Abstract
Antibody responses against antibodies, such as rheumatoid factors, are found in several immunopathological diseases and may play a role in disease pathogenesis. Experience shows that they are usually difficult to induce experimentally. Antibodies specific for immunoglobulin constant regions (anti-allotypic) or for variable regions (anti-idiotypic) have been investigated in animal models; the latter have even been postulated to regulate antibody and T cell responses via network-like interactions. Why and how such anti-antibodies are induced during autoimmune diseases, has remained largely unclear. Because repetitively arranged epitopes in a paracrystalline structure of a viral envelope cross-link B cell receptors efficiently to induce a prompt T-independent IgM response, this study used immune complexes containing viruses or bacteria to evaluate the role of antigen pattern for induction of anti-antibody responses. We present evidence that antibodies bound to strictly ordered, but not to irregularly arranged, antigens dramatically enhance induction of anti-antibodies, already after a single immunization and without using adjuvants. The results indicate a novel link between anti-antibody responses and infectious agents, and suggest a similar role for repetitive self-antigens such as DNA or collagen involved in chronic immunopathological diseases.
Antibodies against the constant and the variable parts of immunoglobulins have been investigated in various studies. Anti-allotypic antibodies directed against heterologous and rheumatoid factors (RF)1 directed against autologous constant immunoglobulin regions have been induced by immunization with immune complexes (IC) that contained antibodies bound to hemocyanin (1), transferrin (2), collagen (3), or LPS (4). Anti-allotypic antibodies were mainly observed in experimental situations after immunization with heterologous immunoglobulin aggregates together with adjuvants (5), whereas RF (6–8) occur under physiological conditions and were shown to have various beneficial effects, such as clearance of IC from the blood (9), enhanced antigen presentation (10), and neutralization of certain pathogens as shown for herpes simplex virus in vitro (11) and trypanosomes in vivo (12). However, RF may be involved in the pathogenesis of synovitis in rheumatoid arthritis (13, 14) and of some immune complex diseases (15), because they can form immune complexes and efficiently activate the complement system (16). In contrast, anti-idiotypic antibodies have been postulated to play a role in the regulation of antibody (17, 18) and T cell responses (19, 20) via network-like interactions. Experimental induction of anti-antibodies in general is difficult and requires adjuvants plus allotypic or species differences (18); therefore, conclusions from these experiments for the role of anti-idiotypic antibodies are limited, and the biological significance of these antibodies is still unclear.
Because there is good evidence that repetitively arranged epitopes in a paracrystalline structure of a viral envelope cross-link B cell receptors efficiently to induce a prompt T-independent IgM response (21), this study attempted to test whether immune complexes with viruses or bacteria exhibiting highly ordered repetitive antigens on their surface may play a role in the induction of anti-antibody responses.
Materials and Methods
Infectious Agents.
VSV serotype Indiana, (VSV-IND, Mudd Summers isolate) and VSV serotype New Jersey, (VSV-NJ, Pringle isolate) were originally obtained from Professor D. Kolakowsky (University of Geneva, Switzerland) and grown on BHK cells in minimal essential medium. UV inactivation was performed as described earlier (22) and monitored by a plaque assay on Vero cells. Recombinant VSV-G protein was produced in a baculovirus expression system as described (23); recombinant baculovirus expressing VSV-G was a gift from Dr. D.H.L. Bishop (NERC Institute of Virology, Oxford, UK). Salmonella typhi strain E.83.728 was provided by F. Sadallah (University of Geneva, Switzerland). Pseudomonas aeruginosa strain Fischer IT-2 was obtained form the Swiss Serum and Vaccine Institute. Both bacteria were grown in tryptic soy (TS) broth at 37°C, quantified on TS agar plates and inactivated as a thin layer in a petri dish by UV irradiation for 10 min (Philips UV lamp, 15 W, distance 8 cm).
Antibodies and IC.
Anti-VSV mAb were obtained by fusion of a VSV-immune spleen from BALB/c mice on day 4 after primary (for IgM-secreting hybridomas) or on day 4 after secondary infection (for IgG-secreting hybridomas). The antibodies WN1 222-5 and WN4 245-2 (both IgG2a) are broadly reactive antiLPS–core antibodies derived from NZB mice (24). The antibodies 99-T2 (IgG2b) and 63-T2 (IgM) are highly specific anti-LPS– O-chain antibodies against Pseudomonas aeruginosa strain Fisher IT-2 and were generated in BALB/c mice (25). IC were generated by incubation of a mixture of UV-inactivated virus or bacteria with the respective antibodies for 1 h at room temperature. IC formation in the VSV model could be demonstrated indirectly by reduction of anti-VSV neutralizing antibody titers in mice immunized with IC compared with mice immunized with VSV alone.
ELISA for Anti-antibody Detection.
We used a sandwich ELISA with the following steps: (a) coating with isotype-specific goat anti– mouse antibody (1 μg/ml; Southern Biotechnologies, Birmingham, AL), (b) blocking with 2% BSA (Fluka, Buchs, Switzerland) in PBS, (c) mAb supernatant (0.2 μg/ml), (d) 20-fold prediluted mouse serum, titrated 1:2 over 11 dilution steps, (e) isotype-specific horseradish peroxidase (HRPO)–labeled goat anti–mouse antibodies(0.5 μg/ml, Southern Biotechnologies), (f) substrate ABTS (2.2′-azino-di-[3-ethylbenzthiazolin-sulfonate (6)], Boehringer Mannheim) and H2O2 (Fluka). Plates were coated over night at 4°C, all other incubations were for 60 to 90 min at room temperature. Between incubations, plates were washed three times with PBS containing 0.5 ml Tween 20 per liter. OD was measured at 405 nm in an ELISA reader. All anti-antibody titers are indicated as -log2 of 20-fold prediluted sera. For Fig. 1, C and D, the dilution step at half maximal OD was determined as shown in Fig. 1 A and then taken as anti-antibody titer. For Fig. 1 E, allotype-specific antiIgM antibodies (Southern Biotechnologies) were used for coating and detection at the same concentrations as described above.
Figure 1.
