the Vβ8.2 Chain in DBA/1LacJ Mice.
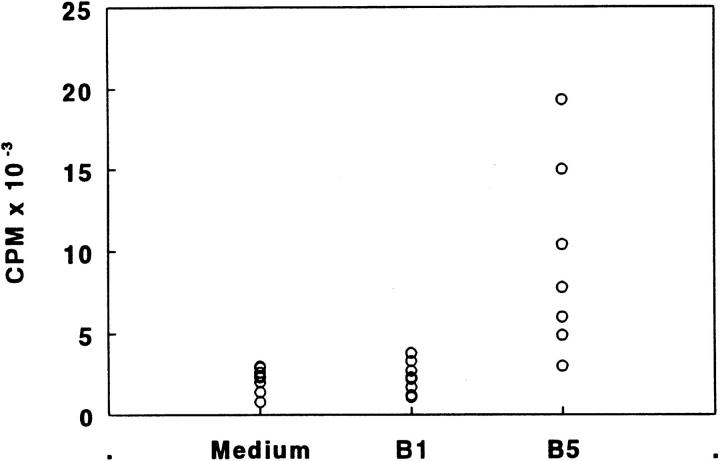
To identify TCR determinants, we have chemically synthesized five overlapping peptides (26–30 mers) encompassing the entire variable region of the TCR Vβ8.2 chain. To characterize the immunogenicity of these TCR peptides, mice were immunized subcutaneously with 7 nmol of individual peptides in CFA. 10 d later, proliferative responses in the draining lymph nodes were assayed in response to an in vitro challenge. We found that only a single peptide (B5) was able to elicit a lymph node proliferative response in DBA/1LacJ mice (Fig. 1). Interestingly, T cells specific for the same TCR peptide, B5, have been shown to regulate autoimmunity to MBP and are primed naturally in B10.PL mice recovering from antigen-induced EAE (4–7). Polyclonal T cell lines specific for B5 did not proliferate in response to an adjacent overlapping peptide B4 (data not shown).
Figure 1.
Immunogenicity of TCR–peptides from the Vβ8.2 chain in DBA/1LacJ mice. DBA/1LacJ mice were immunized with 7 nmol each of the five overlapping TCR–peptides. 10 d later, draining lymph node cells were assayed for proliferation in response to the immunizing peptide at a concentration of 3.5 μM. [3H]thymidine incorporation was measured by standard techniques. Individual responses in two mice are shown. The amino acid sequences of the TCR peptides are given in Materials and Methods.
Vaccination with TCR–Peptide-B5 Reduces Severity and Incidence of CIA.
In an attempt to influence the course of CIA, DBA/1LacJ mice were vaccinated with 7 nmol of B5/IFA twice, before challenge with bCII. Mice in the control group were challenged with PBS/IFA or B4/IFA. The effect of vaccination on the severity as well as on the incidence of arthritis was examined in double-blind experiments. We have performed three different experiments with 5–8 mice in each group. A representative experiment with B5 vaccination is shown in Fig. 2. In Table 2, data pooled from three independent experiments are shown: 34 of 36 mice in the control groups (PBS/IFA and B4/IFA) developed severe arthritis by 8–9 wk after CII immunization, whereas only 6 of 18 mice in the B5-vaccinated group displayed CIA. Importantly, B5-vaccinated mice contracted less severe arthritis than mice in the control group (Table 2). A majority of animals in the control group developed severe arthritic symptoms in both front and hind limbs. In contrast, arthritic symptoms were rarely detected in the front limbs of mice vaccinated with B5. These data demonstrate the efficacy of B5-specific T cells in modulating CIA in DBA/1LacJ mice.
