Abstract
Galectin-1, a β-galactoside binding protein, is produced by thymic epithelial cells and binds to human thymocytes. We have previously reported that galectin-1 induces the apoptosis of activated T lymphocytes. Because the majority of thymocytes die via apoptosis while still within the thymus, we tested whether galectin-1 could induce the apoptosis of these cells. We now report that in vitro exposure to galectin-1 induced apoptosis of two subsets of CD4lo CD8lo thymocytes. The phenotypes of susceptible thymocytes were consistent with that of both negatively selected and nonselected cells. Galectin-1–induced apoptosis was enhanced by preexposure of thymocytes to antibody to CD3, suggesting that galectin-1 may be a participant in T-cell– receptor mediated apoptosis. In contrast, pretreatment of thymocytes with dexamethasone had no effect on galectin-1 susceptibility. We noted that 71% of the cells undergoing apoptosis after galectin-1 treatment had a DNA content greater than 2N, indicating that proliferating thymocytes were most sensitive to galectin-1. We propose that galectin-1 plays a role in the apoptosis of both negatively selected and nonselected thymocytes, and that the susceptibility of thymocytes to galectin-1 is regulated, in part, by entry or exit from the cell cycle.
During T cell maturation in the thymus, only a fraction of thymocytes are selected to survive and ultimately migrate out of the thymus as naïve mature T lymphocytes. The maturation pathway that results in thymocyte survival is termed positive selection (1). Thymic epithelial cells, which express MHC molecules, mediate positive selection. Thymocytes will undergo positive selection when they express a TCR, which can bind with low avidity to self-class I or class II (MHC) (1). During positive selection, thymocytes convert from a CD4+CD8+, double positive (DP)1, phenotype to a CD4+ or a CD8+ single positive (SP), phenotype. In addition thymocytes become CD3hi, CD5hi, and CD69+ during positive selection (2, 3).
The majority of thymocytes will not undergo positive selection, but will die by apoptosis within the thymus (4). Thymocytes that express a functional TCR but are potentially autoreactive will die as a result of negative selection. After negative selection, thymocytes express intermediate levels of CD3 (CD3int) and low levels of CD4 and CD8 (CD4lo, CD8lo) immediately before undergoing apoptosis (5, 6, 7). Thymocytes that do not express a functional TCR cannot undergo positive or negative selection. This nonselected population also dies by apoptosis (4).
Thymocyte maturation requires the participation of thymic epithelial cells and extracellular matrix components (8, 9, 10). We reported the expression of galectin-1, a β-galactoside binding protein, by human thymic epithelial (TE) cells in vivo and in vitro. We further demonstrated that galectin-1 bound to thymocytes and mediated the adhesion of thymocytes to TE cells (11). Recently, we have found that galectin-1 can induce apoptosis of activated T lymphocytes (12).
Galectin-1 is a member of a family of animal lectins that share structural similarities within the carbohydrate binding domain (13, 14). Galectins are also referred to as S-type lectins because these molecules require reduced thiol groups to maintain carbohydrate binding activity. Galectin-1 exists as a homodimer of 14-kD subunits, is evolutionarily conserved, and developmentally regulated. The preferred carbohydrate ligand for galectin-1 is lactosamine (Gal-β1, 4GlcNAc), which can be present on many glycoprotein counterreceptors (14). A number of glycoproteins have been identified that bind to galectin-1, including laminin (15), fibronectin (16), lysosome-associated membrane proteins (LAMPS) (17), and the hematopoietic cell surface membrane proteins CD45 and CD43 (18). Several laboratories have ascribed growth regulatory (19, 20) and/or immunomodulatory activities (21, 22) to galectin-1.
Because galectin-1 is abundant within normal thymus and can mediate the apoptosis of activated T lymphocytes, we undertook this study to determine whether galectin-1 could mediate the apoptosis of human thymocytes. We report that galectin-1 induced the apoptosis of two distinct populations of immature thymocytes. Our data is consistent with a role for galectin-1 in the induction of apoptosis of negatively selected and nonselected thymocytes.
Materials and Methods
Preparation of Recombinant Human Galectin-1.
Galectin-1 was prepared by the method of Couraud et al. (23) in Escherichia coli strain BL21 (DE3) transformed with the expression vector pT7IML-1 (gift of Incyte Pharmaceuticals, Inc., Palo Alto, CA).
Cell Culture and Galectin-1 Treatment.
Human thymocytes were isolated from surgical specimens and passaged over nylon wool as previously described (24). Nylon wool passaged thymocytes were cultured overnight at 37°C in serum-free IMDM with 1% dialyzed BSA, and 85 μg/ml human transferrin (AT-IMDM). The following day, 106 cells in 0.2 ml of AT-IMDM, 1.1 mM dithiothreitol (DTT) were incubated for 5 h at 37°C with 20 μM recombinant, human galectin-1. DTT was added to maintain the carbohydrate binding activity of galectin-1. Samples were adjusted to 0.1 M β-lactose to dissociate aggregated thymocytes, washed in 10 mM phosphate buffer, pH 7.4, 140 mM NaCl (PBS), then either surface stained or fixed with 1% paraformaldehyde for terminal deoxynucleotidyl transferase–mediated dUTP biotin nick end-labeling (TUNEL) to detect apoptotic cells. Samples were surface stained as previously described (11). PE-conjugated CD3 antibody was used in addition to fluorescein-conjugated antibody to discriminate more easily between cells lacking CD3 expression (CD3−) and cells expressing low levels of CD3 (CD3lo) (10). The following mouse anti–human antibodies, directly conjugated to fluorochromes were used: CD3–FITC, CD4–PE, and CD8–peridinin chlorophyll protein (PerCp; Becton Dickinson, San Jose, CA), CD3–PE, CD8–FITC, and CD69–PE (Caltag, South San Francisco, CA). Isotype-matched controls were included for all reagents. After surface staining and washing, cells were either immediately subjected to flow cytometry, or fixed in 1% paraformaldehyde and stored at 4°C for flow cytometry, which was performed within 24 h. All flow cytometry was performed using a Becton Dickinson FACScan® with analysis using Lysis II\xa9 or CELLQuest\xa9 software.