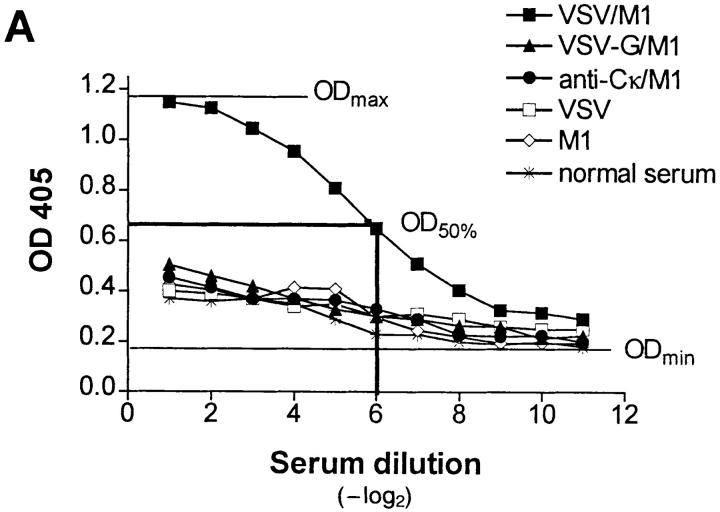
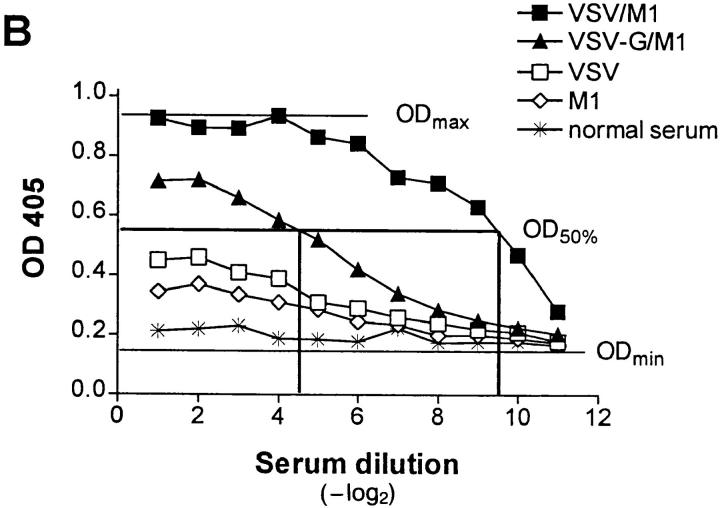
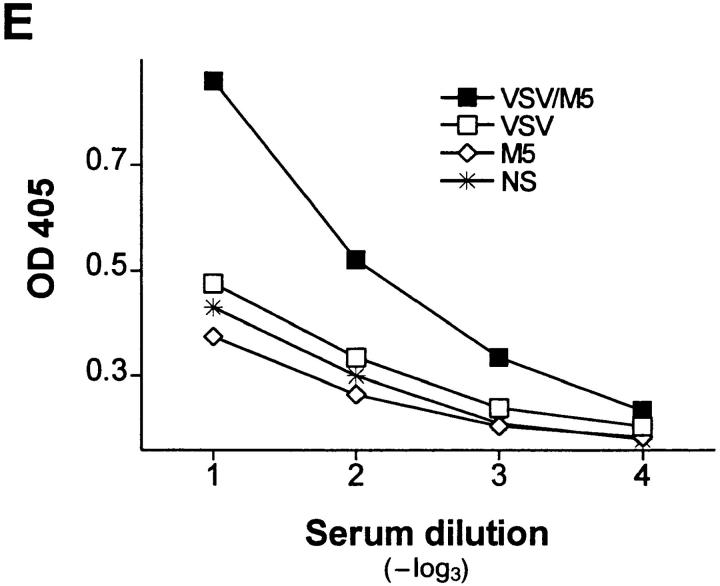
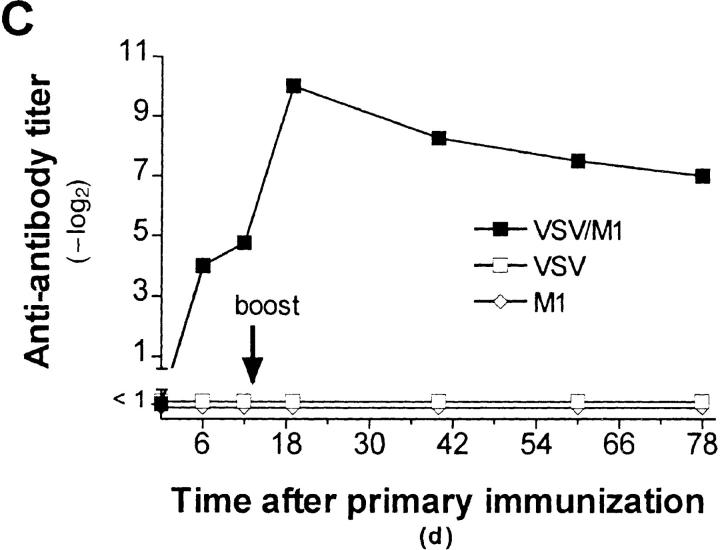
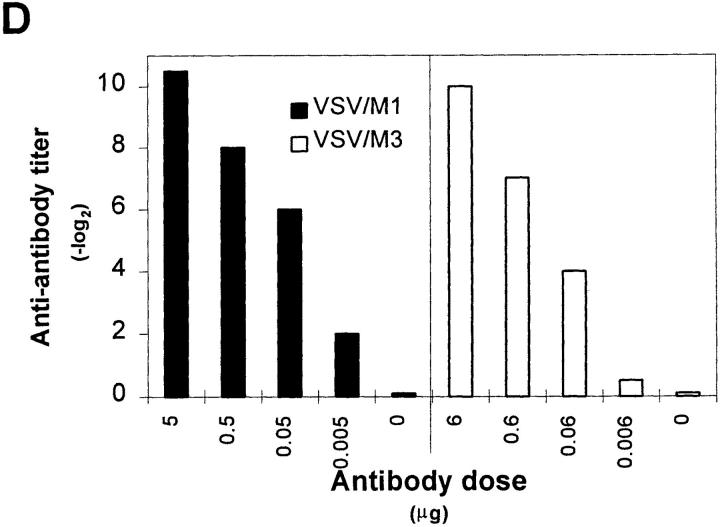
IgG anti-IgM response after immunization with immune complexes versus uncomplexed antigen. (A) BALB/c or (B) A/J mice were immunized twice (interval, 14 d) with 1 μg of the monoclonal anti-VSV-G IgM antibody M1 complexed with 108 PFU UV- inactivated VSV (closed squares), 10 μg of recombinant VSV-G (closed triangles), or 4 μg of rat anti–mouse Cκ antibody (closed circles), with IgM alone (open diamonds) or with VSV alone (open squares). 6 d after secondary immunization, IgG anti-IgM titers were determined by ELISA as described in Materials and Methods. (C) IgG anti-IgM titers of 20-fold prediluted sera were determined over a time period of 80 d after primary immunization as described in A. The same symbols are used. (D) Dose dependence of IgG anti-IgM induction shown by immunization of BALB/c mice with IC made of 108 PFU UV-inactivated VSV and titrated amounts of two different monoclonal anti-VSV-G IgM antibodies M1 (closed bars) and M3 (open bars). (E) C57BL/6 mice were immunized once with 5 μg of the monoclonal anti-VSV-G IgM antibody M5 complexed with 108 PFU UV- inactivated VSV (closed squares), with 5 μg of M5 alone (open diamonds) or with 108 PFU VSV alone (open squares). 4 d later, IgMa-specific IgMb antibodies were determined by ELISA as described in Materials and Methods.
