Abstract
The N-propionylated group B meningococcal polysaccharide (NPrGBMP) mimics a unique protective epitope on the surface of group B meningococci (GBM) and Escherichia coli K1. Using a series of monoclonal antibodies (mAbs) induced by the NPrGBMP–monomeric tetanus toxoid (TT) conjugate vaccine it was demonstrated that mAbs having specificities for both extended and conventional short segments of the NPrGBMP were formed, but only the former were bactericidal, and/or gave passive protection against live challenge by GBM. The failure of mAbs specific for short epitopes to protect was further established when (NeuPr)4–TT was used as the vaccine. Of all the mAbs produced that were specific for short internal segments of the NPrGBMP, none were protective, despite the fact that most of them cross-react with the GBM capsular polysaccharide. In contrast, most of the protective mAbs produced by NPrGBMP– TT did not recognize the group B meningococcal polysaccharide (GBMP) unless it was present in its aggregated high molecular weight form. The bactericidal epitope mimicked by the NPrGBMP was shown to be ubiquitous in the capsule of both GBM and E. coli K1 using immunogold labeling techniques and, because of its unique properties, its identification could be significant in the development of a comprehensive conjugate vaccine against group B meningococcal meningitis. This is because most known human α(2–8)-polysialic acid self-antigens can be accommodated in 30–50 α(2–8)-linked sialic acid residues, which is roughly equivalent to an 11-kD length of the GBMP. It has been hypothesized that the formation of the protective epitope on the surface of GBM is due to the interaction of helical segments of the GBMP with another molecule and that the protective epitope is mimicked by the NPrGBMP. Support for the above hypothesis is provided by the fact that the protective NPrGBMP epitope has a similar unusual length dependency to that of the GBMP epitope.
Group B meningococci (GBM)1 remain a major world health problem and the poor immunogenicity of the group B meningococcal polysaccharide (GBMP) prevents the formulation of a comprehensive polysaccharide-based vaccine against meningococcal meningitis (1). While the covalent coupling of the GBMP to protein carriers to form T-dependent antigens did result in enhanced polysaccharide-specific antibody levels, including antibodies of the IgG isotype, these levels were still generally low and no bactericidal activity was reported (2, 3). It is probable that molecular mimicry is involved in the poor immunogenicity of the GBMP because its capsule, which is also identical to that of Escherichia coli K1, consists of a homopolymer of α(2–8)-linked sialic acid residues (4), and similar structures have been identified in human tissue antigens (5, 6). The sizes of these human tissue antigens range from shorter trimeric fragments found in mammalian gangliosides (7) to well in excess of decameric fragments carried by neural cell adhesion (N-CAM) glycoproteins and other tissues including human tumors (6).
Despite the poor immunogenicity of the GBMP, GBMPspecific antibodies can be produced in special circumstances (8, 9) and it has been established that all these antibodies require a minimum of about nine sialic acid residues for binding to occur (10, 11). It has been proposed that the above antibodies recognize an extended helical form of α(2-8)-polysialic acid on the basis that α(2-8)-polysialic acid and poly(A), with a known propensity to form extended helices, share a common length-dependent epitope (12). Support for this hypothesis has been obtained by potential energy calculations and nuclear magnetic resonance spectroscopy (NMR) studies, which indicate that α(2-8)-polysialic acid can form local helices of this type (13, 14). More convincing evidence was obtained by x-ray diffraction analysis of a Fab fragment of an α(2–8)-polysialic acid–specific monoclonal IgG antibody (mAb 735) in which the binding site was shown to be a groove that could accommodate such an extended helical structure (15).
Currently, there is no vaccine against group B meningococcal meningitis and most efforts to develop an effective vaccine have focused on alternative surface-exposed components such as outer membrane proteins and lipopolysaccharides (8). Many problems associated with the development of vaccines based on these components have been identified, not the least of which is their intrinsic antigenic diversity (8). Because the GBMP is the only conserved antigenic structure on the surface of GBM, a polysaccharidebased vaccine would be the vaccine of choice, provided one could overcome its poor immunogenicity without deleterious autoimmune effects. A novel approach to achieve this goal is to replace the N-acetyl groups of the GBMP with N-propionyl groups before its conjugation to tetanus toxoid (TT) to form a synthetic glycoconjugate vaccine (16). The NPrGBMP–TT conjugate, when administered in CFA, was able to induce high titers of NPrGBMP-specific antibodies in mice, which were highly bactericidal for GBM (16, 17) and passively protective against E. coli K1 (17). It was demonstrated by absorption experiments that the polyclonal NPrGBMP-specific antisera consists of two populations of antibodies, one of which (minor population) crossreacts with the high molecular weight (hmw) GBMP but is not bactericidal, whereas surprisingly the other larger population of hmw GBMP noncross-reactive antibodies contains all the bactericidal activity (18, 19). This evidence indicated that the NPrGBMP mimics a different epitope on the surface of GBM and E.coli K1 than is presented by the hmw GBMP (18, 19). To define further this epitope, we have produced a series of mAbs using an NPrGBMP–TT conjugate as immunogen. Using these mAbs, we have established that the NPr GBMP protective epitope has the same length dependency as the GBMP epitope, and that the natural epitope it mimics is located in the capsular layer of both GBM and E. coli K1 but can be detected in the hmw GBMP.
Materials and Methods
Materials.
Colominic acid, sodium salt (E. coli K1) was obtained from Nacalai Tesque (Kyoto, Japan). Human serum albumin (HSA) was obtained from Sigma Chemical Co. (St. Louis, MO), and TT was obtained from Statenserum Institut (Copenhagen, Denmark). RIBIs complete adjuvant system (RIBI ImmunoChem, Hamilton, MT) was used according to the specifications of the manufacturer. Neisseria meningitidis strains 80–165 (B:2b:P1. Ham) and M992 (B:5:P1.1,7), and E. coli 841743 (018:K1:H7) were obtained from the culture collection of the Laboratory Centre for Disease Control (Ottawa, Canada). The hmw GBMP was prepared and purified from strains M992 and 841743 as previously described (20), except that a GC medium was employed. mAb735 was provided by Professor D. Bitter-Suermann, Medizinische Hochschule (Hannover, Germany). 1H– and 13C–NMR spectra were recorded at 500 and 125 MHz, respectively with a Bruker AMX 500 instrument in the pulsed Fourier-transform mode at 300° K. The polysaccharides and oligosaccharides were twice lyophilized from 99.7% D2O and were examined in the same solvent.