Dexamethasone and CD3 Antibody Pretreatment.
Thymocytes (2.5 × 106 cells/ml) were incubated with 2 μg/ml of soluble antibody to CD3ε (UCHT-1, Dako Corp., Carpinteria, CA), with 20 μM dexamethasone (Calbiochem, La Jolla, CA), or with medium alone at 37°C. After 16 h, galectin-1 or buffer was added. The samples were then incubated, stained, and fixed as described above.
TUNEL Labeling and Assessment of DNA Content.
DNA content and apoptosis were assessed by TUNEL using a modification of the method of Gorczyca (25). Paraformaldehyde-fixed cells were washed in PBS, resuspended in cold (−20°C) 80% ethanol and kept on ice for 30 min. Cells were washed in PBS, then incubated at 37°C for 1 h with terminal deoxytransferase (TdT, 120 U/ml; Promega Corporation, Madison, WI), 1× TdT reaction buffer (provided with enzyme), and biotin–16-2′-deoxyuridine5′-triphosphate (biotin–dUTP, 10 nmol/ml; Boehringer Mannheim, Indianapolis, IN) in a total volume of 50 μl. After washing, incorporated biotin–dUTP was stained with streptavidin–FITC (10 μg/ml, Boehringer Mannheim) in PBS with 1% BSA, 0.05% Triton X-100, and 0.01% NaN3 for 1 h at room temperature. Samples were washed in the same buffer, then in PBS, 1% BSA, and stained for DNA content with 1 μg/ml 7-aminoactinomycin D (7-AAD; Molecular Probes, Eugene, OR), or propidium iodide (PI, Calbiochem, Inc., San Diego, CA) with 0.01% RNAase A, 30 min before flow cytometry was performed. Control samples were treated as above, except the TdT enzyme was omitted.
Results
Galectin-1–induced Apoptosis of Human Thymocytes.
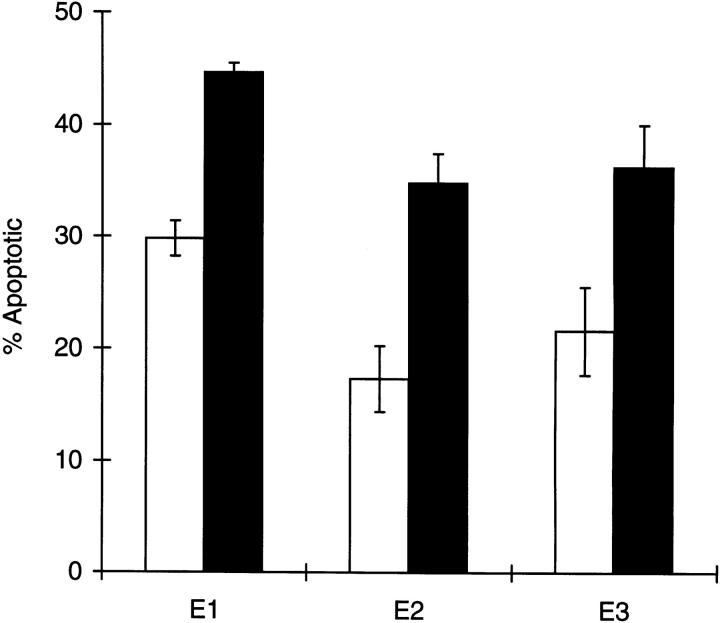
We incubated nylon wool passaged thymocytes with and without galectin-1. Galectin-1 induced apoptosis of a significant fraction of thymocytes (Fig. 1). In galectin-1–treated samples, 37 ± 5.9% (SEM) of the cells were apoptotic with a range of 21–41%. In control samples, treated with ATIMDM, 1.1 mM DTT, an average of 22.2 ± 5.9% of the cells (range, 11–33.5%) were apoptotic. Thus, galectin-1 treatment consistently induced apoptosis of ∼15% of the total thymocyte population.
Figure 1.
Galectin-1 induces apoptosis of thymocytes. Thymocytes were treated with 1.1 mM DTT (open bars), or with 20 μM galectin-1, 1.1 mM DTT (closed bars) in AT-IMDM medium for 5 h at 37° C. The percentage of apoptotic cells in each sample was determined by flow cytometric analysis after TUNEL labeling. The results shown are duplicate determinations from three experiments (E1–E3) ± SD.
