Abstract
Within T cell–rich areas of secondary lymphoid organs, interdigitating dendritic cells recruit antigen-specific T cells that then induce B cells to secrete Igs. This study investigates the possible role(s) of dendritic cells in the regulation of human B cell responses. In the absence of exogenous cytokines, in vitro generated dendritic cells (referred to as Dendritic Langerhans cells, D-Lc) induced surface IgA expression on ∼10% of CD40-activated naive sIgD+ B cells. In the presence of IL-10 and TGF-β, a combination of cytokines previously identified for its capacity to induce IgA switch, D-Lc strongly potentiated the induction of sIgA on CD40-activated naive B cells from 5% to 40–50%. D-Lc alone did not induce the secretion of IgA by CD40-activated naive B cells, which required further addition of IL-10. Furthermore, D-Lc skewed towards the IgA isotype at the expense of IgG, the Ig production of CD40-activated naive B cells cultured in the presence of IL-10 and TGF-β. Importantly, under these culture conditions, both IgA1 and IgA2 were detected. In the presence of IL-10, secretion of IgA2 by CD40-activated naive B cells could be detected only in response to D-Lc and was further enhanced by TGF-β. Collectively, these results suggest that in addition to activating T cells in the extrafollicular areas of secondary lymphoid organs, human D-Lc also directly modulate T cell–dependent B cell growth and differentiation, by inducing the IgA isotype switch.
Dendritic cells (DC)1 transport antigen from their port of entry to the T cell–rich areas of secondary lymphoid organs (for review see reference 1). In these organs, DC present processed antigen to specific T cells that proliferate and differentiate into effector T cells as a consequence of signals transmitted through molecules such as CD40, CD40L, CD80-CD86, and CTLA4-CD28. It is currently believed that these activated-helper T cells turn on specific B cell responses. Whereas dendritic cells clearly contribute to the development of humoral responses (2–4), the extent to which they directly affect B cell growth and differentiation remains to be determined. Studies of the role of human dendritic cells in the regulation of the immune response have been hampered by the problem of getting purified cells in sufficient quantities. With the establishment of techniques allowing their generation in vitro either from hematopoietic progenitors (5, 6) or from monocytes (7, 8), this difficulty is now alleviated. Dendritic Langerhans cells (D-Lc) can be generated in vitro by culturing human CD34+ hematopoietic progenitors in the presence of GMCSF and TNFα (5). These cells express a functional CD40 antigen that induces D-Lc to secrete cytokines (9) and to mature into cells sharing characteristics of interdigitating DC such as the expression of high levels of accessory molecules (9).
Numerous studies have dealt with the molecular mechanisms regulating B and T cell interactions (10, 11). Among those, the receptor/ligand pair, CD40/CD40L, plays a major role (12–14). In humans, CD40 triggering is critical for the induction of isotype switching as best exemplified in the Hyper-IgM syndrome (15, 16) where a genetic alteration of the CD40L results in a deficit of circulating IgG and IgA and the absence of germinal centers. To determine the capacity of a given cytokine to induce isotype switching, we have developed a system in which human tonsillar naive B cells, isolated on the basis of sIgD expression (17), are cultured on surrogate-activated T cells composed of CD40L-transfected fibroblasts (12). In this model, IL-4 or IL-13 induces specific isotype switching towards IgE and IgG4 (18–20), while IL-10 induces the switch towards IgG1 and IgG3 (21, 22). IL-10 may also induce the switch towards IgA as CD40-activated naive sIgD+ B cells were shown to produce IgA, albeit in quantities lower than that of IgG (17). The production of IgA becomes prominent in response to a combination of IL-10 and TGF-β (17).
To determine the possible existence of direct interactions between D-Lc and B cells in a T cell–dependent context, we used in vitro generated D-Lc obtained by culturing CD34+ cells with GM-CSF and TNFα. In this study, we demonstrate that D-Lc skew isotype switching of CD40activated naive B cells towards IgA.
Materials and Methods
Reagents.
The CD40-L–transfected Ltk− cell line (CD40L-L cells) was generated in our laboratory (23). rhGM-CSF (specific activity: 2 × 106 U/mg; Schering-Plough Research Institute, Kenilworth, NJ) was used at a saturating concentration of 200 ng/ml. rhTNFα (specific activity: 2 × 107 U/mg; Genzyme, Boston, MA) was used at an optimal concentration of 2.5 ng/ml (24). rhSCF (specific activity 2 × 105; R&D, Abingdon, UK) was used at optimal concentration of 25 ng/ml. rhIL-10 (107 U/mg; ScheringPlough Research Institute) was used at 200 ng/ml. rhIL-2 (3 × 106 U/mg; Amgen, Thousand Oaks, CA) was used at 20 U/ml. TGF-β1 (R&D) was used at 0.3 ng/ml (unless otherwise stated) and polyclonal anti–TGF-β antibody (R&D) was used at 50 μg/ml. Monoclonal anti–IL-10 receptor antibody (mAb 3F9) was generated by Dr. K. Moore (DNAX Research Institute, Palo Alto, CA) and used at 10 μg/ml. The neutralizing property of mAb3F9 was tested for its capacity to inhibit CD40-activated B cell proliferation and differentiation induced by IL-10 (25).
Isolation of sIgD+ B Lymphocytes.
B cells were isolated from tonsils as described earlier by the Ficoll-Rosetting method (17). Purified sIgD+ B lymphocytes were separated using a preparative magnetic cell sorter (MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to the experimental procedure described in detail by Miltenyi et al. (26). IgD was expressed on >99% of the sIgD+ B cell subpopulation as assessed by fluorescence analysis using a FACScan® (Becton Dickinson & Co., Mountain View, CA).
Purification of Cord–Blood CD34+ Cells.
