Abstract
While it is generally believed that the avidity of the T cell antigen receptor (TCR) for self antigen/major histocompatibility complex (MHC) determines a thymocyte's fate, how the cell discriminates between a stimulus that causes positive selection (survival) and one that causes negative selection (death) is unknown. We have previously demonstrated that glucocorticoids are produced in the thymus, and that they antagonize deletion caused by TCR cross-linking. To examine the role of glucocorticoids during MHC-dependent selection, we examined thymocyte development in organ cultures in which corticosteroid biosynthesis was inhibited. Inhibition of glucocorticoid production in thymi from α/β-TCR transgenic mice resulted in the antigen- and MHC-specific loss of thymocytes that normally recognize self antigen/MHC with sufficient avidity to result in positive selection. Furthermore, inhibition of glucocorticoid production caused an increase in apoptosis only in CD+CD8+ thymocytes bearing transgenic TCRs that recognized self antigen/MHC. These results indicate that the balance of TCR and glucocorticoid receptor signaling influences the antigen-specific thymocyte development by allowing cells with low-to-moderate avidity for self antigen/MHC to survive.
Rigorous selection processes in the thymus prevent nonfunctional or harmful T cells from reaching the periphery. Thymocytes that express a TCR with high avidity for self peptides presented by self MHC-encoded molecules are eliminated by TCR-mediated apoptosis (negative selection). Thymocytes bearing TCRs with lowto-moderate avidity for self peptide/MHC are rescued (positive selection) from the default apoptosis pathway undergone by thymocytes that have not successfully rearranged their TCR genes or that express a receptor with subthreshold avidity for self-MHC (1, 2). How ligandinduced signaling through the TCR can lead to both rescue from death, in the case of positive selection, and death, in the case of negative selection, is unclear.
Occupancy of the glucocorticoid receptor (GR) is a potent means of inducing apoptosis in thymocytes (3–5). However, stimulation with glucocorticoids does not necessarily always result in thymocyte death. Paradoxically, lowto-moderate concentrations of glucocorticoids antagonize TCR-mediated apoptosis of T cell hybridomas and thymocytes (6–8). Furthermore, transgenic mice generated to express antisense transcripts to the 3′ untranslated region of the GR in immature thymocytes revealed that glucocorticoids are critical during at least two points during thymocyte development: progression from the CD4−CD8− to CD4+CD8+ stage, and maintenance of viability at the CD4+CD8+ stage (9). In addition, we have previously shown that thymic epithelial cells produce steroids, and that addition of low concentrations of metyrapone, a selective inhibitor of corticosteroid synthesis (10), to fetal thymic organ culture enhances TCR-mediated deletion, an effect that was reversed by the addition of corticosterone to the cultures (11). Based upon these results, we have proposed that glucocorticoids participate in the antigen-specific development of thymocytes by preventing the TCR-mediated deletion of cells bearing receptors with low-to-moderate avidity for self antigen/MHC. A prediction that follows from this hypothesis is that interfering with glucocorticoid signaling should convert instances of positive selection (TCR interaction with low-to-moderate ligands) to negative selection (deletion by apoptosis) because of the failure of glucocorticoids to antagonize the TCR-mediated signals.
In this report, we address this prediction by analyzing the effect of preventing thymus glucocorticoid biosynthesis on the survival of thymocytes bearing transgenic TCRs of known antigenic specificity. The results indictate that endogenous glucocorticoids prevent thymocyte apoptosis only when the TCR is capable of recognizing self antigen/ MHC with sufficient avidity to normally result in positive selection.
Materials and Methods
Mice and Reagents.
RAG-2−/− H-2d and H-2b mice bearing TCRs specific for H-Y/Db (12) were bred in our facilities by crossing RAG-2−/− mice (H-2b, 129 background, (13)) with H-Y/Db-specific TCR transgenic H-2d mice. TCR transgeneexpressing offspring were bred to RAG-2−/− mice to generate offspring that were RAG-2−/− H-2bxd and expressed the transgenic TCR. These mice were interbred to generate timed pregnants or to establish lines of RAG-2-/- TCR transgenic mice that were homozygous for either H-2d or H-2b. Metyrapone was purchased from ICN Biochemicals (Cosa Mesa, CA). Antibodies used for flow cytometry, anti-CD4, anti-CD8, anti-TCR, and anti-MHC, were purchased from PharMingen (San Diego, CA).
