Abstract
Terminal sialic acids on cell surface glycoconjugates can carry 9-O-acetyl esters. For technical reasons, it has previously been difficult to determine their precise distribution on different cell types. Using a recombinant soluble form of the Influenza C virus hemagglutinin-esterase as a probe for 9-O-acetylated sialic acids, we demonstrate here their preferential expression on the CD4 T cell lineage in normal B10.A mouse lymphoid organs. Of total thymocytes, 8–10% carry 9-O-acetylation; the great majority of these are the more mature PNA−, HSA−, and TCRhi medullary cells. While low levels of 9-O-acetylation are seen on some CD4/CD8 double positive (DP) and CD8 single positive (SP) cells, high levels are present primarily on 80– 85% of CD4 SP cells. Correlation with CD4 and CD8 levels suggests that 9-O-acetylation appears as an early differentiation marker as cells mature from the DP to the CD4 SP phenotype. This high degree of 9-O-acetylation is also present on 90–95% of peripheral spleen and lymph node CD4 T cells. In contrast, only a small minority of CD8 T cells and B cells show such levels of 9-O-acetylation. Among mature peripheral CD4 T lymphocytes, the highly O-acetylated cells are Mel 14hi, CD44lo, and CD45R(exon B)hi, features typical of naive cells. Digestions with trypsin and O-sialoglycoprotease (OSGPase) and ELISA studies of lipid extracts indicate that the 9-O-acetylated sialic acids on peripheral CD4 T cells are predominantly on O-linked mucintype glycoproteins and to a lesser degree, on sialylated glycolipids (gangliosides). In contrast, sialic acids on mucin type molecules of CD8 T cells are not O-acetylated; instead these molecules mask the recognition of O-acetylated gangliosides that seem to be present at similar levels as on CD4 cells. The 9-O-acetylated gangliosides on mouse T cells are not bound by CD60 antibodies, which recognize O-acetylated gangliosides in human T cells. Tethering 9-O-acetylated mucins with the Influenza C probe with or without secondary cross-linking did not cause activation of CD4 T cells. However, activation by other stimuli including TCR ligation is associated with a substantial decrease in surface 9-O-acetylation, primarily in the mucin glycoprotein component. Thus, 9-O-acetylation of sialic acids on cell surface mucins is a novel marker on CD4 T cells that appears on maturation and is modulated downwards upon activation.
Sialic acids (Sia)1 are a family of 9-carbon negatively charged sugars usually found at terminal positions of mammalian cell surface sugar chains. Sias can be attached to underlying glycoconjugates in a variety of linkages, and modified in many different ways (1, 2). These modifications were once thought to be rare, species-specific curiosities. However, recent evidence has shown that some modifications are more widely distributed than previously thought (2, 3). For example, Sias often carry O-acetyl substitutions at the C-7 or C-9 positions (1–4). Since O-acetyl esters at C-7 position will spontaneously migrate to the C-9 position at physiologic extracellular pH (5, 6), 9-O-acetylated Sias predominate on cell surface glycoconjugates (7). Identification of the cells and sialoglycoconjugates bearing 9-Oacetylation has been technically challenging, since these esters are often present on only a fraction of total sialic acids, and biochemical purification steps can be accompanied by varying losses of O-acetylation.
Several studies have shown that 9-O-acetylation can affect the biological properties of the underlying molecules. These esters are commonly seen on cells of neuroectodermal origin and seem to have a role in the migration of developing neurons in the embryonic brain (8, 9). Abrogation of 9-O-acetylation can deleteriously affect development in transgenic mice (10). This modification is also involved in specific recognition of cells by Influenza C virus and coronaviruses (11–13), can modulate activation of complement by the alternate pathway (14, 15), and can mask ligands for I-type lectins that mediate cell adhesion via sialylated ligands e.g., CD22 on B cells (16) and sialoadhesin on tissue macrophages (15, 17).