VSV Neutralization Assay.
VSV-IND neutralizing antibodies (Fig. 2) were determined on the indicated timepoints by a plaque reduction assay on Vero cells as described before (26). Titers are indicated as -log2 of 40-fold prediluted sera.
Figure 2.
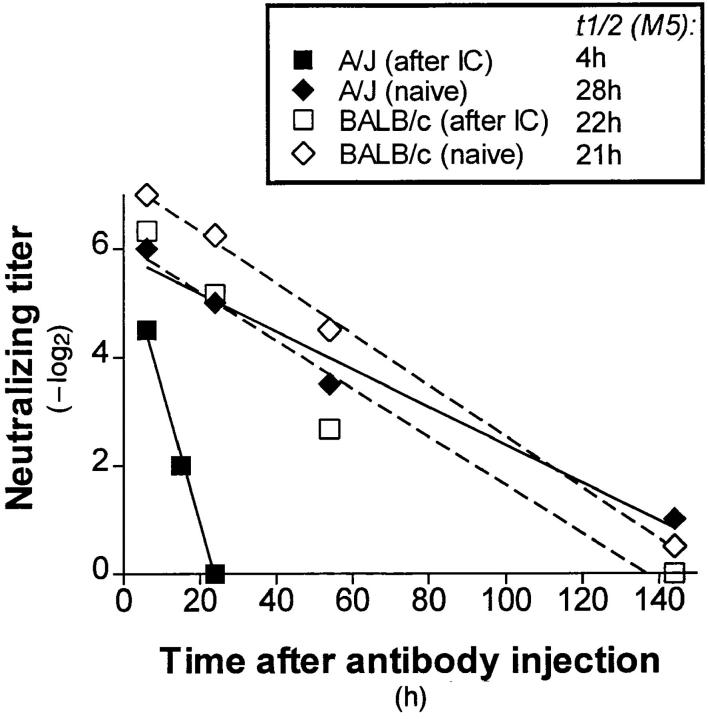
In vivo half-life of an IgM antibody (M5) in mice with or without anti-antibody titers. A/J (closed symbols) or BALB/c mice (open symbols) were twice (interval, 14 d) injected with VSV-NJ/M3 IC to induce anti-antibodies. 10 d after the second injection these mice (squares) and not immunized controls (diamonds) were injected with 5 μg of anti-VSVIND IgM (M5), and VSV-IND neutralizing titers were measured at the indicated timepoints. In vivo half-life of M5 was determined by regression analysis.
VSV-G–huHγ1.
VSV-G–huHγ1 fusion protein was generated following procedures developed by Traunecker and Karjalainen (27) and will be described elsewhere (Bucher, E., U. Kalinke, C. Lopez, T. Fehr, H. Hengartner, and R.M. Zinkernagel, manuscript in preparation). Mouse IgG anti-huIgG titers were determined by ELISA as described in Fig. 1 on plates coated with whole human serum diluted 1:100 in coating buffer. In vivo CD4+ T cell depletion was performed as described in Table 1.
Table 1.
Specificity and Helper T Cell Dependence of Anti-antibodies in BALB/c and A/J Mice
| Experiment* | Mouse strain | Immunization | Anti-antibody titer‡ on plates coated with | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M1§ | M2 | M3 | IgM anti-P.a. | |||||||||||
| 1 | BALB/c | IND/M1 (G7G9C9) | 7 | <1 | <1 | <1 | ||||||||
| IND/M2 (M4C11H12) | <1 | <1 | <1 | <1 | ||||||||||
| NJ/M3 (B3B8H6) | <1 | <1 | 9 | <1 | ||||||||||
| A/J | IND/M1 | 7.5 | 7 | 8 | 6 | |||||||||
| IND/M2 | <1 | <1 | <1 | <1 | ||||||||||
| NJ/M3 | 6 | 6 | 7.5 | 4 | ||||||||||
| M4 | M5 | M6 | IgM anti-P.a. | |||||||||||
| 2 | BALB/c | IND/M4 (M3F2B6) | 5 | <1 | <1 | <1 | ||||||||
| IND/M5 (H2F1C1) | <1 | 7 | <1 | <1 | ||||||||||
| IND/M6 (M4E11G8) | <1 | 1 | 7.5 | <1 | ||||||||||
| A/J | IND/M4 | 6.5 | 4 | 2 | 2 | |||||||||
| IND/M5 | 8 | 9.5 | 5.5 | 6.5 | ||||||||||
| IND/M6 | 3 | 3 | 6 | 2 | ||||||||||
| CD4+ T cells | M3 | M5 | ||||||||||||
| 3 | BALB/c | IND/M5 | Normal | <1 | 8 | |||||||||
| IND/M5 | Depleted‖ | <1 | <1 | |||||||||||
| BALB nu/nu | IND/M5 | Absent | <1 | <1 | ||||||||||
| A/J | NJ/M3 | Normal | 7.5 | 5 | ||||||||||
| NJ/M3 | Depleted‖ | 2 | <1 | |||||||||||
BALB/c (IgMa) and A/J (IgMe) mice were immunized twice with 108 PFU of inactivated VSV complexed with 3 to 8 μg of various anti-VSV-G IgM antibodies (M1–M6) with an interval of 14 d.