Thymic Organ Culture.
Thymic organ cultures were performed as described, in serum-free Nutridoma-SP medium (Boehringer Mannheim Corp., Indianapolis, IN) supplemented with 20 mM Hepes, 100 mM nonessential amino acids, 1 mM sodium pyruvate, and 50 mM 2-mercaptoethanol (organ culture medium) (11). Thymi from 1–2-d-old mice were separated into lobes and cultured with 200 μg/ml of freshly diluted metyrapone, or ethanol control on a Millipore filter floating on a gelfoam sponge in organ culture medium. The sex of each neonate was determined microscopically before removal of the thymus. In some experiments, 10−9M corticosterone, diluted in ethanol, was added to the cultures. Cultures were carried out in 3 ml of medium in 6-well plates. Lobes were harvested after 1 or 3 d of culture and single cell suspensions were prepared for counting and analyzed for CD4, CD8, TCR, and MHC expression, or for TUNEL positivity, by flow cytometry with a FACScan® (Becton Dickinson, Mountain View, CA).
TUNEL Assay.
A modified form of the TUNEL assay (14) was used to detect fragmented DNA in apoptotic thymocytes (9). In brief, thymocytes were formaldehyde-fixed, permeablilized with 0.1% Triton X-100 and 0.1% sodium citrate, and subjected to a modified in situ nick translation using fluorescein-dUTP (Boehringer Mannheim Corp.). Labeled cells were visualized by flow cytometry with a FACScan®. To determine the amount of apoptosis in the DP population, thymocytes were gated for expression of both CD4 and CD8 and analyzed for dUTP-FITC incorporation. Percent specific apoptosis was calculated by the formula:
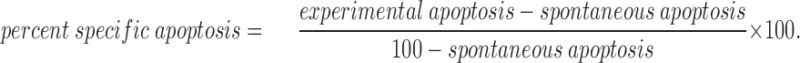 |
Results
Inhibition of Thymic Corticosteroid Production Causes Negative Selection of Thymocytes That Recognize Self with Low Avidity.
The mutual antagonism model of thymocyte selection (6) postulates that in the absence of glucocorticoids, occupancy of TCRs on CD4+CD8+ cells by ligands with either low-to-moderate or high avidity leads to apoptosis (negative selection). In the presence of glucocorticoids, however, thymocytes that recognize ligands with only low- to-moderate avidity would survive and differentiate into CD4+CD8− and CD4−CD8+ thymocytes (positive selection). Therefore, eliminating glucocorticoids during thymocyte development should result in the death of thymocytes that recognize self antigen/MHC with avidity (low-to-moderate) that would otherwise lead to positive selection. To test this, organ cultures were performed with thymi from mice whose cells bear a transgenic α/β-TCR specific for the male H-Y antigen in the context of the H-2b MHC class I molecule (12). Thymocytes from H-2b male mice bearing the H-Y/Db-specific receptor are normally deleted, resulting in a dramatic reduction in the number of CD4+CD8+ (double positive, or DP) cells (12). Thymocytes from female mice are not deleted because they do not express the male H-Y antigen. However, in animals of the H-2b, but not the H-2d, haplotype, DP thymocytes from female mice undergo positive selection and mature into clonotype-bearing CD4−CD8+ cells, indicating that this transgenic TCR recognizes some as yet uncharacterized peptide antigen plus an H-2b-encoded molecule with lowto-moderate avidity (15). Because of allelic exclusion, all TCR-β chains expressed in these transgenic animals are encoded by the β transgene, but incomplete allelic exclusion of the α chain results in a significant number of thymocytes that express endogenous rearranged TCR-α as well as transgenic α (15). To eliminate this complication and ensure that all of the thymocytes in these animals express only the transgenic TCR, RAG-2−/− mice, which lack the rag-2 gene and therefore produce thymocytes that do not rearrange endogenous TCR-α and -β genes (13), were bred to express the transgenic H-Y/Db-specific TCR.