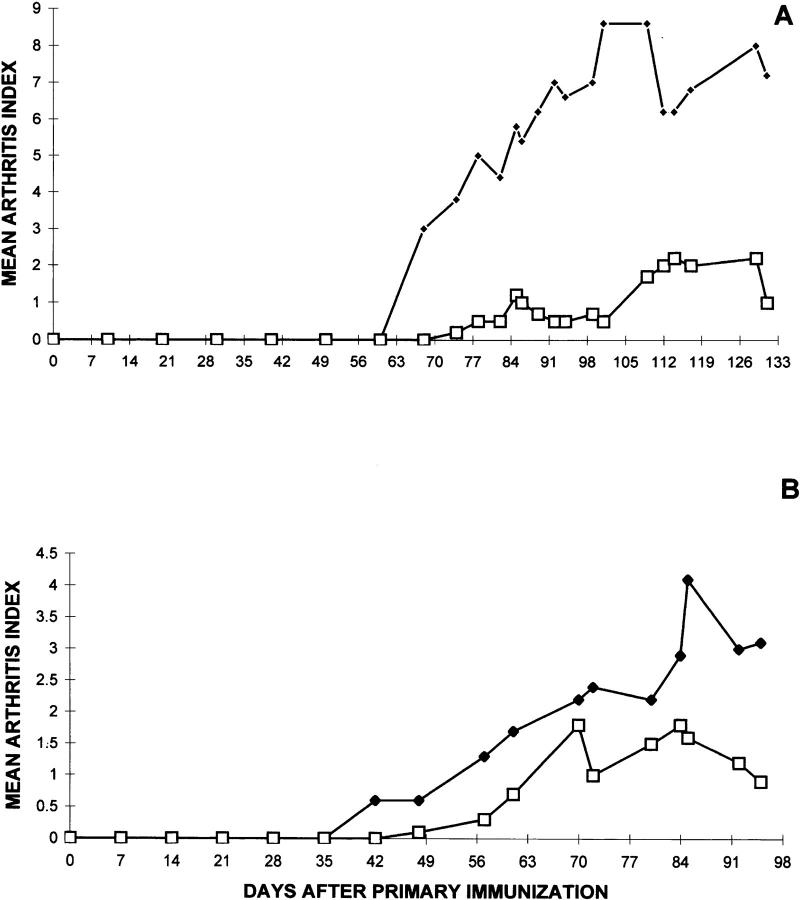
Figure 2.
Vaccination with TCR–peptide B5 reduces severity and incidence of CIA. Groups of DBA/1LacJ mice (eight in each group) were immunized subcutaneously with 100 μg of CII in one hind footpad. Disease was monitored in the three remaining limbs in a double-blind manner by two individuals. Each limb was graded with a score of 1 through 4, the maximum score being 12 for each mouse. The mean arthritis index was determined by summation of the total score of each joint in each group of mice and dividing by the total number of animals in each group. For vaccination experiments, mice were injected with 7 nmol B5/IFA at days −10 and +10 with respect to the primary CII injection (○). Control mice were immunized with PBS/IFA (•).
Table 2.
Vaccination with TCR Peptide B5 Prevents CIA in DBA/1LacJ Mice
| Treatment | CIA | |||
|---|---|---|---|---|
| Incidence | Arthritis severity* | |||
| Control (PBS/IFA or B4/IFA) | 34/36 | 9.8 | ||
| B5/IFA | 6/18 | 2.1 | ||
We have tested several variations of the B5 vaccination protocol described in the legend for Fig. 2. Mice injected only once with 14 nmol of B5 in IFA are not protected from CIA. Furthermore, mice given only a single injection of B5 even at a much higher concentration (50 nmol) are not protected, but rather appear to have increased severity of the disease (data not shown). Finally, we have asked whether treatment with B5 after CII injection can protect mice from CIA. In two independent experiments, DBA/ 1LacJ mice challenged with B5 post-CII priming (days +10, +20, +30, Fig. 3 A; or days +30, +40, +50, Fig. 3 B) show significant amelioration of disease. These data clearly establish that B5-specific T cells are able to down modulate CIA, even after CII-induced disease has been initiated.
Figure 3.
DBA/1LacJ mice challenged with TCR peptide B5, after CII immunization, are significantly protected from CIA. After immunization with bovine CII, mice in each group (n = 9) were challenged with B5/IFA (□) or B4/IFA (♦) on days +10, +20, +30 (A), or days +30, +40, +50 (B) relative to primary immunization with CII/CFA. These immunizations were done as described in the legend for Fig. 2.
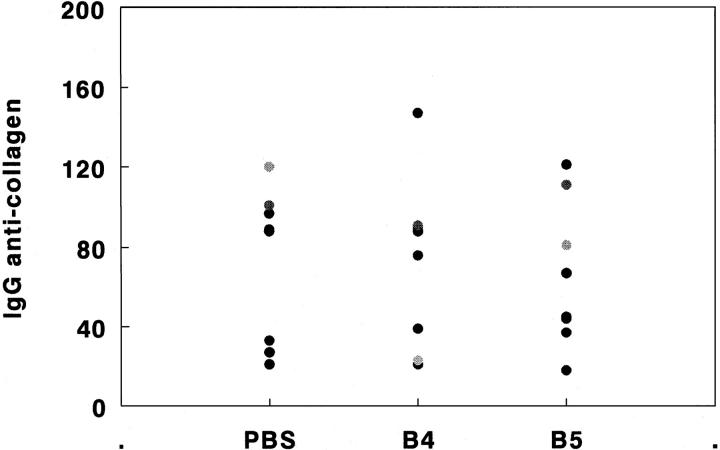
Histologically, joints from untreated or control peptide (B4)-treated animals were severely damaged by the rapidly expanding synovial pannus. Mononuclear cell infiltration, thickening of the synovial membrane, and bone marrow erosion by osteoclasts, as well as cell exudate and polymorphonuclear cell accumulation in the synovial space were present in the controls. In contrast, there were no signs of an inflammatory process in 4 of 5 joints examined from mice vaccinated with B5. Thus, lack of clinical disease in B5-treated mice could be attributed to the absence of inflammation in the synovium (Fig. 4). Interestingly, the anti-CII IgG response in groups treated with B5/IFA or B4/IFA or PBS/IFA did not differ significantly, as shown in Fig. 5. No significant difference in anti-CII titers was seen between arthritic and non arthritic (B5-vaccinated) mice, even though levels in individual mice varied considerably.
