Abstract
The B cell antigen receptor, composed of membrane immunoglobulin (Ig) sheathed by the Igα/Igβ heterodimer plays a critical role in mediating B cell development and responses to antigen. The cytoplasmic tails of Igα and Igβ differ substantially but have been well conserved in evolution. Transfection experiments have revealed that, while these tails share an esssential tyrosine-based activation motif (ITAM), they perform differently in some but not all assays and have been proposed to recruit distinct downstream effectors. We have created transgenic mouse lines expressing chimeric receptors comprising an IgM fused to the cytoplasmic domain of each of the sheath polypeptides. IgM/α and IgM/β chimeras (but not an IgM/β with mutant ITAM) are each independently sufficient to mediate allelic exclusion, rescue B cell development in gene-targeted Igμ− mice that lack endogenous antigen receptors, as well as signal for B7 upregulation. While the (IgM/α) × (IgM/β) double-transgenic mouse revealed somewhat more efficient allelic exclusion, our data indicate that each of the sheath polypeptides is sufficient to mediate many of the essential functions of the B cell antigen receptor, even if the combination gives optimal activity.
The B cell antigen receptor (BCR)1, which is composed of membrane immunoglobulin sheathed by the Igα/ Igβ heterodimer (CD79α/CD79β), mediates the response of the B cell to antigen by initiating transmembrane signaling and driving the internalization of antigen for presentation. The Igα/Igβ sheath is not only necessary for allowing the surface transport of membrane IgM but is also critical for mediating the signaling of the BCR and endocytic activities (1, 2).
Various chimeric receptors have been used to dissect the relative contributions of the Igα and Igβ cytoplasmic domains to BCR function. Because immunoreceptor tyrosine-based activation motifs (ITAMs) (3) present in both sheath polypeptides are central to their signaling activity (4–8), Igα and Igβ may be functionally redundant. Indeed, several transfection studies have failed to reveal significant differences in the signaling activities of Igα and Igβ (6, 7, 9). However, others have found that whereas chimeras containing just the Igα tail could mediate transmembrane signaling in B cell transfectants, analogous Igβ chimeras were impaired to variable extents (4, 10, 11, 12.). This, in addition to the suggestion that the Igα and Igβ cytoplasmic domains bind different downstream effectors (13) and exhibit differences in antigen-presenting activity (14), is consistent with the two chains fulfilling distinct functions.
Experiments to ascertain whether there is a division of function between the Igα and Igβ cytoplasmic domains reveal that in vivo both IgM/α and IgM/β chimeras are able to induce the pro-B to pre-B transition and mediate allelic exclusion (8, 15). Here, we extend the transgenic analysis to the later stages of B cell development, to ask about the triggering of B cell maturation and activation.
Materials and Methods
DNA Constructs, Transfectants and Transgenic Mice.
Plasmids driving the expression of the hen egg lysozyme (HEL)–specific receptors are based on pSV2gpt and pSV2neo. The κ transcription unit, in which the Vκ segment of the mouse D1.3 mAb (16, 17) is linked to rat Cκ, was assembled by exchanging the SacI–XhoI β-globin insert of Lκ-βG (18) for an analogous PCR-generated SacI–XhoI fragment containing D1.3 Vκ. The heavy (H) chain vectors derive from pSV–Vμ1 (19) but with the VNP VH segment replaced by a PCR-generated PstI–BstEII D1.3 VH segment. For the chimeric μ chains, the Cμ region was replaced by Cμ/β or Cμ/βY→ L CH regions (20) or Cμ/α CH regions (20a) (Fig. 1 A).