Umbilical cord blood samples were obtained according to institutional guidelines. Cells bearing CD34 antigen were isolated from mononuclear fractions through positive selection with an anti-CD34 monoclonal antibody (Immu-133.3; Immunotech, Marseille, France) using a preparative magnetic cell sorter (MiniMACS; Miltenyi).
Generation of D-Lc from CD34+ Cells.
Cultures were established in the presence of GM-CSF, TNFα, and rhSCF in medium consisting of RPMI 1640 (GIBCO BRL, Gaithersburg, MD) supplemented with 10% (vol/vol) heat-inactivated FCS (Flow Laboratories, Irvine, UK), 10 mM Hepes (GIBCO BRL), 2 mM l-glutamine (Eurobio, Les Ullis, France) and gentamicin (0.08 μg/ml) (Schering-Plough, Levallois Perret, France). CD34+ cells were seeded for expansion in 25–150-cm2 flasks (Corning Glass, Corning, NY) at 2 × 104 cells/ml. Optimal conditions were maintained by splitting these cultures at day 5 and 10 with medium containing fresh GM-CSF and TNF-α. Cells were routinely collected after 12 d of culture when the cultures contained between 50–90% of CD1a+ dendritic cells (FITC-labeled anti-CD1a mAb; OKT6, Ortho Diagnostic System, Raritan, NJ) (5).
Culture in the CD40L System.
All cultures were performed in Iscove's medium enriched with 50 μg/ml human transferrin, 5 μg/ml bovine insulin, 0.05% BSA (all from Sigma Chemical Co., St. Louis, MO), 5% FCS (Flow Laboratories), 2 mM l-glutamine (Eurobio) and gentamicin (0.08 μg/ml) (Schering-Plough). Unless otherwise stated, for B cell proliferation and Ig secretion, 5 × 104/ml sIgD+ B cells and 5 × 104/ml D-Lc (irradiated at 3,400 rad or non irradiated) were cultured in the presence of 1.25 × 104/ml irradiated (7,500 rad) CD40L-transfected L cells (CD40L-L cells) cells in a final volume of 200 μl. For B cell proliferation, cells were pulsed 24 h with [3H]TdR at day 6. [3H]TdR uptake was measured by standard liquid scintillation counting techniques after harvesting. For phenotypic studies, the same concentration of cells was used for 7 d in a final volume of 1 ml.
RNA Isolation and PCR Amplification.
Total RNA was extracted from 0.5 to 1 × 106 of each cell type studied: Epstein-Barr virus (EBV) cell line (JY), T cell clone (MT9), in vitro generated dendritic cells, sIgD+ B cells alone and cocultured with dendritic cells. RNA was isolated by the guanidine thiocyanate method (27). Total RNA was reverse transcribed into cDNA using a random hexamer primer (Pharmacia, Upsala, Sweden) and SuperScript RNase H− reverse transcriptase (GIBCO BRL) in a 20 μl final volume. The PCR was performed as described in standard protocols with 0.5 μl of the cDNA preparation, 0.1 mM of each dNTP, 0.1 μg of each primer and 2.5 U TaqI DNA polymerase (Perkin Elmer, Norwalk, CT) in 100 μl PCR buffer (Perkin Elmer). The temperature profile used in 35 cycles of amplification was 1 min at 94°C, 1 min at 60°C and 2 min at 74°C per cycle. Oligonucleotide primers used for the PCR amplification were: sense CD3 5′-AAGATGGTTCGGTACTTCTGACTTGTG-3′, antisense CD3 5′-GTAGAGCTGGTCATTGGGCAACAGAGT-3′, and sense β-actin 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′, antisense β-actin 5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′. The size of the PCR products were 567 and 661 bp for CD3 and β-actin, respectively.
Immunoglobulin Quantification.
For immunoglobulin quantification, supernatants were harvested after 14 d of culture and IgG, IgA and IgM levels were determined by ELISA as described elsewhere (28). For titration of human IgA1, flat-bottomed microtiter plates are first coated with a rabbit anti–human IgA (dilution 1:104; Behring, Rueil-Malmaison, France), in carbonate buffer (pH 9.6). After incubation for 4 h at 37°C, the plates are then washed and saturated in PBS (0.05% Tween, 5% FCS) and incubated overnight at 4°C. Dilutions of culture supernatants in PBS (0.05% Tween, 5% FCS) are then added to plates and incubated for 2 h at room temperature. After washing, plates are incubated with mouse anti–human IgA1 (dilution of ascite at 1:103; Nordimmune, The Netherlands) for 2 h at room temperature. Plates are then washed and a goat anti–mouse IgG (Dako, Glostrup Denmark) coupled to phosphatase alcaline is added at a final dilution of 1:2,000 in PBS (0.05% Tween, 2% FCS) during 2 h at room temperature. After washing, p-nitrophenyl phosphate in 1 M diethanolamine-HCl buffer, pH 9.8, is added to plates, then allowed to react at 37°C for 30–90 min before measurement of the optical density at 405 and 490 nm with an automated device (VMAX ELISA reader; Molecular Devices, Palo Alto, CA).
For titration of human IgA2, flat-bottomed microtiter plates are first coated with a rat anti–mouse IgG1 (mAb LO-MG1-13; University of Louvain, Brussels, Belgium) at 5 μg/ml in carbonate buffer (pH 9.6). After incubation for 4 h at 37°C, the plates are washed in PBS (0.05% Tween) and incubated with mouse anti–human IgA2 (dilution of ascite at 1:103; Nordimmune) overnight at 4°C. Plates are washed and dilutions of culture supernatants in PBS (0.05% Tween, 4% nonfat milk) are added to plates and incubated for 2 h at room temperature. After washing, plates are incubated with a rabbit anti–human IgA coupled to phosphatase alcaline added at a final dilution of 1:1,000 in PBS (0.05% Tween, 4% nonfat milk) during 2 h at room temperature. After washing, substrate to phosphatase alcaline is added and optical density is measured as described above. For IgA1 and IgA2 quantitations, standards composed of 50% IgA1 kappa myeloma (BP086; The Binding Site, LTD, Birmingham, UK) and 50% IgA1 lambda myeloma (BP087) or of 50% IgA2 kappa myeloma (BP088) and 50% IgA2 lambda myeloma (BP089) are used as references. The limits of sensitivity are 4 and 8 ng/ml for IgA1 and IgA2, respectively.