IgG anti-IgM titers are indicated as −log2 of 20-fold prediluted sera on day 6 after booster injection and are determined as described in Fig. 1 A.
ELISA plates were coated with a goat anti–mouse IgM as a catcher antibody for the different IgM hybridoma supernatants M1–M6 (anti-VSV) and 63-T2 (anti–Pseudomonas aeruginosa [P.a.]).
CD4+ T cells were depleted in vivo by intraperitoneal injection of 1 mg of the anti-CD4 antibody YTS 191.6 on days −3 and −1 before immunization.
Results
Induction of Anti-antibodies by VSV–IgM Complexes.
A strictly repetitive paracrystalline order of antigen in a viral envelope with a spacing of 5 to 10 nm has been shown to facilitate B cell responses, even to self-antigen (28). To evaluate whether antibodies bound to such highly organized antigens might also be presented in an ordered and repetitive fashion and therefore could induce anti-antibodies, BALB/c mice were immunized with IC of autologous antibodies bound to vesicular stomatitis virus (VSV) particles exhibiting paracrystalline strictly repetitive glycoproteins (G) in their envelope. Control groups were immunized with irregularly complexed or monomeric antibodies. While in vitro–generated IC containing 1 μg of a monoclonal IgM antibody M1 against VSV-G and 108 PFU of UV-inactivated VSV particles efficiently induced anti-antibodies, IC with the same amount of antibody M1 either irregularly complexed with the recombinant VSV-G protein spontaneously aggregating in tail-to-tail micelles (28a) or cross-linked with a rat anti–mouse Cκ antibody did not (Fig. 1 A). Similar results were obtained in A/J and C57BL/6 mice, but in this situation, IgG anti-IgM antibodies could also be induced by the poorly organized IC containing recombinant VSV-G, although at much lower titers than with the highly repetitive complex (Fig. 1 B). When BALB/c mice were boosted once after 14 d, only those treated with virus/antibody complexes exhibited IgM-specific IgG titers of 1:3,000, which did not drop significantly over a period of 80 d (Fig. 1 C). They were dependent on the antibody dose used for generation of IC, as shown for two different monoclonal IgM (Fig. 1 D). In similar experiments using different VSV-specific monoclonal IgG instead of IgM antibodies, no anti-antibodies were induced. This might be explained as follows: (a) the potentially immunogenic variable domains of IgG molecules binding to the virus surface are not easily accessible to B cells, or (b) clearance and processing of IgG- and IgMcontaining IC is distinct, because the former, but not the latter, exhibit easily accessible Fc domains that could bind to Fc receptors of macrophages, which would then lead to faster clearance of the IC. Also VSV infection itself did not induce anti-antibodies, probably because the virus only abortively replicates extraneuronally in mice and is rapidly eliminated within 1 d (29), before neutralizing IgM antibodies, which could lead to IC formation, are measured.
Specificity of Anti-antibodies Induced by VSV–IgM Complexes.
To determine the specificity of these anti-antibodies, we immunized BALB/c mice (IgMa) or A/J mice (IgMe) with IC formed with six different monoclonal VSV neutralizing IgM (M1–M6), which were isolated from BALB/c mice. M3 is specific for the distinct serotype VSV-New Jersey (VSV-NJ), the others for VSV Indiana (VSV-IND). Analysis of these sera on ELISA plates coated with M1 to M6 revealed that BALB/c mice produced anti-antibodies exclusively specific for the IgM used for immunization, whereas sera of A/J mice recognized any IgM as long as they were of BALB/c origin (Table 1). A monoclonal IgM of A/J origin as well as IgM of normal A/J serum was not recognized by either of the sera (data not shown). Therefore, the anti-antibodies induced in BALB/c mice are idiotype specific, whereas those induced in A/J mice were allotype-specific. One antibody (M2) failed to induce anti-antibodies (Table 1, Experiment 1); this correlated with its 10–100-fold lower neutralizing capacity and IC formation in vitro compared with the other IgM (data not shown). Both BALB/c antiidiotypic and A/J anti-allotypic antibodies were IgGs and, therefore, T helper cell dependent; this is demonstrated by the failure of CD4+ T cell–depleted or of athymic nude mice to respond with anti-antibodies of IgG isotype (Table 1, Experiment 3). Anti-allotypic antibodies were also found in C57BL/6 mice (IgMb), and in this case we were even able to detect an IgMa allotype-specific response on day 4 after primary immunization with IC (titers of 1:100), but not after immunization with virus or antibody alone (Fig. 1 E). This response could be prolonged by additional use of LPS (data not shown).
Next, it was tested whether the specificity of induced anti-antibodies as shown in ELISA could also be demonstrated in an independent in vivo read out. For this purpose, A/J and BALB/c were immunized twice with IC containing 108 PFU inactivated VSV-NJ and 5 μg of the antibody M3 to induce anti-antibodies. 10 d later these mice were treated with a specific IgM anti-VSV-IND antibody (M5), that did not cross-react with VSV-NJ. This enabled us to measure the half life of M5 by determining the neutralizing titer of the sera against VSV-IND (Fig. 2). In A/J mice, the half-life of M5 was reduced from 28 to 4 h, whereas in BALB/c mice it was comparable to unimmunized controls; this confirmed the presence of antibodies against the constant region of BALB/c immunoglobulin in A/J, but not in BALB/c mice. Thus, in this model situation anti-allotypic antibodies could be shown to modulate serum IgM antibodies by forming IC in vivo. We were not able to use the same experimental approach to assess modulation by anti-idiotypic antibodies, because the immunization with IC itself induced specific long-lived virus-neutralizing antibodies of IgG type.