Taking advantage of the binding and catalytic property of the Influenza C virion's hemagglutinin-esterase (CHE) protein for 9-O-acetylated sialic acids, we had earlier engineered a recombinant soluble form of the extracellular domain of CHE (CHE-Fc) with a human IgG1 Fc region fused to the carboxyl end (3). Treatment of this molecule with diisopropylfluorophosphate (DFP) selectively abrogates its esterase activity (CHE-FcD) while preserving the binding property (3, 18). Thus, the probe is a bifunctional tool for either selectively removing (CHE-Fc) or specifically detecting (CHE-FcD) 9-O-acetyl esters. In studying mouse peripheral leukocytes with this probe, we noted high binding to CD4 T cells and much less binding to CD8 T cells. Apart from the CD4 molecule itself, we are unaware of any other cell surface marker that is selectively expressed on CD4 cells in comparison to CD8 cells. We therefore decided to further delineate the presence and distribution of 9-O-acetylated sialic acids on cells of the murine T cell lineage. Indeed, we find that 9-O-acetylation of sialic acids is a novel cell surface marker which appears during differentiation of CD4 single positive T cells in the thymus, persists at high levels on these cells in the periphery, and decreases in expression upon cellular activation. We have also used the enzymes trypsin and O-sialoglycoprotease (OSGPase) (19) to explore the distribution of these modified sialic acids on the different classes of glycoconjugates found on CD4 cell surfaces. The latter enzyme is particularly useful because it selectively cleaves mucin-type glycoproteins with clustered sialylated O-linked sugar chains (20), such as CD43 and some CD45 forms (21).
Materials and Methods
Mice.
4–6-wk-old B10.A mice were purchased from The Jackson Laboratories (Bar Harbor, ME). All studies were done with freshly isolated cells from 6–8 wk old B10.A mice.
Antibodies and Probes.
Fluoresceinated anti-human IgG secondary antibodies were used to detect the binding of the CHEFcD probe to the cells. FITC, phycoerythrin or tricolor conjugated rat monoclonal antibodies against murine CD4 and CD8 were purchased from Caltag Laboratories (San Francisco, CA). Binding of biotinylated rat monoclonal antibodies (from PharMingen, San Diego, CA) against murine CD25, CD69, CD5, Mel 14, CD45RB, CD43, and Pgp-1 was detected using appropriately conjugated streptavidin in all flow cytometric assays. The antiCD43 monoclonal antibody S7 recognized the 115-kD isoform of CD43. The 23G2 clone of the anti CD45RB monoclonal antibody was used in all FACS® staining and Western blots. Binding of anti-CD45RB and CD43 antibodies to proteins in Western blots was detected using Neutravidin-alkaline phosphatase conjugate purchased from Pierce (Rockford, Illinois). The anti-mouse CD3 hamster hybridoma 2C11was prepared in ascites form and used to stain cells expressing the TCR. The rat anti–mouse heat stable antigen (HSA) was used as a culture supernatant from the J11D hybridoma. JONES antibody (a mouse IgM recognizing 9-O-acetylated GD3) (9) was purchased from Sigma Chem. Co. (St. Louis, MO) while 27A, a mouse IgG (22) with the same specificity as JONES, was obtained from cell culture supernatants of hybridomas provided by M. Farquhar (UCSD). The soluble chimeric protein CHE-Fc, consisting of the extracellular domain of influenza C hemagglutinin-esterase fused to the Fc portion of human IgG1 was generated and characterized as described (3). The modified form CHE-FcD was generated by treating CHE-Fc with 1 mM DFP to inactivate the esterase activity as described (3). CHE-Fc specifically releases 9-O-acetyl esters from Sias (i.e., it functions as an esterase), whereas CHE-FcD specifically recognizes and binds to 9-O-acetylated Sias (3). Fluoresceinated peanut agglutinin lectin (PNA) was obtained from EY Laboratories Inc. (San Mateo, CA).
Flow Cytometric Analysis of 9-O-Acetylation on Cells.
