Abstract
T helper cell (Th) 1, but not Th2, effectors undergo rapid Fas/Fas ligand (FasL)-mediated, activation-induced cell death upon restimulation with antigen. Unequal apoptosis is also observed without restimulation, after a longer lag period. Both effectors undergo delayed apoptosis induced by a non–Fas-mediated pathway. When Th1 and Th2 effectors are co-cultured, Th2 effectors survive preferentially, suggesting the responsible factor(s) is intrinsic to each population. Both Th1 and Th2 effectors express Fas and FasL, but only Th2 effectors express high levels of FAP-1, a Fas-associated phosphatase that may act to inhibit Fas signaling. The rapid death of Th1 effectors leading to selective Th2 survival provides a novel mechanism for differential regulation of the two subsets.
Activation-induced cell death (AICD)1 in CD4+ Th has been widely studied. It is the mechanism of peripheral deletion of CD4+ cells and is implicated in the loss of effectors during infectious diseases, especially those caused by viruses (1, 2). The antigen-induced deletion of Th is often accompanied by an imbalance in Th1 and Th2. HIV infection leads to a progressive loss of Th1 by Fas/Fas ligand (FasL)–mediated AICD, whereas normal levels of Th2 cytokine production after stimulation are seen both early and very late in AIDS (3). Some models of acquired tolerance are also associated with selective loss of Th1 and persistence of Th2 (4, 5). The mechanisms underlying these imbalances in Th subsets remain unclear. Th2 predominance is also seen at high antigen doses in response to some model antigens and to some infectious antigens (6, 7). Th1 lines have been reported to be more susceptible to AICD than Th2 lines, but the susceptibility of T cell subsets obtained without prolonged culture is unclear (8).
During a primary response, resting naive cells are selected by a specific antigen to proliferate and differentiate into large, activated effector cells with the capacity to rapidly produce high titers of cytokines after restimulation (9). Effectors behave differently from naive cells. They are more easily triggered (10), they proliferate directly to cytokines, and they have been reported to be highly susceptible to AICD (11–13).
The pattern of cytokines produced by an effector population upon reencounter with antigen will in large part determine its function. The two most polarized patterns of cytokine production, Th1 (characterized by production of IL-2, IFN-γ, TNF-α, and TNF-β) and Th2 (production of IL-4, -5, -6, -10, and -13) were originally reported in long-term clones of CD4 T cells (14). Activated or memory T cell populations from animals usually secrete a broader variety of cytokines (9). However, dramatic polarization can be achieved by adding IFN-γ or IL-12 on one hand, or IL-4 on the other, to naive CD4 T cells responding to antigen in vitro, resulting in the respective generation of Th1 and Th2 effectors in 4–5 d of culture (15–17). The polarization of short-term lines is often very stable; polarized Th1 and Th2 effectors generated from the naive CD4 T cells derived from TCR transgenic (Tg) mice upon transfer to adoptive hosts, develop into resting memory cells that retain their polarization for up to a year without exposure to antigen (18).
Th1 and Th2 lines differ in their activation requirements (19, 20), in their abilities to be anergized (19, 20), and in their susceptibility to AICD (8). However, the reported distinctions in AICD have been seen predominantly in cell lines and not in populations derived from short-term cultures (8, 21, 22). Death of activated T cells is often mediated by interactions between Fas and FasL (8, 22–25), but an alternate pathway involving interaction of TNF-α with TNFR has also been described (26, 27). We previously reported that unstimulated Th2 effectors persist up to a week in culture in the absence of additional stimulation, and that even after restimulation with antigen, they did not undergo substantial levels of apoptosis until 4–5 d (13). This time course for AICD is much longer than those usually reported for bulk activated T cells, T cell lines (21, 22), or T cell hybridomas (23, 24).
To evaluate whether there are physiologically relevant functional differences in AICD between Th1- and Th2polarized T cells and to investigate the bases of such distinctions, we have compared well-polarized effectors generated by short-term culture from highly purified naive CD4 populations derived from TCR Tg mice. These populations are analogous to effectors generated in vivo in response to antigen (28) and they do not have the disadvantage of having been selected in vitro by multiple restimulation, which is likely to select for cells that are relatively resistant to cell death. Our results indicate that Th1, but not Th2, effectors undergo a rapid Fas/FasL-mediated AICD upon reactivation with antigen. This difference between Th1 and Th2 effectors may be responsible for the predominance of Th2 effectors in a number of disease states.
Material and Methods
Mice.
H-2b/k or H-2k/k Vβ3/Vα11 and TCR Tg mice (15) were used at 3–6 mo of age and bred in the animal facilities at University of California, San Diego (La Jolla, CA). C3H.HeJ (C3H), C3H.MRL-Faslpr (lpr), and sex- and age-matched C3H/HeJFasLgld (gld) mice were used at 6–12 wk (purchased from Jackson Labs., Bar Harbor, ME).
Reagents.
Medium for all in vitro cultures was complete RPMI supplemented with penicillin (200 μg/liter), streptomycin (200 μg/liter), and glutamine (4 μM) plus 10% selected FCS from Hyclone Labs. (Logan, UT). Pigeon cytochrome c fragment 88-104 (PCCF), used as antigen in all experiments, was synthesized by the Peptide Synthesis Facility (University of California, San Diego, La Jolla, CA) and purified by HPLC. Con A was from ICN ImmunoBiologicals (Lisle, IL). Recombinant IL-2 (human, 10–20 U/ml, from the Biological Response Modifiers Program, National Institutes of Health, Bethesda, MD) or an equivalent dose of murine IL-2 derived from the X63–IL-2 line transfected with the murine IL-2 gene were used interchangeably and had equivalent effects. Anti-Fas antibody (Jo2) or control antibody (hamster IgG) (both from PharMingen, La Jolla, CA) and fluorescein (FL)- conjugated goat anti–hamster IgG (Fab′)2 fraction (CALTAG, South San Francisco, CA) were used to detect Fas expression on the cell surface. Fas.Fc, a fusion protein of murine Fas and the Fc region of human IgG (23), was used to block the FasL/Fas-mediated interaction. Human IgG was used as a control in experiments using Fas.Fc. The vital fluorescent dyes 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes Inc., Eugene, OR; reference 29) and PKH26 (Sigma Chemical Co., St. Louis, MO) were used to stain and visualize effector populations which were then detected by FACScan® analysis (Becton Dickinson, San Jose, CA).
Cell Preparations.
Purified naive CD4+ T cells from Tg mice or from C3H, lpr, or gld mice were isolated as described previously (17). In brief, spleen cells were passed over nylon columns to remove B cells, adherent cells, and activated cells. Populations were then treated with anti-CD8 and anti-HSA (J11D) plus complement (C′). The purified cell populations obtained were >90% CD4+ resting cells of naive phenotype. APC for effector generation were prepared by T cell depletion of spleen cells with two anti-Thy1.2 Ab (F7D5 and HO13.14), anti-CD4 (RL172.4), and anti-CD8 (HO2.2 and AD4) plus C′ and treatment with 50 μg/ml of mitomycin C. For restimulation of effectors, DCEK-ICAM (intracellular adhesion molecule) cells, a fibroblast line transfected with I-Ek and ICAM-1 that also expresses high levels of B7.1 (10), were mitomycin C–treated and used as APC.
CD4 effectors were generated as previously reported (15, 18). Purified naive CD4+ cells (3 × 105/ml) from Tg mice were cultured with PCCF and APC, whereas CD4+ cells from non-Tg mice were stimulated with Con A (2 μg/ml) with APC. IL-2 and either IL-12 and anti-IL-4 (11B11, 10 μg/ml) for polarization to Th1 effectors, or IL-4 and anti-IFN-γ (XMG1.2, 10 μg/ml) for Th2 effectors, were added at the initiation of culture to achieve optimal growth and complete polarization (18). After 5 d, Th1 and Th2 effectors were harvested, and in some cases, low density cells were enriched by Percoll gradient separation. Effectors were restimulated at 5 × 105/ml with PCCF (5 μM) and APC (1.7 × 105/ml) (transgenics), or with Con A (2 μg/ml) and APC. According to the design of the experiment, recultured effector cells were harvested at different days, counted, and further analyzed for evidence of DNA damage or ability to be stimulated to produce cytokines.
Cytokine Analysis.
Titers of IL-2, IL-4, IL-5, and IFN-γ were determined from supernatants collected 18–24 h after restimulation of T cells as described previously (18, 28). In brief, IL-2 was measured by bioassay with the NK cell line. IL-4, IL-5, and IFN-γ levels were determined by an ELISA assay.
Labeling of Effector Cells with Fluorescent Dyes.
Th1 effectors were labeled with the vital dye CFSE as previously described (29). In brief, 5 × 107 Th1 effectors in 1 ml PBS were incubated with 1 mM CFSE at 37°C for 10 min. 10 ml of cold PBS was added to stop the reaction. Labeled cells were washed once and counted. Th2 effectors were labeled with PKH26. 2 × 107 Th2 effectors in 1 ml of diluent were incubated with 1 ml of 4 × 106 M PKH26 at 25°C for 2–5 min. 2 ml of serum was then added for 1 min to stop the staining reaction, followed by addition of 4 ml of medium. Labeled cells were washed three times and counted. Control experiments indicated effectors, and naive T cells stained with both dyes retained high and equal viability (Zhang, X., unpublished data). Labeled effector cells (Th1, Th2, or 1:1 mix) were then plated at 5 × 105/culture and restimulated as in other experiments. After 1 d, the entire culture was harvested, cell viability and recovery was determined, and the whole culture was subjected to FACS® analysis. In some experiments, parallel cultures were restimulated with PCCF and APC, and supernatants were collected 24 h later for determination of cytokine titers.