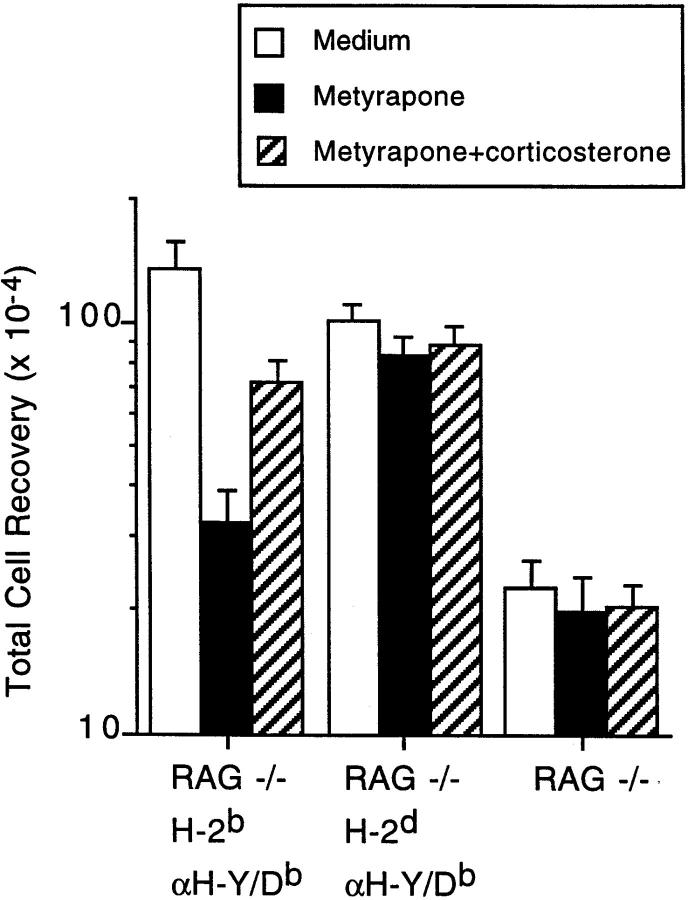
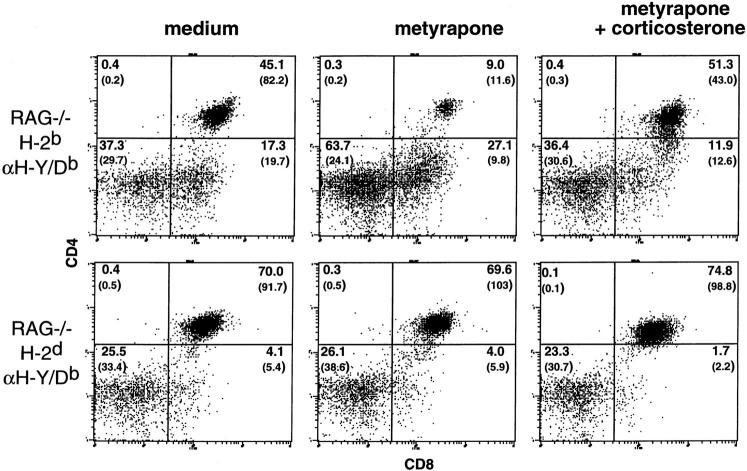
To address what effect a decrease in corticosteroid production would have on the antigen-specific fate of DP thymocytes, RAG-2−/− H-2bxd TCR females and males were bred and thymi from 1-2-d-old offspring were used in organ culture experiments. Thymocyte development is delayed in SCID mice and RAG-2−/− mice that express a transgenic TCR; thymi of these newborn mice have the developmental phenotype of thymi from fetal d17-18 of normal mice (16 and our unpublished observation). We have previously shown that glucocorticoids play a role in the DN to DP transition (9). In newborn RAG-2−/− TCR transgenic mice, however, the large majority of thymocytes have already reached the DP stage, allowing us to address the effects of metyrapone on antigen-specific selection. The thymi were separated into three pieces and cultured in the absence or presence of metyrapone. Metyrapone is a selective inhibitor of the enzyme P450c11 that blocks the conversion of biologically inactive deoxycorticosteroids to the active forms, corticosterone or cortisol, and has been used clinically to treat hypercortisolism and as a test for the pituitary's response to decreased levels of plasma cortisol (10). Thymic organs cultures were performed in serumfree medium to avoid the addition of any steroids present in fetal calf serum or any steroid-binding proteins. The addition of metyrapone caused a dramatic decrease in thymocyte recovery (<20% of the medium control) in H-2b thymi from female animals (Fig. 1). This was largely due to the loss of DP cells, as the number of CD4−CD8− (double negative, DN) cells was the same in the medium and metyrapone-treated groups (Fig. 2). As in previous studies (9, 11), the effect of metyrapone was significantly reversed by the addition of a physiologic concentration (10−9 M) of free corticosterone. To determine if occupancy of the transgenic TCR by ligand was required for the decrease in cell recovery, thymi from H-2d littermates, which do not express a ligand recognized