Induction of Anti-antibodies by Bacteria–IgG Complexes.
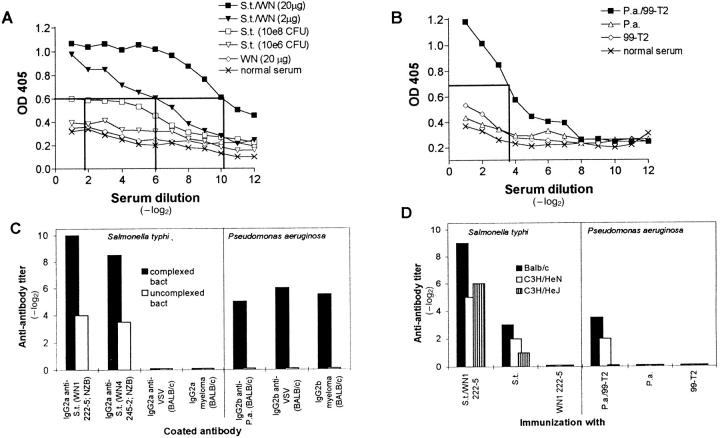
To evaluate whether these findings hold true not only for VSV, but also for bacteria exhibiting highly repetitive antigens on their surface, IC formed with gram negative bacteria (exhibiting regularly spaced LPS molecules) and anti-LPS antibodies were tested. 108 CFU of Salmonella typhi (S.t.) (Fig. 3 A) or Pseudomonas aeruginosa (P.a.) (Fig. 3 B) were UV-inactivated and complexed with a monoclonal IgG2a (origin: NZB) or IgG2b (origin: BALB/c) anti-LPS antibody, respectively, to immunize BALB/c mice. After 14 d, these mice were boosted once, and 6 d later IgG anti-IgG antibodies were found in both situations. The induction of these anti-antibodies was again dependent on the antibody dose used to form IC, as shown for S.t. (Fig. 3 A). With the highest dose (108 CFU) immunization with bacteria alone also induced some anti-antibodies, but at much lower titers than with IC. To test whether these results reflected polyclonal B cell activation by the bacterial LPS, we immunized LPS-responder (C3H/HeN, BALB/c) and LPS-nonresponder (C3H/HeJ) mice with IC or noncomplexed antibodies (Fig. 3 D) and tested the BALB/c sera on different antibodies of the same isotype (Fig 3 C). The results indicate: (a) Efficient LPS-mediated stimulation of B cells in the case of P.a., which, however, depended on the presence of IC; the induced anti-antibodies were of rheumatoid factor type because they recognized any autologous IgG2b of BALB/c origin (Fig. 3 C). (b) Successful induction of specific anti-allotypic antibodies by IC with S.t., which recognized only heterologous NZB-derived IgGs (Fig. 3 C) and were independent of B cell activation by LPS (Fig. 3 D). In the case of P.a., 38% (18 of 48) of the mice immunized twice with identical complexes died upon the second immunization from a shock-like syndrome compared with only 4% (2 of 48) of the control groups that were immunized with bacteria, purified LPS, or antibody only; this in vivo finding confirmed the presence of antiantibodies measured in vitro and, in addition, suggested a pathogenic function of RF-like anti-antibodies.
Figure 3.
IgG anti-IgG titers after immunization with antibodies complexed to Salmonella typhi (S.t.) and Pseudomonas aeruginosa (P.a.). (A) BALB/c mice were immunized twice (interval 14 d) with 108 CFU of S.t. complexed with 20 μg (closed squares) or 2 μg (closed triangles) of the anti-LPS antibody WN1 222-5 (IgG2a) or with 108 (open squares) or 106 (open triangles) CFU of S.t. alone or with 20 μg of antibody WN1 222-5 alone (open diamonds). 6 d after the second injection, IgG2b plus IgG1 anti-IgG2a titers in 20-fold prediluted sera were determined in a sandwich ELISA. (B) BALB/c mice were immunized twice with 108 PFU of P.a. complexed with 7 μg of the anti-LPS antibody 99-T2 (IgG2b) (closed squares), with 108 CFU of P.a. alone (open triangles) or with 7 μg of 99-T2 alone (open diamonds). IgG2a plus IgG1 anti-IgG2b titers of 20-fold prediluted sera were determined in a sandwich ELISA. (C) Sera of BALB/c mice immunized twice with IC or with bacteria alone as described in A or B were tested on ELISA plates coated with different antibodies of the corresponding isotype, and anti-antibody titers were determined as described in Fig 1. (D) BALB/c, LPS-responsive C3H/HeN, and LPS-nonresponsive C3H/HeJ mice were immunized twice with IC, and anti-antibody titers were determined 6 d after booster injection as described in the legend to Fig 1. Bars in C and D represent geometrical means of 2–3 animals per group. Standard deviation was within ± one dilution step. Results of one out of three comparable experiments are shown.
Immunogenicity of Multivaltent Versus Bivalent IC.