Synthesis of NPr GBMP.
NPrGBMP was prepared as previously described (16) with modifications resulting in improved yields of product. Colominic acid (500 mg) was N-deacetylated using 2M NaOH (10 ml) containing NaBH4 (100 mg) for 6.5 h at 110°C. The N-deacetylated polysaccharide was treated directly with propionic anhydride (10 eq) in small installments while maintaining the pH at 9.5 with the use of an autotitrator and 2N NaOH. The solution was then exhaustively dialyzed against dH2O at 4°C and lyophilyzed. Complete N-propionylation was confirmed by integrating appropriate signals in the 1H–NMR spectrum of the product (16).
Synthesis of a 11-kD NPrGBMP–TT.
The NPrGBMP was activated as previously described (16) by selective periodate oxidation (100 eq/polysaccharide) of the nonreducing terminus, followed by size fractionation of the polysaccharide on a Bio-Gel A.5 column (Bio-Rad, Richmond, California). Selection of a 11-kD fragment with a narrow size range (10–12 kD) was accomplished by screening the resultant fractions using SEC–HPLC (Superose-12, Pharmacia Biotech, Uppsala, Sweden), and comparing them with a calibration curve constructed using a series of salic acid (NeuAc)n oligosaccharides. The choice of an 11-kD fragment was based on the fact that mouse polyclonal antisera induced by this vaccine, rather than others synthesized using longer or shorter lengths of NPrGBMP, was optimal in terms of bactericidal activity (data not shown). The oxidized 11-kD fragment (10 mg), referred to henceforth as NPrGBMP, was then combined with freshly purified monomeric (2) TT (3 mg) in a solution of phosphate buffer (0.2 M; pH 8.1) containing NaBH3CN (6 mg) and incubated at 37°C for 5 d. The material was purified by gel filtration chromatography on Bio-Gel A.5. Sialic acid content was determined by the method of Svennerholm (21) and protein was determined using a modified bicinchoninic acid protein microassay (Pierce, Rockford, IL). The final conjugate was found to contain 30% sialic acid (wt/wt), which is equivalent to six chains of polysaccharide per TT molecule. In a similar manner, equivalent 11-kD fragments NPrGBMP and GBMP were conjugated to HSA for use as coating antigens.
Synthesis of an α(2–8)-linked (NeuPr)4–TT.
The N-propionylated α(2–8)-linked sialooligosaccharides (NeuPr)n were obtained as previously described (10), except that the partial hydrolysis was optimized for the production of (NeuPr)10. This was determined by both anion exchange HPLC (Mono-Q) and SEC–HPLC (Superdex G-75) using appropriate oligosaccharide standards. In brief, the NPrGBMP (500 mg) was hydrolyzed in NaOAc buffer (0.2 M; pH 5.1) for 30 min at 70°C, and the oligomers were separated on a DEAE Sephadex A-25 ion exchange column (Cl-form) (Pharmacia) using a NaCl gradient elution. The degree of polymerization and purity of the oligomers was also confirmed by 1H– and 13C–NMR. The purified (NeuPr)4 oligosaccharide (10 mg; 4 mg/ml) was activated using NaIO4 (2 eq) at 4°C for 60 min, and activated (NeuPr)4 was purified on a Sephadex G-10 (Pharmacia) desalting column. Oxidized (NeuPr)4 (2 mg) was combined with monomeric TT (5 mg; 45:1 molar ratio) in sodium phosphate buffer (0.33 ml; 0.2 M; pH 8.1) containing NaBH3CN (7 mg) and heated at 37°C for 5 d. The (NeuPr)4–TT conjugate was purified on a Bio-Gel A.5 column in PBS buffer (pH 7.6). The final conjugate contained 4.3% sialic acid or six chains of (NeuPr)4 per TT molecule. A (NeuPr)4–HSA conjugate was also synthesized as a coating antigen essentially as described above for the (NeurPr)4–TT conjugate.
Preparation of a (NeurPr)4–HSA Coating Antigen Via Conjugation of (NeuPr)5 Through the Reducing Terminus ([NeurPr]4RE–HSA).
Purified (NeuPr)5 oligosaccharide (9 mg; 5.5 umol) was combined with HSA (5 mg; 0.074 μmol) and NaBH3CN (8.7 mg; 0.14 mmol) and the mixture was dissolved in 250 μl of borate buffer (0.2 M; pH 9.2). The mixture was heated at 37°C for 5 d with periodic monitoring by SEC–HPLC (Superose-12; Pharmacia). At the end of the 5-d interval, the conjugate was purified by passage of the mixture over a column of Bio-Gel A.5 (1.6 × 50 cm) equilibrated in PBS. The high molecular weight peak was collected, dialyzed against dH2O, and lyophilized to 8.7 mg of (NeuPr)4RE–HSA.
Preparation of MAbs.
NPrGBMP–TT and (NeuPr)4–TT were injected intraperitoneally into two groups of five BALB/c mice (6–8 wk; Charles River, St. Constant, Canada). Each mouse received 2 μg of sialic acid in 0.2 ml of RIBIs complete adjuvant per injection. The mice were boosted with an equivalent amount of vaccine on day 21, test bled via the tail vein on day 29, and the mouse with the highest antibody titer to the homologous antigen was selected for a final intravenous boost on day 35 with the vaccine in saline (0.1 ml). 3 d after intravenous boost, the mice were splenectomized and the spleen cells were fused with Sp2/O myeloma cells using a 10:1 ratio of spleen cells to myeloma cells. The resulting hybridomas were screened by ELISA with a panel of HSA conjugates and the selected hybridomas were cloned by limiting dilution. For the production of ascites fluids, pristane primed BALB/c mice were injected intraperitoneally with 2 × 106 cells in 0.5 ml DMEM and the resulting fluid was collected and pooled daily. MAbs were purified from ascitic fluid by affinity chromatography on protein A– or G–Sepharose columns (Pharmacia). The final concentrations of the purified mAbs were determined using the modified bicinchoninic acid protein microassay.
ELISA for Measuring Direct Binding of mAb.