by the transgenic TCR (15), were evaluated. In contrast to the H-2b thymi, metyrapone had little if any effect when added to organ culture of thymi with the nonselecting haplotype (Figs. 1 and 2), or to RAG-2−/− nontransgenic thymi, which consist of only CD4−CD8− cells (13). Notably, the addition of metyrapone to female H-2b RAG-2−/− transgenic thymi lowered cell recovery to levels almost equal to that from RAG-2−/− nontransgenic thymi cultured in medium alone (Fig. 1). Similar results were obtained in experiments performed with thymi from female SCID mice expressing the transgenic H-Y/Db-specific TCR (data not shown). Together, these results demonstrate that inhibition of endogenous corticosterone production results in a marked loss of thymocytes that have TCRs with functionally significant (as judged by their ability to mediate positive selection) avidity for self but not those that do not.
Figure 1.
Effect of absence of glucocorticoids on thymocyte development in TCR transgenic RAG-2−/− mice with selecting or non-selecting haplotypes. Thymi from 1-2-d-old RAG2−/− H-Y/Db-specific TCR transgenic littermates homozygous for either H-2d (n = 18) or H-2b (female only, n = 8), or RAG-2−/− non–TCR transgenic mice (n = 14), were separated into three pieces and cultured in medium alone or with 200 μg/ml of freshly diluted metyrapone, in the presence or absence of 10−9 M corticosterone.
Figure 2.
Specific loss of CD4+CD8+ thymocytes from TCR transgenic RAG-2−/− H-2b mice after metyrapone treatment and its prevention by corticosterone. Thymi from 1-2-dold female RAG-2−/− H-Y/ Db-specific TCR transgenic littermates homozygous for either H-2d or H-2b were separated into three pieces and cultured in medium alone or with 200 μg/ ml of freshly diluted metyrapone, in the presence or absence of 10−9 M corticosterone. The percentage of each subpopulation and total cell recoveries (×104, in parentheses) are shown in each quadrant.
Inhibition of Thymic Corticosteroid Synthesis Results in Apoptosis of Only Thymocytes That Bear TCRs That Recognize Self Antigen/MHC.