Figure 4.

The infiltration of inflammatory cells into the synovium and cartilage or bone erosion does not occur in B5-treated mice. Four mice from each group treated with PBS/IFA, B4/IFA, or B5/IFA, were killed 12–14 wk after collagen II immunization. Hind limbs were formalinfixed, decalcified, embedded in paraffin, and stained in hematoxylin and eosin. Tissue sections were examined and results are illustrated with a representative example from each group.
Figure 5.
In B5-treated mice, the IgG anti-collagen II response is not inhibited. DBA/1LacJ mice were vaccinated with B5/IFA, B4/IFA, or PBS/IFA as described in the legend for Fig. 2. Individual serum samples, collected 8 wk after CII injection, were analyzed for the presence of antiCII IgG, using a standard ELISA assay. For the quantitation of IgG responses, a standard curve was generated using a hyperimmune antiserum to CII. The values are represented in arbitrary units reflecting titers of the anti-CII antibody in different groups of mice.
Induction of a Proliferative Response to B5 During CIA.
Although after peptide immunization (see Fig. 1), a proliferative response was detected only to a single TCR–peptide, B5, in DBA/1LacJ mice, it was important to determine whether B5-specific T cells are activated physiologically during the course of disease, in the absence of any challenge with the peptide itself. Mice were challenged with CII for the induction of CIA. 35 d after primary immunization with CII, at which time mice had already developed arthritic symptoms, peripheral T cells proliferated in vitro in response to B5 (Fig. 6). Proliferative responses to any of the other TCR peptides from the Vβ8.2 chain were not detected. Also, nonimmunized mice as well as mice immunized with HEL showed no response to B5.
Figure 6.
Physiological induction of a proliferative response to B5 during CIA. DBA/1LacJ mice were immunized with CII for the induction of CIA. 35 d later, splenic cells were assayed for proliferation in response to a concentration (7 μM) of different TCR peptides, B1 and B5. [3H]thymidine incorporation was measured by liquid scintillation counting. There was no significant response to B5 in nonimmunized mice, nor in mice challenged with HEL, 35 d after immunization. Individual responses of eight individual mice are shown.
The dynamics of spontaneous activation of B5-specific T cells was studied further by following spontaneous proliferation to B5 in lymph node and spleen cells isolated from DBA/1LacJ mice (three mice in each group) 2, 10, 20, 30, 40, and 50 days after CII injection. In contrast with studies in the EAE model, the proliferative response to B5 was relatively lower (stimulation index [S.I.] of 2.5–3.0) until day 30. Interestingly, responsiveness to B5 continues to increase even beyond day 50 (S.I. of 4–5). An indolent expansion of B5-reactive T cells could perhaps explain the chronic nature of CIA, in that Treg are not able to expand quickly enough to control the damage induced by CII- reactive T cells.
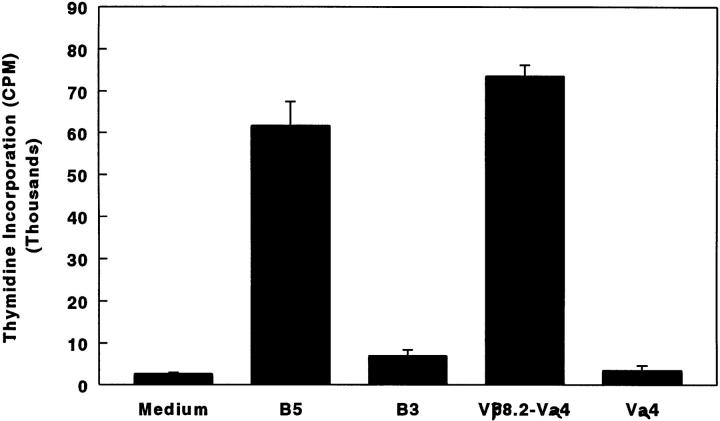
T Cell Determinant(s) Within B5 Are Naturally Processed and Presented.