TUNEL Staining for Detection of Nicked DNA.
DNA damage was determined using a method adapted from Gorczyca et al. as described (30). Effectors were harvested at different times after restimulation, stained with rat anti–murine CD4 conjugated with PE (PE–anti-CD4), fixed in 1% formaldehyde, and then stored in 70% ethanol at 0–4°C. For analysis, cells were incubated at 106 in 50 ml of buffer containing 100 U/ml terminal deoxynucleotidyl transferase (TdT) and 10 mM biotin-16-dUTP (both from Boehringer Mannheim, Indianapolis, IN) followed by FL-streptavidin staining. Gated CD4+ cells were analyzed by FACScan® using Lysys II software. In some experiments, as indicated, only live CD4+ cells with high forward and low side scatter were analyzed.
Fas and FasL Detection.
Fas expression was assessed by staining. Effector cells (106) were incubated with either anti-Fas antibody (Jo2, PharMingen) or control antibody (hamster IgG), and then counterstained with FL goat anti–hamster IgG (Fab′)2 fraction. Cells were washed again and stained with PE–anti-CD4. Gated CD4+ cells were analyzed for Fas expression by FACScan®. For detection of cell surface expression of FasL, cells were stained with a direct PE-conjugated anti-FasL Ab (Kay-10; provided by PharMingen). As a control, cells were stained with a PE-conjugated, isotype-matched Ab not expected to specifically stain CD4 T cells (PE-conjugated anti–human CD8, Sigma Chemical Co.). Cells were counterstained as above with FL anti-CD4. Functional FasL expression was quantitated in a cytotoxicity assay by determining the ability of effector cells to cause DNA fragmentation in Fas-expressing target cells. The parental L1210 cells (Fas−) or the cells transfected with murine Fas (Fas+), gifts of Pierre Golstein (Institut National de la Santè et de la Recherche Mèdicale, Marseille, France), were labeled with 5 μCi [3H]thymidine at 106/ml for at least 2 h at 37°C and used as target cells. Target cells at 2 × 104 in 100 μl were cultured with 100 μl of appropriately diluted effector cells in each well of flat-bottom 96well plates with either coated anti-CD3 (2 μg/ml, overnight) or PCCF plus APC as stimuli. A1.1 cells, activated by anti-CD3, were used as a positive control (23). The plates were harvested using a Skatron cell harvester after 9 h incubation at 37 °C and [3H]thymidine-labeled unfragmented DNA was counted in a liquid scintillation counter. The percentage of DNA fragmentation was calculated with formula: % DNA fragmentation = 100 × (1 − cpm experimental group/cpm control group). Assays were performed in triplicate.
RNase Protection Assay for Fas, FasL, and Fas-associated Phosphatase 1 Messenger RNA.
RPA was performed using a commercial RPA kit (Ambion Inc., Austin, TX) according to the manufacturer's protocol. In brief, Fas, FasL (provided by P. Golstein, Institut National de la Santè et de la Recherche Mèdicale, Marseille, France, and S. Nagata, Osaka Bioscience Institute, Osaka, Japan, respectively), murine Fas-associated phosphatase 1 (FAP-1) cDNA (31), and β-actin cDNA (positive control), were cloned using pBluescript II and [32P]UTP-labeled antisense riboprobes were generated. 10 × 106 of Th1 and Th2 effectors were stimulated with antigen/APC and total RNA was isolated after 0, 1, 3, 5, 8, and 24 h. 10 μg RNA of each sample was hybridized with indicated radiolabeled antisense RNA transcripts, and then digested with RNase A/T1. Samples were then separated by urea/SDS-PAGE and gel was exposed to x-ray film.
Results
Th1, but not Th2, Effectors Undergo Rapid AICD.
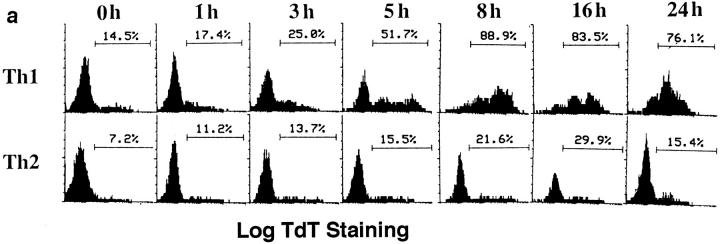
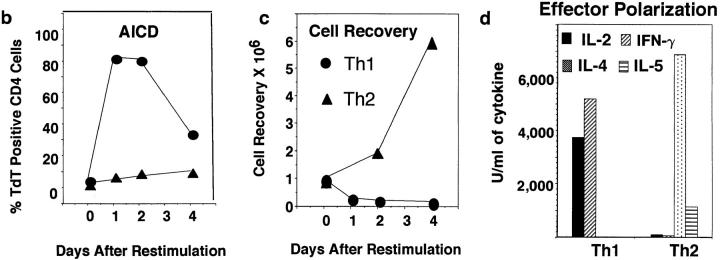
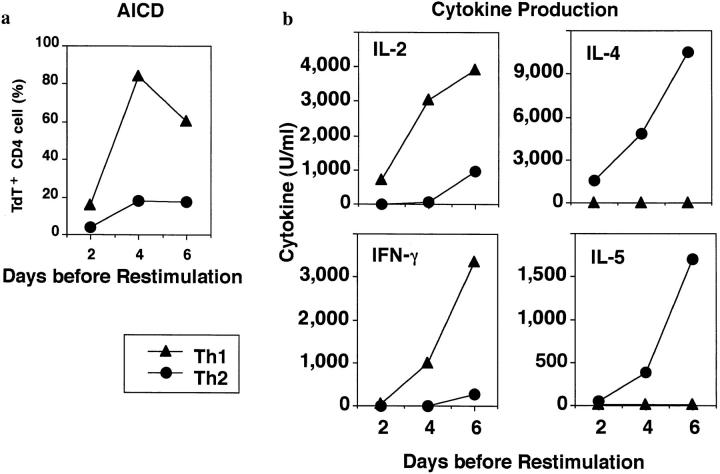
Addition of cytokines to antigen-stimulated, naive CD4 T cells cultured in vitro for just a few days will direct their differentiation into highly polarized Th1 and Th2 effectors (15–17). Because such effectors have not been subjected to selective effects of long-term culture, they are likely to represent physiologically polarized T cell effector subsets which arise in primary immune responses. We directly compared the AICD of Th1 and Th2 effectors generated from highly purified naive CD4 populations derived from AND TCR transgenic mice which express TCR specific for PCCF, presented by I-Ek (18). Effectors were harvested after 4–5 d of response to antigen in vitro and were then restimulated with peptide antigen, PCCF, and APC in vitro. As shown in Fig. 1 a, many antigen-restimulated Th1 effectors displayed detectable DNA fragmentation after 3–5 h indicative of commitment to apoptosis, and the great majority of Th1 effectors were apoptotic by 8 h. In contrast, only low levels of DNA strand breaks could be detected in the Th2 population throughout the first 24 h. Effector cells restimulated with antigen typically showed two, clearly separate, populations as assessed by FACS® analysis of forward versus side scatter. Here we show the percentage of TdT+ (indicating TdT had access to fragmented DNA) CD4 cells in the high forward and low side scatter population, i.e., the portion of the population that had not already died. Analysis of the rate of apoptosis over a longer period indicated that high levels of DNA fragmentation were seen at both 1 and 2 d after restimulation in Th1 effectors, while Th2 effectors showed little TUNEL staining throughout this time period (Fig. 1 b). By 4 d, the population of cells remaining in the Th1 group were less apoptotic, suggesting that selection for cells resistant to apoptosis had occurred. Indeed, Th1 effector cultures showed a rebound in cell recovery after 4 d when they were given IL-2 (not shown). By 4 d, the Th2 population was just beginning to show increased DNA fragmentation (Fig. 1 b), and as seen in earlier studies (13), substantial levels of Th2 apoptosis were seen by day 7 (not shown). The rapid death of Th1 without a corresponding apoptosis among Th2 effectors resulted in a marked difference in cell recovery with Th1 effectors showing very low recoveries after 1–4 d in the range of 40–60%, whereas Th2 effectors increased over sixfold (Fig. 1 c). The increase in Th2 number reflects their proliferation in response to restimulation (13). Th1 effectors also begin to synthesize DNA. Even at day 1, when most of Th1 effectors stained in the TUNEL assay (Fig. 1, a and b) and showed other signs of impending death such as shifts in forward versus side scatter and incorporation of propidium iodide (not shown), the incorporation of 3H[TdR] was nearly equivalent. (In a representative experiment, Th1 incorporation 1 d after stimulation and a 6-h labeling was a mean of 70,420 cpm against a background of 743 cpm, while Th2 incorporated 172,543 cpm over a background of 2127 cpm). As expected, the Th1 incorporation of radiolabeled nucleotides decreases with time as the majority of cells undergo apoptosis. The starting Th1 and Th2 effector populations were highly polarized with regard to their abilities to produce cytokines as illustrated in Fig. 1 d. Similar results have been obtained in all polarized Th1 and Th2 effector experiments.
Figure 1.