The wells of Corning microtiter plates (Corning Glass Works, NY) were coated at 37°C for 1 h with various HSA conjugates (1 μg/100 μl PBS/ well) or by the hmw GBMP after a precoating of the wells with BSA (1 μg/100 μl PBS/well). The plates were then washed (three times) with PBS and Tween-20 (0.05%) (Allen Fisher Assoc., Haddonfield, NJ), followed by blocking with 200 μl of 1% BSA in PBS for 1 h at room temperature. The contents of the wells were removed and serial dilutions (100 μl/well) of either culture supernatant, ascites, or purified mAb in 3% BSA were performed, followed by incubation for 60 min at ambient temperature. After washing (three times, PBS and Tween-20), 100 μl of a 1:2,000 dilution in 3% BSA of a peroxidase-labeled goat anti–mouse IgG(H + L) or IgG + IgM antibody (Kirkegaard & Perry Laboratories, Gaithersburg, MD) was added and the plates were incubated for 1 h at room temperature. The wells were then reacted after washing (three times, PBS and Tween-20; one time, PBS) with 50 μl of TMB and peroxide substrate (Kirkegaard & Perry Laboratories) and allowed to develop for 10 min before stopping with 1 M H3PO4 (50 μl). The plates were read at 450 nm using a BioTek multiscan ELISA plate reader. Isotyping experiments were performed using an ELISA system as described above with the substitution of peroxidase-labeled goat anti–mouse isotype-specific antibodies (Southern Biotechnology Associates, Birmingham, AL).
Competitive Inhibition ELISA with NeuPr Oligosaccharides.
NPrGBMP–HSA or (NeuPr)4RE–HSA was coated on the wells of a Corning microtiter plate (1 μg/100 μl PBS/well) for 1 h at 37°C. The plate was washed (three times) with PBS/Tween-20 (0.05%) and the wells were blocked with 200 μl of 1% BSA in PBS for 1 h at 37°C. Concurrently, a second microtiter plate containing serial twofold dilutions in PBS of NeuPr oligosaccharides (50 μl total vol) was mixed with 50 μl of a previously determined dilution of mAb in 3% BSA that yielded a OD450 = 1. This mixture was incubated at 4°C for 1 h and the contents of the plate were transferred to the original blocked plate with absorbed NPr antigen. The plate was then incubated at 4°C for 1 h, followed by washing (three times) with PBS/Tween-20. A 1:10,000 dilution in 3% BSA of goat anti–mouse IgG(H + L) (100 μl/ well) was added to the wells and the plate was incubated at room temperature for 1 h. The plate was washed (three times) with PBS/Tween-20, followed by PBS alone. Substrate consisting of TMB-peroxide was added (50 μl/well) and the plate was allowed to develop for 10 min, quenched with 50 μl of 1M H3PO4 and OD measurements at 450 nm were taken.
Bactericidal Assays.
The bactericidal assay was carried out in tissue culture 96-well microtiter plates (Corning). GBM (strain 80–165 B:2b:P.1) was grown overnight on chocolate agar plates (QueLab, Montreal, Quebec, Canada) at 37°C under a 5% CO2 atmosphere, followed by inoculating a second plate and incubating it for 5 h. Appropriate dilutions of culture supernatent or of the purified mAb were made directly in the plate using HBSS containing 1% casein hydrolyzate, diluted to a final volume of 50 μl/well. A suspension of GBM in HBSS, 1% casein hydrolyzate was made giving an OD490 = 0.29 and a final working dilution of bacteria was prepared by a further 1:20,000 dilution. Freshly thawed baby rabbit complement was added (20 μl) to each well, followed by 30 μl of the working dilution of bacteria (2,500 CFU/well). The plate was then shaken at 37°C for 1 h. The contents of each well was then mixed before plating (10 μl) on to chocolate agar. The agar plates were incubated overnight at 37°C, 5% CO2 and the number of CFU were counted. The percent of killing was calculated relative to the mean values of either HBSS control wells or culture supernatant medium in the following manner: percentage of killing = (CFUcontrol − CFUmAb / CFUcontrol) × 100.
Passive Protection Experiments.
Groups of five female CF1 mice (8–10 wk; Charles River, St. Constant, Quebec, Canada) were injected intravenously with 200 μl of known concentrations of purified mAbs in sterile PBS. After 1 h, each group of mice was challenged with an intraperitoneal injection (500 μl) containing 800–1,200 CFU of GBM (80–165) per ml of brain heart infusion/PBS. After 5 h, the blood was harvested from the individual mice by cardiac puncture, and 10 μl of blood was plated onto chocolate agar plates. The plates were incubated at 37°C under 5% CO2 and the number of CFUs were determined 15–20 h later. The percent of clearance of bacteria was calculated relative to control mice receiving either an irrelevant antiserum or PBS as follows: percent of clearance = (CFUcontrol − CFUmAb / CFUcontrol) × 100.
Electron Microscopy.
GBM (M992) and E. coli K1 (841743) were grown on Columbia broth plates (QueLabs) and suspensions of the bacteria in Hepes buffer (Research Organics, Inc., Cleveland, OH), 0.1 M, pH 6.8, were prepared. Nickel 200 mesh grids coated with Formvar and carbon were floated on 100 μl droplets for 30 s. Excess liquid was blotted off, and then the grids processed for immunolabeling as previously described (22). Then the grids were subjected to the following sequential procedures. They were blocked with BSA in 1% PBS for 30 min before incubation with either mAb 735, 6B9, or 13D9. The grids were then reblocked (three times for 3 min) with 1% BSA in PBS. The grids were then reacted for 1 h with a protein A gold solution (Sigma) diluted 1:50. They were then washed with PBS (three times for 3 min) and finally with ultrapure water. The grids were then viewed in a Philips EM300, operating at 60 kV with the liquid nitrogen cold trap in place.
Results
Preparation and Screening of mAbs.