FACS® Analysis.
For cytofluorometry, FITC-labeled rabbit polyclonal anti-IgA antibody was obtained from Dakopatts, FITClabeled goat polyclonal anti-IgG antibody was obtained from Kallestad (Austin, TX). Viable cells were gated by non-incorporation of propidium-iodide. For IgA subclasses staining, FITCconjugated anti-human IgA1 or anti-human IgA2 (Nordimmune) were used. The specificity of the signal was demonstrated using a cloned EBV transformed cell line expressing only IgA1 generated in our laboratory (VLMB325). For IgA2, a cloned EBV cell line (HEL) expressing only IgA2 was established from a donor being homozygous for a deletion encompassing A1-GP-G2-G4-E reported elsewhere (29). Competition experiments were done using 50 μg/ml of human IgA1κ myeloma (BP086) + IgA1λ myeloma (BP087) or 50 μg/ml of human IgA2κ myeloma (BP088) + IgA2λ myeloma (BP089) and then staining of each cell lines with FITC-conjugated anti-IgA1 or anti-IgA anti-IgA2 antibodies were performed. Labeling of the sIgA1+ EBV clone (VLMB325) was totally inhibited by IgA1λ + κ myelomas but not IgA2κ + λ myelomas, while the labeling of the sIgA2+ EBV clone (HEL) was specifically inhibited by the IgA2κ + λ myelomas but not IgA1κ + λ myelomas (Fig. 1).
Figure 1.
Surface IgA1 and IgA2 labelings are specific. Cells (5 × 105) from a sIgA1+ EBV clone (VLMB 325) and from a sIgA2+ EBV clone (HEL) were stained, respectively, with FITC-labeled anti-sIgA1 and -sIgA2 mAbs (dotted lines). Competition experiments were done by preincubating FITC-labeled anti-sIgA1 or anti-sIgA2 (right) together with 50 μg/ml human IgA1λ + κ myelomas (left) or with 50 μg/ml human IgA1λ + κ myelomas, which were then used to stain each EBV clone (black lines). Controls with unrelated mAb of the same isotype were overlapped in each panel (gray histograms).
Results
Dendritic Cells Increase the Proliferation of CD40-activated Naive sIgD+ B Cells.
We first studied whether the proliferation of CD40-activated naive sIgD+ B cells was affected by the addition of D-Lc (dendritic cells generated by culturing CD34+ progenitors in the presence of GM-CSF and TNF-α for 12 d). As shown in Fig. 2 A, addition of 103 D-Lc increased DNA synthesis of 104 CD40-activated naive B cells. Maximum DNA synthesis was observed with 104 D-Lc, the highest density of D-Lc tested (concentrations above 104 D-Lc resulted in medium impoverishment). The stimulatory effect of 104 D-Lc appeared at early time points (day 4), increased until day 6 and then diminished (Fig. 2 B). Similar results were obtained when D-Lc were either irradiated or not (Fig. 2, A and B). Naive B cells did not proliferate in response to D-Lc when CD40L-L cells were omitted (data not shown).
Figure 2.
In vitro generated D-Lc enhance CD40-activated naive B cell proliferation. 5 × 104/ml highly purified sIgD+ B lymphocytes were cultured in a final volume of 200 μl over 1.25 × 104/ml irradiated CD40L-L cells in the absence or with increasing numbers of D-Lc (irradiated or not) without exogenous cytokine. Thymidine uptake, determined after 6 d (A), or at different indicated time points (B), is expressed as mean ± SD of triplicate cultures. (C) DNA synthesis of 5 × 104/ml CD40-activated sIgD+ B cultured 6 d in the absence or presence of 5 × 104/ml in vitro generated D-Lc with or without IL-10 (200 ng/ml), TGF-β (0.3 ng/ml), or both. (D) For enumeration experiments, 5 × 104/ml sIgD+ B were cultured for 6 d in the presence or absence of 5 × 104/ml D-Lc, with or without IL-10 (200 ng/ml) and increasing doses of TGF-β (0.1, 0.3, 1, and 3 ng/ml). Viable cells that were not colored with Trypan blue dye were enumerated at day 6. One representative experiment is shown out of 4 in A and B, 6 in C, and 2 in D.
CD40-activated naive B cells showed enhanced proliferation in response to IL-10. Addition of D-Lc slightly enhanced IL-10–induced B cell proliferation (Fig. 2 C). TGF-β neither enhanced nor inhibited the proliferation of CD40activated naive B cells in the presence or absence of IL-10 with or without D-Lc (Fig. 2 C). The enhanced DNA synthesis observed in response to the addition of D-Lc, resulted in increased numbers of viable B cells as measured after 6 d (Fig. 2 D). Note that the numbers of viable B cells recovered from cultures performed with TGF-β (0.1–3 ng/ml) were not markedly decreased (Fig. 2 D).
Dendritic Cells Induce CD40-activated Naive sIgD+ B Cells to Express Surface IgA1 and IgA2.