Kinetics of AICD of Th1 and Th2 effectors restimulated with antigen. Effectors specific for PCCF were generated in vitro from naive CD4+ cells of H-2k/k AND TCR Tg mice (Vβ3/Vα11; see Materials and Methods) After 5 d, Th1 and Th2 effectors were harvested, and low density cells obtained by Percoll gradient separation were restimulated at 5 × 105/ml with PCCF (5 μM) and APC (1.7 × 105/ml). (a) Kinetics of Th1 and Th2 AICD. Effector cells restimulated for different times as above were stained with TdT and dUTP for detection of nicked DNA (TUNEL method), followed by biotinylated UTP, and then FITC-streptavidin. Gated CD4+ cells were analyzed for DNA strand breaks (TdT+ cells) by FACScan® analysis. The results are the representative of six experiments. (b) Longterm kinetics of AICD. Effectors were harvested at 1, 2, 3, and 4 d after restimulation, and apoptosis in live cells determined by TdT staining as described above. (c) Kinetics of cell recovery. The cultures harvested in Fig. 1 b were also analyzed for number of viable effector cells determined counting trypan blue excluding cells. Results are expressed as the ratio of viable CD4 T cells recovered per culture per CD4 T cells plated at time of restimulation. (d) Effector polarization. Cytokines were measured in the supernatants collected 18–24 h after restimulation of effector cells generated from Tg mice. IL-2 titers (filled bar) were determined by bioassay. IFN-γ, IL-4, and IL-5 titers were determined by ELISA as described in Materials and Methods.
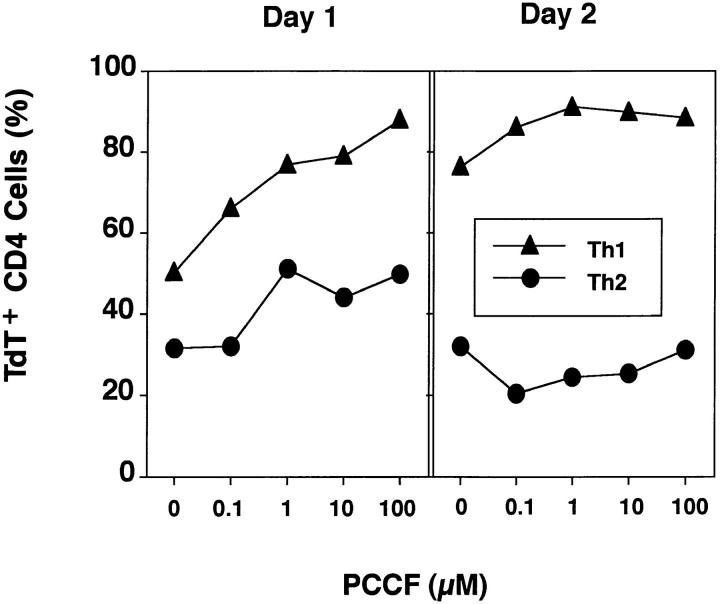
An earlier report indicated that Th2 lines would undergo as much apoptosis as Th1 lines when high doses of antigen were used to stimulate activated T cells (32). To investigate whether the difference between Th1 and Th2 effectors was dependent on antigen dose, we examined the effect of peptide dose on the AICD of Th1 and Th2 effectors after 1 and 2 d of culture (Fig. 2). In this experiment, because of the many cultures that were harvested simultaneously, healthy blasts were not isolated by lympholyte separation as in most other experiments, and therefore the background of death in the Th2 population was significantly higher, indicating some cells had become committed to die during the initial culture period. Both Th1 and Th2 effectors showed increased AICD with high dose of antigen on day 1, but the percentage of apoptotic cells was much higher in the Th1 than Th2 effectors at each dose. By day 2, there was substantial AICD in Th1 effectors, even in the absence of additional antigen (0 μM PCCF). The kinetics of cell death in the absence of antigen restimulation is shown below in more detail. High doses of antigen increased AICD only slightly after 2 d of restimulation of Th1 effector cultures, but Th2 effectors were resistant to AICD at all concentrations of peptide. Thus, Th1 effectors were much more sensitive to AICD than Th2 effectors over a broad range of antigen concentrations.
Figure 2.
Effect of antigen dose on Th1 and Th2 AICD. Th1 or Th2 effector cells (5 × 105) and 1.7 × 105 DCEK APC were incubated in 1 ml of medium and restimulated with graded concentrations of PCCF. After 1 and 2 d, cells were harvested from each culture and stained with TdT and PE-CD4. The percentage of TdT+ CD4+ T cells was determined as above. Results are representative of three experiments.
Th1, but Not Th2, Effectors Are Also Susceptible to Early Programmed Cell Death.
We next examined the kinetics of Th1 and Th2 apoptosis in the absence (APC only) compared to the presence (APC plus PCCF) of restimulation with antigen. Stimulation of Th2 effectors with optimal peptide concentration induced apoptosis 1–2 d sooner than culture without antigen, and a similar pattern was seen within the Th1 population (Fig. 3). However, as before, the onset of apoptosis in Th1 effectors occurred more quickly than in Th2 effectors, where it was delayed several days. Thus, Th1 effector cells undergo more rapid death, both in the absence (programmed cell death) and presence of antigen (AICD), and stimulation by antigen induces apoptosis to occur more rapidly in both Th populations.
Figure 3.
Th1 effectors undergo more rapid apoptosis with and without restimulation. Th1 and Th2 effector cells at 5 × 105 per culture were stimulated with 1.7 × 105 of APC in 1 ml of medium with or without PCCF (5 μM) for 1, 2, 4 and 7 d. At each time, cultures were harvested and stained with TdT and CD4 as above. The appearance of TdT+ CD4 cells in Th1 (triangles) and Th2 (circles) effector cultures stimulated with antigen and APC (closed) or with APC without antigen (open) was determined. The results are the representative of three experiments.
Preferential Th2 Effector Survival Is an Intrinsic Property of Th2 Effectors.
Several mechanisms could be suggested to account for this preferential death of Th1 effectors. Th1 effectors might preferentially express a cell surface receptor such as Fas, which when engaged by FasL, could induce death. Conversely, Th2 effectors might produce a cytokine(s) which protects effectors from death. Alternatively, there could be an intrinsic difference in susceptibility of the cells to death, such as expression within the cell of components that either promote or block pathways leading to apoptosis. In support of the first two possible mechanisms, it has been reported that a Th1 clone could induce apoptosis of a Th2 clone when Th1 and Th2 clones were mixed (8), and we and others reported that some cytokines such as IL-2 and TGF-β (13) and IL-10 (33) can protect T cells from AICD.
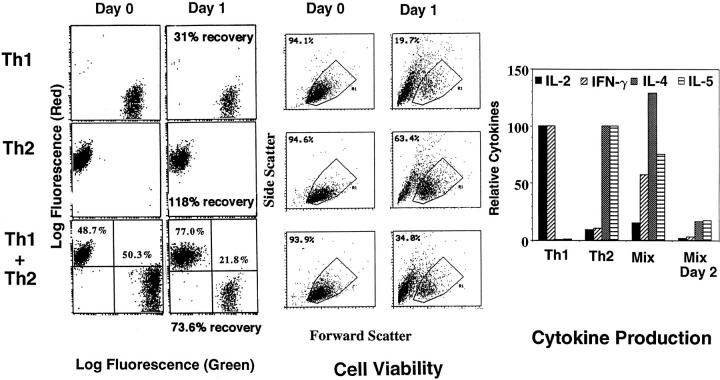
To explore the mechanisms responsible for unequal susceptibility of Th1 and Th2 effectors to cell death, we labeled Th1 and Th2 effectors with CFSE and PKH26, respectively. These vital dyes fluoresce strongly with green and red emissions, respectively, and are well retained by live cells for extended periods of time (29) and through several cell divisions. The stained cells can be directly detected by flow cytometry (see Fig. 4, Day 0). As shown in Fig. 4 (Day 0), Th1 and Th2 effectors were labeled and then cultured separately (top and middle), or together in equal numbers (bottom), either with APC alone or antigen plus APC.
Figure 4.
Differential survival of Th1 and Th2 in mixed culture. Th1 effector cells were labeled with CFSE (Green) and Th2 were labeled with PKH26 (Red) using conditions designed to retain full cell viability. Aliquots of labeled Th1, Th2, and a 50:50 mix of the two were analyzed for red and green fluorescence (left) and cell viability as determined by forward and side scatter (middle; Day 0). The three populations were then cultured at 5 × 105 in 1 ml of medium with 1.7 × 105 of APC in the presence of PCCF for 1 d. After 1 d, supernatants were removed and each culture was harvested and counted. An aliquot was analyzed for red and green fluorescence (Day 1). Cell recovery per culture, based on the recovered cells numbers, was determined by trypan blue exclusion and is indicated below each day 1 fluorescence plot. The percentage of gated, dye-labeled cells not undergoing apoptosis, as determined by forward versus side scatter, is indicated within the cell viability plot (middle). Cytokine titers were measured as in Fig. 1 d in the supernatants (right).