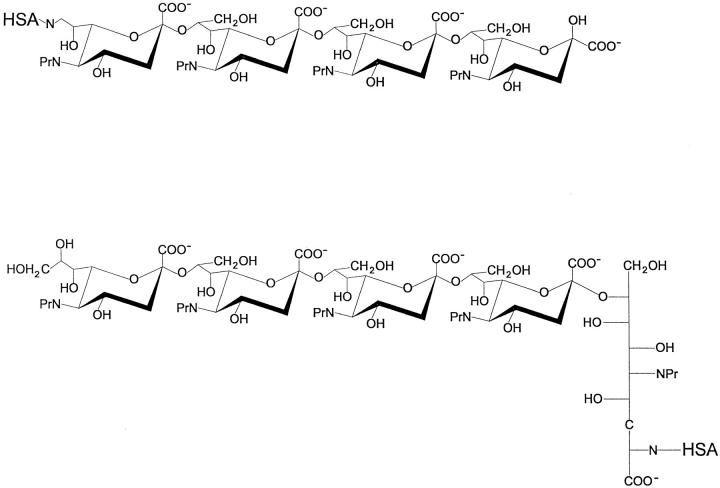
For screening mAbs produced by the NPrGBMP–TT, an NPrGBMP–HSA conjugate having the same length as NPrGBMP was employed. 69 hybridomas induced by NPrGBMP–TT were identified and subcloned. In earlier studies (18) using a similar NPrGBMP–TT vaccine to induce polyclonal antisera in mice, the presence of antibodies specific to extended segments of NPrGBMP was detected. This phenomenon is associated with immune responses to the GBMP (10, 11) and has been attributed to immune selection where only antibody responses to extended helical segments of the GBMP have been reported (11). Antibody responses to shorter segments of the NPrGBMP were not looked for in previous polyclonal studies, but by screening the panel of hybridomas with (NeuPr)4–HSA, a single clone (6B9) with a specificity for a conventional shorter epitope was detected. Owing to the paucity of mAbs with a specificity for shorter epitopes, a second series of hybridomas were generated using a vaccine synthesized with (NeuPr)4. Thus, it was anticipated that (NeuPr)4–TT would force an antibody response to shorter segments of the NPrGBMP. mAbs having a specificity for short segments of the NPrGBMP may be directed towards either terminal or internal residues of the polymer, therefore, because our interest was focused on the latter, the following screening strategy was employed. In addition to (NeuPr)4–HSA, where (NeuPr)4 was linked directly through its nonreducing end, (NeuPr)4RE–HSA was synthesized in such a way as to link (NeuPr)4 to HSA through the opposite end. To accomplish this, (NeuPr)5 was linked directly to HSA through its reducing end to HSA by reductive amination, thus transforming its terminal reducing NeuPr residue into an open chain spacer. The structures of the screening antigens are shown in Fig. 1, and because they differ in structure at both ends, it was rationalized that any mAbs reacting with both screening antigens would probably be specific for internal segments of the NPrGBMP. Using the above strategy, 75 hybridomas induced by (NeuPr)4– TT were identified and subcloned.
Figure 1.
Structures of the (NeuPr)4 screening antigens linked through the nonreducing end ([NeuPr]4–HSA) (top), and through the reducing end ([NeuPr]4RE–HSA) (bottom).
Distribution of Isotypes.
Isotyping 56 clones produced by (NeuPr)4–TT and 42 clones produced by NPrGBMP–TT was carried out to assess both the clonality of the positive hybridomas as well as to aid in the selection of clones for more extensive studies. The breakdown of the distribution of isotypes raised to both immunogens is given in Table 1. 56 clones stemming from the (NeuPr)4–TT fusion were screened, leading to a relatively even distribution of IgG1- (23%), IgG2a (29%), and IgG2b (41%) antibody isotypes with relatively few IgG3 (7%) secreting clones identified. The isotype distribution found among 42 clones obtained from the NPrGBMP–TT fusion differed significantly, with a bias toward IgG1 secreting clones (57%), followed by approximately equal amounts of IgG2a (24%) and IgG3 (17%), with only one IgG2b secreting clone identified.
Table 1.
Initial Screening of Immunological Properties of mAbs Produced by (NeuPr)4–TT and NPrGBMP–TT
| Characteristic | (NeuPr)4–TT | NPrGBMP–TT | ||||
|---|---|---|---|---|---|---|
| Isotype | IgG1 | 13/56 | 24/42 | |||
| IgG2a | 16/56 | 10/42 | ||||
| IgG2b | 23/56 | 1/42 | ||||
| IgG3 | 4/56 | 7/42 | ||||
| Antigenic specificity | NPrGBMP | 8/8 | 29/29 | |||
| (NeuPr)4 | 8/8 | 1/29 | ||||
| (NeuPr)4RE | 8/8 | 1/29 | ||||
| GBMP | 7/8 | 2/29 | ||||
| Bactericidal activity | IgG1 | 0/3 | 0/22 | |||
| IgG2a | 0/6 | 6/8 | ||||
| IgG2b | 0/14 | 1/1 | ||||
| IgG3 | 0/3 | 7/7 |
Antigenic Specificity of mAbs.
8 mAbs from the (NeuPr)4– TT fusion and 29 from the NPrGBMP–TT fusion were selected from the initial screening of clones, ensuring that all isotypes were represented. The antigenic specificity of these mAbs was determined using a direct binding ELISA with NPrGBMP–HSA, (NeuPr)4–HSA, and (NeuPr)4RE– HSA coating antigens. In addition, a GBMP–HSA coating antigen was used in the assay to identify any mAbs that cross-reacted with the GBMP. The results of these experiments are shown in Table 1. Those mAbs deemed to be specific for an antigen were characterized by at least 100fold greater titer towards the homologous antigen relative to other antigens. All eight mAbs produced by (NeuPr)4– TT reacted equally well with (NeuPr)4–HSA, (NeuPr)4RE– HSA, and NPrGMBP–HSA, thus indicating that the short epitopes expressed on (NeuPr)4 were probably present in internal segments of NPrGBMP. In addition, seven of eight of the above mAbs cross-reacted with GBMP–HSA to the extent of ⩾90% of the reaction with the homologous antigen, only one of the eight being specific for NPrGBMP– HSA. Of the twenty-nine mAbs produced by NPrGBMP– TT, all reacted with the homologous NPrGBMP–HSA, but only one of them reacted with (NeuPr)4–HSA. This mAb (6B9) also reacted equally well with (NeuPr)4RE, and thus probably originated from an epitope situated on an internal segment of the NPrGBMP rather than on its terminus. In contrast with the mAbs produced by (NeuPr)4–TT, those produced by NPrGBMP–TT were much less crossreactive with GBMP–HSA. Only 2 of 29 mAbs tested were cross-reactive, the degree of cross-reactivity being much less (<8%). From the mAbs, listed in Table 1, five produced by (NeuPr)4–TT and eight produced by NPrGBMP-TT were selected for further studies. They were selected to ensure a comprehensive rather than a statistical representation of isotypes and antigenic specificities. The individual antigenic specificities of the selected mAbs are shown in Table 2, together with data obtained using the hmw GBMP as an additional screening antigen. On the basis of the introduction of this latter antigen to the screening process a clear differentiation in the antigenic specificities of mAbs produced by NPrGBMP–TT and (NeuPr)4–TT could be made. While all of the mAbs produced by the former vaccine, with the exception of one (6B9), reacted exclusively with the hmw GBMP, those produced by the latter vaccine reacted equally well with both the GBMP and the hmw GBMP.