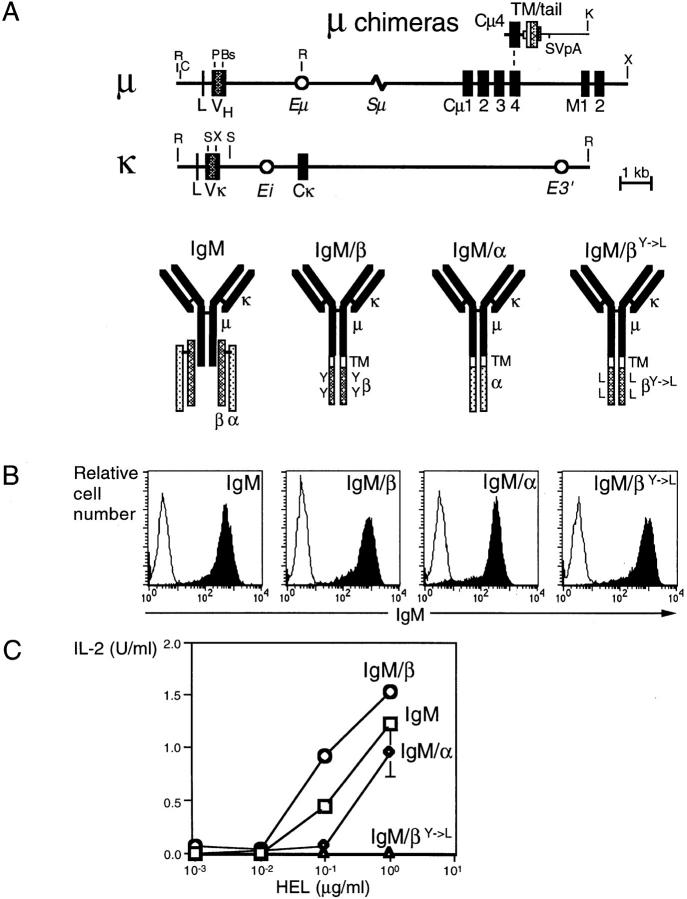
Figure 1.
Antigen induces IL-2 secretion from the A20 transfectants. (A) Schematic representation of the transgene constructs and predicted structures of the receptors (R, EcoRI; C, ClaI; P, PstI; Bs, BstEII; K, KpnI; X, XhoI; S, SacI). (B) Flow cytometric analysis of transfected receptors in A20 by staining for surface IgM; unfilled histograms depict the unstained controls. (C) Mean levels of IL-2 secreted by A20 transfectants after incubation with hen egg lysozyme (HEL) for 24 h. Standard errors were below 0.5 U/ml except where indicated.
DNA was introduced into the mouse A20 B cell lymphoma by electroporation and stably transfected clones selected in DMEM, 10% FCS, 50 μM 2-ME containing G418 and mycophenolic acid. Transgenic mice were established by coinjection into zygotes from (CBA/Ca × C57BL/6)F1 mice of an EcoRI fragment containing the light (L) chain transcription unit together with either a ClaI–XhoI (wild-type IgM) or ClaI–KpnI (chimeric IgM) fragment for the H chain; founders were then bred with (CBA/Ca × C57BL/6)F1s or crossed into a μMT background (21). For all four constructs, multiple founders were identified that carried H or L chain transgenes. All chimeric H chain constructs were present in 10–20 copies. In the case of wild-type HEL-specific IgM, two founders that had cointegrated the H and L transcription units were studied and gave similarly effective allelic exclusion, although only one of the two founders was bred into a μMT background. For the IgM/β chimera, two founders positive for both H and L expression were identified but only the first founder was used in subsequent studies as the second founder segregated the H and L transgenes on breeding. With IgM/βY→ L and IgM/α, only a single positive H+L founder was obtained in each case.
Flow Cytometry.
Analysis was performed on Becton Dickinson FACScan® or FACScalibur® using LYSYS II or CELLQuest software. FITC-conjugated and biotinylated goat anti-IgM, and PE-conjugated rat anti-IgD were from Southern Biotechnology (Birmingham, AL); FITC–RA3-6B2 (rat anti-B220[CD45R]), PE-conjugated B3B4 (rat anti-CD23), biotinylated 53-2.1 (rat anti-Thy1.2[CD90]) and purified 7G6 (rat anti-CD21/35[CR2/1]) were from PharMingen (San Diego, CA); PE-conjugated RA36B2 and RED670–streptavidin were from GIBCO BRL (Paisley, UK); FITC–streptavidin was from Amersham (Amersham, UK); and PE–streptavidin was from Jackson ImmunoResearch (West Grove, PA). Cells making the E5.2 monoclonal anti-D1.3 idiotype antibody (22) were a gift from R. Poljak. The flow cytometric analyses illustrated are representative of multiple individual animals aged 2–4 mo old.
B Cell Activation.