To determine the stage of differentiation at which thymocytes were most susceptible to galectin-1, we examined the viable cells in galectin-1–treated and control samples for expression of a number of cell surface markers. We observed that galectin-1 treatment eliminated a fraction of the CD3− population, as indicated by comparing absolute numbers of cells within the region defined by M1 in Fig. 2 A. We also consistently saw a decrease in the population expressing intermediate levels of CD3 (CD3int, Fig. 2 A; M3). The same samples were stained by three-color staining using CD3–FITC, CD4–PE, and CD8–PerCP. The results shown in Fig. 2 B indicate that galectin-1 selectively eliminated a subset of the CD4+ CD8+ double-positive (DP) cells that were dim for both markers. CD3 staining in these samples (data not shown) was consistent with that shown in Fig. 2, A and C. By comparing absolute numbers of cells in control and galectin-1–treated samples, we determined that none of the CD4−CD8− double-negative (DN) cells were lost (data not shown). This indicates that the CD3− cells that were susceptible to galectin-1 were expressing CD4 and CD8, and were not in the triple negative fraction. Galectin-1 also eliminated thymocytes from the CD5lo (data not shown) and CD69− (Fig. 2 C) populations. In summary, the thymocyte populations most susceptible to galectin-1–induced apoptosis were CD3int or CD3− and CD4loCD8loCD5loCD69−.
Figure 2.
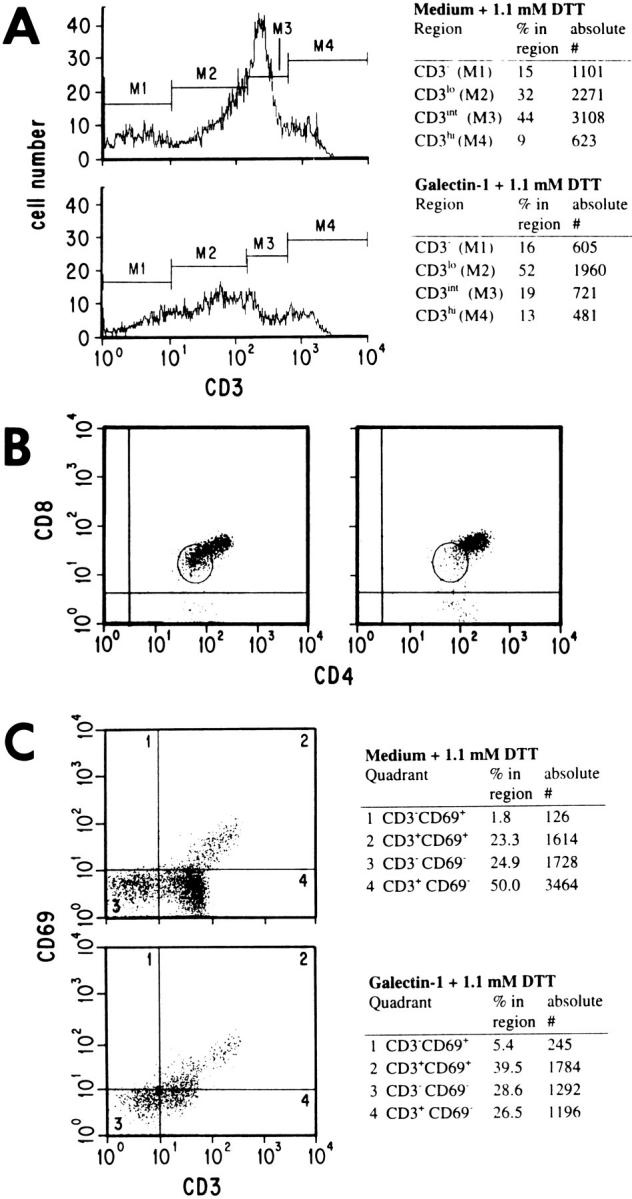
Distinct subsets of immature thymocytes were susceptible to galectin-1. Cells treated with galectin-1 or DTT only were surface stained for CD3–PE (A), CD4–PE and CD8–PerCP (B), or CD3–FITC and CD69–PE (C). The population within the region circled in B indicates the CD4loCD8lo population, which comprised 41.2% of the DP cells in the control sample (left) and 17.3% of the DP cells after galectin-1 treatment (right). In A and C absolute numbers of cells and the percentage in each marker region or quadrant are indicated in the tables to the right of the figures. 10,000 events (live plus dead cells) were acquired for each sample in A, and C, and 5,000 events for each sample in B. Nonviable cells were excluded from the data shown, by selective gating based on light scatter profiles. In B and C, appropriate isotype control antibodies were used to set the cursors.
The Effects of Dexamethasone or CD3 Stimulation on Galectin-1 Susceptibility.