After 1 d, whole cultures were harvested, viability and cell recovery were determined, and recovery of labeled cells was determined by flow cytometry (Fig. 4, Day 1). As expected, the number of cells in the Th1 effector population decreased (to 31% of initial recovery) when they were cultured separately with peptide, whereas the Th2 population remained at levels near those originally plated (118% recovery). Mixed cultures had intermediate total cell recoveries (73.6%). A similar pattern was seen in the analysis of cell viability of gated, dye-labeled cells by forward versus side scatter depicted in Fig. 4 (middle, Cell Viability). In Th2 cultures, viability of CD4 cells was 63%, whereas the viability in the Th1 cultures was only 20%. When equal numbers of Th1 and Th2 effectors were mixed with APC in the presence of antigen, the percentage of Th1 effectors decreased from an initial representation of 50.3 to 21.8%, whereas that of the Th2 increased from 48.7% to 77.0%, changing the ratio of Th2 to Th1 effectors from 1.00 at day 0 to 3.50 at day 2. Mixed cultures also had an intermediate viability (34%). If effectors were cultured with APC without antigen, there was little difference in the ratio of Th1 versus Th2 after 24 h, although the Th2 cells came to dominate culture after 2–3 d (not shown). To substantiate the functional significance of the apparent selective Th2 survival, initial populations (Th1 alone, Th2 alone and mixed, plus the mix after 1 d) were restimulated with PCCF plus APC, and supernatants were collected after 24 h and assayed for cytokines. Results shown in Fig. 4 (right) show that while the mixed cultures initially made both Th1 and Th2 cytokines, after restimulation for 2 d only, Th2 cytokines were still made in substantial amounts. Thus, Th1 and Th2 effectors underwent AICD at their own rate regardless of whether they were in separate or in mixed cultures. This suggests that Th1 effectors do not kill bystander Th2 effector cells (fratricide) and that cytokines released by stimulated Th2 effectors do not rescue Th1 effectors within the time frame of these experiments. Similar results were seen in two repeat experiments.
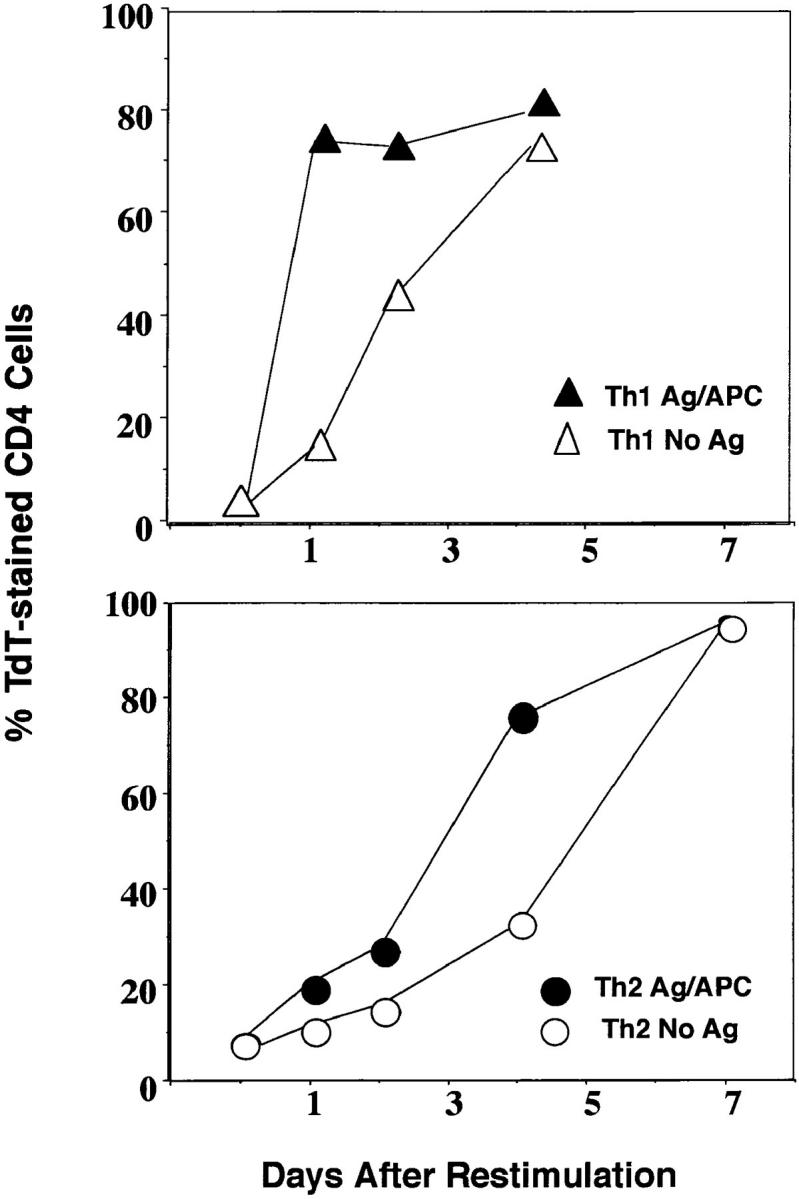
Development of the Susceptibility to AICD Occurs with the Same Time Course as Generation of Effectors.
To further evaluate the unequal acquisition of susceptibility to AICD, we cultured naive CD4+ T cells with antigen plus APC in our standard conditions for generating Th1 and Th2 effectors. Our previous studies had suggested that acquisition of CD4 effector behavior, as determined by the levels of cytokines produced upon restimulation and other phenotypic and functional properties, showed a lag phase of 2–3 d, and then rose sharply both in vitro (34) and in vivo (28). We stimulated naive CD4 T cells under polarizing conditions and removed aliquots at different times during the generation of effectors. Harvested cells were restimulated with antigen and APC. The level of AICD was determined and the cytokines in the supernatants were tested to monitor whether the Th were capable of cytokine production (Fig. 5). Naive cells (day 0) were highly resistant to AICD and produced only low levels of IL-2, with no detectable IL-4, IL-5, or IFN-γ, as detailed in previous studies (16; day 0 not shown). Analysis by restimulation 2 d after initiation of effector generation cultures indicated that cells from both cultures displayed the phenotype of activated T cells with high CD44, lower L-selectin, high CD45RB, and high IL-2 receptor (not shown). However, after 2 d (Fig. 5, day 2), both groups of cells produced only low titers of cytokines upon restimulation, indicating that T cells were not yet fully differentiated into effectors. Also after 2 d, both populations showed similar and low AICD. After 4 d of in vitro differentiation, both Th1 and Th2 cultures secreted large amount of cytokines after restimulation (Fig. 5, right, individual cytokines produced) and the effectors were highly polarized into Th1 or Th2 patterns. By 4 d, they also exhibited unequal susceptibility to AICD. A majority of Th1 effectors (66–92%) were committed to apoptosis after antigen stimulation, whereas only ∼20% of Th2 effector underwent AICD (Fig. 5, right). By day 6, cytokine production was at its highest per given number of cells stimulated, and the differences in AICD persisted. Thus, even though cytokine production per se does not seem to be responsible for the unequal susceptibility to AICD as indicated by the mix experiment in Fig. 4, there is kinetic correlation between the onset of Th1 effector function and development of susceptibility to AICD.
Figure 5.
Onset of AICD susceptibility and effector function. Naive CD4+ cells (3 × 105/ml) from Tg mice were cultured with PCCF/APC plus IL-2 (20 U/ml) and either IL-12 and antiIL-4 (11B11, 10 μg/ml) to induce polarization to Th1 effector (•), or IL-4 and anti-IFN-γ (XMG1.2, 10 μg/ml) to induce polarized Th2 effectors (▴). After 2, 4, and 6 d of differentiation in vitro, effectors were harvested from Th1 and Th2 conditions. (a) Kinetics of development of AICD. Cells were harvested from effector generation cultures at different times and restimulated with PCCF/APC for 1 d. Apoptosis was determined by TdT staining. (b) Cytokine production. Cells were harvested from effector generation cultures at different times and restimulated with PCCF/ APC. Supernatants were removed after 18–24 h, and cytokine titers were determined.
The Rapid AICD of Th1 Effectors Is Mediated by Fas/FasL Pathway.