Table 2.
Immunological Properties of Selected mAbs Induced by (NeuPr)4–TT and NPrGBMP–TT
| Vaccine | Clone | Isotype | Antigenic Specificity* | Epitope size | Bactericidal activity | Passive protection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NPrGBMP | (NeuPr)4 | (NeuPr)4RE | GBMP | mw GBMP | ||||||||||||||||
| (NeuPr)4–TT | 10B9 | IgG1 | +≳ | + | + | + | + | Short | NB | 0% | ||||||||||
| 7C11 | IgG1 | + | + | + | + | + | Short | NB | 0% | |||||||||||
| 11G1 | IgG2a | + | + | + | + | + | Short | NB | 0% | |||||||||||
| 1F1 | IgG2b | + | + | + | + | + | Short | NB | 20% @ 50 μg | |||||||||||
| 11F3 | IgG3 | + | + | + | + | + | Short | NB | 11% @ 50 μg | |||||||||||
| NPrGBMP–TT | 13A8 | IgG1 | + | −‡ | − | − | + | Extended | NB | 97% @ 100 μg | ||||||||||
| 76% @ 50 μg | ||||||||||||||||||||
| 56% @ 2 μg | ||||||||||||||||||||
| 6E10 | IgG1 | + | − | − | − | − | Extended | NB | 40% @ 50 μg | |||||||||||
| 13A11 | IgG1 | + | − | − | −§ | + | Extended | NB | 90% @ 50 μg | |||||||||||
| 13D9 | IgG2a | + | − | − | − | +‖ | Extended | 1.2 μg/ml | 99% @ 100 μg 92% @ 10 μg | |||||||||||
| 79% @ 2 μg | ||||||||||||||||||||
| 6B9 | IgG2a | + | + | + | − | − | Short | NB | 0% @ 50 μg | |||||||||||
| 12C5 | IgG2b | + | − | − | − | + | Extended | 23 μg/ml | 87% @ 100 μg | |||||||||||
| 82% @ 50 μg | ||||||||||||||||||||
| 12E12 | IgG3 | + | − | − | − | + | Extended | 14 μg/ml | — | |||||||||||
| 14F10 | IgG3 | + | − | − | −§ | + | Extended | 1.2 μg/ml | 98% @ 100 μg | |||||||||||
| GBM | 735¶ | IgG2a | + | − | − | + | + | Extended | 2.0 μg/ml | — | ||||||||||
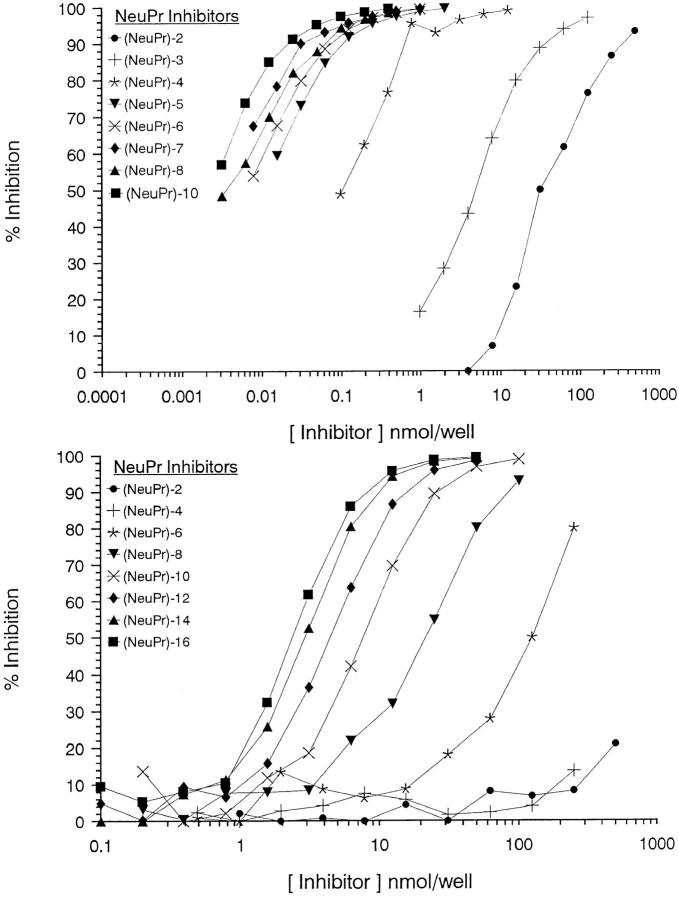
Antigenic specificity of mAbs based on the use of screening antigens does not provide precise information on the epitope size and, therefore, to achieve this, each of the individual mAbs listed in Table 2 was subjected to competitive ELISA experiments similar to those previously described (19). When oligomers of increasing size were used to inhibit the binding of mAbs produced by NPrGBMP– TT to NPrGBMP–HSA, all the mAbs except one (6B9) gave similar inhibition curves. The inhibition curves of 13D9, typical of the majority of the mAbs, together with 6B9 are shown in Fig. 2. With the majority of mAbs, the inhibitory properties of the oligomers maximized at (NeuPr)14–16 and the epitopes were thus designated as extended in Table 2. In contrast, when oligomers of increasing size were used to inhibit the binding of 6B9 to NPrGBMP–HSA the inhibition maximized at (NeuPr)5, and the epitope was defined as short in Table 2. All the mAbs produced by (NeuPr)4–TT gave similar inhibition profiles to 6B9, except that as expected, based on the fact that the vaccine is composed of only four NeuPr residues, the inhibitory properties of the oligomers maximized at (NeuPr)4.