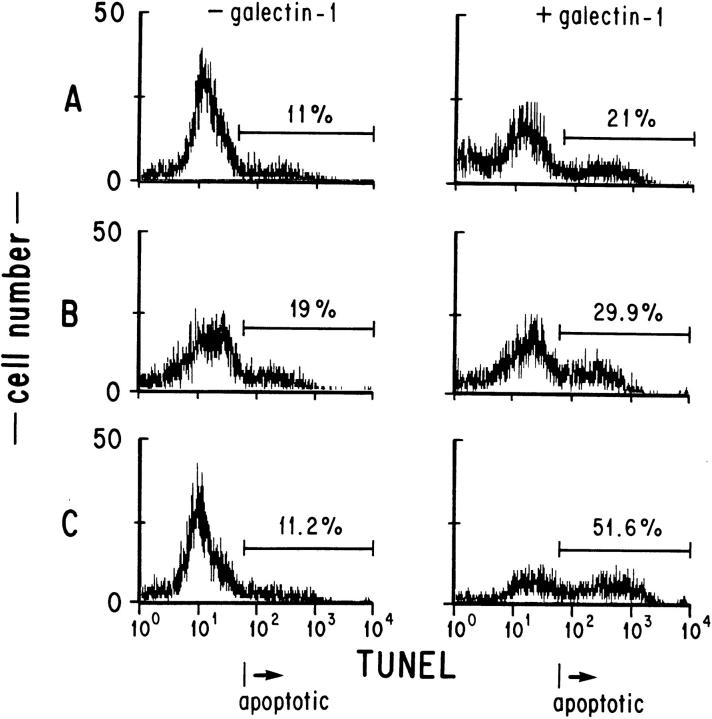
The apoptotic effects of CD3 stimulation and steroids antagonize each other when the treatments are given simultaneously (26, 27). To determine whether galectin-1 induces apoptosis via a pathway that interacts or overlaps with that involved in apoptosis induced by steroids or CD3 antibody, we pretreated thymocytes for 16 h with dexamethasone, or with CD3 antibody before exposure to galectin-1 (Fig. 3). As expected, the level of apoptosis in dexamethasone pretreated samples was higher than in the nontreated controls (19% and 11%, respectively). However, after correcting for background apoptosis, dexamethasone pretreatment did not alter the fraction of the total population that was susceptible to galectin-1 (10% as compared with 10.9% in controls). These data indicate that galectin-1–induced apoptosis of thymocytes was independent of steroid pretreatment.
Figure 3.
Galectin-1–induced apoptosis of thymocytes was enhanced by preincubation with CD3 antibody, but not by preincubation with dexamethasone. Thymocytes were preincubated with medium (A), 20 μM dexamethasone (B), or with 1 μg/ml CD3 antibodies (C) for 16 h before a 5-h exposure to DTT alone, or to galectin-1 plus DTT. The percentage of apoptotic cells was determined by flow cytometry after TUNEL staining. The results shown are representative of duplicate determinations.
In contrast, pretreatment of the thymocytes with soluble antibody to CD3 did not alter the background level of apoptosis, but did result in a fourfold increase in the fraction of cells susceptible to galectin-1–induced apoptosis (40% above control levels as compared with 10% without CD3 antibody pretreatment). These results demonstrate that engagement of the TCR with CD3 antibodies before galectin-1 exposure increased the sensitivity of thymocytes to galectin-1.
Proliferating Cells Are Most Sensitive to Galectin-1.
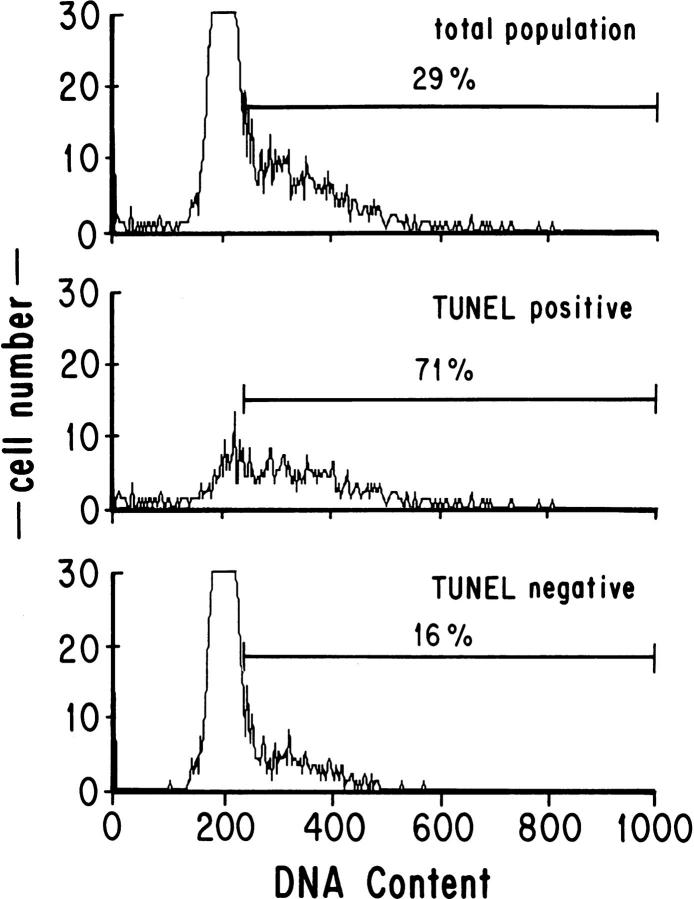
Previous results from our laboratory showed that galectin-1 induces apoptosis of peripheral T cells that are activated and proliferating, whereas resting peripheral T cells are not susceptible to galectin-1–induced apoptosis (12). Others have reported cell cycle–dependent differences in the susceptibility of cells to apoptosis in a number of systems (25, 28, 29, 30). To determine at which stage(s) of the cell cycle thymocytes were undergoing apoptosis, we examined the DNA content of galectin-1–treated and –untreated thymocytes. By selective gating of TUNEL labeled thymocytes, we found that in untreated samples, the majority of apoptotic thymocytes (74%) had a DNA content greater than 2N, characteristic of S, G2, or M phase cells (data not shown). When a greater fraction of the total population was induced to undergo apoptosis by galectin-1, a comparable percentage (71%, Fig. 4) of the TUNEL-positive (apoptotic) cells had a DNA content greater than 2N. In contrast, 29% of the total sample and 16% of the TUNEL-negative cells had a DNA content greater than 2N (Fig. 4). DNA content was not altered by the TUNEL labeling procedure (data not shown). This observation strongly suggests that thymocytes that are progressing through the cell cycle are most sensitive to apoptosis and most susceptible to galectin-1.