Production of IL-2 from A20 transfectants (105 cells in 200 μl) cultured for 24 h in medium containing various concentrations of HEL was monitored by providing the culture supernatant to IL-2–dependent HT2 cells (2.5 × 104 cells in 100 μl medium). The viability of the HT2 cells was determined after 24 h as previously described (20) and the assay calibrated with recombinant IL-2 standards.
To monitor proliferative responses, splenocytes that had been depleted of erythrocytes by hypotonic lysis were cultured in triplicate aliquots (2 × 105 in 200 μl) in RPMI, 10% FCS, 50 μM 2-ME in the presence of 1 μg/ml LPS (Sigma, Poole, UK) for 48 h before pulsing with 0.5 μCi [3H]thymidine for 15 h and scintillation counting.
To monitor CTLA4 binding, spleen cells depleted of erythrocytes were cultured (106 cells/ml) in medium in the presence or absence of 10 μg/ml F(ab′)2 goat anti–mouse IgM (Jackson ImmunoResearch) for 24 h before staining with mCTLA4–Hγ1 fusion protein (gift from P. Lane) and FITC-conjugated goat anti– human IgG (Jackson ImmunoResearch).
BrdU Uptake.
Mice were given two intraperitoneal injections 4 h apart of 1 mg BrdU (5-bromo-2-deoxyuridine) (Sigma) in PBS and the drinking water supplemented with 1 mg/ml BrdU for 72 h following the protocol of Torres et al. (23). Spleens were removed and, after staining for CD45R(B220) and either D1.3 idiotype or IgM, the cells were fixed and permeabilized with 70% ethanol and 1% paraformaldehyde, 0.01% Tween-20 in PBS, treated with DNaseI and stained with FITC-conjugated antiBrdU antibody (Becton Dickinson, San Jose, CA) for cytofluorimetric analysis.
Results
The IgM/α and IgM/β but not IgM/βY→ L Chimeras Signal in A20.
To discriminate between the functions of Igα and Igβ cytoplasmic domains, we constructed a set of plasmids encoding either wild-type or chimeric HEL-specific receptors. The chimeras are composed of mouse μ and rat κ Ig chains directly linked through a hydrophobic transmembrane segment to the cytoplasmic domains of either Igα, Igβ, or a mutated Igβ whose ITAM tyrosines are substituted by leucines (IgM/α, IgM/β, or IgM/βY→ L; Fig. 1 A). The transmembrane segment (which derives from the H-2Kb gene) confers sheath-independent surface transport (24) and the receptors do not show detectable association with endogenous Igα or Igβ chains (20).
The plasmids encoding the various receptors were transfected into the A20 B cell lymphoma; the transfectants all stained for IgM although there were some differences in the brightness with the IgM/α chimera being the least well transported to the cell surface (Fig. 1 B). The IgM/α and IgM/β chimeras, as well as the wild-type IgM receptor, were able to initiate signaling after antigen binding as judged by the production of IL-2 from transfectants of the A20 lymphoma (Fig. 1 C). However, the signaling activity was abolished by mutation of the ITAM tyrosines in the Igβ cytoplasmic domain.
Receptor Expression in Transgenic Mice.
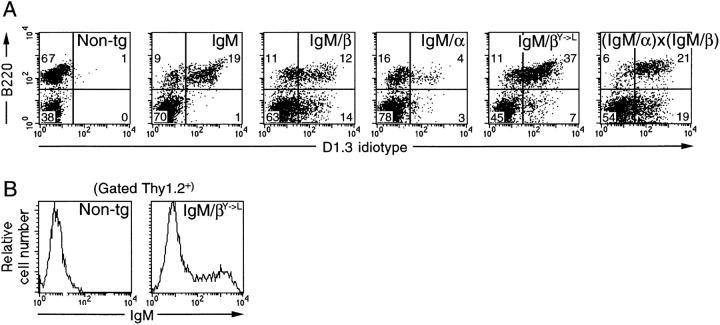
The transcription units encoding the various receptors were introduced into the germline of transgenic mice. Cytofluorimetric analysis of spleen cells with anti-D1.3 idiotype antibody (Fig. 2 A) as well as with anti-rat κ and labeled HEL (data not shown) revealed that they were all expressed on the B cell surface, although the IgM/α staining was weaker than that of the other receptors. With the mice bearing the chimeric receptors, the receptors were also expressed on some CD45R(B220)− cells. These correspond to a subpopulation of T cells (Fig. 2 B), probably reflecting the expression pattern of the IgH enhancer in transgenic mice (25). (The absence of surface expression of the wild-type transgenic IgM receptor in this subpopulation is consistent with the fact that wild-type IgM, but not the IgM chimeras, requires endogenous Igα/ Igβ for surface transport).