Figure 2.
ELISA inhibition of the binding of NPrGBMP–HSA to mAb 6B9 (top) and mAb 13D9 (bottom) using a series of α(2–8)-linked homooligosaccharides (NeuPr)n.
Bactericidal Activity of mAbs.
From an initial qualitative bactericidal screening (Table 1), purified mAbs selected for more rigorous characterization were subjected to quantitative bactericidal assays and the results of these assays are listed in Table 2. The assays were carried out using a modification of a published procedure (18) in which significantly more organisms (2,500 CFU) per test were used, resulting in a shift towards the detection of mAbs with the strongest bactericidal activity. From an analysis of the bactericidal results listed in Table 2, one can reach the definite conclusion that there is a strong correlation between mAbs that recognize an extended epitope and their ability to kill GBM by bacteriolysis. The only exceptions to the rule are mAbs 13A8, 6E10, and 13A11, which can only be differentiated from the mAbs above by the fact that they are all of the IgG1 isotype. There is evidence that mouse antibodies of this isotype are less able to fix complement and this is amply confirmed from an initial screen of bactericidal activity of 38 mAbs produced by NPrGBMP–TT in which none of 22 mAbs of the 1gG1 isotype that were detected were bactericidal (Table 1). The other observation of interest is that none of the mAbs, regardless of isotype, which recognise a short epitope, whether induced by either (NeuPr)4– TT or NPrGBMP–TT, demonstrated any bactericidal activity against GBM in this assay (Table 2).
Passive Protection Induced by mAbs.
Purified mAbs were assayed for their ability to clear GBM-infected mice upon their passive transfer. The results are expressed as percent reduction in GBM brought about by a known amount of purified mAb and they are listed in Table 2. All of the mAbs that recognize a short epitope, whether induced by either (NeuPr)4–TT or by NPrGBMP–TT, were not able to clear the GBM effectively from infected mice. As expected, strongly bactericidal mAbs, all of which recognize extended epitopes, were effective in clearing GBM from infected mice. In addition, all mAbs of the nonbactericidal IgG1 isotype that recognize an extended epitope were also able to clear GBM from infected mice, although for mAb 6E10 clearance was the least effective. To determine the relative effectiveness of bactericidal mAb 13D9 to clear GBM from infected mice as compared with nonbactericidal mAb 13A8, their ability to clear GBM with reducing quantities of mAbs was investigated. The results shown in Table 2 indicate that the effectiveness of the nonbactericidal mAb 13A8 to clear GBM was reduced much faster than that of the bactericidal mAb 13D9.
Surface Expression of Extended NPrGBMP-specific Epitopes.
Immunogold labeling experiments were carried out with mAbs 735, 13D9, and 6B9 on both GBM and E. coli K1 and the results are shown in Fig. 3. E. coli K1 was included in the experiment because it has the same α(2–8)-polysialic acid capsule as GBM. 13D9 and 6B9 were chosen because both were produced using the same NPrGBMP–TT vaccine and both do not recognize GBMP. In addition, they are both of the same isotype (IgG2a). They differ in the fact that while 13D9 recognizes an extended epitope on NPrGBMP, 6B9 recognizes a short epitope on the same polymer. For a positive control mAb 735 was used, the specificity of which is known to be for an extended helical epitope of 10 contiguous sialic units in the GBMP (15). A uniform distribution of mAb 735 was detected on the surface of E. coli K1 and GBM (Fig. 3, A and D), thus indicating the presence of the extended helical epitope of the GBMP on the surface of both organisms. A similar result was obtained using mAb 13D9, although the density of gold labeling was reduced for both E. coli K1 and GBM (Fig. 3, B and E) when compared with that obtained with mAb 735. No mAb 6B9 was detected on either E. coli K1 or GBM (Fig. 3, C and F).
Figure 3.
Immunogold labeling of Escherichia coli K1 using mAbs 735 (A), 13D9 (B), and 6B9 (C), and of group B Neisseria meningitidis using mAbs 735 (D), 13D9 (E), and 6B9 (F).
Discussion
Because the GBMP is the most conserved antigenic structure on GBM, a polysaccharide-based vaccine would be the vaccine of choice provided one could overcome its poor immunogenicity (8). In addition, one would also have to consider that the antibody generated would not lead to pathological consequences because the lack of immunogenicity is attributed to structural mimicry between the GBMP and human tissue antigens (6, 7). One way to achieve this goal is to use a synthetic vaccine composed of the N-propionylated (NPr) form of the GBMP, which when conjugated to TT, induces in mice high titer antibodies that are bactericidal for all GBM (16, 17). Based on previous evidence that the hmw GBMP failed to absorb out the bactericidal activity from the above polyclonal antisera it was concluded that NPrGBMP–TT produced two distinct populations of antibodies, only one of which was associated with the hmw GBMP, while the other was associated with a unique bactericidal epitope on the surface of GBM (18). Using a series of mAbs induced by NPrGBMP–TT, we were able to examine mAbs having a variety of individual immune properties. Although our results are still consistent with the original hypothesis of the presence of two distinct epitopes on the surface of GBM, the nature of the bactericidal epitope needs to be redefined due to the fact that we were able to detect both epitopes on the hmw GBMP in a direct binding assay.
One interesting finding that emerges from our data is that while most of the mAbs induced by NPrGBMP–TT recognize an epitope located only on extended segments of the NPrGBMP ([NeuPr]14–15 residues by inhibition), one was able to bind to shorter internal regions ([NeuPr]4–5 residues by inhibition) of the NPrGBMP. Thus, in contrast with GBMP-specific antibodies, which up to now have been shown only to recognize extended helical epitopes (13, 15), NPrGBMP-specific antibodies that recognize both extended epitopes and conventional short epitopes can be detected. It is not unreasonable to suppose that these epitopes could have their origins in the internal extended helical and random coil forms of an intrinsically flexible NPrGBMP (8, 13). NMR studies and potential energy calculations demonstrate that the NPrGBMP can form extended helices, similar to those identified in the GBMP (14), but so far we have no X-ray data, as in the case of the GBMP, to support this hypothesis. However, some supporting serological evidence is available, because mAb735 that recognizes an extended helical form of the GBMP (15) also cross-reacts strongly with NPrGBMP (Table 2), as has been demonstrated previously (11). Comparison of the epitope size with protective properties of the mAbs also provides another important observation, because only mAbs that bind to extended NPrGBMP epitopes were protective against GBM as defined by bactericidal activity and passive protection studies. This was also true for mAbs of the IgG1 isotype, which while not bactericidal, were still able to passively protect mice against challenge with live GBM, provided the mAb bound to extended NPrGBMP epitopes.