The expression of cell surface 9-O-acetylated sialic acids was examined by flow cytometric analysis on a FACScan® machine. Freshly obtained live cells from B10.A mouse thymus, lymph nodes and spleen were prepared after hypotonic lysis of RBCs and 1 × 106 cells were washed and suspended in ice cold PBS with 1% bovine serum albumin and 0.1% sodium azide (staining buffer). The CHE-FcD probe was allowed to bind to the cells after being immunocomplexed with FITC or phycoerythrin conjugated goat anti– human secondary antibody. CHE-FcD (3 μg) was incubated with a 1 in 100 dilution of the secondary antibody at 4°C overnight. This pre-complexing step increases the overall avidity of the probe towards 9-O-acetylated sialic acids. The CHE-FcD + anti-human IgG Fc immunocomplex was allowed to bind to the cells for 2 h on ice. For all double and triple-color stainings, the CHE-FcD probe was allowed to bind to the cells before staining with the remaining antibodies for 30 min at room temperature. The CHE-Fc was used under identical conditions as a negative control for CHE-FcD binding. In studies where cells had to be de-O-acetylated before staining, CHE-Fc was used at a final concentration of 10 μg/ml in a volume of 50 μl of staining buffer containing 3–5 × 106 cells and incubated at 37°C for 1 h. For trypsinization, cells were incubated in 0.1% trypsin with 0.5 mM EDTA in Hank's balanced salt solution for 1 h in a 37°C water bath, then washed twice with cold PBS before staining. The loss of anti-CD4 binding was used to confirm trypsin activity. For treatment with OSGPase (Cedarlane Laboratories Ltd., Ontario, Canada) 5 × 106 live cells in 100 μl of staining buffer were incubated with 5 μl of the reconstituted enzyme at 37°C for 1 h. Cells were then washed twice with cold PBS before staining. Decrease in CHE-FcD binding to murine erythroleukemia cells following OSGPase treatment was used as a positive control for this enzyme (data not shown). Following staining, cells were washed with excess staining buffer and fixed in PBS containing 1% formaldehyde (fixing buffer). Stained fixed cells were kept in dark at 4°C and analyzed by flow cytometry the same day.
Purification of CD4 T Cells.
Fresh mouse lymphocytes from either spleen or lymph nodes were prepared after hypotonic lysis of RBCs and separation of dead cells by Ficoll Hypaque density centrifugation. Cells were stained with rat anti–mouse CD4 conjugated with magnetic beads from Miltenyi Biotec, Inc. (Auburn, CA). This antibody was used at a final dilution of 1:10 to stain 10 million live cells in a volume of 100 μl on ice for 30 min. CD4 cells were then separated using a magnetic cell sorter (MACS). The purity of the sorted population, confirmed by flow cytometry, was ∼90%. In some cases, CD4 cells were separated on a Becton Dickinson FACSorter® after staining with tricolor conjugated rat anti–mouse CD4.
Activation of T Cells.
Either total cells or FACSorted® CD4 T cells from mouse lymph nodes were activated in EHAA medium (from GIBCO BRL, Gaithersburg, MD) supplemented with 10% heat inactivated FBS, 1% l-glutamine, 5 × 10−5 M 2-ME, 1% penicillin and streptomycin and 10 mM Hepes (referred hereafter as EHAA growth medium) at 37°C in a humidified atmosphere with 5% CO2 in the presence of 2 ng per ml of PMA and 300 nM ionomycin. Typically 5 × 106 cells were cultured in 2 ml of medium per well in a 6-well plate. Aliquots of cells were then analyzed by flow cytometry for levels of expression of 9-O-acetylation on subsequent days. In other experiments, either total lymph node cells or 0.5 × 106 CD4 T cells mixed with 2.5 × 106 mitomycin-treated syngenic mouse spleen cells in 200 μl of EHAA growth medium were placed in 96-well plates coated with 50 ng 2C11 mAb to cross-link the TCR complex and activate the cells.
Studies to Check for CHE-FcD Induced Activation.