Figure 4.
Cells in S, G2, or M (with a DNA content greater than 2N) were most susceptible to apoptosis mediated by galectin-1. Thymocytes were treated with galectin-1, then stained for apoptosis by nick end-labeling (TUNEL) and for DNA content with propidium iodide. Apoptotic cells are TUNEL positive. The numbers given indicate the percentage of each population with a DNA content greater than 2N and were determined using the marker as indicated.
Discussion
We have investigated the effects of galectin-1 on thymocyte survival and have examined the surface phenotype of thymocyte populations before and after galectin-1 treatment. Our results indicate that, after correction for background apoptosis, exogenous galectin-1 induced apoptosis of 15% of human thymocytes (Fig. 1). This is a significant fraction when one considers that the thymocytes were exposed to galectin-1 in vivo less than 48 h before use in this study. Additionally, we found that the susceptible thymocytes were all of an immature phenotype.
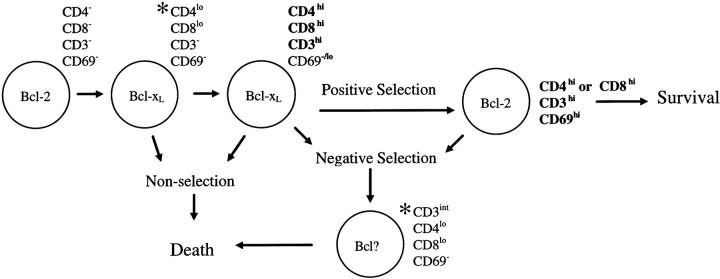
Susceptibility to galectin-1 correlated with a number of events that occur during thymocyte differentiation. The phenotype of galectin-1–sensitive cells and how galectin-1 susceptibility relates to thymocyte differentiation is shown schematically in Fig. 5. Examination of the surface phenotype of thymocytes before and after galectin-1 treatment suggests galectin-1 may be involved in apoptosis of both nonselected and negatively selected thymocytes.
Figure 5.
Model indicating galectin-1–sensitive populations within the human thymus. Asterisk indicates a galectin-1–sensitive phenotype.
There were primarily two galectin-1–susceptible populations as determined by flow cytometry. One of the two populations was CD3−CD4loCD8loCD69− (Fig. 2). The expression of CD4 and CD8 normally occurs during thymocyte ontogeny subsequent to the expression of the TCR β chain (31, 32). Therefore, those DP-susceptible thymocytes that were detected as CD3− by flow cytometry had already attempted TCR rearrangement. The population that carries this surface phenotype has been shown to express the intracellular regulator of apoptosis, Bcl-xL, but not Bcl-2 (33, 34). Within this group may be cells that express the pre-T α chain but have unsuccessfully rearranged a TCR β chain (35). Because this population did not express detectable levels of surface CD3, these cells could not undergo negative selection. It is reasonable to propose that these cells represent the nonselected population, i.e., those thymocytes that fail to express a functional TCR and die because they are not positively selected. Our observation that DP, CD3− thymocytes are sensitive to galectin-1 suggests a role for galectin-1 in apoptosis due to nonselection.
The second susceptible population was CD3intCD4lo CD8loCD69−. This same phenotype precedes apoptosis after negative selection (5). This population may also include thymocytes that are nonselected because they express a nonfunctional TCR. Although the viable CD69+ population was not reduced by galectin-1 treatment, 58% of the apoptotic cells were found to express low levels of CD69 (CD69lo, data not shown). This fraction of CD69lo apoptotic thymocytes is similar to that observed by Kersh and Hedrick (5) for murine thymocytes undergoing negative selection. Our data suggest that a fraction of the CD69− thymocytes became CD69lo upon treatment with galectin-1. This issue warrants further investigation.
Treatment of thymocytes in vitro with antibody specific for the CD3ε chain mimics antigen stimulation and is used as a model for negative selection (36). CD3 antibody treatment of thymocytes increases the CD3intCD4loCD8lo population, as does antigen engagement during negative selection (5, 6). Using in vitro models, specific peptide and MHC binding or CD3 antibody stimulation alone was not sufficient to induce apoptosis, and a second, unknown, signal was required as well (6, 7). This second signal can be delivered by some antigen-presenting cell lines, thymic epithelial cells, or dendritic cells (6, 7). Previous attempts to identify a molecule that mediates this second signal have yielded conflicting results (6, 36, 37). Galectin-1 is potentially a candidate molecule as a provider of a second (apoptotic) signal needed for negative selection. Our observations that CD3intCD4loCD8lo cells were eliminated by galectin-1 treatment and that galectin-1 susceptibility was enhanced by CD3 engagement (Fig. 3) support this hypothesis, and suggest a role for galectin-1 in negative selection.