Because of the paucity of mAbs induced by NPrGBMP– TT that bound to short epitopes, and our need to evaluate more of them, additional mAbs were made using (NeuPr)4– TT. These mAbs were screened to try to eliminate any that might be specific for terminal segments of the NPrGBMP. Interestingly, none of these mAbs were bactericidal for, or had any significant ability to protect passively against, live GBM even though all of them cross-reacted extensively with both the GBMP and the hmw GBMP. We have no explanation for this, but this evidence strongly supports our original observation that only mAbs with a specificity for an extended NPrGBMP epitope are protective against GBM. Another important conclusion that can be reached from the (NeuPr)4–TT data is that vaccines based on short segments of the NPrGBMP would be completely inappropriate for human use, both from the point of view of efficacy and the fact that they also produce antibodies that are highly cross-reactive with the GBMP. However, it is important to note that the implications of this cross-reaction, if any, are not known at this time. Contrary to mAbs induced by (NeuPr)4–TT, those induced by NPrGBMP–TT reacted almost exclusively with the hmw GBMP and not with the GBMP. The exceptions are mAb 6B9, the specificity of which is exclusive for short segments of NPrGBMP, and mAbs 13A11 and 14F10, which also cross-react to a limited extent with the GBMP, presumably through a shared extended epitope. Thus, all the mAbs in Table 2 can be classified into two major epitopic specificities based on their cross-reactions with GBMP and the hmw GBMP.
The presence of these two different epitopes in the capsular layer of GBM and E. coli K1 was confirmed by visualization of the binding of selected mAbs to these bacteria using an immunogold-labeling technique. mAb 735, which is known to bind to an extended helical segment of the GBMP having a minimum of 10 NeuAc residues (15), was detected in the capsular layer of both GBM and E. coli K1. However, mAb 735 also cross-reacts with NPrGBMP and probably binds to both epitopes. Bactericidal mAb 13D9, which bound to an extended NPrGBMP epitope, but did not bind to GBMP, was also detected on both GBM and E. coli K1. However, the labeling was less dense than that produced by mAb 735, which probably reflects the unique specificity of mAb 13D9, which binds only to the bactericidal epitope mimicked by NPrGBMP, and not to GBMP alone. MAb 6B9, which was of the same isotype as 13D9 but was specific for conventional short NPrGBMP epitopes, did not bind to either organism, which provides an explanation for its lack of bactericidal activity.
Based on the close structural resemblance between the NPrGBMP and the GBMP, it has been suggested that the bactericidal epitope on GBM is closely associated with the GBMP (8, 19). Additional evidence presented in this paper, such as the length dependency of NPrGBMP epitope, is consistent with this hypothesis. However, this hypothesis is not consistent with a previous finding in which it was observed that although GBM and E. coli K1 were able to absorb out the bactericidal activity from a polyclonal mouse antiNPrGBMP–TT serum (19) the hmw GBMP was not (18). Interestingly, in recent immunization studies in nonhuman primates, the bactericidal activity induced by an NPrGBMP– rPorB conjugate could be inhibited by the hmw GBMP obtained from E. coli K1, although it was much less efficient as an inhibitor than 10-kD NPrGBMP (23). Perhaps the above anomalies are indicative of the relative instability of the bactericidal epitope, which while seemingly abundant on the surface of GBM and E. coli K1 (Fig. 3, B and E) is less so in the isolated hmw GBMP. This instability could conceivably be attributed to the extraction and purification procedures used to prepare the hmw GBMP.
The identification of the bactericidal epitope on the hmw GBMP and the fact that it cannot be expressed on an 11-kD length of the GBMP indicates that it is only expressed on the aggregated form of the GBMP (24). It has been proposed that NPrGBMP mimics a complex intermolecular epitope on the surface of GBM that involves the close association of extended helical portions of the GBMP with another molecule (19). It is tempting to propose that this other molecule is the phosphoglycerolipid component of the hmw GBMP, which is situated at the terminus of individual short α(2–8)-polysialic chains equivalent to the GBMP, and causes the chains to aggregate (24). Thus, the bactericidal epitope could involve either direct participation of the lipid component or indirect participation, where by generating the GBMP aggregates, it also stabilizes other complex, possibly conformational, epitopes. Certainly, it has been demonstrated that bactericidal NPrGBMP-specific antibodies can be absorbed by short chains of α(2–8)-polysialic acid provided they are linked to long spacers on a solid surface (19). In conclusion, the evidence above clearly defines two capsular epitopes, one based on the GBMP and the other on the hmw GBMP. Should this latter epitope be unique to the surface of GBM and E. coli K1, it would be of great significance to the development of a vaccine against group B meningitis. This is because current studies indicate that most mammalian α(2–8)-polysialic antigens are of ∼50 NeuAc residues or less (6) and could be easily accomodated in the GBMP. That the hmw GBMP epitope is unique to the bacteria is supported by the fact that NPrGBMP–TT readily induces in mice large quantities of mAbs with a specificity for the hmw GBMP, whereas the number that cross-react to any extent with the GBMP is minimal.
Acknowledgments
We thank Mr. R. Harris of the Guelph Regional Scanning Transmission Electron Microscope Facility for immunolabeling and elecron microscopy.
Footnotes
This is National Research Council of Canada publication Number 39555 and the work was supported in part by North American Vaccines, Beltsville, MD.
1 Abbreviations used in this paper: GBM, group B meningococci; GBMP, group B meningococcal polysaccharide (11 kD); hmw, high molecular weight; HSA, human serum albumin; N-CAM, neural cell adhesion; NeuPr, N-propionylated sialic acid; NMR, nuclear magnetic resonance spectroscopy; NPrGBMP, N-propionylated GBMP (11 kD); TT, monomeric tetanus toxoid.