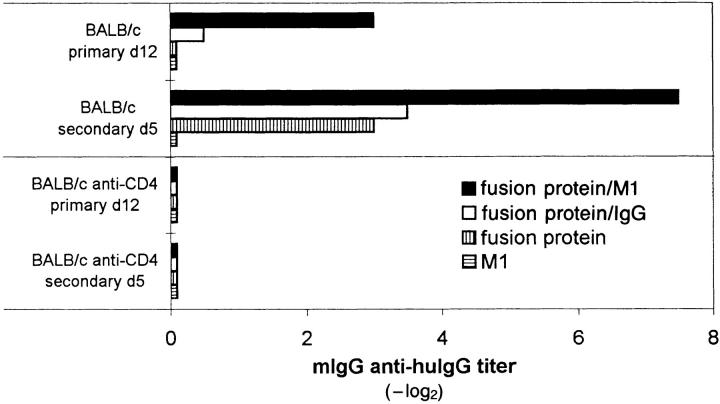
The notion that antibodies bound to repetitively ordered viral or bacterial antigens induced anti-antibodies was further tested with a sort of inversed IC. Complexes were formed between a VSV-G–specific decavalent IgM (M1) or a bivalent IgG2a antibody (VI49; reference 30) as core of the complex that binds a fusion protein of VSV-G plus constant part of the human IgG1 H chain (huHγ1) molecule. These IC display the Fc portions of huIgG1 as repetitive domains and form under optimal conditions decameric (with IgM) or dimeric (with IgG) complexes. In primary and secondary immune responses of BALB/c mice, the fusion protein complexed with the decavalent IgM induced much higher titers of anti-antibodies to huHγ1 than bivalent IgG-complexed or the noncomplexed fusion protein alone (Fig. 4). These anti-antibodies were of IgG isotype and T-help dependent, as shown by the negative effect of in vivo CD4+ T cell depletion. Although a very rigid IC structure cannot be assumed in this model, the results show that antibody responses to a foreign constant IgG region, which involves species differences, can be markedly enhanced by multimeric aggregation compared with dimers or monomers. Importantly, they may even suggest that IgM-bound antigen in turn binding IgG may be able to induce anti-IgG antibodies.
Figure 4.
Anti-antibody response upon immunization with a recombinant VSV-G–huHγ1 fusion protein alone or complexed with anti-VSV-G IgM or IgG antibodies. BALB/c mice were immunized with 10 μg of the fusion protein VSV-G-huHγ1 alone (vertically striped bars), the fusion protein complexed with 1 μg of M1 (closed bars) or 1 μg of a monoclonal VSV-G–specific IgG2a antibody (E5A7C9; open bars) or with 1 μg of M1 alone (horizontally striped bars). Mouse IgG (mIgG) anti-human IgG titers of 20-fold prediluted sera were determined on day 12 after primary and on day 5 after secondary immunization (day 18). Bars represent geometrical means of two to three mice per group. Standard deviation was within ± one dilution step.
In conclusion, the presented data suggest that anti-antibodies may be induced by antibodies bound to highly ordered repetitive antigens, but not by antibodies bound to oligo- or monomeric antigens. Formation of IC was necessary for induction of anti-allotypic, anti-idiotypic and RFlike antibodies. However, when allotypic (Fig. 1 B) or species differences (Fig. 4) were the target, anti-antibodies could be induced by poorly organized aggregates, whereas anti-idiotypic antibodies were only induced with highly ordered IC (Fig. 1 A). Induction of RF could only be demonstrated in the Pseudomonas model (Fig. 3 B), where in addition to repetitive antigen order the B cell stimulator LPS was necessary (LPS alone or IC containing purified LPS were not sufficient). These findings confirm that autoreactive B cells including those with specificity for membranebound self-antigens (e.g., immunoglobulin) may not be deleted (28, 31) and perhaps exert biological effects. It will be interesting to evaluate the relevant differences to various other membrane-bound self-antigens that cause deletion of autoreactive B cells (32–34).
Our findings indicate a novel link between anti-antibody responses and infectious agents or repetitive self-antigens. The role of highly ordered multimeric antigen patterns for efficient B cell activation has been tested systematically in several model situations involving linear (flagellin [35]; haptenated polymers [36]) or particulate antigens (Hepatitis B core antigen [37] or VSV [26]). Most infectious agents including viruses, bacteria, and parasites expose highly ordered repetitive antigenic epitopes on their surface. They are able to induce efficient T-independent IgM responses by cross-linking B cell receptors (21, 26, 38, 39). This study now demonstrates that the same rules seem to apply for induction of anti-antibodies. These anti-antibodies were readily induced after only two injections of repetitive IC involving mAbs, but without using adjuvants. In view of the observation that protective anti-Haemophilus influenzae (40) and anti-viral antibodies (30) are of restricted or virtually monoclonal specificity, our results with mAbs against infectious agents can probably be generalized. These findings may explain the secretion of RF during virus infections (influenza [41]), rubella, CMV, EBV, HIV) (6) and how the same configuration together with or without LPS may sustain anti-antibody responses in the course of bacterial infections (such as bacterial endocarditis, tuberculosis, or syphilis; for review see 42). Especially chronic infections with persistence of the pathogen during an ongoing antibody response would fulfill the conditions for IC formation in vivo, as it is described for herpesviruses (43, 44), HIV (45, 46), or mycobacteria (47, 48). In addition, the results in BALB/c mice could suggest that, instead of RF, anti-idiotypic antibodies may enhance immunopathology found in reactive forms of arthritis, which are apparently RF negative. Also, repetitive self antigens (e.g., collagen II [49] in rheumatoid lesions, DNA [50] in SLE) exposed after primary or secondary tissue injury may induce anti-antibodies by formation of highly repetitive immune complexes. This may explain why RF secretion is such a typical manifestation accompanying these diseases (15).
Acknowledgments
We thank S. Ehl, P. Klenerman, and M. van den Broek for helpful discussions and critical reading of the manuscript, and A. Althage for excellent technical support.
This work was supported by the Swiss National Science Foundation (grant no. 31-32195.91 to R.M. Zinkernagel) and the Kanton of Zürich.
Footnotes
1 Abbreviations used in this paper: HRPO, horseradish peroxidase; IC, immune complex; P.a., Pseudomonas aeruginosa; RF, rheumatoid factors; S.t., Salmonella typhi; TS, trypic soy; VSV, vesicular stomatitis virus; VSV-G, VSV glycoprotein; VSV-G–huHγ1, fusion protein of VSV-G with human IgG1 heavy chain constant regions; VSV-IND, VSV serotype Indiana; VSV-NJ, VSV serotype New Jersey.