If galectin-1 induces apoptosis after negative selection, then thymic epithelial (TE) cells may participate in negative selection to a greater extent than has previously been proposed. It is known that TE cells synthesize galectin-1 (11), can induce apoptosis of T cells (7, 38) and can mediate negative selection (39, 40). In our laboratory, we observed that 53% of MOLT-4 cells that bound to TE cells on glass cover slips were apoptotic after 4 h at 37°C, whereas only 7% of CEM cells bound to TE cells were apoptotic (data not shown). This is intriguing in light of our previous observation that MOLT-4 cells are sensitive to galectin-1–induced apoptosis, whereas CEM cells are not (12). Although TE cells can mediate negative selection, dendritic cells have been reported to do this more efficiently (2). If galectin-1 is required for negative selection, one would predict that dendritic cells may also synthesize galectin-1, or else acquire it from the extracellular milieu. This remains to be investigated.
Three distinct pathways leading to apoptosis of thymocytes have been identified (41). The first requires the expression of the tumor suppressor protein p53 and can be induced by DNA damage. The other two pathways, one induced by steroid treatment, the other by TCR engagement, are p53 independent (41–43). It has been reported that DP thymocytes do not express p53 (31). Our data showing that only DP thymocytes are susceptible to galectin-1 suggest that galectin-1 also induces apoptosis by a p53 independent pathway.
Galectin-1 may be a component of TCR-mediated apoptosis. Ligation of CD3 primed thymocytes for galectin1–induced apoptosis and resulted in an increased fraction of cells that were susceptible to galectin-1 (Fig. 3). This data is similar to that found using murine thymocytes treated with CD3 antibody and galectin-1 (Vespa, G.N.R., and M.C. Miceli, manuscript in preparation). This synergism between CD3 antibody and galectin-1 suggests that galectin-1 may be a normal component in the process by which TCR engagement induces apoptosis. Engagement of the TCR, as occurs during negative selection, may induce intracellular expression of apoptotic machinery, which is then activated by galectin-1. This hypothesis is supported by the kinetics of CD3-mediated apoptosis, which is slow (10–18 h) when compared with galectin-1–induced apoptosis, which occurs rapidly (1–4 h).
Galectin-1–induced apoptosis was independent of that induced by steroids. In contrast with the effects of CD3 antibody pretreatment, we observed no effect of dexamethasone pretreatment on galectin-1 susceptibility (Fig. 3). Our observation that dexamethasone neither antagonizes, nor augments galectin-1–mediated apoptosis indicates that the mechanisms leading to steroid and galectin-1–mediated apoptosis can operate independently.
Galectin-1 susceptibility may be regulated by entry of a vulnerable cell into the cell cycle. We observed that galectin-1 preferentially kills proliferating cells. This observation is consistent with a previous proposal that the susceptibility of thymocytes to apoptosis is controlled by entry or exit from the cell cycle (44). We found that only two subsets of the DP thymocytes were susceptible to galectin-1 (Fig. 2). Whereas the majority of DP thymocytes are not dividing, 10–15% of the total thymocyte population is composed of DP cells that are undergoing cell division (45, 46). This number corresponds to the average fraction of thymocytes that we found were susceptible to apoptosis by galectin-1 (15%). Thus, the previously reported size of the population of proliferating DP thymocytes is sufficient to account for the fraction of galectin-1–sensitive thymocytes that we observed.
Entry into the cell cycle is unlikely to be the only factor that determines galectin-1 susceptibility. Thymocytes, which are in direct contact with TE cells during maturation, may be in continuous contact with galectin-1 in vivo (11). However, proliferating thymocytes do not all undergo apoptosis simultaneously in vivo or in vitro. A number of additional factors potentially influence the response of thymocytes to galectin-1. These factors include (a) the expression of specific cell surface glycoprotein counter receptors recognized by galectin-1; (b) the glycosylation of those specific counter receptors; (c) the expression of Bcl-2 family members; and (d) the presence of intracellular apoptotic machinery. The latter may be influenced by TCR engagement, which occurs during negative and positive selection. All of these factors would affect galectin-1 binding and signal transduction, and most likely combine to determine the susceptibility of specific thymocyte populations to galectin-1. It is important to note that lactosamine, the carbohydrate ligand for galectin-1, can be found in varying amounts on more than one cell surface glycoprotein counter receptor. In addition, the dimeric nature of galectin-1 would permit intermolecular and intramolecular cross-linking of cell surface glycoproteins. Thus, a response to galectin-1 may require the expression of more than a single glycoprotein species on the cell surface.
Based on the data presented above and the constitutive expression of galectin-1 in normal thymus, we hypothesize that galectin-1 is a participant in the elimination of nonselected and possibly negatively selected cells during normal thymocyte maturation. A galectin-1 knock out mouse has been generated (47). This model may be useful for further investigation of the role of galectin-1 in thymocyte development.