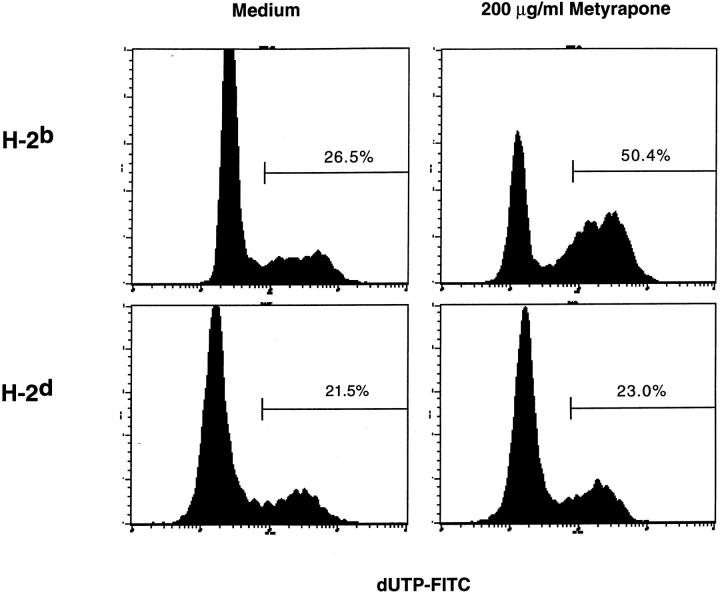
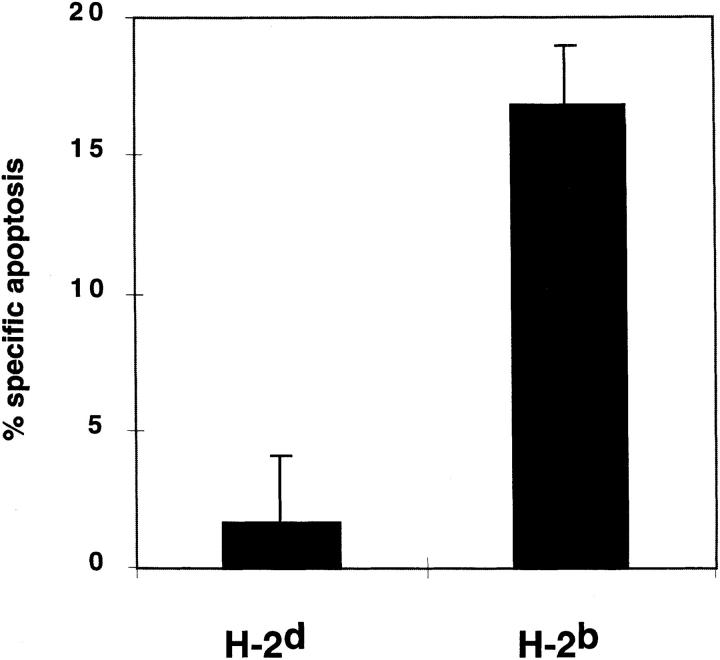
Antigen-specific thymocyte deletion is due to the induction of apoptotic cell death (17, 18). Therefore, experiments were performed to determine if the decrease in thymocyte number when corticosteroid production was inhibited was due to apoptosis of DP thymocytes. Cells undergoing apoptotic death acquire single- and double-strand DNA breaks, which can be detected by techniques in which labeled oligonucleotides are incorporated into the damaged DNA. We have used a modified version of the original TUNEL assay in which harvested thymocytes are stained with antibodies to cell surface molecules such as CD4 and CD8, permeabilized, and incubated with DNA polymerase I, allowing the incorporation of fluoresceinated dUTP into nicked DNA. The cells are analyzed by flow cytometry and the number of TUNELpositive cells quantitated. A representative example of profiles of thymocytes from a RAG-2−/− H-2b or a RAG2−/− H-2d thymus cultured in the absence or presence of metyrapone for 24 h is shown in Fig. 3. In the nonselecting H-2d haplotype, the background level of spontaneous cell death was unaffected by the addition of metyrapone. In contrast, metyrapone caused a substantial increase in DP TUNEL-positive cells compared to medium alone in DP thymocytes of the H-2b haplotype. When the data from multiple experiments were averaged (Fig. 4), it was found that there was little if any increase in spontaneous apoptosis in DP cells when thymi from RAG-2−/− H-2d mice expressing the H-Y/H-Db-specific TCR were cultured with the corticosteroid-synthesis inhibitor. In contrast, in thymi of H-2b littermates there was an induction of almost 20% specific apoptosis. Therefore, inhibition of local corticosteroid production in the thymus specifically causes the apoptotic death and deletion of DP cells only when their TCRs recognize self.
Figure 3.
Modified TUNEL assay for thymocyte apoptosis. Thymi from 1-2-d-old female RAG-2−/− H-Y/Db-specific TCR transgenic mice homozygous for either H-2d or H-2b were separated into individual lobes and cultured in medium alone or with 200 μg/ml of freshly diluted metyrapone. After 18 h, thymocytes were analyzed for expression of CD4 and CD8 and evaluated for apoptosis on the FACScan® using a modified TUNEL assay to detect nicked DNA. To determine the amount of apoptosis in the DP population, thymocytes were gated for expression of both CD4 and CD8 and analyzed for dUTPFITC incorporation.