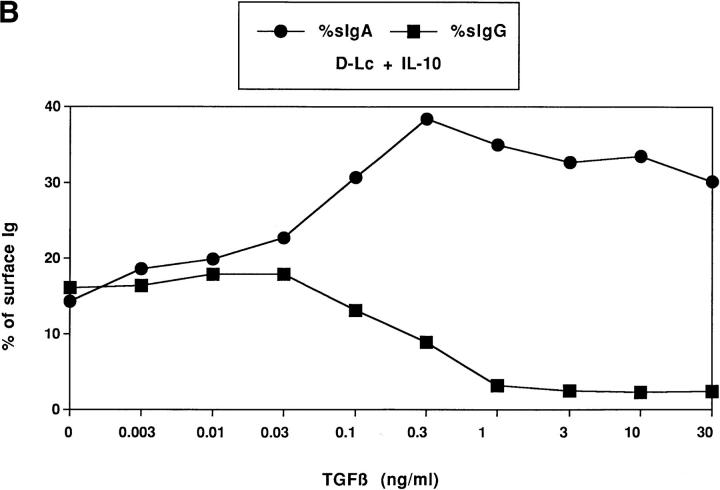
After 7 d of CD40 activation, the frequency of cultured naive B cells expressing sIgA was always lower than 1–2% (Fig. 3 A). Upon addition of either DC alone or a combination of TGF-β and IL-10, 6.5–10% of naive B cells expressed sIgA. Neither IL-10 nor TGF-β alone were able to induce naive B cells to express sIgA. However IL-10, and to a lower extent TGF-β, can enhance sIgA expression induced by D-Lc. D-Lc potentiated strikingly the induction of sIgA on CD40activated naive B cells induced by a combination of IL-10 and TGF-β from 6.5 up to 41.7% (mean 35 ± 15%, n = 6). 0.3 ng/ml of TGF-β induced an optimal increase of the frequency of sIgA B cells generated in response to D-Lc and IL-10 (Fig. 3 B). With regard to sIgG, IL-10 alone appeared to be a strong inducer (from 3.3 to 11.6%), whereas D-Lc alone displayed virtually no effect. Yet, D-Lc enhanced IL-10 induced expression of sIgG (from 11.6 to 16.9%) (Fig. 3 A). Importantly, D-Lc with TGF-β, inhibited IL-10–induced sIgG expression at concentrations that stimulated sIgA expression (Fig. 3 B). Further analysis of the sIgA subclasses shows that 18.8% of CD40-activated naive B cells expressed sIgA1, whereas 7.9% expressed sIgA2 after culture in the presence of IL-10, TGF-β, and D-Lc (Fig. 3 C).
Figure 3.
D-Lc induce CD40-activated naive B cells to express sIgA. 5 × 104/ml sIgD+ B lymphocytes were cultured in a final volume of 1 ml in the absence or presence of 5 × 104/ml D-Lc over 1.25 × 104/ml irradiated CD40L-L cells without exogenous cytokine or with IL-10 (200 ng/ ml), TGF-β (0.3 ng/ml) or both (A), or with IL-10 and increasing doses of TGF-β (B). B cells were harvested after 7 d of culture and stained with rabbit polyclonal FITC-labeled anti-IgA or anti-IgG antibodies (A, B) or FITC-labeled anti-IgA1 or -IgA2 mAbs (C) and analyzed by FACScan®. Dead cells which incorporated propidium iodide were excluded. Histograms are presented (A) or percentages of sIgG and sIgA positively stained cells (B). One representative experiment out of six (A) and one out of three (B) are shown.
Induction of sIgA by Dendritic Cells Is Partially Dependent on Endogenous TGF-β.
As TGF-β and IL-10 can be readily produced by CD40-activated B cells (30, 31), we questioned whether D-Lc–induced sIgA could be dependent on the endogenous production of these cytokines. As shown in Fig. 4, D-Lc–induced sIgA on B cells was not affected by the addition of a neutralizing anti–IL-10 receptor antibody. In contrast, addition of a neutralizing anti–TGF-β polyclonal antibody significantly inhibited D-Lc–induced sIgA on B cells. The combination of anti–IL-10 receptor and anti–TGF-β antibodies had no further inhibitory effect than that observed with the anti–TGF-β antibody alone. The neutralizing properties of the antibodies were demonstrated by the fact that (a) addition of anti–IL-10R mAb blocked IL-10–induced sIgA expression in the presence of D-Lc and (b) addition of anti–TGF-β blocked the potentiating effect of TGF-β in response to D-Lc and IL-10. Finally, a toxic effect of anti–TGF-β antibody was excluded, as high dose of TGF-β added exogenously reversed the inhibitory effect of the anti–TGF-β antibody (data not shown). Thus endogenous TGF-β, but not IL-10, partially contribute to the capacity of D-Lc to induce surface IgA expression on CD40-activated naive B cells in the absence of exogenous cytokines. However, our results indicate that D-Lc provide an additional important sIgA inducing signal that acts in concert with IL-10 and TGF-β to induce the expression of surface IgA on almost half the naive B cells.
Figure 4.
Induction of sIgA by D-Lc is partially dependent on endogenous TGF-β. 5 × 104/ml sIgD+ B lymphocytes were cultured in a final volume of 1 ml in the absence or presence of 5 × 104/ml D-Lc over 1.25 × 104/ml irradiated CD40L-L cells without exogenous cytokine or with IL-10 (200 ng/ml), TGF-β (0.3 ng/ml) or both. To neutralize IL-10 or TGF-β, a rat monoclonal anti–IL-10 receptor antibody (mAb3F9) or a rabbit polyclonal anti–TGF-β antibody (anti–TGF-β) were used at 10 or 50 μg/ml, respectively. B cells were harvested after 7 d of culture and stained with rabbit polyclonal FITC-labeled anti-IgA and analyzed by FACScan®. Dead cells that incorporated propidium iodide were excluded. One representative experiment out of six is shown.
Dendritic Cells Skew Cytokine-dependent Ig Secretion towards IgA.