References
- 1.Nemazee DA. Immune complexes can trigger specific, T cell–dependent, autoanti-IgG antibody production in mice. J Exp Med. 1985;161:242–256. doi: 10.1084/jem.161.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coulie PG, Van Snick J. Rheumatoid factor (RF) production during anamnestic immune responses in the mouse. III. Activation of RF precursor cells is induced by their interaction with immune complexes and carrier-specific helper T cells. J Exp Med. 1985;161:88–97. doi: 10.1084/jem.161.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmdahl R, Nordling C, Rubin K, Tarkowski A, Klareskog L. Generation of monoclonal rheumatoid factors after immunization with collagen II–anti-collagen II immune complexes. An anti-idiotypic antibody to anti-collagen II is also a rheumatoid factor. Scand J Immunol. 1986;24:197–203. doi: 10.1111/j.1365-3083.1986.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 4.Kanoh M, Utsumi S, Hino T. Induction of rheumatoid factors in mice by immune complexes of bacterial lipopolysaccharide with mouse IgG antibody. Eur J Immunol. 1986;16:63–68. doi: 10.1002/eji.1830160113. [DOI] [PubMed] [Google Scholar]
- 5.Spring SB, Nisonoff A. Allotypic markers on Fab fragments of mouse immunoglobulins. J Immunol. 1974;113:470–478. [PubMed] [Google Scholar]
- 6.Schrohenloher, R.E., and W.J. Koopman. 1993. Rheumatoid factor. In Arthritis and Allied Conditions. D.J. McCarty and W.J. Koopman, editors. Lea and Febiger, Philadelphia/ London. 861–876.
- 7.Carson, D.A. 1993. Rheumatoid factor. In Textbook of Rheumatology. W.N. Kelley, E.D. Harris, S. Ruddy, and C.B. Sledge, editors. W.B. Saunders Company, Philadelphia. 155–163.
- 8.Carson DA, Chen PP, Kipps TJ. New roles for rheumatoid factor. J Clin Invest. 1991;87:379–383. doi: 10.1172/JCI115007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Snick JL, Van Roost E, Markowetz B, Cambiaso CL, Masson PL. Enhancement by IgM rheumatoid factor of in vitro ingestion by macrophages and in vivo clearance of aggregated IgG or antigen–antibody complexes. Eur J Immunol. 1978;8:279–285. doi: 10.1002/eji.1830080412. [DOI] [PubMed] [Google Scholar]
- 10.Roosnek E, Lanzavecchia A. Efficient and selective presentation of antigen–antibody complexes by rheumatoid factor B cells. J Exp Med. 1991;173:487–489. doi: 10.1084/jem.173.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Notkins, A.L. 1971. Infectious virus–antibody complexes: interaction with anti-immunoglobulins, complement, and rheumatoid factor. J. Exp. Med. 134(Suppl):41s–51s. [PubMed]
- 12.Clarkson AB, Jr, Mellow GH. Rheumatoid factor–like immunoglobulin M protects previously uninfected rat pups and dams from Trypanosoma lewisi. Science (Wash DC) 1981;214:186–188. doi: 10.1126/science.7025211. [DOI] [PubMed] [Google Scholar]
- 13.Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16:265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
- 14.del Puente A, Knowler WC, Pettitt DJ, Bennett PH. The incidence of rheumatoid arthritis is predicted by rheumatoid factor titer in a longitudinal population study. Arthritis Rheum. 1988;31:1239–1244. doi: 10.1002/art.1780311004. [DOI] [PubMed] [Google Scholar]
- 15.Hughes, G.R.V. 1987. Connective Tissue Diseases. Blackwell Scientific Publications, Oxford. 1–286.
- 16.Winchester RJ, Agnello V, Kunkel HG. Gamma globulin complexes in synovial fluids of patients with rheumatoid arthritis. Partial characterization and relationship to lowered complement levels. Clin Exp Immunol. 1970;6:689–706. [PMC free article] [PubMed] [Google Scholar]
- 17.Jerne NK. Towards a network theory of the immune system. Ann Inst Pasteur Immunol. 1974;125C:373–389. [PubMed] [Google Scholar]
- 18.Eichmann K. Expression and function of idiotypes on lymphocytes. Adv Immunol. 1978;26:195–254. doi: 10.1016/s0065-2776(08)60231-x. [DOI] [PubMed] [Google Scholar]
- 19.Binz H, Wigzell H. Antigen-binding idiotypic T lymphocyte receptors. Contemp Topics Immunobiol. 1977;7:113. doi: 10.1007/978-1-4684-3054-7_4. [DOI] [PubMed] [Google Scholar]
- 20.Pereira P, Bandeira A, Coutinho A, Marcos M-A, Toribio M, Martinez-A C. V-region connectivity in T cell repertoires. Annu Rev Immunol. 1989;7:209–249. doi: 10.1146/annurev.iy.07.040189.001233. [DOI] [PubMed] [Google Scholar]
- 21.Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell–independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? . Eur J Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, Bast C, Hengartner H, Zinkernagel RM. Immunogenicity of a viral model vaccine after different inactivation procedures. Med Microbiol Immunol. 1994;183:95–104. doi: 10.1007/BF00277160. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura Y, Possee RD, Overton HA, Bishop DHL. Baculovirus expression vectors: the requirements for high level expression of proteins, including glycoproteins. J Gen Virol. 1987;68:1233–1250. doi: 10.1099/0022-1317-68-5-1233. [DOI] [PubMed] [Google Scholar]
- 24.Di Padova FE, Brade H, Barclay GR, Poxton IR, Liehl E, Schuetze E, Kocher HP, Ramsay G, Schreier MH, McClelland DB, et al. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharides. Infect Immun. 1993;61:3863–3872. doi: 10.1128/iai.61.9.3863-3872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang, A.B., U. Bruderer, E. Fuerer, J.W. Larrick, and S.J. Cryz. 1990. Immunoprotective capacities of human and murine monoclonal antibodies recognizing serotype-specific and common determinants of gram-negative bacteria. In Therapeutic Monoclonal Antibodies. C.A.K. Borrebaeck and J.W. Larrick, editors. Stockton Press, New York. 223–234.