Acknowledgments
We wish to thank Drs. H. Spits, M.C. Miceli, S.M. Hedrick, and L. Goodglick for critical review of the manuscript. We also wish to thank Drs. M.C. Miceli and G.N.R. Vespa for very helpful discussions. We are grateful to the staff of the Jonsson Cancer Center Flow Cytometry core laboratory for technical assistance.
This work was supported by training grant no. CA09056 from USPHS to N.L. Perillo, by National Institutes of Health grant no. AG104015 through the Claude D. Pepper Older American Independence Center, and grants from the Concern Foundation and the University of California Cancer Research Coordinating Committee to L.G. Baum, and by National Institutes of Health grant no. HD29341 to C.H. Uittenbogaart.
Footnotes
1 Abbreviations used in this paper: 7-AAD, 7-amino actinomycin D; DP, double positive; DN, double negative; DTT, dithiothreitol; LAMPS, lysosome associated membrane proteins; PI, propidium iodide; SP, single positive; TdT, terminal deoxytransferase; TE, thymic epithelial; TUNEL, terminal deoxynucleotidyl transferase–mediated dUTP nick end-labeling.
References
- 1.von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 2.Jenkinson EJ, Anderson G, Owen JJT. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamashita I, Nagata T, Tada T, Nakayama T. CD69 cell surface expression identifies developing thymocytes which audition for T cell antigen receptor–mediated positive selection. Int Immunol. 1993;5:1139–1150. doi: 10.1093/intimm/5.9.1139. [DOI] [PubMed] [Google Scholar]
- 4.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature (Lond) 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 5.Kersh GJ, Hedrick SM. Role of TCR specificity in CD4 versus CD8 lineage commitment. J Immunol. 1995;154:1057–1068. [PubMed] [Google Scholar]
- 6.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+thymocytes. J Immunol. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 7.Hiramine C, Hojo K, Koseto M, Nakagawa T, Mukasa A. Establishment of a murine thymic epithelial cell line capable of inducing both thymic nurse cell formation and thymocyte apoptosis. Lab Invest. 1990;62:41–54. [PubMed] [Google Scholar]
- 8.Anderson G, Owen JJT, Moore NC, Jenkinson EJ. Thymic epithelial cells provide unique signals for positive selection of CD4+CD8+thymocyte in vitro. J Exp Med. 1994;179:2027–2031. doi: 10.1084/jem.179.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson G, Moore NC, Owen JJT, Jenkinson EJ. Cellular interactions in thymocyte development. Annu Rev Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- 10.Galy A, Verma S, Barcena A, Spits H. Precursors of CD3+CD4+CD8+cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymic development. J Exp Med. 1993;178:391–401. doi: 10.1084/jem.178.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart C, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med. 1995;181:877–887. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perillo NL, Pace KE, Seilhamer JJ, Baum LG. Apoptosis of T lymphoid cells mediated by galectin-1. Nature (Lond) 1995;378:736–739. doi: 10.1038/378736a0. [DOI] [PubMed] [Google Scholar]
- 13.Barondes WH, Cooper DNW, Gitt MA, Leffler H. Galectins: structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807–20810. [PubMed] [Google Scholar]
- 14.Leffler H, Barondes SH. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian β-galactosides. J Biol Chem. 1986;261:10119–10126. [PubMed] [Google Scholar]
- 15.Zhou Q, Cummings RD. The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch Biochem Biophys. 1990;281:27–35. doi: 10.1016/0003-9861(90)90408-q. [DOI] [PubMed] [Google Scholar]
- 16.Ozeki Y, Matsui T, Yamamoto Y, Funahashi M, Hamako J, Titani K. Tissue fibronectin is an endogenous ligand for galectin-1. Glycobiology. 1995;5:255–261. doi: 10.1093/glycob/5.2.255. [DOI] [PubMed] [Google Scholar]
- 17.Do K-Y, Smith DF, Cummings RD. Lamp-1 in CHO cells is a primary carrier of poly-N-acetyllactosamine chains and is bound preferentially by a mammalian S-type lectin. Biochem Biophys Res Comm. 1990;173:1123–1128. doi: 10.1016/s0006-291x(05)80902-7. [DOI] [PubMed] [Google Scholar]
- 18.Pace, K., and L. Baum. 1996. Identification and characterization of T cell surface counter-receptors for galectin-1. Glycobiology. 6:745. (Abstr.)