Figure 2.
Expression of transgenes. (A) Flow cytometric analysis of spleens of transgenic mice. Cells were stained with FITC-conjugated anti-D1.3 idiotype and PE-conjugated anti-B220 and gated for propidium iodide exclusion. Numbers indicate percentage of splenocytes in each quadrant (Non-tg, nontransgenic). (B) Transgene expression on T cells. Splenocytes were stained with FITC-conjugated anti-IgM, biotinylated anti-Thy1.2, and PE– streptavidin; the profiles present the IgM staining of Thy1.2+ gated cells.
IgM/α and IgM/β but not IgM/βY→ L Drive B Cell Development.
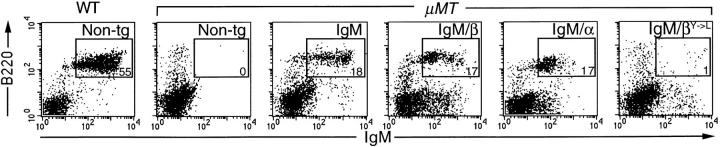
Signals transmitted through membrane Ig are required for B cell development; μMT mice that carry a targeted disruption of the μ membrane exon are B cell deficient (21). To see whether the individual Igα and Igβ cytoplasmic domains of the IgM chimeras were sufficient to signal for B cell maturation, the various transgenic lines were bred into a homozygous μMT background. It was immediately evident that the HEL-specific IgM BCR as well as the IgM/α and IgM/β chimeras all had a significant effect on B cell development; their presence led to a substantial (around two log) increase in serum IgG levels as compared with nontransgenic μMT controls. (Serum IgG is routinely detected in reconstituted μMT mice and is presumably encoded by Ig-transgenic B cells that also carry productive VHDJH integrations on an endogenous μMT allele that has switched to a downstream isotype). The reconstitution of peripheral B cell development was confirmed by cytofluorimetric analysis of spleen cells: the presence of either the IgM/α or the IgM/β chimera was sufficient to yield splenic IgM+ B220+ cells with a functional ITAM being essential for this activity (Fig. 3).
Figure 3.
The IgM, IgM/α and IgM/β, but not IgM/βY→ L, transgenes reconstitute the splenic B cell pool in μMT mice. Splenocytes were stained with FITC-conjugated anti-IgM and PE-conjugated anti-B220; the numbers denote the percentage of B220+ IgM+ cells within gated region and are representative of multiple animals (WT 57 ± 7% [n = 8]; IgM 26 ± 19% [n = 4]; IgM/β 15 ± 6% [n = 5]; IgM/α 15 ± 7% [n = 8]). WT, wild-type background; μMT, homozygous μMT background; Non-tg, nontransgenic.
Phenotype of the Transgenic B Cells.