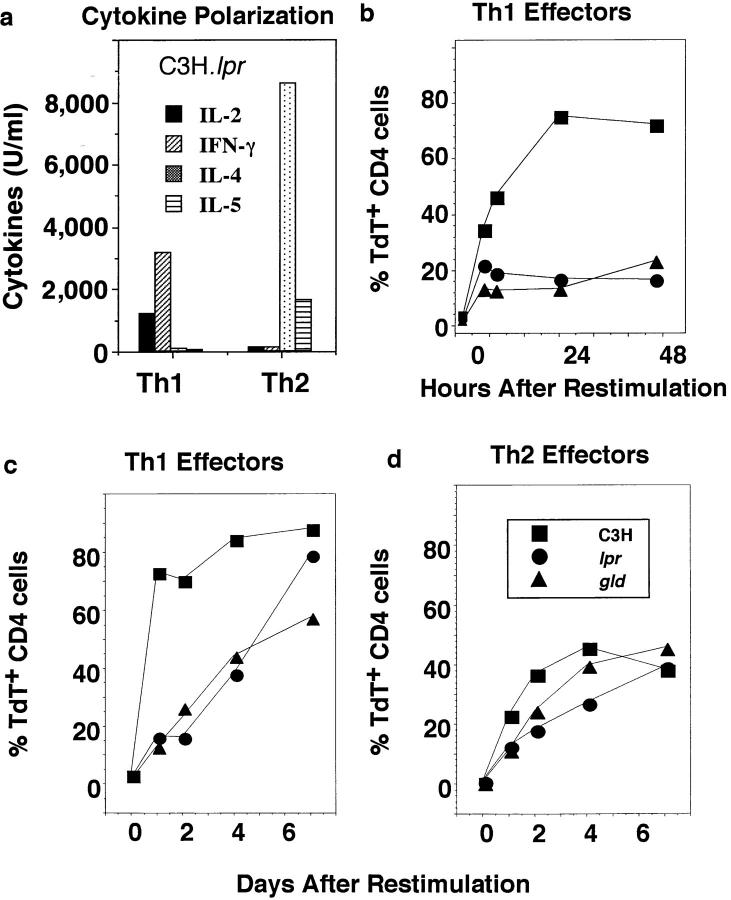
To investigate whether effector cell death was mediated by a Fas/FasL interaction, we used polyclonal stimulation to generate Th1 and Th2 effectors from mice with mutations in Fas (lpr) and FasL (gld), and compared them to effectors from wild-type C3H mice. We used the polyclonal mitogen Con A plus APC to stimulate effector generation (9), since the donors were not TCR Tgs. The Th1 and Th2 effectors generated were as well polarized as antigen-generated Th1 and Th2 effectors, as indicated in Fig. 6 a, which shows the cytokines produced by the polarized effectors generated from C3H.lpr mice. Effectors generated from wild-type and C3H.gld mice were equally polarized (not shown). Differences in AICD were especially marked at early time points (Fig. 6 b). Th1 effectors derived from wild-type C3H mice underwent rapid apoptosis after restimulation, as indicated by dramatic increases in TUNEL staining from 0–24 h (Fig. 6 b), whereas TUNEL staining in Th2 populations was very low during this time (see Fig. 6 d). In contrast, Th1 effectors derived from either the Fas (lpr) or FasL (gld) mutant strains underwent little apoptosis (Fig. 6 b). The lack of apoptosis in the Fas– and FasL–deficient effectors was reflected in a sizable increase in cell recovery of about fourfold over the 2-d period, during which time wild-type effectors were recovered in reduced numbers (not shown). By 4–6 d, mutant-derived Th1 effectors began to exhibit DNA fragmentation with substantial numbers of apoptotic cells evident by day 7 (Fig. 6 c). Thus, a second non-Fas/FasL pathway of cell death was operating after several days. Wild-type Th2 effector populations exhibited a delayed death (Fig. 6 d) resembling the non–Fasmediated Th1 death. Th2 effectors derived from lpr and gld animals were only slightly resistant to this second phase of apoptosis. Thus, a second phase of Fas-independent apoptosis was evident in both Th1 and Th2 effector populations. Therefore, the rapid AICD that Th1, but not Th2, effectors undergo is mediated, at least in large part, by a Fas/FasL interaction, while a slower, Fas-independent mechanism is responsible for delayed AICD and is present in both subsets.
Figure 6.
Role of Fas and FasL in effector AICD. Th1 and Th2 effector cells were generated in vitro from naive CD4+ cells obtained from C3H (▪), C3H.MRL-Faslpr (•), and C3H/HeJ-FasLgld (▴) mice by culture with Con A (2 μg/ml) and DCEK-ICAM APC in the presence of IL-2 plus either IL-12 and anti–IL-4 (11B11) or IL-4 and anti–IFN-γ, respectively. After 5 d, well-polarized Th1 or Th2 effectors (5 × 105/ml) were recovered and stimulated with Con A (2 μg/ml) and APC (1.7 × 105/ml) again for hours or days as indicated. At the various times after restimulation, cultures were harvested. Viability was determined and samples were stained with TdT as described above. (a) Cytokine polarization. In parallel cultures, the effector populations were restimulated as above and supernatants were harvested 18–24 h after restimulation for determination of cytokine titers. The cytokine pattern of Th1 and Th2 effectors from wild-type C3H and C3H.lpr mice were very similar to those from gld mice (not shown). IL-2 was tested by bioassay, and IFN-γ, IL-4, and IL-5 by ELISA. (b) Kinetics of Th1 AICD. Th1 effector cells from each group were stained with PE–anti-CD4 and TdT/dUTP. The percentage of TdT+ among gated CD4 effector cells harvested at 0, 5, 8, 24, and 48 h from C3H (▪), lpr (•), and gld (▴) mice was determined. (c and d) Longterm kinetics of AICD. The percentage of TdT+ gated CD4 cells in Th1 (c) and Th2 (d) effector mice were determined 1, 2, 4, and 7 d after restimulation.
Further Evidence for the Role of Fas/FasL in Activation-induced Th1 Apoptosis.
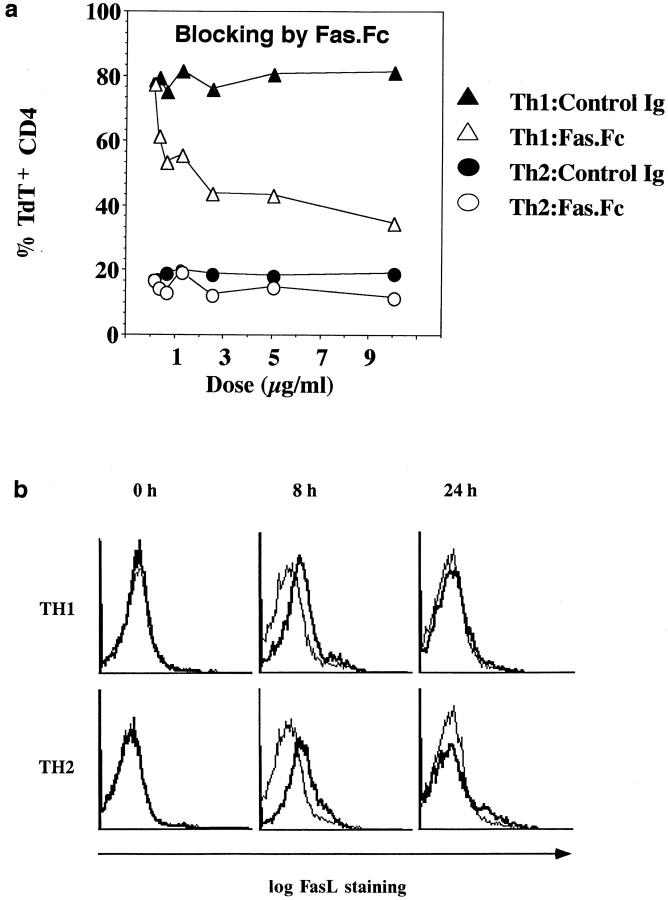
To substantiate the role of Fas in Th1, but not Th2, effector death, Jo2 antibody to Fas was added to both Th1 and Th2 effectors during a 2-d culture, with hamster Ig as a control. Only Th1 effector death was influenced, and it was blocked >50% by the addition of soluble Ab at 10 μg/ml (not shown). To further explore the roles of Fas, we also tested whether Fas.Fc would selectively block activation-induced Th1 effector death. Th1 and Th2 effectors were generated from TCR Tg CD4 T cells as before, and effectors were restimulated for 2 d with PCCF plus APC in the presence of various doses of a Fas.Fc fusion protein or control human IgG. Results are shown in Fig. 7 a. The apoptosis of Th1 effectors was blocked substantially by the soluble Fas construct. The minimal apoptosis of Th2 effectors was not exhibited by the Fas.Fc. Altogether, this study provides compelling evidence that Th1 undergo a rapid, Fas/FasL mediated apoptosis, but that Th2 effectors are resistant to being killed by this mechanism.
Figure 7.
Role of FasL in Th1 death. Th1 and Th2 effectors were generated from AND mice as in previous experiments. (a) Inhibition of Th1 death by Fas.Fc. The ability of Fas.Fc to block the apoptosis of Th1 and Th2 effectors was determined. Aliquots of 5 × 105/ml Th1 or Th2 effectors were cultured with PCCF/APC and several concentrations of either Fas.Fc fusion protein or the control human IgG. 2 d after restimulation, cells in each culture were harvested and stained with TdT and PE–antiCD4 to analyze apoptosis. TdT+ cells from the CD4+ population were analyzed. (b) Kinetics of FasL expression. Cell surface expression of FasL on Th1 and Th2 effector cells, restimulated with PCCF and APC for 0, 8, or 24 h, was assessed by staining. Effectors harvested at each time point were stained with FITC–anti-CD4 and PE–anti-FasL (bold lines) or PE– anti-CD8 (light lines) as control.
Fas and FasL Expression of Th1 and Th2 Effectors Is Similar.
An earlier study reported that Th1 cell lines express somewhat higher levels of FasL than Th2 lines and suggested that this may account for unequal susceptibility to AICD of the two types of lines (8). However, there are also examples in which both Fas and FasL are expressed by T cells, but the T cells are not committed to death (31). Therefore, we further examined the kinetics of expression of FasL and Fas by Th1 and Th2 effectors before and after restimulation. First, we used the anti-FasL antibody to visualize FasL expression on effectors. The expression of FasL on effectors (Fig. 7 b, 0 h) was not detectable, indicating either that effectors do not initially express FasL, or that expression is below the limits of detection using this method. However, by 8 h after stimulation, expression of FasL was seen and was equivalent on Th1 and Th2 effectors. By 24 h, levels of FasL were again reduced to undetectable levels. A functional analysis of FasL expression is shown below.