References
- 1.Wyle FA, Artenstein MS, Brandt BL, Tramont DL, Kasper DL, Altieri P, Berman SL, Lowenthal JP. Immunological response of man to group B meningococcal polysaccharide antigens. J Infect Dis. 1972;126:514–522. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 2.Jennings HJ, Lugowski D. Immunochemistry of groups A, B, and C meningococcal polysaccharide–tetanus toxoid conjugates. J Immunol. 1981;127:1011–1018. [PubMed] [Google Scholar]
- 3.Bartolini A, Norelli F, Ceccarini C, Rappuoli R, Constantino P. Immunogenicity of meningococcal B polysaccharide conjugated to tetanus toxoid or CRM197 via adipic acid dihydrazide. Vaccine. 1995;13:463–470. doi: 10.1016/0264-410x(94)00007-a. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith ICP. Structural determination of the sialic acid polysaccharide of Neisseria meningitisserogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975;250:1926–1932. [PubMed] [Google Scholar]
- 5.Finne J, Finne V, Deagostini-Bazin H, Goridis C. Occurrence of α-2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983;112:482–487. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 6.Troy FA. Polysialylation: from bacteria to brains. Glycobiology. 1992;2:5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- 7.Ando S, Yu RK. Isolation and characterization of two isomers of brain tetrasialogangliosides. J Biol Chem. 1979;254:12224–12229. [PubMed] [Google Scholar]
- 8.Jennings HJ. N-Propionylated group B meningococcal polysaccharide glycoconjugate vaccine against group B meningococcal meningitis. Int J Infect Dis. 1996;1:158–164. [Google Scholar]
- 9.Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D. NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coliK1 and group B meningococci. Proc Natl Acad Sci USA. 1985;82:1194–1198. doi: 10.1073/pnas.82.4.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennings HJ, Roy R, Michon F. Determinant specificities of the groups B and C polysaccharides of Neisseria meningitidis. . J Immunol. 1985;134:2651–2657. [PubMed] [Google Scholar]
- 11.Häyrinen J, Jennings HJ, Raff HV, Rougon G, Hanai N, Gerardy-Schahn R, Finne J. Antibodies to polysialic acid and its N-propyl derivative: binding properties and interaction with human embryonal brain glycopeptides. J Infect Dis. 1995;171:1481–1490. doi: 10.1093/infdis/171.6.1481. [DOI] [PubMed] [Google Scholar]
- 12.Kabat EA, Liao J, Osserman F, Gamian A, Michon F, Jennings HJ. The epitope association with the binding of the capsular polysaccharide of the group B meningococcus and of Escherichia coliK1 to a human monoclonal macroglobulin, IgM NOV. J Exp Med. 1988;168:699–711. doi: 10.1084/jem.168.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisson J-R, Baumann H, Imberty A, Pérez S, Jennings HJ. Helical epitope of the group B meningococcal α(2–8)-linked sialic acid polysaccharide. Biochemistry. 1992;31:4996–5004. doi: 10.1021/bi00136a012. [DOI] [PubMed] [Google Scholar]
- 14.Baumann H, Brisson J-R, Michon F, Pon R, Jennings HJ. Comparison of the conformation of the epitope of α(2–8)-polysialic acid with its reduced and N-acetyl derivatives. Biochemistry. 1993;32:4007–4013. doi: 10.1021/bi00066a022. [DOI] [PubMed] [Google Scholar]
- 15.Evans SV, Sigurskjold BW, Jennings HJ, Brisson J-R, To R, Tse WC, Altman E, Frosch M, Weisgerber C, Kratzin HD, et al. Evidence for the extended helical nature of polysaccharide epitopes. The 2.8 Å resolution structure and thermodynamics of ligand binding of an antigen binding fragment specific of α(2–8)-polysialic acid. Biochemistry. 1995;34:6737–6744. doi: 10.1021/bi00020a019. [DOI] [PubMed] [Google Scholar]
- 16.Jennings HJ, Roy R, Gamian A. Induction of meningococcal group B polysaccharide-specific IgG antibodies in mice by using an N-propionylated B polysaccharide– tetanus toxoid conjugate vaccine. J Immunol. 1986;137:1708–1713. [PubMed] [Google Scholar]
- 17.Ashton FE, Ryan JA, Michon F, Jennings HJ. Protective efficacy of mouse serum to the N-propionyl derivative of meningococcal group B polysaccharide. Microbiol Pathog. 1989;6:455–458. doi: 10.1016/0882-4010(89)90087-9. [DOI] [PubMed] [Google Scholar]
- 18.Jennings HJ, Gamian A, Ashton FE. N-propionylated group B meningococcal polysaccharide mimics a unique epitope on group B Neisseria meningitidis. . J Exp Med. 1987;165:1207–1211. doi: 10.1084/jem.165.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings HJ, Gamian A, Michon F, Ashton FE. Unique intermolecular bactericidal epitope involving the homosialopolysaccharide capsule on the cell surface of group B Neisseria meningitidis and Escherichia coliK1. J Immunol. 1989;142:3585–3591. [PubMed] [Google Scholar]
- 20.Bundle DR, Jennings HJ, Kenny CP. Studies on the group-specific polysaccharide of Neisseria meningitidisserogroup X and an improved procedure for its isolation. J Biol Chem. 1974;249:4797–4801. [PubMed] [Google Scholar]
- 21.Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol–hydrochloric acid method. Biochim Biophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- 22.Beveridge, T., T.T. Popkin, and R.M. Cole. 1994. Electron microscopy: In Methods for General and Molecular Bacteriology. P. Gerhanrdt, R.G.E. Murray, W.A. Wood, and N.R. Krieg, editors. American Society for Microbiology, Washington, DC. 42–71.
- 23.Tai JY, Michon F, Fusco PC, Blake MS. Preclinical evaluation of a novel group B meningococcal conjugate vaccine that elicits bactericidal activity in both mice and non-human primates. J Infect Dis. 1997;175:364–372. doi: 10.1093/infdis/175.2.364. [DOI] [PubMed] [Google Scholar]
- 24.Gotschlich EC, Fraser BA, Nishimura O, Robbins JB, Liu TY. Lipid on capsular polysaccharides of gram negative bacteria. J Biol Chem. 1981;256:8915–8921. [PubMed] [Google Scholar]