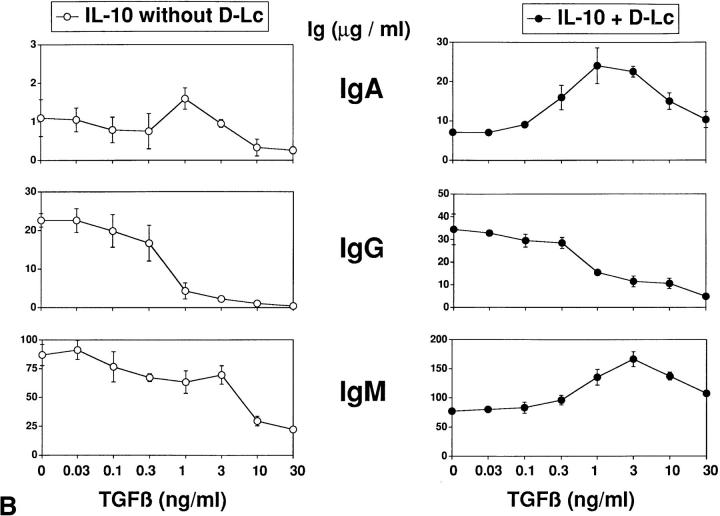
sIgD+ B cells triggered solely through their CD40 antigen did not secrete detectable Ig (17 and Fig. 5 A) and further addition of D-Lc did not result in significant increases of IgA, IgG, and IgM (Fig. 5 A). However, D-Lc considerably enhanced (10–20-fold) the secretion of IgA induced by IL-10 used alone or in combination with TGF-β while the production of IgM and IgG were less significantly affected (Fig. 5 A). In the absence of D-Lc, 0.3–3 ng/ml TGF-β enhanced the IL-10–induced IgA synthesis of CD40-activated sIgD+ B cells, while inhibiting IL-10– induced IgG and IgM synthesis (17 and Fig. 3 B). In the presence of D-Lc, TGF-β increased IL-10–induced IgM synthesis by twofold but retained its strong inhibitory effect on the secretion of IgG (Fig. 5 B). D-Lc potentiated the secretion by CD40-activated sIgD+ B cells of IgA1 and importantly, induced the secretion of IgA2 in response to IL-10 (Table 1). Addition of TGF-β to cultures further enhanced the secretion of both IgA subclasses.
Figure 5.

D-Lc enhance cytokine-induced Ig secretion of CD40-activated naive B cells. 5 × 104/ml sIgD+ B lymphocytes were cultured for 14 d in a final volume of 200 μl in the presence or absence of 5 × 104/ml D-Lc over 1.25 × 104/ml irradiated CD40L-L cells without exogenous cytokine or with IL-10 (200 ng/ml), TGF-β (1 ng/ml), or both (A), or with IL-10 and increasing doses of TGF-β (B). IgA, IgG, or IgM levels in culture supernatants were measured in specific ELISAs and results are expressed as mean ± SD of triplicate cultures. One representative experiment out of eight (A) and three (B), respectively, are shown.
Table 1.
D-Lc Can Drive B Cells to Secrete Both IgA1 and IgA2 in Response to Cytokines
| Experiment | IgA1 (ng/ml) | IgA2 (ng/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Without D-Lc | With D-Lc | Without D-Lc | With D-Lc | |||||||
| 1 | IL-10 | 413 ± 350 | 2456 ± 1116 | 0 | 58 ± 20 | |||||
| IL-10 + TGFβ | 725 ± 283 | 6051 ± 812 | 5 ± 2 | 180 ± 69 | ||||||
| 2 | IL-10 | 1125 ± 117 | 6339 ± 432 | 6 ± 2 | 169 ± 14 | |||||
| IL-10 + TGFβ | 2091 ± 215 | 8429 ± 1113 | 12 ± 4 | 307 ± 41 | ||||||
| 3 | IL-10 | 181 ± 84 | 9492 ± 966 | 0 | 583 ± 174 | |||||
| IL-10 + TGFβ | 507 ± 344 | 15816 ± 3844 | 0 | 806 ± 287 | ||||||
5 × 104/ml slgD+ B lymphocytes were cultured for 14 d in a final volume of 200 μl in the presence or absence of 5 × 104/ml D-Lc over 1.25 × 104/ml irradiated CD40L-L cells with IL-10 (200 ng/ml) or with IL-10 + TGFβ (1 ng/ml). IgA1 and IgA2 levels in culture supernatants were measured in specific ELISAs as described in Materials and Methods. Results are expressed as mean ± SD of triplicate cultures.
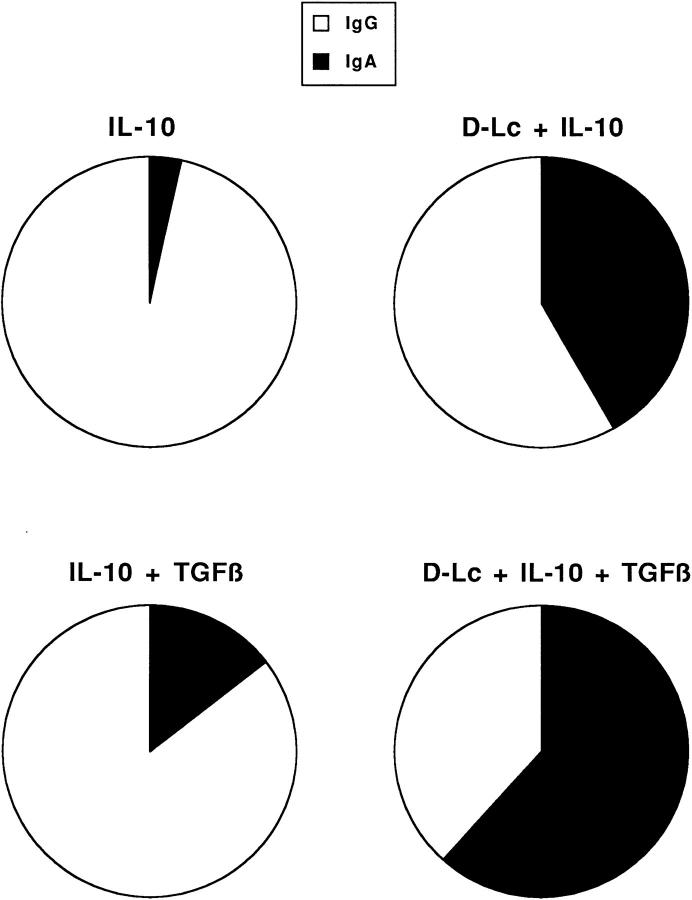
Overall, D-Lc preferentially favored IgA synthesis induced by IL-10 alone or in combination with TGF-β at the expense of IgG. This is illustrated in Fig. 6 which is a compilation of the results of 23 experiments performed with IL-10 and 8 experiments performed with IL-10 and TGF-β over a two-year period, using different batches of tonsillar sIgD+ B cells and D-Lc CD34+-derived from distinct donors.