The existence of primed B5-specific T cells in animals with CIA suggests that T cells specific for TCR determinant(s) are physiologically primed. To examine further immunodominance as well as the naturally processed form of TCR determinants, soluble TCR-recombinant single-chain TCRs containing appropriate Vα and Vβ chains (26), produced in Escherichia coli and provided by Dr. E. Sally Ward (Dallas, TX), were used to challenge DBA/ 1LacJ mice. An in vitro recall response was tested with various Vβ chain peptides. In parallel, TCR molecules containing a Vβ chain other than Vβ8.2 were tested for specificity, to establish whether B5-reactive T cells could be stimulated by TCR molecules containing the appropriate Vβ domain. As shown in Fig. 7, lymph node cells from B5-primed mice gave good proliferative recall to the immunizing peptide B5, as well as to soluble recombinant scTCR molecules containing the Vβ8.2 chain (Vβ8.2–Vα4). There was no proliferative recall to a different TCR peptide (B3) or to scTCR molecules lacking a Vβ8.2 chain, such as Vα4. These data clearly established that TCR determinants within B5 are processed and presented following CII injection.
Figure 7.
TCR determinant(s) within B5 are naturally processed and presented. DBA/1LacJ mice were immunized with 7 nmol of B5 in CFA. 10 d later, draining lymph node cells were assayed for proliferation in response to varying concentration of different antigens (3.5–14 μM). [3H]thymidine incorporation, measured by standard techniques, at an optimum concentration, 7 μM, is shown. Responses in lymph node cells pooled from two mice are shown. B5 and B3 are synthetic TCR peptides, whereas Vβ8.2–Vα4 and Vα4 are scTCR molecules containing both V domains or only a single V domain, respectively.
Discussion
The studies reported in this paper demonstrate a remarkably parallel strategy in the regulation controlling bovine CIA in H-2q mice and the previously examined MBP- induced EAE in H-2u mice. In EAE, the major disease- inducing, MBP-specific T cells are highly restricted in their TCR V gene usage, predominantly using the Vβ8.2 gene segment. A variety of experiments point to potent regulation by naturally primed CD4 T cells that recognize a framework 3 region determinant of the Vβ8.2 chain. Here, we show that TCR peptide–specific T cells are implicated in the physiological control of CII-reactive T cells, which mediate autoimmune arthritis in DBA1/LacJ mice. Our data support earlier studies (21–23) suggesting the in vivo involvement of Vβ8.2 T cells in CIA induced by CII, which is accompanied by activation of T cells specific for a determinant derived from the framework 3 region (peptide B5, amino acids 76–101) of the Vβ8.2 chain. Vaccination with TCR peptide B5 before (Fig. 2) or after CII immunization (Fig. 3) protects mice significantly from arthritis and prevents inflammation in the synovia. With the use of recombinant scTCR protein containing the Vβ8.2 chain, we were able to determine that the framework 3 region determinant is immunodominant and can be processed and presented from the whole Vβ8.2 chain.
Earlier studies from some laboratories (21–23), but not from others (27) showed that CII-reactive T cell hybridomas preferentially use the TCR Vβ8.2 gene segment and also that anti-Vβ8.2 treatment resulted in a significant reduction in the incidence of arthritis in DBA/1LacJ mice. Consistent with the role of Vβ8.2 T cells, our data demonstrate that mice treated with the superantigen, SEB, but not with SEA, 10 d before antigenic challenge, are significantly protected from CIA (Table 1), presumably by exhaustive activation (25) of potential disease-causing T cell precursors. SEB appears to exacerbate disease when given after CII injection, perhaps by expanding/activating a previously primed CII-reactive Vβ8.2 T cell population. These studies further confirm the involvement of Vβ8.2 T cells in CII-induced arthritis in DBA/1LacJ mice.