CHE-FcD probe in amounts ranging from 0.5 to 3 μg was allowed to bind overnight at 4°C on to Staph protein A–coated 96-well plates. 0.5 × 106 lymphocytes from peripheral lymph nodes were then incubated with 200 μl of EHAA growth medium per coated well. Triplicate wells were set up for each amount of CHE-FcD. An identical setup with CHE-Fc was done as a negative control. 2 d later, the cells were pulsed with 1 μCi/well of [3H]thymidine and the following day the cells were harvested onto glass fiber filtermats. The amount of incorporated radioactivity was determined on a betaplate reader (Wallac 1205; Wallac Inc., Gaithersburg, MD). Cross-linking with 2C11 as described above was done as a positive control. In another set of experiments, CHE-FcD was allowed to bind to CD4 T cells in solution without cross-linking its ligands. Assays were once again done in triplicate for each amount of CHE-FcD in 96-well flat bottomed plates with 0.1 × 106 purified CD4 cells and 0.5 × 106 mitomycin-treated syngenic mouse splenic cells added in 200 μl of EHAA growth medium per well. The final concentration of CHE-FcD in the wells varied from 10–100 μg/ml. Similar amounts of CHE-Fc were used as a negative control. To see whether CHE-FcD binding to cells either potentiated or diminished the response to other activating stimuli, cells were stimulated pharmacologically using PMA or by cross-linking the TCR with an anti-CD3 antibody 2C11. The stimulation was carried out using increasing doses of the stimulating agent over a range that gave concomitant increases in activation. CHE-Fc or CHE-FcD (50 μg/ml final) was used in combination with the varying amounts of PMA or anti-CD3, and compared to cells being activated in the absence of CHE-Fc or CHE-FcD. Cells were incubated for 4 d before being pulsed with 1 μCi/well of [3H]thymidine. The incorporated radioactivity was quantitated as described earlier.
Extraction of Glycoproteins and Glycolipids.
Membrane proteins were prepared from spleen and lymph node cells remaining after hypotonic lysis of red blood cells. The cells were washed with 1× PBS three times followed by hypotonic lysis in the presence of 20 mM Hepes, pH 7.4, and a cocktail of protease inhibitors. They were disrupted by nitrogen cavitation and spun at 500 g at 4°C to remove nuclei and unbroken cells. The cloudy supernatant was then spun at 100,000 g at 4°C to obtain a total membrane pellet. The membranes were then extracted with 1% Triton X-100 in 1× Tris-buffered saline, pH 8.0 (TBS), separated from the membrane debris by recentrifugation at 100,000 g, and the protein concentration was determined by Bradford assay. Typically 600– 700 μg of membrane proteins could be obtained from 3.5 × 108 cells and were adjusted to ∼1–2 μg/μl for use in Western blots and immunoprecipitation assays. To purify gangliosides, the washed cell pellets were sequentially extracted by 10–20 vol CHCl3/MeOH (2:1, 1:1, and 1:2 vol/vol), using brief homogenization. The extracts were pooled, dried down, and brought up in CHCl3/MeOH/water in the ratio of 4:8:5.6. After phase separation, the gangliosides were enriched in the hydophilic upper phase. This was dried down, resuspended in methanol, and kept at −20°C until use.
ELISA Plate Assay for 9-O-Acetylation on Gangliosides.
Total ganglioside extracts prepared as above were studied by lipid ELISA as described previously (23). In brief, lipids were applied to the plates in 45% methanol, allowed to dry, and then blocked with 5% BSA in PBS overnight. The effects of base (de-O-acetylation) on reactivity were assessed by treatment with 0.1 M NaOH at 4°C for 30 min (these conditions do not cause any loss of bound gangliosides). After treatment, the plates were extensively washed with PBS, blocked with 2% BSA in PBS for 1 h, and then incubated with CHE-FcD (20 μg/ml) for 2 h. After washing three times with 1% BSA, a horseradish peroxidase-conjugated goat anti–mouse IgG was added (at 1:1,000 dilution) for 1 h. After washing further, the reaction was developed as described above. Background levels determined with the secondary antibody alone were subtracted.
Western Blot Assays.