Figure 6.
D-Lc favor IgA secretion to the expense of IgG. The effect of D-Lc on the ratio of IgA versus IgG produced by CD40-activated naive B cells cultured with IL-10 alone or in combination with TGF-β is schematically represented. The figure represents the results of 23 experiments for culture with IL-10 and 8 for IL-10 plus TGF-β with or without D-Lc. These results are the compilation of different cultures performed over a two-year period using different donors of sIgD+ B cells and D-Lc.
Effects on B Cell Responses Are Strictly due to Dendritic Cells.
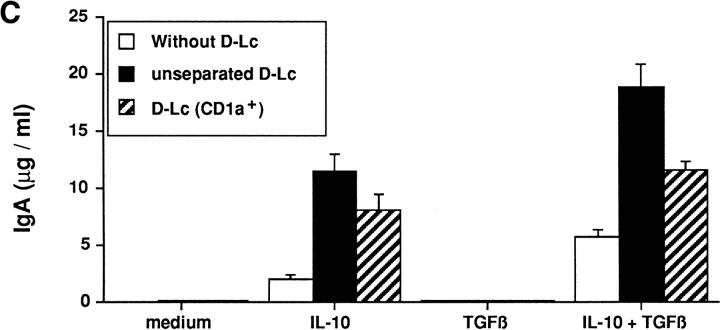
Dendritic cells generated in vitro by culturing hematopoietic progenitors for 12 d in GM-CSF, TNF-α, and SCF contain between 50–90% of CD1a+ cells. To exclude the contribution of nondendritic cells, dendritic cells were sorted on the basis of CD1a expression. As shown in Fig. 7 A, purity of CD1a-sorted dendritic cells reached 95% (ranging from 95 to 97%, n = 3). CD1a-sorted Dendritic cells were capable to induce comparable levels of sIgA on CD40-activated sIgD+ B cells after 7 d of coculture in the presence of IL-10 and TGF-β than those obtained with unseparated D-Lc (30.1 versus 35.6%) (results obtained with CD1asorted Dendritic cells: mean 29.2 ± 0.8%, n = 3) (Fig. 7 B). In addition, as observed with unseparated D-Lc, CD1asorted Dendritic cells were able to potentiate IL-10-induced IgA secretion by CD40-activated sIgD+ B cells (Fig. 7 C) that was further increased in the presence of TGF-β.
Figure 7.
Effects on B cell responses are strictly due to D-Lc. (A) Day 0, sorting of CD1a+ dendritic cells. (B) Day 7, induction of sIgA by CD1a+-sorted dendritic cells. (C) Day 14, CD1a+-sorted dendritic cells potentiate cytokine-induced IgA secretion. After 12 d maturation of CD34+ hematopoietic progenitors into dendritic cells, D-Lc were sorted on the basis of CD1a expression (A). 5 × 104/ml sIgD+ B lymphocytes were cultured in a final volume of 1 ml in the absence or presence of 5 × 104/ml unseparated or sorted CD1a+D-Lc over 1.25 × 104/ml irradiated CD40L-L cells without exogenous cytokine or with IL-10 (200 ng/ml), TGF-β (0.3 ng/ml) or both. Surface IgA on B cells was analyzed after 7 d culture with IL-10 and TGF-β (B) and IgA secretion was measured at day 14 (C) as previously described.
To exclude a possible contribution to those effects of contaminating T cells expanded by allogeneic D-Lc, the presence of T cells was analyzed using the sensitive RTPCR for CD3 mRNA. As shown in Fig. 8, CD3 mRNA could not be detected within (a) in vitro-generated D-Lc, (b) MACS-purified sIgD+ B cells, (c) cocultures of D-Lc and sIgD+ B cells even in the presence of IL-2. Likewise, no CD3 mRNA could be detected in cocultures of D-Lc and sIgD+ B cells in the presence of IL-10 with or without TGF-β (data not shown). Contaminating T cells could not be detected under such culture conditions, thus demonstrating that the results reported herein are only dependent upon the interaction of Dendritic cells with CD40-activated sIgD+ B cells.
Figure 8.
T cells were never detected whatever culture conditions tested. Total RNA was extracted from 0.5–1 × 106 of each cell type studied: JY (EBV cell line), MT9 (T cell clone), unseparated D-Lc, sIgD+ B cells alone or cocultured 7 d at 5 × 104/ml in a final volume of 2 ml in the absence or presence of 5 × 104/ ml D-Lc over 1.25 × 104/ml irradiated CD40L-L cells without exogenous cytokine or with IL-2 (20 U/ml). Total RNA was transcribed into cDNA and 35 cycles PCR were performed with CD3 primer (lanes 2, 4, 6, 8, 10, 12, 14) or with β-actin primer (lanes 1, 3, 5, 7, 9, 11, 13) as positive control. The size of the PCR products are 567 bp for CD3 and 661 bp for β-actin.
Discussion
The present study shows that DC skew humoral immune response towards a mucosal type as demonstrated by the induced switch and secretion of IgA1 and IgA2 by CD40-activated naive B cells.