It is a striking characteristic of experimentally induced autoimmune diseases in mice (and the Lewis rat) bearing the Vβ8.2 gene that the autoimmune response appears to focus on T cells utilizing the TCR Vβ8.2 gene segment. In the Lewis rat, MBP induced a remarkably homogeneous response in which the induced T cells were specific for peptides 68–88 and most used the TCR Vβ8.2 gene segment (28). In H-2u mice, the response to MBP focuses on the NH2-terminal peptide, Ac1-9, and again, the TCR Vβ8.2 gene segment is predominantly used (3, 4). In the Lewis rat, experimental autoimmune neuritis (EAN), induced by the myelin P-2 protein or peptides 53–78 of this protein, and experimental autoimmune uveoretinitis (EAU) induced by S antigen or interphotoreceptor retinoid binding protein (IRBP), are both characterized by the high frequency of antigen-specific T cells using the Vβ8.2 gene segment in their TCRs (29, 30). EAU in B10.A mice is not as well characterized with regard to T cell usage, but there is evidence that T cells using TCR Vβ8.2 are also a major component in this autoimmune disease (31). In BALB/c mice, which are resistant to active EAE induction by MBP, T cell clones specific for peptides 59–76 of mouse MBP have been isolated that induce EAE upon transfer to naive recipients (32). These clones are I-Ad–restricted and mainly use the TCR Vβ8.2 gene segment. Models of Sjogren's syndrome have been developed in several autoimmuneprone strains (NZB × NZW)F1, MRL/lpr, and MRL(+/+) and one strain (NFS/sld) not prone to autoimmunity (33, 34). The disease is characterized by lymphocytic infiltration of the lacrimal glands and infiltrating T cells have been identified bearing the TCR Vβ8.2 gene segment. In NOD mice, early induction of diabetes by a single injection of cyclophosphamide is prevented by treatment with anti-Vβ8.2 (35). In the autoimmunity-prone mouse strain, MRL/lpr, there is amelioration of clinical autoimmunity by treatment with anti-Vβ8.2 or with SEB. Finally, with regard to CIA, although some groups have failed to demonstrate an effect of anti-Vβ8.2 (27), in other studies a clear-cut inhibition of CIA was shown. Clearly, our studies indicate a major involvement of T cells utilizing Vβ8.2 in CII-induced arthritis in DBA/1LacJ mice. It is not yet known why autoantigenspecific Vβ8-expressing T cells play a major role in mediating different experimental autoimmune diseases in rodents carrying Vβ8 genes and also why Vβ8-expressing T cells constitute such a major proportion (10–30%) of the peripheral T cell repertoire.
For this reason, it is of interest to consider EAE induction in SJL (H-2s) and CIA in BUB (H-2q) mice, because both these strains are of the TCRVβa haplotype and lack Vβ8 genes. In SJL/J mice, MBP-induced EAE is characterized by T cells specific for peptides 89–101/As. There is oligoclonality with predominant usage of the Vβ4 or Vβ17a gene segment (36). In BUB mice, CII injection results in inflammatory polyarthritis. TCR usage of T cells isolated from arthritic joints appears to be limited to Vβ3 and Vβ10 gene segments (19). These results suggest that oligoclonality need not be restricted to the TCR Vβ8 gene family and may be characteristic of many autoimmune T cell responses. It is significant that BUB mice, which lack the Vβ8.2 gene, contract severe CIA in contrast with the minimal response in DBA/1LacJ mice in which Vβ8.2-bearing T cells have been greatly reduced by SEB treatment (Table 1) or by anti-Vβ8 treatment. Because genes coding for TCR Vβ chains used in the BUB mice are present in DBA/1LacJ mice, it would appear that in Vβ8.2+ mice, a functional hierarchy is established, perhaps during early development, in which Vβ3 or Vβ10 TCR specific for CII are excluded.
It is remarkable that the B10.PL (H-2u) mouse and the DBA/1LacJ (H-2q) mouse appear to use apparently similar regulatory mechanisms in the physiological control of EAE and CIA. In each case, a regulatory circuit is spontaneously activated during the course of the disease after antigenic challenge. T cells specific for the framework 3 region determinant derived from the Vβ8.2 chain are also spontaneously primed and involved in regulating EAE in the Lewis rat model (37). It is interesting that despite the differences in species, MHC haplotypes, background genes, and disease systems, T cells specific for the TCR determinants within the same framework 3 region (B5 TCR–peptide, amino acids 76–101), but not necessarily the same determinant, become activated spontaneously. There could be at least three relevant aspects to the broad use of B5, in particular, that could explain its predominant use in regulatory circuitry. First, the likelihood is that B5 is efficiently processed in a variety of mouse strains and becomes readily available, leading to its immunodominance within the Vβ8.2 TCR. Consistent with this, lymph node cells from both B10.PL and DBA/1LacJ mice challenged with B5 respond to recombinant single-chain TCR molecules containing the entire Vβ8.2 chain in recall proliferation assays. This indicates that this TCR determinant can be processed and presented from the whole Vβ8.2 chain. Second, the possibility exists that B5 possesses one or more promiscuous determinants, able to bind to various class II MHC molecules. Third is the unusual feature of an apparent inability to induce tolerance. Notably, we have not been able to induce proliferative tolerance to this TCR peptide in neonatal DBA/1LacJ mice or in the B10.PL mouse model (7; our unpublished observations). In contrast, proliferative tolerance to all other immunogenic TCR peptides or peptides from any other antigen tested (from MBP, HEL, cyt C) could be successfully induced after neonatal exposure.