- 19.Wells V, Mallucci L. Identification of an autocrine negative growth factor: mouse β-galactoside-binding protein is a cytostatic factor and cell growth regulator. Cell. 1991;64:91–97. doi: 10.1016/0092-8674(91)90211-g. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka L, Ohno S, Kawasaki H, Suzuki K. Overexpression of a β-galactoside binding protein causes transformation of BALB3T3 fibroblast cells. Biochem Biophys Res Comm. 1991;179:272–279. doi: 10.1016/0006-291x(91)91365-j. [DOI] [PubMed] [Google Scholar]
- 21.Levi G, Tarrab-Hazdai R, Teichberg VI. Prevention and therapy with electrolectin of experimental autoimmune myasthenia gravis in rabbits. Eur J Immunol. 1983;13:500–507. doi: 10.1002/eji.1830130613. [DOI] [PubMed] [Google Scholar]
- 22.Offner H, Celnik B, Bringman TS, Casentini-Borocz D, Nedwin GE, Vandenbark AA. Recombinant human β-galactoside binding lectin suppresses clinical and histological signs of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1990;28:177–184. doi: 10.1016/0165-5728(90)90032-i. [DOI] [PubMed] [Google Scholar]
- 23.Couraud P-O, Casentini-Borocz D, Bringman TS, Griffith J, McGrogan M, Nedwin GE. Molecular cloning, characterization and expression of a human 14 kDa lectin. J Biol Chem. 1989;264:1310–1316. [PubMed] [Google Scholar]
- 24.Vollger LW, Uittenbogaart CH. Interleukin-7 promotes the generation of phenotypically mature CD45RA positive human thymocytes in vitro. Cytokine. 1993;5:157–168. doi: 10.1016/1043-4666(93)90055-a. [DOI] [PubMed] [Google Scholar]
- 25.Gorczyca W, Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. The cell cycle related differences in susceptibility of HL-60 cells to apoptosis induced by various antitumor agents. Cancer Res. 1993;53:3186–3192. [PubMed] [Google Scholar]
- 26.Zacharchuk CM, Mercep M, Chakraborti P, Simons SS, Jr, Ashwell JD. Programmed T lymphocyte death: cell activation and steroid-induced pathways are mutually antagonistic. J Immunol. 1990;145:4037–4045. [PubMed] [Google Scholar]
- 27.Iwata M, Hanaoka S, Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991;21:643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- 28.Cotter PG, Lennon SV, Glynn JG, Martin SJ. Cell death via apoptosis and its relationship to growth, development and differentiation of both tumour and normal cells. Cancer Res. 1990;10:1153–1160. [PubMed] [Google Scholar]
- 29.Dou QP, An B, Will PL. Induction of a retinoblastoma phosphatase activity by anticancer drugs accompanies p53-independent G1 arrest and apoptosis. Proc Natl Acad Sci USA. 1995;92:9019–9023. doi: 10.1073/pnas.92.20.9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: BCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang DM, Lenardo J, Zuniga-Pflucker JC. p53 prevents maturation to the CD4+CD8+stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J Exp Med. 1996;183:1923–1928. doi: 10.1084/jem.183.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkai Y, Koyasu L, Nakayama K-I, Murphy KM, Loh DY, Reinherz EL, Alt FW. Restoration of T cell development in RAG-2–deficient mice by functional TCR transgenes. Science (Wash DC) 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 33.Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. J Immunol. 1993;151:2546–2554. [PubMed] [Google Scholar]
- 34.Ma A, Pena JC, Chang B, Margosian E, Davidson L, Alt FW, Thompson CB. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramiro AR, Trigueros C, Marquez C, San JL, Millan, Toribio ML. Regulation of pre-T cell receptor (pTα-TCRβ) gene expression during human thymic development. J Exp Med. 1996;184:519–530. doi: 10.1084/jem.184.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Page DM, Kane LP, Onami TM, Hedrick SM. Cellular and biochemical requirements for thymocyte negative selection. Semin Immunol. 1996;8:69–82. doi: 10.1006/smim.1996.0010. [DOI] [PubMed] [Google Scholar]
- 37.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown KM, Spirito S, Basch RA. Thymic stromal cells in culture: Establishment and characterization of a line which is cytotoxic for normal thymocytes and produces hematopoietic growth factor(s) Cell Immunol. 1991;134:442–457. doi: 10.1016/0008-8749(91)90316-4. [DOI] [PubMed] [Google Scholar]
- 39.Pircher H, Brduscha K, Steinhoff U, Kasai M, Mizuochi T, Zinkernagel RM, Hengartner H, Kyewski B, Muller K-P. Tolerance induction by clonal deletion of CD4+8+thymocytes in vitro does not require dedicated antigen-presenting cells. Eur J Immunol. 1993;23:669–674. doi: 10.1002/eji.1830230315. [DOI] [PubMed] [Google Scholar]
- 40.Robey E, Fowlkes BJ. Selective events in T cell development. Annu Rev Immunol. 1994;12:675–705. doi: 10.1146/annurev.iy.12.040194.003331. [DOI] [PubMed] [Google Scholar]
- 41.Lane DP. A death in the life of p53. Nature (Lond) 1993;362:786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- 42.Lowe SW, Schmitt SM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature (Lond) 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 43.Clarke AR, Purdie DA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature (Lond) 1993;362:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 44.Pilarski LM. Adhesive interaction in thymic development: does selective expression of CD45 isoforms promote stage-specific microclustering in the assembly of functional adhesive complexes on differentiating T lineage lymphocytes? . Immunol Cell Biol. 1993;71:59–69. doi: 10.1038/icb.1993.6. [DOI] [PubMed] [Google Scholar]
- 45.Scollay, R., and D.I. Godfrey. 1995. Thymic emigration: conveyor belts or lucky dips? Immunol. Today. 16:268–273. [DOI] [PubMed]
- 46.Alvarez-Vallina L, Gonzalez A, Gambon R, Kreisler M, Diaz-Espada R. Delimitation of the proliferative stages in the human thymus indicates that cell expansion occurs before the expression of CD3 (T cell receptor) J Immunol. 1993;150:8–16. [PubMed] [Google Scholar]
- 47.Poirier F, Robertson EJ. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development. 1993;119:1229–1236. doi: 10.1242/dev.119.4.1229. [DOI] [PubMed] [Google Scholar]