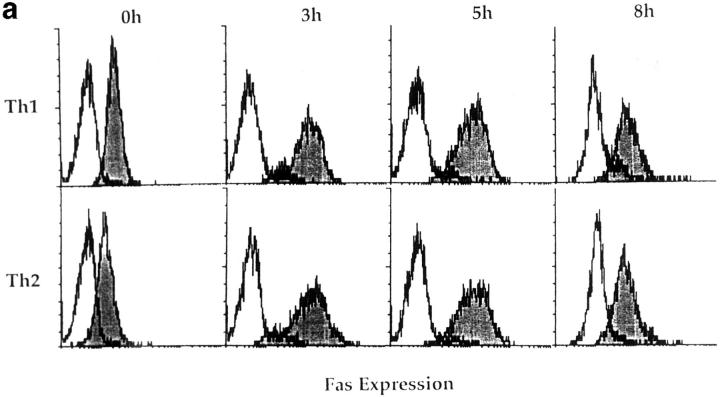
To further explore the expression of the components of the Fas pathway, we stained effectors both immediately after generation and at several time points for expression of Fas with the Jo2 Ab. As seen in Fig. 8 a, Th1 and Th2 effectors initially expressed equivalent low levels of cell surface Fas at the time of harvest (0 h). Fas expression increased in both populations 3 and 5 h after restimulation, and then declined slightly by 8 h. Again, the levels of expression by Th1 and Th2 effectors were comparable.
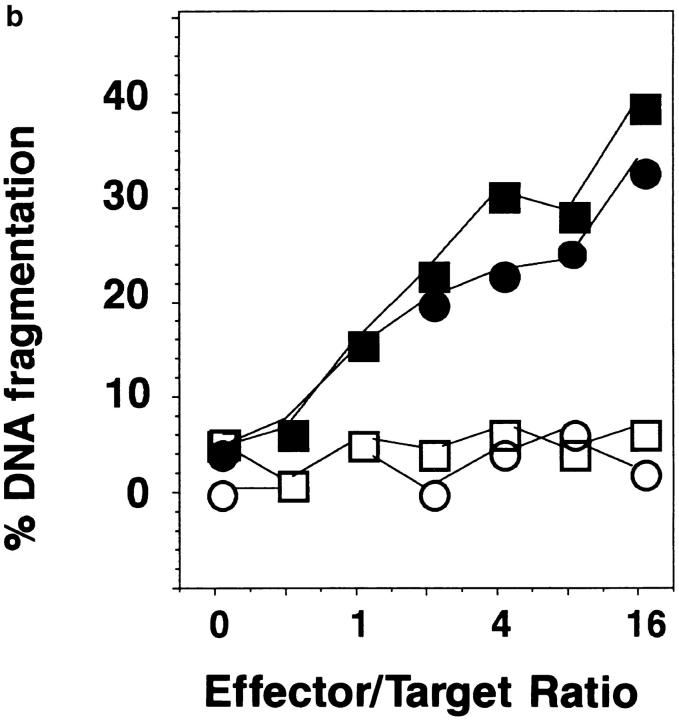
Figure 8.
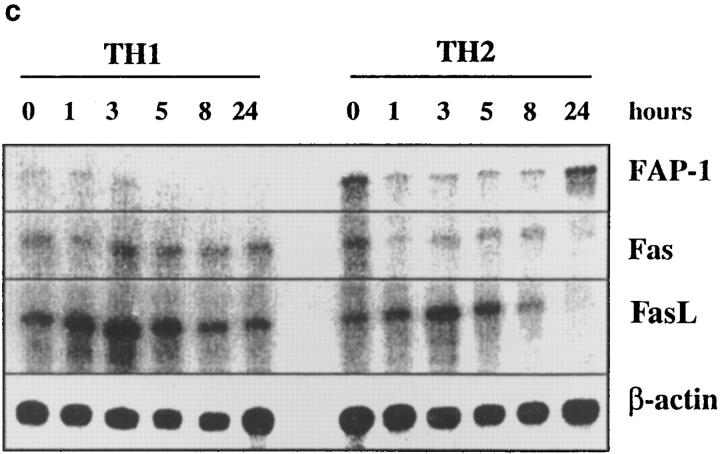
Fas, FasL, and FAP-1 expression in Th1 and Th2 effectors. Th1 and Th2 effectors were generated from naive CD4 cells from AND mice as before, and aliquots were restimulated with PCCF and APC in vitro. Cells were collected at different time points after restimulation. (a) Fas expression by effectors. Th1 and Th2 effector cells restimulated for 0, 3, 5, or 8 h were stained with either hamster anti–mouse Fas antibody (Jo2, shaded peaks) or hamster IgG (open peaks), followed by FL-conjugated goat anti–hamster IgG (Fab′)2 fraction. Cells were counterstained with PE–anti-CD4. Gated CD4+ cells were analyzed for Fas expression by FACScan®, using Lysys II software. (b) Fas-mediated killing by effectors. Functional FasL expression was determined by assessing the ability of Th1 and Th2 effector cells (not restimulated) to cause DNA fragmentation in Fas+ target cells as described in Materials and Methods. Parental L1210 cells (Fas−, ○ or □) or the same line transfected with murine Fas (Fas+, • or ▪) were labeled with 5 μCi [3H]thymidine and used as target cells. 2 × 104/100 μl targets were cultured with 100 μl of appropriately diluted Th1 (○, •) or Th2 (□, ▪) effector cells stimulated with anti-CD3. A1.1 cells, activated by anti-CD3, were used as positive control. The percentage of DNA fragmentation was calculated from thymidine counting with formula. Assays were performed in triplicate and means are shown. (c) Expression of Fas, FasL, and FAP-1 mRNA. RNA was isolated from effectors restimulated with PCCF/APC for 0, 1, 3, 5, 8, and 24 h. An RPA was run on the isolated RNA using a commercial RPA kit (Ambion Inc.) according to the manufacturer's protocol. Fas, FasL, murine FAP-1 cDNA, and β-actin cDNA (positive control), were cloned using pBluescript II. Total RNA was isolated from 10 × 106 Th1 and Th2 restimulated effectors. 10 μg RNA from each sample was hybridized with indicated radiolabeled antisense RNA transcripts, and then digested with RNase A/T1. Samples were then separated by urea/SDS-PAGE and gel was exposed to x-ray film. Results are representative of two similar experiments.
Because the initial staining of Th1 and Th2 effectors before restimulation for FasL was too low to make a definitive conclusion as to levels, we also assessed functional FasL expression by measuring the abilities of Th1 and Th2 effectors to kill Fas+ versus Fas− targets (Fig. 8 b). Th1 and Th2 effectors were equivalent in their ability to induce apoptosis in the Fas+ targets, whereas neither killed targets not expressing Fas, indicating similar functional expression of FasL in both Th1 and Th2 effectors. Together, these experiments emphasize that levels of expression of Fas or FasL is unlikely to be the major factor responsible for the resistance of Th2 effectors to AICD.
FAP-1 Is Expressed at Higher Levels in Th2 Effectors.
We therefore sought another explanation for this difference in Th1 and Th2 susceptibility to apoptosis. Since Bcl-2 and Bcl-xL function as important regulators of apoptosis (35), we examined the expression of these two molecules on Th1 and Th2 effectors. Th1 and Th2 effectors expressed comparable Bcl-2 and Bcl-xL (not shown) before and after antigen stimulation, which suggested that these two molecules do not play an important role in the differential susceptibility of Th1 and Th2 effectors (Zhang, X., L. Tsui, and S.L. Swain, unpublished data). A recent report indicated that FAP-1 can block the Fas-mediated death signaling cascade and protect some cells from AICD (31), so we examined the expression of FAP-1 messenger RNA (mRNA) by RNAse protection and also examined levels of Fas and FasL mRNA expression (Fig. 8 c). mRNA levels for both Fas and FasL were initially comparable in the RNA from Th1 and Th2 effectors, consistent with the staining and function studies above. Both Fas and FasL expression were retained in the Th1 effectors over the next 24 h, whereas expression of both RNAs were decreased in Th2 effectors after stimulation. FasL mRNA remained high for at least 5–8 h in both populations, although FasL was downregulated more rapidly in Th2, becoming barely detectable by 24 h (Fig. 8 c). Moreover, since activation-induced, Fas/FasLmediated apoptosis occurs rapidly (within 12 h) in Th1 effectors, the small differences in expression of Fas and FasL at later time points noted above could not be expected to account for the difference in susceptibility to AICD.
We observed a dramatic difference between Th1 and Th2 effectors in their expression of FAP-1 mRNA (Fig. 8 c) that correlated inversely with apoptosis. Unstimulated Th2 effectors expressed easily detectable FAP-1 mRNA, whereas Th1 effectors express very little. After restimulation, FAP-1 mRNA declined in Th1 effectors from very low levels to undetectable levels, but remained at easily detectable levels in Th2 effectors. By 24 h, FAP-1 levels in Th2 effectors were high, while Fas and FasL were low. Thus, Th1 and Th2 effectors show a marked difference in expression of FAP-1, which could account for their differences in rapid Fas-induced AICD both in the presence and absence of restimulation with antigen. Similar results were seen in a repeat RPA assay.
Altogether, the results of cell surface expression of Fas and FasL, mixtures of Th1 and Th2 effectors, functional activity of FasL, and presence of RNA for Fas and FasL suggest that although rapid AICD in Th1 cells is mediated by the Fas/FasL pathway, the resistance of Th2 cells cannot be simply explained by the absence or low levels of Fas or FasL, and that some additional intrinsic difference is responsible for their failure to commit suicide. Since FAP-1 has been implicated in blocking the Fas/FasL mediated death pathway, the observed difference in FAP-1 expression by Th2 cells is an obvious candidate for a difference which might be partly or wholly responsible for the unequal death of the Th1 and Th2 effector populations.