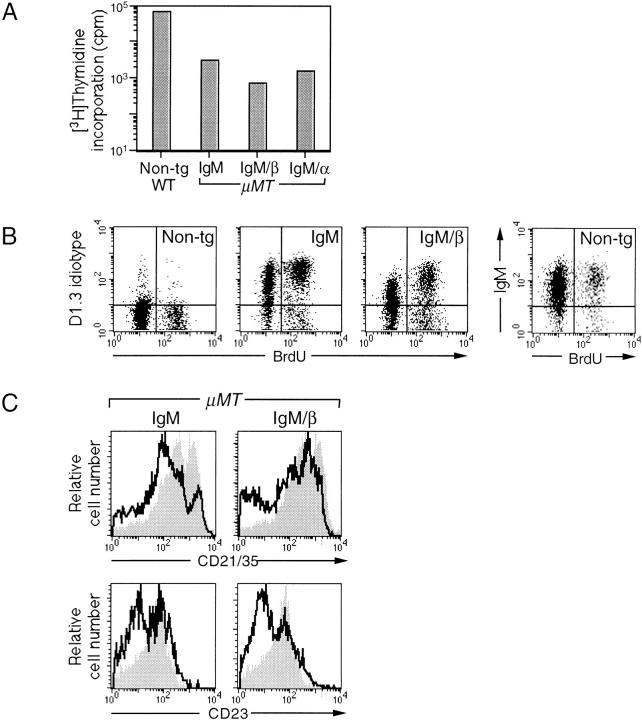
Although these analyses revealed that the IgM chimeras were able to drive the maturation of a splenic B cell population, we found that the reconstituted B cells proliferated extremely poorly on in vitro culture with LPS (Fig. 4 A). Indeed, compared with normal controls, the transgenic B cells exhibited a two- to fivefold increase in cell death during a 24-h in vitro culture. The diminished proliferative response is not unique to the IgM chimeras: it is also evident in mice transgenic for the wild-type anti-HEL IgM (Fig. 4 A). An altered turnover of the transgenic B cells is also apparent in vivo: BrdU labeling revealed that animals carrying the transgenic receptors had an increased proportion of rapidly turning over splenic B cells (Fig. 4 B). With regard to surface markers, splenic B cells from mice transgenic for both the wildtype IgM and the IgM chimeras revealed a shift to lower expression of the mature B cell markers, CD21/35 and CD23 (Fig. 4 C) as compared with normal mice, though CD22 expression was unaffected. Thus, in view of their diminished proliferative response, increased in vivo turnover and decreased expression of CD21/35 and CD23, it is evident that the splenic B cells whose development is driven by the transgenic anti-HEL receptors do not show the same distribution of maturational development as splenic B cells in normal mice. However, this is an effect of all the anti-HEL transgenes and does not indicate compromised signaling by either of the individual IgM chimeras.
Figure 4.
Phenotypic analysis of the transgenic B cells. (A) The transgenic B cells proliferate poorly. After 48-h culture in the presence of LPS, the splenocytes from wild-type, nontransgenic mice (WT, Non-tg), or transgenic mice crossed into a μMT background were pulsed with [3H]thymidine for 15 h before scintillation counting. Bars show mean counts and standard errors were >10% of means. Counts after incubation in medium alone were 1,000–1,500 cpm for nontransgenic and 200–300 cpm for transgenic cells. (B) Turnover of transgenic B cells. After uptake of BrdU (5-bromo-2-deoxyuridine) for 72 h, spleen cells were stained with PE-conjugated anti-B220 and biotinylated anti-D1.3 idiotype or anti-IgM (nontransgenic only) and RED670–streptavidin. After fixation and permeabilization, cells were stained with a FITC-conjugated antiBrdU antibody. The analyses (10,000 gated cells) are presented for cells that have been gated as B220+. Whereas 10–20% of the IgM+ cells in nontransgenic mice had incorporated BrdU, the figure increased to 40– 50% of the D1.3 idiotype+ cells in the transgenic lines. (C) Splenic B cells from IgM and IgM/β express lower levels of mature B cell markers CD21/35 and CD23. Histograms show splenic B cells from transgenic mice crossed into a μMT background overlaid on nontransgenic controls (shaded). For CD21/35 expression, splenocytes were stained with FITC– anti-IgM, PE–anti-B220, and biotinylated anti-CD21/35 (revealed by RED670–streptavidin), gating by lymphocyte scatter and B220+ IgM+. For CD23 expression, cells were stained with FITC–anti-B220 and PE– anti-CD23, gating by scatter and B220+.
Upregulation of B7 by the IgM/α and IgM/β Chimeras.
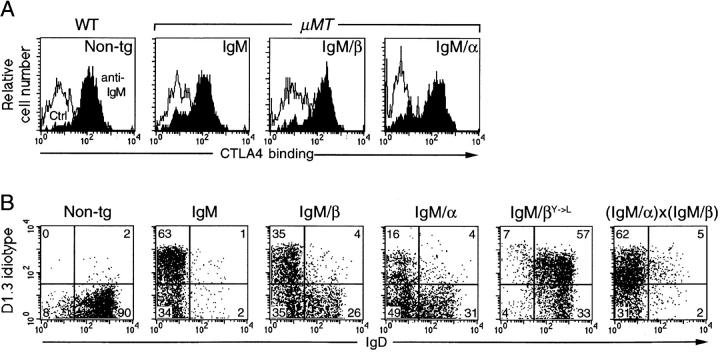
To monitor signaling by the IgM chimeras in the transgenic B cells, we sought to measure the proliferative responses to anti-IgM and IL-4. However, as with LPS, the responses were very poor; therefore, we used upregulation of the B7 costimulatory molecules as our readout. Splenic B cells from the transgenic mice were cultured for 24 h with anti-IgM and upregulation of B7 was judged by staining with a CTLA4–Ig fusion protein (Fig. 5 A). Triggering the receptors with HEL or anti-D1.3 idiotype antibody gave analogous results but additionally revealed that the IgM/βY→ L receptor was signaling defective (data not shown).