The mechanism by which B5-specific Treg mediate protection from CIA is not yet clear. Preliminary data indicate that B5 vaccination does not result in deletion of most Vβ8.2-expressing T cells in DBA/1LacJ mice. This is in contrast with the mechanism suggested in BUB mice, where immunization of mice with peptide 25–46 derived from the TCR Vβ10 chain resulted in deletion of most T cells bearing TCR Vβ10, presumably by induction of an anti-Vβ10 antibody response (38). Whether T cells specific for the TCR Vβ10–peptide are spontaneously primed and are physiologically involved in regulation of CIA in BUB mice remains unknown. Similarly, it is not known whether TCR determinants on other Vβ (and Vα) chains are naturally involved in such regulation (38, 39) or whether the immune system focuses on T cells expressing the Vβ8.2 chain to reestablish effective regulation of autoimmunity. Currently, experiments are in progress to investigate further whether CII-specific and Vβ8.2-bearing T cells are deleted, or deviated to produce noninflammatory cytokines, e.g., IL-4, after B5-induced regulation. Our preliminary data indicate that regulatory mechanisms in EAE, as well as in the CIA system, result in antigen-specific immune deviation (Kumar, V., unpublished observations). CIA appears to be mediated by CII-specific Th1-like cells, with antigen-specific Th2 cells being protective.
With regard to regulation of EAE in B10.PL mice, regulatory T cells are specific for the peptide sequence derived from the framework 3 region of the Vβ8.2 chain, and does not include the D–J region sequence, i.e., a sequence on all Vβ8.2 TCRs is recognized. Nevertheless, the vast majority of Vβ8.2 cells are unaffected by vaccination with B5 or the scTCR containing the Vβ8.2 chain (4–7, Kumar, V., E. Coulsell, B. Ober, G. Hubbard, E. Sercarz, and E. Sally Ward, manuscript submitted for publication). In our preliminary studies in the CIA model, only activated antigenspecific T cells appear to be downregulated after vaccination of mice with B5, suggesting a similar mechanism to that involved in the EAE model. These findings are consistent with earlier indications that it is only activated T cells which are subject to interaction with Treg (40). With relation to the treatment of chronic autoimmune diseases, it is noteworthy that vaccination of mice with TCR peptide B5 as late as 30 d after CII immunization leads to a significant inhibition in the incidence and severity of arthritis (Fig. 3). It is likely that the common regulatory principles we have described in the control of two distinct experimental autoimmune conditions define a generalized TCR centered regulatory mechanism employed by the immune system. This mechanism may be exploitable through specific therapeutic strategies in several human autoimmune diseases.
Acknowledgments
We thank Dr. E. Sally Ward for providing recombinant single chain TCR proteins and Dr. Thomas Goldschmidt for help in establishing a reproducible scoring system for CIA.
Footnotes
1 Abbreviations used in this paper: bCII, bovine collagen II; CIA, collagen IIinduced arthritis; CII, collagen II; EAE, experimental autoimmune encephalomyelitis; EAN, experimental autoimmune neuritis; EAU, experimental autoimmune uveoretinitis; IRBP, interphotoreceptor retinoid binding protein; MBP, myelin basic protein; NOD, nonobese diabetic; SEA, staphylococcal enterotoxin A; SEB, staphylococcal enterotoxin B; S.I., stimulation index.