Discussion
Our results identify a major functional difference between short-term, in vitro–cultured Th1 and Th2 effectors which can lead to the preferential survival of Th2 effectors and may be relevant to a number of in vivo disease states in which Th2 dominance is seen. Our studies clearly show that short-term, polarized effector T cells differ dramatically in their susceptibility to apoptosis. Thus, while Th1 effectors undergo rapid Fas/FasL-mediated AICD 5–12 h after restimulation and also die within 2 d without restimulation, Th2 effectors escape this fate despite expression of both Fas and FasL, remaining resistant to death for 4–7 d in the presence or absence of antigen. Both types of effectors are susceptible to delayed, non–Fas-mediated death. This unequal susceptibility to death is apparently an intrinsic property of the subsets, since there is also selective loss of the Th1 population in mixed cultures of Th1 and Th2 effectors. The susceptibility to rapid Fas/FasL-mediated apoptosis develops as naive cells become Th1-polarized effectors. Interestingly, Th2 effectors express higher FAP-1 mRNA, suggesting that FAP-1 may act to prevent Fas/FasL-mediated cell death in Th2 effectors both before and after antigen stimulation. This provides the first instance where expression of FAP-1 in untransfected cells expressing Fas and FasL is correlated with resistance to Fas-mediated apoptosis, and provides a potential mechanism for inhibiting apoptosis.
The failure of several other groups to see differences similar to those illustrated here between Th1 and Th2 effectors, may be due to the use of effector populations that are not as well polarized as the ones used in the studies reported here (Figs. 1 and 6). We have optimized protocols for achieving polarization, and have found that use of highly purified naive cells from TCR Tg mice, which are uncontaminated by even low numbers of activated cells, and low density cultures result in the most well-polarized population. We have seen dramatic differences in AICD between Th1 and Th2 effectors, such as those shown, in 14 out of 14 experiments. The susceptibility of CD4 cells to rapid AICD develops only after >2 d of culture of naive cells under Th1-polarizing conditions, as the cells with naive phenotype shift to expression of an effector phenotype characterized both by shifts in expression of cell surface markers and by their capacity to secrete high titers of Th1 cytokines upon restimulation (Fig. 5). We have also found that effectors generated in IL-2 alone (without IL-12), which secrete higher IL-2 and lower IFN-γ than Th1, but no detectable IL-4 and IL-5, also undergo similar rapid AICD as Th1 effectors (not shown), and thus suggest that the susceptibility to AICD may be the default pattern and that only the most well-polarized Th2 effectors are resistant.
Fas/FasL coexpression and interaction leading to apoptosis is one of the main pathways responsible for the AICD of activated CD4+ T cells (22–24, 36, 37). The rapid AICD in Th1 effector cells is clearly mediated in large part by Fas/ FasL, since Th1 effectors from mice deficient either in Fas (lpr) or in FasL (gld) are resistant to the rapid AICD (Fig. 6), whereas the delayed death of Th1 and Th2 death are not affected. Moreover, the rapid AICD of Th1 effectors is largely blocked either by soluble Ab to Fas or by Fas.Fc fusion protein in soluble form (Fig. 7). However, differences in Fas and FasL expression on effectors do not appear to be responsible for the dramatic differences between Th1 and Th2 AICD seen in the first 24–48 h. Th1 and Th2 effectors express equivalent levels of Fas (Fig. 8), both initially and for the first 8 h, by which time the Th1, but not the Th2, effectors exhibit striking DNA damage that presumably indicates they are already committed to apoptosis. The expression of Fas is increased in Th effectors after stimulation (Fig. 8), which could contribute to the faster rate of apoptosis in Th1 effectors after antigen stimulation. FasL expression is undetectable by Ab staining of either Th1 or Th2 effectors before restimulation, but increases to detectable levels by 8 h (Fig. 7). However, functional FasL expression is detectable since both effectors can mediate equivalent induction of apoptosis in Fas+, but not Fas−, targets (Fig. 8 b). The analysis of mRNA expression indicates that FasL expression may be selectively downregulated among Th2 effectors by 24 h, which could contribute to the continuing resistance of Th2 effectors to apoptosis, but this difference is insufficient to explain the large difference in DNA damage and cell death during the first 12 h of culture after restimulation. An earlier report indicated that FasL was expressed at higher levels on Th1 lines (8), and also found short-term Th2 lines expressed substantially more FasL than Th2 clones. Another study reported that Fas/FasL were expressed not only by activated Th1, but also by some Th2 clones (37). Thus, we conclude that the expression of Fas and FasL is required for rapid effector death but not sufficient for such death, and the differences in Fas or FasL expression are not likely to be primarily responsible for the unequal death in the Th1 and Th2 effectors reported here.
FAP-1 can interact with the intracellular tail of Fas and inhibit Fas signal transduction, according to experiments in which FAP-1 expression is forced in Jurkat cells (31). The correlation of FAP-1 mRNA expression with Th2 resistance to apoptosis provides the first evidence that regulation of FAP-1 expression may be an important mechanism determining T effector fate. Our results indicate that the expression of FAP-1 is inversely correlated with the differential AICD and programmed cell death of Th1 and Th2 effectors (Fig. 8 c), supporting a model in which FAP-1 expression is responsible for the resistance of AICD in Th2 cells. The fact that Th1 and Th2 effectors differ only in the Fas/FasL-mediated rapid death pathway, but not in the non–Fas-mediated slower death pathway is consistent with a specific difference of the Fas-mediated pathway such as would be seen with FAP-1. Further studies to determine what induces FAP-1 expression and how closely it is correlated with Th2 cytokine polarization and resistance to cell death should shed further light on factors that are active in conferring Th2 resistance to apoptosis.
The studies reported here support a model in which an intrinsic difference(s) between Th1 and Th2 subsets is responsible for their unequal susceptibilities to Fas-mediated AICD. Either mixed or cultured separately, Th1 and Th2 effectors died at the same rate (Fig. 4) so that after 1 d, Th2 predominate in mixed cultures, which also become unable to produce Th1 cytokines, IL-2, and IFN-γ in significant amounts. The Th1 plus Th2 effector mix experiments suggest that Th1 effectors do not indiscriminately kill bystander Th2 effector cells, ruling out an indiscriminate fratricidal mechanism, such as was suggested previously for Th1 cell lines (8). Earlier studies had indicated that Fas/FasL interactions could mediate cell death by a cell autonomous mechanism (23, 37) and the rapid Th1 apoptosis seen here may depend on such a mechanism. The mix experiments suggest that production of and response to Th2 cytokines was not responsible for resistance of Th2 effectors to apoptosis. An additional argument against a role for cytokines in determining the unequal death of Th1 and Th2 effectors are the facts that cytokine production by effectors peaks only 6–12 h after restimulation (8), whereas Th1 effectors have considerable commitment to cell death even at 5 h, as indicated by DNA fragmentation detected by TUNEL assay (Fig. 1). It is also notable that even though the Th1 effectors are in the midst of dying as just noted, they nonetheless produce and secrete high levels of cytokines (Figs. 5 and 6) and incorporate radiolabeled nucleotides at high rates as if they were going to divide. All indications are that the DNA breaks detected by the TUNEL staining are well correlated with true cell death and cell disappearance, since very low Th1 cell recovery follows Th1, but not Th2, restimulation (Fig. 1).
It is noteworthy that both Th1 and Th2 effectors which are cultured without antigen die more slowly than when they are restimulated; however, the Th1 effectors nonetheless undergo death much sooner than the Th2 effectors (Fig. 3). One possible scenario is that in effectors that are not restimulated, levels of FasL are sufficient only for occasional induction of apoptosis (Fig. 7 b), and that restimulation leads to rapid expression of FasL (probably from intracellular stores), as well as higher Fas expression (Fig. 8 a) allowing a high level of Fas/FasL interaction.
The unequal death of the two effector subsets might potentially be due to any number of intrinsic differences between the subsets. Therefore, we searched for evidence that the expression of other components in death pathways were responsible. Several studies have suggested that the presence of Bcl-2 on one hand (38, 39), or Bcl-xL(35) on the other, can protect T cells from apoptosis. But others have not found a role for Bcl-2 in blocking effector T cells from AICD (40, 41). van Parijs et al. concluded that induction of Bcl-xL by costimulation could protect naive T cells that die by a non–Fas-mediated pathway, but not activated T cells that use a Fas/FasL mechanism (41). We find that Bcl-2 and Bcl-xL expression is comparable in the Th1 and Th2 effectors (Zhang, X., L. Tsui, and S.L. Swain, unpublished data), implying that differential expression of these proteins by Th2 and Th1 effectors is not likely to be responsible for the differences in cell death observed. Of course other proteins which negatively regulate the Fasmediated death pathway might play a major or ancillary role in protecting Th2 effectors.
We recently showed that the cytokines, IL-2, and TGFβ1 were able to prevent Th2 effector death (13). We find that restimulated Th1 effectors are also rescued in the presence of TGF-β1 (our manuscript in preparation), providing a potential mechanism for Th1 effector expansion and suggesting that the effect of TGF-β is on a common apoptosis pathway shared by Fas– and non–Fas-mediated mechanisms of apoptosis. However, because the onset of death of Th1 effectors is rapid (within a few hours), they will have much less opportunity than their Th2 counterparts to encounter cytokines or other agents which might interfere with or block apoptosis and allow their expansion.