- 26.Fehr T, Bachmann MF, Bluethmann H, Kikutani H, Hengartner H, Zinkernagel RM. T-independent activation of B cells by Vesicular stomatitis virus: no evidence for the need of a second signal. Cell Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- 27.Traunecker A, Schneider J, Kiefer HR, Karjalainen K. Highly efficient neutralization of HIV with recombinant CD4–immunoglobulin molecules. Nature (Lond) 1989;339:68–70. doi: 10.1038/339068a0. [DOI] [PubMed] [Google Scholar]
- 28.Bachmann MF, Hoffmann U, Rohrer, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science (Wash DC) 1993;262:1448–1451. doi: 10.1126/science.8248784. [DOI] [PubMed] [Google Scholar]
- 28a.Petri WA, Jr, Wagner RR. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979;254:4313–4316. [PubMed] [Google Scholar]
- 29.Wagner, R.R. 1987. The Rhabdoviruses. Plenum Press, New York. 1–544.
- 30.Kalinke U, Bucher EM, Ernst B, Oxenius A, Roost HP, Geley S, Kofler R, Zinkernagel RM, Hengartner H. The role of somatic mutation in the generation of the protective humoral immune response against vesicular stomatitis virus (VSV) Immunity. 1996;5:639–652. doi: 10.1016/s1074-7613(00)80277-0. [DOI] [PubMed] [Google Scholar]
- 31.Murakami M, Tsubata T, Okamoto M, Shimizu A, Kumagai S, Imura H, Honjo T. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature (Lond) 1992;357:77–80. doi: 10.1038/357077a0. [DOI] [PubMed] [Google Scholar]
- 32.Nemazee DA, Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature (Lond) 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 33.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature (Lond) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 34.Tighe H, Heaphy P, Baird S, Weigle WO, Carson DA. Human immunoglobulin (IgG) induced deletion of IgM rheumatoid factor B cells in transgenic mice. J Exp Med. 1995;181:599–606. doi: 10.1084/jem.181.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldmann M, Basten A. The relationship between antigenic structure and the requirement for thymusderived cells in the immune response. J Exp Med. 1971;134:103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dintzis RZ, Middleton MH, Dintzis HM. Inhibition of anti-DNP antibody formation by high doses of DNP-polyacrylamide molecules; effects of hapten density and hapten valence. J Immunol. 1985;135:423–427. [PubMed] [Google Scholar]
- 37.Schödel F, Peterson D, Zheng J, Jones JE, Hughes JL, Milich DR. Structure of hepatitis B virus core and e-antigen. A single precore amino acid prevents nucleocapsid assembly. J Biol Chem. 1993;268:1332–1337. [PubMed] [Google Scholar]
- 38.Burns WH, Billups LC, Notkins AL. Thymus dependence of viral antigens. Nature (Lond) 1975;256:654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- 39.Szomolanyi-Tsuda E, Welsh RM. T cell–independent antibody-mediated clearance of polyoma virus in T cell–deficient mice. J Exp Med. 1996;183:403–411. doi: 10.1084/jem.183.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barington T, Hougs L, Juul L, Madsen HO, Ryder LP, Heilmann C, Svejgaard A. The progeny of a single virgin B cell predominates the human recall B cell response to the capsular polysaccharide of Haemophilus influenzaetype b. J Immunol. 1996;157:4016–4027. [PubMed] [Google Scholar]
- 41.Fazekas G, Rajnavolgyi E, Kurucz I, Sintar E, Kiss K, Laszlo G, Gergely J. Isolation and characterization of IgG2a-reactive autoantibodies from influenza virus–infected BALB/c mice. Eur J Immunol. 1990;20:2719–2729. doi: 10.1002/eji.1830201229. [DOI] [PubMed] [Google Scholar]
- 42.Williams, R.C. 1988. Rheumatoid factors in subacute bacterial endocarditis and other infectious diseases. Scand. J. Rheumatol. 75 (Suppl.):300–308. [DOI] [PubMed]
- 43.Ferraro AS, Newkirk MM. Correlative studies of rheumatoid factors and anti-viral antibodies in patients with rheumatoid arthritis. Clin Exp Immunol. 1993;92:425–431. doi: 10.1111/j.1365-2249.1993.tb03415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchiya N, Williams RC, Jr, Hutt-Fletcher LM. Rheumatoid factors may bear the internal image of the Fc gamma-binding protein of herpes simplex virus type 1. J Immunol. 1990;144:4742–4748. [PubMed] [Google Scholar]
- 45.Schuval SJ, Bonagura VR, Ilowite NT. Rheumatologic manifestations of pediatric human immunodeficiency virus infection. J Rheumatol. 1993;20:1578–1582. [PubMed] [Google Scholar]
- 46.Medina-Rodriguez F, Guzman C, Jara LJ, Hermida C, Albourek D, Cervera H, Miranda JM, Fraga A. Rheumatic manifestations in human immunodeficiency virus positive and negative individuals: a study of 2 populations with similar risk factors. J Rheumatol. 1993;20:1880–1884. [PubMed] [Google Scholar]
- 47.Chavez-Legaspi M, Gomez-Vazquez A, Garcia-De I La Torre. Study of rheumatic manifestations and serologic abnormalities in patients with lepromatous leprosy. J Rheumatol. 1985;12:738–741. [PubMed] [Google Scholar]
- 48.Kardjito T, Grange JM. Immunological and clinical features of smear-positive pulmonary tuberculosis in East Java. Tubercle. 1980;61:231–238. doi: 10.1016/0041-3879(80)90043-4. [DOI] [PubMed] [Google Scholar]
- 49.Holmdahl R, Andersson M, Goldschmidt TJ, Gustafsson K, Jansson L, Mo JA. Type II collagen autoimmunity in animals and provocations leading to arthritis. Immunol Rev. 1990;118:193–232. doi: 10.1111/j.1600-065x.1990.tb00817.x. [DOI] [PubMed] [Google Scholar]
- 50.Izui S, Eisenberg RA. Circulating anti-DNA– rheumatoid factor complexes in MRL/1 mice. Clin Immunol Immunopathol. 1980;15:536–551. doi: 10.1016/0090-1229(80)90065-3. [DOI] [PubMed] [Google Scholar]