Figure 4.
Inhibition of thymic corticosteroid production induces apoptosis of DP thymocytes bearing TCRs with low-to-moderate avidity for self antigen/MHC. Thymi from 1-2-d-old female RAG-2−/− H-Y/ Db-specific TCR transgenic mice homozygous for either H-2d (n = 10) or H-2b (n = 10) were separated into lobes and cultured in medium alone or with 200 μg/ml of freshly diluted metyrapone. After 18 h, the amount of apoptosis in the DP population was determined on the FACScan® by gating for expression of both CD4 and CD8 and analysis of dUTP-FITC incorporation.
Discussion
Positive selection of thymocytes is an essential step in the formation of the T cell antigen-specific repertoire. It is generally believed that it is the avidity of TCRs for self antigen/MHC that determines the fate of immature (1, 2, 19, 20). Low to moderate avidity interactions result in thymocyte survival and differentiation (positive selection), while high avidity interactions result in apoptosis (negative selection). Given that there will be a continuum of TCR avidities for self, some mechanism must be present to set the threshold that distinguishes positive from negative selection. How the cell interprets the avidity of the TCR– ligand interaction is unknown. One possibility is that there are qualitative differences in the signals generated in response to TCR–ligand interactions of different avidities (21–23). The different signals generated would result in either rescue from, or induction of, apoptosis. Another possibility is that varying the avidity of the TCR–ligand interaction results in a quantitatively, but not qualitatively, variable signal, which induces thymocytes to both differentiate and undergo apoptosis. A third, not mutually exclusive, possibility is that another receptor-mediated stimulus, such as that mediated by the glucocorticoid receptor, may prevent apoptosis and allow positive selection to occur. The observation that glucocorticoids can antagonize TCRmediated apoptosis in T cell hybridomas and thymocytes has provided the basis for a mutual antagonism model of thymocyte development (6–8). This model proposes that the quantitative balance between TCR and GR signaling sets the threshold of TCR avidity for self that distinguishes positive from negative selection. Whereas low-to-moderate avidity TCR interactions are antagonized by glucocorticoids, allowing positive selection to proceed, high-avidity TCR interactions produce a signal too potent to be antagonized. Death by neglect may result from GR stimulation in the absence of TCR-mediated signaling.
The results in this report support the mutual antagonism model by demonstrating that TCR avidity for self antigen/ MHC is in fact not the only factor that determines the fate of immature thymocytes: inhibition of glucocorticoid production caused the apoptotic death of thymocytes bearing a transgenic α/β-TCR that would normally have been positively selected, but had little effect in mice that lacked expression of the appropriate MHC. Although the mechanism for the antagonism of TCR-mediated thymocyte death by glucocorticoids is not yet known, there are several clues that can be taken from previous work with T cell hybridomas and mature peripheral T cells. Glucocorticoids inhibit activation-induced apoptosis in these cells by inhibiting the upregulation of Fas ligand (24, 25). While Fas and Fas ligand do not appear to have a similar role in the TCRmediated death of thymocytes (26), a study with mice in which CD30, a member of the gene superfamily that includes Fas, was deleted by homologous recombination has suggested that this molecule may be required for antigenspecific thymocyte deletion (27). Among the many possibilities, corticosteroids may regulate, for example, the expression of CD30 or its ligand.
The data presented here demonstrate that locally-produced corticosteroids are important for preventing TCR occupancy by low-to-moderate avidity ligands from causing apoptotic death (negative selection). Since the rescued cells go on to be positively selected (15), thymic-derived corticosteroids are likely to influence the peripheral antigen-specific T cell repertoire. This may be directly testable by analyzing the immune response of mice in which the thymocyte response to glucocorticoids is blunted because they express antisense GR driven by the proximal lck promoter (9). Such studies are currently being pursued in our laboratory.
Acknowledgments
We thank Dr. E. Shores for providing the TCR transgenic mice, Dr. J. Zuniga-Pflucker for providing the RAG-2−/− mice, and Drs. A. Singer, R. Hodes, R. Germain, L. King, A. Weissman, A. Rosenberg, and E. Shores for critical review of this manuscript.