Figure 5.
In vitro activation and allelic exclusion. (A) Upregulation of CTLA4 binding following a 24-h culture with antiIgM or medium alone (Ctrl). Cultured splenocytes from transgenic mice in a μMT background (or wild-type nontransgenic controls, WT, Non-tg) were stained with PE–anti-B220 and an mCTLA4–Hγ1 fusion protein, which was visualized with FITC–anti-human IgG. The profiles were gated for B220+ cells that excluded propidium iodide. (B) Exclusion of endogenous IgD. Splenocytes were stained with FITC–antiB220, PE–anti-IgD, and biotinylated anti-D1.3 plus RED670– streptavidin. Cells were gated for B220+ and numbers denote percentage of splenic B cells within each quadrant.
The IgM/α and IgM/β Chimeras Give Partial Allelic Exclusion but the Combination is Optimal.
The ability to mediate the feedback regulation of endogenous Ig gene rearrangement (allelic exclusion) provided another parameter for comparing the signaling ability of the different receptors. Allelic exclusion in the transgenic mice was readily monitored by determining the proportion of D1.3 idiotype+ B cells that coexpress IgD and gave similar conclusions to staining with anti-μ allotype antibodies. It will be seen (Fig. 5 B) that the exclusion mediated by the wild-type HEL receptor is significantly greater than that mediated by the IgM/α and IgM/β chimeras. The IgM/βY→ L receptor is ineffective in exclusion with all D1.3 idiotype+ B cells coexpressing an endogenous rearrangement. We crossed the IgM/α and IgM/β chimeras to see whether coexpression of the two receptors yielded more complete exclusion. The double-transgenic mice exhibited greatly decreased expression of endogenous Ig gene rearrangements (Fig. 5 B) despite the fact that there was no significant increase in the abundance of transgenic IgM on the B cell surface (see Fig. 2 A).
Discussion
The results show that both the IgM/α and IgM/β chimeras can broadly perform many of the major in vivo functions of the complete BCR. Furthermore, the ability to drive the maturation and activation of peripheral B cells is dependent upon the ITAM; this parallels previous findings on pre-B cell development (8, 15).
Transfection experiments using cell lines have revealed that both Igα and Igβ are needed for surface transport of membrane Ig (26, 27). Thus, mice carrying targeted disruptions of Igβ cannot express surface Ig and are B cell deficient (28). In contrast, both the IgM/α and IgM/β chimeras described here allow extensive B cell maturation because, by virtue of their mutant transmembrane sequences, these chimeras can be transported to the B cell surface without an attendant Igα/Igβ sheath. The IgM/β chimera performs slightly better than the IgM/α chimera in several of the assays but this may simply reflect the more efficient surface transport of the IgM/β chimera.
Therefore, our results so far do not lend significant support to the idea that Igα and Igβ cytoplasmic domains perform distinct autonomous functions within the context of the intact BCR. Nevertheless, it is clear that the chimeras are not as effective as the complete BCR. Thus, the (IgM/α) × (IgM/β) double transgenic mouse is considerably more efficient than its single transgenic parents in effecting allelic exclusion of endogenous Ig gene rearrangement. This is consistent with cell line transfection experiments indicating cooperativity between the two cytoplasmic domains with the heterodimer giving a stronger signal than the component homodimers (29). Indeed, the structural conformation of the heterodimer could differ substantially from that of the homodimers and this could lead to differences in the kinetics of phosphorylation or efficacy of effector protein (e.g., Syk) recruitment, as well as in the sensitivity to antigen binding.