Our present observation significantly extends earlier studies performed with mouse and human B cells. As observed with T cell–activated mouse B cells, DC can induce CD40 (T cell)-activated human B cells to switch towards IgA (32– 35). In this context, this present study brings about important new information: (a) DC have a direct effect on B cells, provided that B cells are activated through their CD40. (b) in contrast to the unique IgA isotype in mice, humans bear two IgA subclasses whose differential in vivo expression suggests distinct regulation. Our data indicate an important role for DC in allowing the production of IgA2, an isotype that naive B cells cannot be induced to secrete in response to cytokines (IL-10 and TGF-β) alone. (c) In vitro generated human DC share properties with DC isolated from mouse secondary lymphoid organs such as spleen and Peyers patches.
Although studies with human B cells indicated that both IL-10 and TGF-β are involved in regulating the switch towards IgA (17, 30), the IgA switch capacity of DC cannot be solely ascribed to either or both of these cytokines. Antibodies neutralizing IL-10 did not affect DC-induced IgA expression, while neutralizing anti–TGF-β only partially reduced the responses. Indeed, DC were reproducibly found to induce the IgA switch more efficiently than the combination of TGF-β and IL-10. The unique nature of DC IgA switching activity was also illustrated by the synergy observed in sIgA induction when supplementing DC to the combination of IL-10 and TGF-β. The DC molecule(s) that induce(s) the IgA switch remain(s) to be determined and preliminary experiments indicate that the effect cannot be simply reproduced using cell-free supernatants from D-Lc (activated or not), neither by separating physically D-Lc from naive B cells.
Although DC are able to provide CD40-activated naive B cells with the necessary signals to induce surface IgA expression, they are not able to directly induce naive B cells to differentiate into IgA-secreting cells. However, when IL-10 is added to provide the critical signal for this differentiation to occur, then DC considerably enhance Ig secretion. Interestingly, in the presence of IL-10 and TGF-β, DC skewed Ig secretion towards IgA and importantly secretion of both IgA1 and IgA2 subclasses is upregulated. Our study analyses isotype switching to IgA1/IgA2 down to the expression/secretion of the proteins, while earlier studies on human B cells have essentially focused on the induction of the germline and mature α1/α2 transcripts. These molecular studies have provided some insights into the complex regulation of IgA subclasses expression with regard to cytokines/T cell signals. Naive B cells can be induced to express: (a) germline α1 and α2 transcripts in response to either TGF-β or CD40 triggering, (b) mature α1 transcripts in response to either a combination of TGF-β and IL-10 or CD40 triggering, (c) mature α2 transcripts in response to a combination of TGF-β, IL-10, and CD40 triggering (30, 36). Our results regarding the cell surface expression and secretion of IgA1 and IgA2 proteins show that (a) cell surface expression and secretion of IgA1 can be induced in response to a combination of IL-10 and CD40 triggering (with or without exogenous TGF-β); and (b) cell surface expression and secretion of IgA2 can be induced with a combination of IL-10, dendritic cells and CD40 triggering (with or without exogenous TGF-β). Thus DC provide a critical signal for the expression and the secretion of IgA2 protein. However, the present data do not permit to determine whether DC enhance an event that is otherwise under the detection threshold or whether DC provide a unique signal for expression/secretion of IgA2.
Our current demonstration of a direct functional effect of DC on IgA switch of CD40-activated naive B cells further extends our recent studies that showed that DC also induce CD40-activated (a) memory B cells to secrete high amounts of IgG and IgA and (b) naive B cells to produce considerable levels of IgM in response to IL-2 (37). Taken together, these effects may reflect physiological events occurring in vivo. First, the interdigitating dendritic cells within T cell–rich areas may be involved in the differentiation of activated B cells into plasma blast cells that subsequently accumulate within medullary cords. Second, the numerous DC found in the gut lining (38) may contribute to the switching towards IgA1 and IgA2, which characterizes mucosal responses. Importantly, the recent identification of a new population of DC located within germinal centers (39), a critical site for isotype switching (40), suggests that some DC may directly be involved in the regulation of isotype switching.
Acknowledgments
We gratefully acknowledge the expert editorial assistance of Sandrine Bonnet-Arnaud and Nicole Courbière and Daniel Lepot for the illustrations. We are also indebted towards Dr. Ahmed Helal and Professor Girard LeFranc who generously provided us with peripheral blood from a donor homozygous for a deletion encompassing A1-GP-G2-G4-E. We are grateful to doctors from clinics and hospitals in Lyon who provide us with umbilical cord blood samples and tonsils.