References
- 1.Sprent J, Lo D, Gao EK, Ron Y. T cell selection in the thymus. Immunol Rev. 1988;101:172–190. doi: 10.1111/j.1600-065x.1988.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. Acquisition of immunologic self-tolerance. Cell. 1989;57:1073–1077. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- 3.Smith CA, Williams GT, Kingston R, Jenkins EJ, Owen JJT. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature (Lond) 1989;337:181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- 4.Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature (Lond) 1980;284:555–557. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- 5.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;32:38–42. [PubMed] [Google Scholar]
- 6.Zacharchuk CM, Mercep M, Chakraborti P, Simons SS, Ashwell JD. Programmed T lymphocyte death: Cell activation- and steroid-induced pathways are mutually antagnoistic. J Immunol. 1990;145:4037–4043. [PubMed] [Google Scholar]
- 7.Zacharchuk, C.M., M. Mercep, and J.D. Ashwell. 1991. Thymocyte activation and death: A mechanism for molding the T cell repertoire. In Antigen and Clone-specific Immunoregulation. Vol. 636. R.J. Edelson, editor. New York Academy of Sciences, New York. 52–60. [DOI] [PubMed]
- 8.Iwata M, Hanaoka S, Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: A possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991;21:643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- 9.King LB, Vacchio MS, Hunziker R, Margulies DH, Ashwell JD. A targeted glucocorticoid receptor antisense transgene increases thymocyte apoptosis and alters thymocyte development. Immunity. 1995;5:647–656. doi: 10.1016/1074-7613(95)90135-3. [DOI] [PubMed] [Google Scholar]
- 10.Haynes, Jr., R.C. 1990. Adrenocorticotropic hormone: adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In The Pharmacological Basis of Therapeutics. A.G. Gilman, T.W. Rall, A.S. Nies, P. Taylor, editors. Pergamon Press, New York. 1431–1462.
- 11.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature (Lond) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 13.Shinkai Y, Rathburn G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2 deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 14.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature (Lond) 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 16.Takahama Y, Sugaya K, Tsuda S, Hasegawa T, Hashimoto Y. Regulation of early T cell development by the engagement of TCR-beta complex expressed on fetal thymocytes from TCR-beta transgenic scid mice. J Immunol. 1995;154:5862. [PubMed] [Google Scholar]
- 17.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlo thymocytes in vivo. Science (Wash DC) 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 18.Surh CD. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature (Lond) 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 19.Ashton-Rickardt PG, Bandeira A, Delaney JR, VanKaer L, Pircher HP, Zinkernagel RM, Tonegawa S. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 20.Hogquist KA, Gavin MA, Bevan MJ. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 21.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signalling: altered phospho-ζ and lack of Zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 22.Madrenas J, Wange RL, Wang JL, Isolov N, Samelson LE, Germain RN. ζphosphorylation without Zap-70 activation induced by TCR antagonists or partial agonists. Science (Wash DC) 1995;267:515–518. doi: 10.1126/science.7824949. [DOI] [PubMed] [Google Scholar]
- 23.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. J Exp Med. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Mercep M, Ware CF, Ashwell JD. Fas and activation-induced Fas ligand mediate apoptosis of T cell hybridomas: inhibition of Fas ligand expression by retinoic acid and glucocorticoids. J Exp Med. 1995;181:1673–1682. doi: 10.1084/jem.181.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Minucci S, Ozato K, Heyman RA, Ashwell JD. Efficient inhibition of activation-induced Fas ligand up-regulation and T cell apoptosis by retinoids requires occupancy of both retinoid X receptors and retinoic acid receptors. J Biol Chem. 1995;270:18672–18677. doi: 10.1074/jbc.270.31.18672. [DOI] [PubMed] [Google Scholar]
- 26.Singer GG, Abbas AK. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 27.Amakawa R, Hakem A, Kundig TM, Matsuyama T, Simard JJ, Timms E, Wakeham A, Mittruecker HW, Griesser H, Takimoto H, et al. Impaired negative selection of T cells in Hodgkin's disease antigen CD30-deficient mice. Cell. 1996;84:551–562. doi: 10.1016/s0092-8674(00)81031-4. [DOI] [PubMed] [Google Scholar]