However, although the chimeric HEL-specific receptors do not perform as well as the wild-type IgM BCR in driving the reconstitution of a splenic B cell compartment in μMT mice, the difference is relatively small and the impaired B cell maturation is certainly not nearly as dramatic as that observed by Torres et al. (23) in mice carrying a targeted disruption of the mb-1 gene that leads to the synthesis of a BCR with a truncated Igα tail. The different performance of the various compromised BCRs in driving pre-B and B cell development could well be accounted for by a requirement for differing qualities of signal at the various maturational checkpoints. It will obviously be interesting to correlate the differentiative potential of the various mutant BCRs with their biochemical signaling activities.
Acknowledgments
We thank S. Davies and T. Langford for animal handling, G. Williams for advice, V. Aluvihare for cells, A. Riddell for flow cytometry, the Howard Hughes Medical Institute for an international research scholars award, and R. Poljak, K. Rajewsky and P. Lane for provision of materials.
This work was supported by an international research scholars award from the Howard Hughes Medical Institute.
Footnotes
1 Abbreviations used in this paper: BCR, B cell antigen receptor; BrdU, 5-bromo-2-deoxyuridine; H, heavy; HEL, hen egg lysozyme; ITAM, immunoreceptor tyrosine-based activation motif; L, light.
References
- 1.Pleiman CM, D'Ambrosio D, Cambier JC. The B-cell antigen receptor complex: structure and signal transduction. Immunol Today. 1994;15:393–399. doi: 10.1016/0167-5699(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 2.DeFranco AL. Structure and function of the B cell antigen receptor. Annu Rev Cell Biol. 1993;9:377–410. doi: 10.1146/annurev.cb.09.110193.002113. [DOI] [PubMed] [Google Scholar]
- 3.Reth M. Antigen receptor tail clue. Nature (Lond) 1989;338:383–384. [PubMed] [Google Scholar]
- 4.Sanchez M, Misulovin Z, Burkhardt AL, Mahajan S, Costa T, Franke R, Bolen JB, Nussenzweig M. Signal transduction by immunoglobulin is mediated through Igα and Igβ. J Exp Med. 1993;178:1049–1055. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaswinkel H, Reth M. Dual role of the tyrosine activation motif of the Ig-α protein during signal transduction via the B cell antigen receptor. EMBO (Eur Mol Biol Organ) J. 1994;13:83–89. doi: 10.1002/j.1460-2075.1994.tb06237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams GT, Peaker CJ, Patel KJ, Neuberger MS. The α/β sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping or for serine/threonine–kinase recruitment. Proc Natl Acad Sci USA. 1994;91:474–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taddie JA, Hurley TR, Hardwick BS, Sefton BM. Activation of B- and T-cells by the cytoplasmic domains of the B-cell antigen receptor proteins Ig-α and Ig-β. J Biol Chem. 1994;269:13529–13535. [PubMed] [Google Scholar]
- 8.Papavasiliou F, Misulovin Z, Suh H, Nussenzweig MC. The role of Igβ in precursor B cell transition and allelic exclusion. Science (Wash DC) 1995;268:408–411. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 9.Law DA, Chan VWF, Datta SK, DeFranco AL. B-cell antigen receptor motifs have redundant signalling capabilities and bind the tyrosine kinases PTK72, Lyn and Fyn. Curr Biol. 1993;3:645–657. doi: 10.1016/0960-9822(93)90062-s. [DOI] [PubMed] [Google Scholar]
- 10.Kim KM, Alber G, Weiser P, Reth M. Differential signaling through the Ig-α and Ig-β components of the B cell antigen receptor. Eur J Immunol. 1993;23:911–916. doi: 10.1002/eji.1830230422. [DOI] [PubMed] [Google Scholar]
- 11.Choquet D, Ku G, Cassard S, Malissen B, Korn H, Fridman WH, Bonnerot C. Different patterns of calcium signaling triggered through two components of the B lymphocyte antigen receptor. J Biol Chem. 1994;269:6491–6497. [PubMed] [Google Scholar]
- 12.Cassard S, Choquet D, Fridman WH, Bonnerot C. Regulation of ITAM signaling by specific sequences in Ig-β B cell antigen receptor subunit. J Biol Chem. 1996;271:23786–23791. doi: 10.1074/jbc.271.39.23786. [DOI] [PubMed] [Google Scholar]