Footnotes
1 Abbreviations used in this paper: CD40L, CD40 ligand; CD40L-L cells, CD40L-transfected L cells; DC, dendritic cells; D-Lc, dendritic Langerhans cells; sIg, surface immunoglobulins; IL-10, interleukin 10; TGF-β, transforming growth factor β.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Inaba K, Granelli-Piperno A, Steinman RM. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and man. Proc Natl Acad Sci USA. 1983;80:6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inaba K, Witmer MD, Steinman RM. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells, during primary antibody responses in vitro. J Exp Med. 1984;160:858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sornasse T, Flamand V, de Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Oberdan L, Moser M. Antigen-pulse dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caux C, Dezutter-Dambuyant C, Schmitt D, Banchereau J. GM-CSF and TNF-α cooperate in the generation of dendritic Langerhans cells. Nature (Lond) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 6.Reid CDL, Stackpoole A, Meager A, Tikerpae J. Interactions of tumor necrosis factor with granulocytemacrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+progenitors in human bone marrow. J Immunol. 1992;149:2681–2688. [PubMed] [Google Scholar]
- 7.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caux C, Massacrier C, Vanbervliet B, Dubois B, van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark EA, Ledbetter JA. How B and T cells talk to each other. Nature (Lond) 1994;367:425–428. doi: 10.1038/367425a0. [DOI] [PubMed] [Google Scholar]
- 11.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–360. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its Ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbaugh D, Ochs HD, Noelle RJ, Ledbetter JA, Aruffo A. The role of CD40 and its ligand in the regulation of the immune response. Immunol Rev. 1994;138:23–37. doi: 10.1111/j.1600-065x.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 14.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand GP39. Ann Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 15.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyperIgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 16.Kroczek RA, Graf D, Brugnoni D, Giliani S, Korthuer U, Ugazio A, Senger G, Mages HW, Villa A, Notarangelo LD. Defective expression of CD40 ligand on T cells causes “X-linked immunodeficiency with hyper-IgM (HIGM1)”. Immunol Rev. 1994;138:39–59. doi: 10.1111/j.1600-065x.1994.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 17.Defrance T, Vanbervliet B, Brière F, Durand I, Rousset F, Banchereau J. Interleukin 10 and transforming growth factor β cooperate to induce anti-CD40–activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992;175:671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gascan H, Gauchat JF, Roncarolo MG, Yssel H, Spits H, de Vries JE. Human B cell clones can be induced to proliferate and to switch to IgE and IgG4 synthesis by interleukin 4 and a signal provided by activated CD4+T cell clones. J Exp Med. 1991;173:747–750. doi: 10.1084/jem.173.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousset F, Garcia E, Banchereau J. Cytokineinduced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabara HH, Fu SM, Geha RS, Vercelli D. CD40 and IgE: synergism between anti-CD40 monoclonal antibody and interleukin 4 in the induction of IgE synthesis by highly purified human B cells. J Exp Med. 1990;172:1861–1864. doi: 10.1084/jem.172.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malisan F, Brière F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. IL-10 induces IgG isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brière F, Servet-Delprat C, Bridon JM, Saint-Remy JM, Banchereau J. Human interleukin 10 induces naive sIgD+ B cells to secrete IgG1 and IgG3 . J Exp Med. 1994;179:757–762. doi: 10.1084/jem.179.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrone P, Neidhardt EM, Garcia E, Galibert L, van Kooten C, Banchereau J. Fas ligation induces apoptosis of CD40-activated human B lymphocytes. J Exp Med. 1995;182:1265–1273. doi: 10.1084/jem.182.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caux C, Saeland S, Favre C, Duvert V, Mannoni P, Banchereau J. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+hematopoietic progenitor cells. Blood. 1990;75:2292–2298. [PubMed] [Google Scholar]
- 25.Liu, Y., R. de Waal Malefyt, F. Brière, C. Parham, J.M. Bridon, J. Banchereau, K. Moore, and J. Xu. 1997. The Epstein-Barr virus interleukin-10 (IL-10) homolog is a selective agonist with impaired binding to the IL-10 receptor. J. Immunol. In press. [PubMed]
- 26.Miltenyi S, Müller W, Weichel W, Radbruch A. High gradient magnetic cell separation with Macs. Cytometry. 1990;11:231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Defrance T, Vanbervliet B, Pène J, Banchereau J. Human recombinant IL-4 induces activated B lymphocytes to produce IgG and IgM. J Immunol. 1988;141:2000–2005. [PubMed] [Google Scholar]
- 29.Wiebe V, Helal A, Lefranc MP, Lefranc G. Molecular analysis of the T17 immunoglobulin CH multigene deletion (del A1-GP-G2-G4-E) Hum Genet. 1994;93:520–528. doi: 10.1007/BF00202816. [DOI] [PubMed] [Google Scholar]
- 30.Kitani A, Strober W. Differential regulation of Cα1 and Cα2 germ-line and mature mRNA transcripts in human peripheral blood B cells. J Immunol. 1994;153:1466–1477. [PubMed] [Google Scholar]
- 31.Burdin N, van Kooten K, Galibert L, Abrams JS, Wijdenes J, Banchereau J, Rousset F. Endogenous IL-6 and IL-10 contribute to the differentiation of CD40- activated human B lymphocytes. J Immunol. 1995;154:2533–2544. [PubMed] [Google Scholar]
- 32.Schrader, C.E., and J.J. Cebra. 1993. Dendritic Cell Dependent expression of IgA by clones in T/B microcultures. In Dendritic Cells in Fundamental and Clinical Immunology. Kamperdijk, editors. Plenum Press. New York. 59–64. [DOI] [PubMed]
- 33.Schrader CE, Geroge A, Kerlin RL, Cebra JJ. Dendritic cells support production of IgA and other non-IgM isotypes in clonal microculture. Int Immunol. 1990;2:563–570. doi: 10.1093/intimm/2.6.563. [DOI] [PubMed] [Google Scholar]
- 34.Cebra, J.J., N.A. Bos, E.R. Cebra, C.F. Cuff, G.J. Deenen, F.G.M. Kroese, and K.E. Shroff. 1994. Development of Components of the mucosal immune system in SCID recipient mice. In In Vivo Immunology. E. Heinen, editors. Plenum Press, New York. 255–259. [DOI] [PubMed]
- 35.Spalding DM, Griffin JA. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986;44:507–515. doi: 10.1016/0092-8674(86)90472-1. [DOI] [PubMed] [Google Scholar]
- 36.Islam KB, Nilsson L, Sideras P, Hammarström L, Smith CIE. TGF-β1 induces germ-line transcripts of both IgA subclasses in human B lymphocytes. Int Immunol. 1991;3:1099–1106. doi: 10.1093/intimm/3.11.1099. [DOI] [PubMed] [Google Scholar]
- 37.Dubois B, Vanbervliet B, Fayette J, Massacrier C, van Kooten C, Brière F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer's patch. J Exp Med. 1996;183:237–247. doi: 10.1084/jem.183.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grouard G, Durand I, Filgueira L, Banchereau J, Liu YJ. Dendritic cells capable of stimulating T cells in germinal centers. Nature (Lond) 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- 40.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers isotype switching of immunoglobulin genes occurs after onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]