Differences in the susceptibility of Th1 and Th2 subsets to AICD can be expected to have a major impact on the course of immune responses. If in vivo Th1 effector populations that reencounter antigen rapidly produce cytokines and then die, it would bring an end to the effector response. The in vivo behavior of Th1 effectors in the presence or absence of restimulation with antigen has yet to be clearly recorded, but some viral infections similarly produce such a rapid exhaustion of the effector response (42). Based on our in vitro results, we predict that Th2 effectors will also behave differently in vivo, which could result in a shift in the response towards a more Th2 pattern with time in vivo. It may be one of the mechanisms of rapid loss of Th1, but not Th2, via Fas/FasL-mediated AICD in progressive HIV infection (3), and of selective loss of Th1 in the tolerance induced in vivo by antigen without adjuvants (4, 5). Clearly, further experiments will have to be done to analyze whether such a mechanism of selective Th1 death is responsible in vivo, but it is clearly an attractive possibility. Selective Th1 effector death in vitro may also partly explain the selective Th2 polarization which occurs when high doses of antigen are used to stimulate naive cells to develop into effectors (6; Rogers, P., X. Zhang, and S.L. Swain, manuscript submitted).
Thus, the difference in rates of AICD represents a clear distinction between Th1 and Th2 subsets, which provides one of the clearest differences (other than cytokine production) that differentiate between Th1 and Th2 effectors generated in short-term, in vitro cultures, and it provides a new clue to potential mechanisms that could contribute to the imbalance between Th subsets in many chronic diseases where massive or repeated antigen exposure may deplete Th1-polarized effectors leading to Th2 predominance.
Footnotes
The authors are thankful to S. Nagata and P. Golstein for providing valuable reagents.
This research was supported by National Institutes of Health grants AI37935 and AI26887 to S.L. Swain, GM52735 to D.R. Green, CA72994 and ACS IM414 to J.C. Reed, and a fellowship from the U.S. Army Breast Cancer Program to T. Sato. T. Brunner is a fellow of the Swiss Society for Med. Biol. Fellowships.
1 Abbreviations used in this paper: AICD, activation-induced cell death; CFSE, 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester; FasL, Fas ligand; ICAM, intracellular adhesion molecule; FAP-1, Fas-associated phosphatase 1; FL, fluorescein; mRNA, messenger RNA; PCCF, pigeon cytochrome c fragment 88-104; RPA, RNase protection assay; TdT, terminal deoxynucleotidyl transferase; Tg, transgenic.
X. Zhang and T. Brunner contributed equally to this work.
References
- 1.Fleischer B. Superantigens produced by infectious pathogens: molecular mechanism of action and biologic significance. Int J Clin Lab Res. 1994;24:193–197. doi: 10.1007/BF02592461. [DOI] [PubMed] [Google Scholar]
- 2.Soudeyns H, Rebai N, Pantaleo GP, Ciurli GP, Boghossian T, Sekaly RP, Fauci AS. The T cell receptor V beta repertoire in HIV-1 infection and disease. Semin Immunol. 1993;5:175–185. doi: 10.1006/smim.1993.1021. [DOI] [PubMed] [Google Scholar]
- 3.Estaquier J, Idziorek T, Zou W, Emilie D, Farber CM, Bourez JM, Ameisen JC. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)– mediated apoptosis of CD4+T cells from human immunodeficiency virus–infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burstein HJ, Shea CM, Abbas AK. Aqueous antigens induce in vivo tolerance selectively in IL-2 and IFNγ-producing (Th1) cells. J Immunol. 1992;148:3687–3691. [PubMed] [Google Scholar]
- 5.De Wit D, van Mechelen M, Ryelandt M, Figueiredo AC, Abramowicz D, Goldman M, Bazin H, Urbain J, Leo O. The injection of deaggregated gamma globulins in adult mice induces antigen-specific unresponsiveness of T helper type 1 but not type 2 lymphocytes. J Exp Med. 1992;175:9–14. doi: 10.1084/jem.175.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+T helper cell phenotype development in a T cell receptor–αβ– transgenic model. J Exp Med. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretscher PA, Wei G, Menon JN, BielefeldtOhmann H. Establishment of stable, cell-mediated immunity that makes susceptible mice resistant to Leishmania major. . Science (Wash DC) 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 8.Ramsdell F, Seaman MS, Miller RE, Picha KS, Kennedy MK, Lynch DH. Differential ability of Th1 and Th2 T cells to express Fas ligand and to undergo activation-induced cell death. Int Immunol. 1994;6:1545–1553. doi: 10.1093/intimm/6.10.1545. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg AD, English M, Swain SL. Distinct regulation of lymphokine production is found in fresh versus in vitro primed murine helper T cells. J Immunol. 1990;144:1800–1807. [PubMed] [Google Scholar]
- 10.Dubey C, Croft M, Swain SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J Immunol. 1996;157:3280–3289. [PubMed] [Google Scholar]
- 11.Cohen J. Exponential growth in apoptosis. Immunol Today. 1995;16:346–348. doi: 10.1016/0167-5699(95)80153-7. [DOI] [PubMed] [Google Scholar]
- 12.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature (Lond) 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X, Giangreco L, Broome E, Dargan CM, Swain SL. Control of CD4 effector fate: transforming growth factor β-1 and interleukin 2 synergize to prevent apoptosis and promote effector expansion. J Exp Med. 1995;182:699–709. doi: 10.1084/jem.182.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffman RL, Seymour BW, Lebman DA, Hiraki DD, Christiansen JA, Shrader B, Cherwinski HM, Savelkoul HF, Bond MW, Finkelman FD. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 15.Swain SL, Bradley LM, Croft M, Tonkonogy S, Atkins G, Weinberg AD, Duncan DD, Hedrick SM, Dutton RW, Huston G. Helper T cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 16.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 17.O'Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. doi: 10.1016/0952-7915(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 18.Swain SL. Generation and in vivo persistence of polarized Th1 and Th2 memory cells. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 19.Swain SL, Croft M, Dubey C, Haynes L, Rogers MP, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 20.Gajewski TF, Lancki DW, Stack R, Fitch FW. “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell J. Activation-induced death of mature T cells in the regulation of immune responses. Curr Opin Immunol. 1995;7:382–388. doi: 10.1016/0952-7915(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 22.Singer GG, Carrera AC, Marshak-Rothstein A, Martinez C, Abbas AK. Apoptosis, Fas and systemic autoimmunity: the MRL-lpr/lprmodel. Curr Opin Immunol. 1994;6:913–920. doi: 10.1016/0952-7915(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 23.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green D. Cell-autonomous Fas (CD95)/ Fas–ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 24.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature (Lond) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 25.Critchfield JM, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science (Wash DC) 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 26.Sarin A, Conan-Cibotti M, Henkart PA. Cytotoxic effect of TNF and lymphotoxin on T lymphoblasts. J Immunol. 1995;155:3716–3718. [PubMed] [Google Scholar]
- 27.Zheng L, Fisher G, Miller R, Pschon J, Lynch DH, Lenardo M. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature (Lond) 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 28.Bradley LM, Duncan DD, Tonkonogy S, Swain SL. Characterization of antigen-specific CD4+ effector T cells in vivo: immunization results in a transient population of MEL- 14−, CD45RB−helper cells that secretes interleukin 2 (IL-2), IL-3, in IL-4, and interferon γ. J Exp Med. 1991;174:547–559. doi: 10.1084/jem.174.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies: Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 30.Gorczyca W, Bigman K, Mittelman A, Ahmed T, Gong J, Melamed MR, Darzynkiewicz Z. Induction of DNA strand breaks associated with apoptosis during treatment of leukemias. Leukemia (Baltimore) 1993;7:659–670. [PubMed] [Google Scholar]
- 31.Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science (Wash DC) 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Murphy K, Loh DY, Weaver C, Russell JH. Differential activation of antigen-stimulated suicide and cytokine production pathways in CD4+T cells is regulated by the antigen-presenting cell. J Immunol. 1993;150:3832–3842. [PubMed] [Google Scholar]
- 33.Taga K, Chretien J, Cherney B, Diaz L, Brown M, Tosato G. Interleukin-10 inhibits apoptotic cell death in infectious mononucleosis T cells. J Clin Invest. 1994;94:251–260. doi: 10.1172/JCI117315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swain SL, Huston G, Tonkonogy S, Weinberg A. Transforming growth factor-β and IL-4 cause helper T cell precursors to develop into distinct effector helper cells that differ in lymphokine secretion pattern and cell surface phenotype. J Immunol. 1991;147:2991–3000. [PubMed] [Google Scholar]
- 35.Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. Bcl-x, a Bcl-2–related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 36.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, Nakao K, Nagata S. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154:3806–3813. [PubMed] [Google Scholar]
- 37.Dhein J, Walczak H, Baumler C, Debatin KM, Krammer PH. Autocrine T-cell suicide mediated by APO1/(Fas/CD95) Nature (Lond) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 38.Mor F, Cohen IR. IL-2 rescues antigen-specific T cells from radiation or dexamethasone-induced apoptosis. Correlation with induction of Bcl-2. J Immunol. 1996;156:515–522. [PubMed] [Google Scholar]
- 39.Noel PJ, Boise LH, Green JM, Thompson CB. CD28 costimulation prevents cell death during primary T cell activation. J Immunol. 1996;157:636–642. [PubMed] [Google Scholar]
- 40.Memon SA, Moreno MB, Petrak D, Zacharchuk CM. Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol. 1995;155:4644–4652. [PubMed] [Google Scholar]
- 41.van Parijs L, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–328. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 42.Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]