References
- 1.Heber-Katz E, Acha-Orbea H. The V-region hypothesis: evidence from autoimmune encephalomyelitis. Immunol Today. 1989;10:164–169. doi: 10.1016/0167-5699(89)90174-6. [DOI] [PubMed] [Google Scholar]
- 2.Acha-Orbea H, Mitchell DJ, Timmermann L, Wraith D, Tausch GS, Waldor MK, Zamvil SS, McDevitt HO, Steinman L. Limited heterogeneity of T cell receptors from lymphocyte mediating autoimmune encephalomyelitis allows specific immune intervention . Cell. 1988;54:263–273. doi: 10.1016/0092-8674(88)90558-2. [DOI] [PubMed] [Google Scholar]
- 3.Urban JL, Kumar V, Kono DH, Gomez C, Horvath SJ, Clayton J, Ando DG, Sercarz EE, Hood L. Restricted use of T cell receptor V genes in murine autoimmune encephalomyelitis raises possibilities for antibody therapy. Cell. 1988;54:577–592. doi: 10.1016/0092-8674(88)90079-7. [DOI] [PubMed] [Google Scholar]
- 4.Kumar V, Sercarz E. T cell regulatory circuitry: antigen-specific and TCR–idiopeptide-specific T cell interactions in EAE. Int Rev Immunol. 1993;9:287–297. doi: 10.3109/08830189309051212. [DOI] [PubMed] [Google Scholar]
- 5.Kumar V, Sercarz E. The involvement of T cell receptor peptide-specific regulatory CD4+T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993;178:909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V, Sercarz E. Dysregulation of potentially pathogenic self reactivity is crucial for the manifestation of clinical autoimmunity. J Neurosci Res. 1996;45:334–339. doi: 10.1002/(SICI)1097-4547(19960815)45:4<334::AID-JNR2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Kumar V, Stellrecht K, Sercarz E. Inactivation of CD4 regulatory T cells results in chronic autoimmunity. J Exp Med. 1996;184:1609–1617. doi: 10.1084/jem.184.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen: an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuart JM, Townes AS, Kang AH. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- 10.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunization against heterologous type II collagen induces arthritis in mice. Nature (Lond) 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 11.Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- 12.Holmdahl R, Jansson L, Andersson M, Larsson E. Immunogenetics of type II collagen autoimmunity and susceptibility to collagen arthritis. Immunology. 1988;65:305–310. [PMC free article] [PubMed] [Google Scholar]
- 13.Ranges GE, Sriram S, Cooper SM. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985;162:1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahn E, Trentham DE. Experimental synovitis induced by collagen-specific T cell lines. Cell Immunol. 1989;118:491–503. doi: 10.1016/0008-8749(89)90396-1. [DOI] [PubMed] [Google Scholar]
- 15.Kakimoto K, Katsuiki M, Hirofuji T, Iwata H, Koga T. Isolation of T cell line capable of protecting mice against collagen-induced arthritis. J Immunol. 1988;140:78–83. [PubMed] [Google Scholar]
- 16.Lider O, Karin N, Shinitzky M, Cohen IR. Therapeutic vaccination against adjuvant arthritis using autoimmune T cells treated with hydrostatic pressure. Proc Natl Acad Sci USA. 1987;84:4577–4580. doi: 10.1073/pnas.84.13.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee S, Haqqi TM, Luthra HS, Stuart JM, David CS. Possible role of Vβ T cell receptor genes in collagen induced arthritis in mice. J Exp Med. 1988;167:832–839. doi: 10.1084/jem.167.3.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haqqi TM, David CS. T-cell receptor Vβgenes repertoire in mice possible role in resistance and susceptibility to type II collagen-induced arthritis. J Autoimmunity. 1990;3:113–121. doi: 10.1016/0896-8411(90)90135-f. [DOI] [PubMed] [Google Scholar]
- 19.Haqqi TM, Banerjee S. Limited heterogeneity in T cell receptor V beta chain gene expression in arthritic joints of BUB/BnJ (H-2q) mice, a T cell receptor Vβ astrain. Ann NY Acad Sci. 1995;756:221–224. doi: 10.1111/j.1749-6632.1995.tb44517.x. [DOI] [PubMed] [Google Scholar]
- 20.Haqqi TM, Anderson GD, Banerjee S, David CS. Restricted heterogeneity in T-cell antigen receptor Vβ gene usage in the lymph nodes and arthritic joints of mice. Proc Natl Acad Sci USA. 1992;89:1253–1255. doi: 10.1073/pnas.89.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman GE, Toda M, Kanagawa O, Hood LE. Characterization of the T cell receptor repertoire causing collagen arthritis in mice. J Exp Med. 1993;177:387–395. doi: 10.1084/jem.177.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiocchia G, Boissier MC, Fournier C. Therapy against murine collagen-induced arthritis with T cell receptor Vβ-specific antibodies. Eur J Immunol. 1991;21:2899–2905. doi: 10.1002/eji.1830211202. [DOI] [PubMed] [Google Scholar]