- 13.Clark MR, Campbell KS, Kazlauskas A, Johnson SA, Hertz M, Potter TA, Pleiman C, Cambier JC. The B cell antigen receptor complex: association of Ig-α and Ig-β with distinct cytoplasmic effectors. Science (Wash DC) 1992;258:123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- 14.Bonnerot C, Lankar D, Hanau D, Spehner D, Davoust J, Salamero J, Fridman WH. Role of B cell receptor Igα and Igβ subunits in MHC class II–restricted antigen presentation. Immunity. 1995;3:335–347. doi: 10.1016/1074-7613(95)90118-3. [DOI] [PubMed] [Google Scholar]
- 15.Papavasiliou F, Jankovic M, Suh H, Nussenzweig MC. The cytoplasmic domains of immunoglobulin (Ig) α and Igβ can independently induce the precursor B cell transition and allelic exclusion. J Exp Med. 1995;182:1389–1394. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amit AG, Mariuzza RA, Phillips SE, Poljak RJ. Three-dimensional structure of an antigen–antibody complex at 6 Å resolution. Nature (Lond) 1985;313:156–158. doi: 10.1038/313156a0. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature (Lond) 1990;348:552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- 18.Yelamos J, Klix N, Goyenechea B, Lozano F, Chui YL, Gonzalez A, Fernandez, Pannell R, Neuberger MS, Milstein C. Targeting of non-Ig sequences in place of the V segment by somatic hypermutation. Nature (Lond) 1995;376:225–229. doi: 10.1038/376225a0. [DOI] [PubMed] [Google Scholar]
- 19.Neuberger MS. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO (Eur Mol Biol Organ) J. 1983;2:1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel KJ, Neuberger MS. Antigen presentation by the B cell antigen receptor is driven by the α/β sheath and occurs independently of its cytoplasmic tyrosines. Cell. 1993;74:939–946. doi: 10.1016/0092-8674(93)90473-4. [DOI] [PubMed] [Google Scholar]
- 20a.Aluvihare, V.R., A.A. Khamlichi, G.T. Williams, L. Adorini, and M.S. Neuberger. 1997. Acceleration of intracellular targetting of antigen by the B cell antigen receptor: importance depends on the nature of the antigen/antibody interaction. EMBO (Eur. Mol. Biol. Organ.) J. In press. [DOI] [PMC free article] [PubMed]
- 21.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell–deficient mouse by targeted disruption of the membrane exon of the immunoglobulin μ chain gene. Nature (Lond) 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 22.Fields BA, Goldbaum FA, Ysern X, Poljak RJ, Mariuzza RA. Molecular basis of antigen mimicry by an anti-idiotope. Nature (Lond) 1995;374:739–742. doi: 10.1038/374739a0. [DOI] [PubMed] [Google Scholar]
- 23.Torres RM, Flaswinkel H, Reth M, Rajewsky K. Aberrant B cell development and immune response in mice with a compromised BCR complex. Science (Wash DC) 1996;272:1804–1808. doi: 10.1126/science.272.5269.1804. [DOI] [PubMed] [Google Scholar]
- 24.Williams GT, Dariavach P, Venkitaraman AR, Gilmore DJ, Neuberger MS. Membrane immunoglobulin without sheath or anchor. Mol Immunol. 1993;30:1427–1432. doi: 10.1016/0161-5890(93)90104-j. [DOI] [PubMed] [Google Scholar]
- 25.Cook GP, Meyer KB, Neuberger MS, Pettersson S. Regulated activity of the IgH intron enhancer (Eμ) in the T lymphocyte lineage. Int Immunol. 1995;7:89–95. doi: 10.1093/intimm/7.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature (Lond) 1990;343:760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- 27.Venkitaraman AR, Williams GT, Dariavach P, Neuberger MS. The B-cell antigen receptor of the five immunoglobulin classes. Nature (Lond) 1991;352:777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- 28.Gong S, Nussenzweig MC. Regulation of an early developmental checkpoint in the B cell pathway by Igα. Science (Wash DC) 1996;272:411–414. doi: 10.1126/science.272.5260.411. [DOI] [PubMed] [Google Scholar]
- 29.Luisiri P, Lee YJ, Eisfelder BJ, Clark MR. Cooperativity and segregation of function within the Ig-α/β heterodimer of the B cell antigen receptor complex. J Biol Chem. 1996;271:5158–5163. doi: 10.1074/jbc.271.9.5158. [DOI] [PubMed] [Google Scholar]