- 23.Moder KG, Luthra HS, Griffiths M, David CS. Prevention of collagen induced arthritis in mice by deletion of T cell receptor Vβ8 bearing T cells with monoclonal antibodies. Br J Rheumat. 1993;32:26–30. doi: 10.1093/rheumatology/32.1.26. [DOI] [PubMed] [Google Scholar]
- 24.Kappler JW, Staerz U, White J, Marrack PC. Self tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature (Lond) 1988;332:35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- 25.Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- 26.Ward ES. Secretion of T cell receptor fragments from recombinant E. Coli cells. J Mol Biol. 1992;239:885–890. doi: 10.1016/0022-2836(92)90455-s. [DOI] [PubMed] [Google Scholar]
- 27.Goldschmidt TJ, Jansson L, Holmdahl R. In vivoelimination of T cells expressing specific T-cell receptor Vβ chains in mice susceptible to collagen-induced arthritis. Immunology. 1990;69:508–514. [PMC free article] [PubMed] [Google Scholar]
- 28.Burns FR, Li X, Shen N, Offner H, Chou YK, Vandenbark AA, Heber-Katz E. Both rat and mouse T cell receptors specific for the encephalitogenic determinant of myelin basic protein use similar Vα and Vβ chain genes even though the major histocompatibility complex and encephalitogenic determinants being recognized are different. J Exp Med. 1989;169:27–39. doi: 10.1084/jem.169.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang XM, Esch TR, Clark L, Gregorian S, Rostami A, Otvos L, Jr, Heber-Katz E. Neuritogenic Lewis rat T cells use TCR β chains that include a new TCR Vβ8 family member. Immunogenetics. 1994;40:266–270. doi: 10.1007/BF00189971. [DOI] [PubMed] [Google Scholar]
- 30.Egwuagu CE, Mahdi RM, Nussenblatt RB, Gery I, Caspi RR. Evidence for selective accumulation of Vβ8 T lymphocytes in experimental autoimmune uveoretinitis induced with two different retinal antigens. J Immunol. 1993;151:1627–1636. [PubMed] [Google Scholar]
- 31.Rizzo LV, Silver P, Wiggert B, Hakim F, Gazzinelli RT, Chan C, Caspi RR. Establishment and characterization of a murine CD4 T cell line and clone that induce experimental autoimmune uveoretinitis in B10.A mice. J Immunol. 1996;156:1654–1660. [PubMed] [Google Scholar]
- 32.Abromson-Leeman S, Alexander J, Bronson R, Carroll J, Southwood S, Dorf M. Experimental autoimmune encephalomyelitis–resistant mice have highly encephalitogenic MBP-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J Immunol. 1995;154:388–398. [PubMed] [Google Scholar]
- 33.Takahashi M, Mimura Y, Hamano H, Haneji N, Yanagi K, Hayashi Y. Mechanism of the development of autoimmune dacryoadenitis in the mouse model for primary Sjogren's syndrome. Cell Immunol. 1996;170:54–62. doi: 10.1006/cimm.1996.0133. [DOI] [PubMed] [Google Scholar]
- 34.Hayashi Y, Hamano H, Haneji N, Ishimaru N, Yanagi K. Biased T cell receptor Vβ gene usage during specific stages of the development of autoimmune sialadenitis in the mrl/lprmouse model of Sjogren's syndrome. Arthritis Rheum. 1995;38:1077–1084. doi: 10.1002/art.1780380809. [DOI] [PubMed] [Google Scholar]
- 35.Bacelj A, Charlton B, Mandel TE. Prevention of cyclophosphamide-induced diabetes by anti-Vβ8 T cell receptor monoclonal antibody therapy in NOD/Wehi mice. Diabetes. 1989;38:1492–1495. doi: 10.2337/diab.38.11.1492. [DOI] [PubMed] [Google Scholar]
- 36.Padula SJ, Lingenheld EG, Stabach PR, Chou C-HJ, Kono DH, Clark RB. Identification of Vβ4bearing T cells in SJL/J mice. Further evidence for the V region disease hypothesis? . J Immunol. 1991;146:879–883. [PubMed] [Google Scholar]
- 37.Vainiene M, Celnik B, Vandenbark AA, Hashim GA, Offner H. Natural immunodominant and EAEprotective determinant within the Lewis rat Vβ8.2 sequence include CDR2 and framework 3 idiotopes. J Neurosci Res. 1996;43:137–145. doi: 10.1002/(SICI)1097-4547(19960115)43:2<137::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 38.Haqqi TM, Qu X, Anthony D, Ma J, Sy M. Immunization with T cell receptor Vβ chain peptides deletes pathogenic T cells and prevents the induction of collagen- induced arthritis in mice. J Clin Invest. 1996;97:2849–2858. doi: 10.1172/JCI118741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosloniec EF, Brand DD, Whittington KB, Stuart JM, Ciubotaru M, Ward ES. Vaccination with a recombinant V alpha domain of a TCR prevents the development of collagen-induced arthritis. J Immunol. 1995;155:4504–4511. [PubMed] [Google Scholar]
- 40.Lohse AW, Mor F, Karin N, Cohen IR. Control of experimental autoimmune encephalomyelitis by T cells responding to activated T cells. Science (Wash DC) 1989;244:820–822. doi: 10.1126/science.2471264. [DOI] [PubMed] [Google Scholar]