Membrane proteins were extracted as described above from total lymphocytes harvested from mouse spleen and lymph nodes. SDS-PAGE was done under reducing conditions in 7.5% gels. The electrophoresed material was transferred to nitrocellulose membranes, blocked with 5% bovine serum albumin in 1× TBS for 2 h at room temperature and then incubated with the primary probe overnight at 4°C on a rocking platform. CHE-FcD was used at a concentration of 5 μg/ml and the biotinylated monoclonals S7 (anti-CD43 115-kD isoform) and 23G2 (anti-CD45RB) were used at a concentration of 1 μg/ml in 1X TBS containing 1% bovine serum albumin. After this the membranes were washed thrice with 0.05% Tween 20 in 1× TBS for 30 min and incubated with the secondary reagents for 1 h at room temperature. Bound biotinylated antibodies were detected with 1:5,000 dilution of Neutravidin-alkaline phosphatase (Pierce Chem. Co., Rockford, IL) and CHE-FcD binding was assayed with 1:2,500 dilution of goat anti–human Fc conjugated with alkaline phosphatase (Bio-Rad, Richmond, CA). The membranes were washed thrice with 0.05% Tween 20 in 1× TBS for 30 min and then with 1× TBS and put in alkaline phosphatase buffer (pH 9.5) before adding the substrates NBT/BCIP at a 1: 100 dilution. The reaction was allowed to progress for 5–15 min before washing with water and drying.
Selective Precipitation of 9-O-Acetylated Glycoproteins from Membrane Extracts.
100–150 μg of the membrane protein extract was first precleared with protein A–Sepharose beads by end over end rolling at 4°C for 2 h. Meanwhile, 75 μg of CHE-FcD was allowed to complex with 50 μl of protein A–sepharose by end over end rolling at 4°C for the same length of time. The whole mixture was then incubated overnight with the precleared sample in a volume of 500 μl with end over end rolling at 4°C. The beads were then spun, washed in TBS, pH 7.4 and 6.8, successively and boiled in SDS sample buffer with 2-mercaptoethanol. The eluted proteins were loaded on SDS-PAGE gel and probed with antiCD45RB and anti-CD43 monoclonals as described here. The CHE-Fc molecule served as a negative control for the proteins specifically bound by CHE-FcD. The immunoprecipitates were also reprobed with CHE-FcD to confirm their reactivity to check the percentage of total 9-O-acetylated proteins that was precipitated.
Results
9-O-Acetylation of Sias on the Cell Surface of Mature Murine Thymocytes.
Flow cytometry analysis of dispersed thymocytes after CHE-FcD staining showed that 8–10% have surface 9-O-acetylation (Fig. 1 A, middle). Positively staining cells are very heterogenous with respect to their degree of 9-O-acetylation in a range of ∼3 logs of fluorescence intensity. Double-color analysis with PNA and CHE-FcD showed that almost all of the 9-O-acetyl positive cells have the more mature PNA− phenotype expected of medullary thymocytes (Fig. 1 B). This fits with our previous study, in which staining of murine thymus sections with the CHEFcD probe was noted predominantly in the medullary region (16). The specificity of CHE-FcD staining was confirmed by the markedly lower binding of CHE-Fc (Fig. 1 A, left), the residual staining is probably because cells with very high 9-O-acetylation can give a weak signal even with the esterase-active probe (3). Finally, binding with CHE-FcD was substantially abrogated when cells were treated with CHE-Fc esterase before staining (Fig. 1 A, right). In most of the subsequent studies, these CHE-Fc controls were not routinely done.
Figure 1.
Expression of 9-O-acetylation on murine thymocytes. (A) Single-color flow cytometry showing cell surface 9-O-acetylation on total thymocytes. Thymocytes were stained with CHE-FcD or CHE-Fc as indicated within the panels, followed by phycoerythrin-conjugated goat anti–human IgG. One aliquot was pretreated with CHE-Fc to remove 9-O-acetyl groups before staining with CHE-FcD. The dashed line indicates the background level of staining with the secondary antibody alone. (B) Double-color flow cytometry comparing PNA and CHE-FcD staining on total thymocytes. Thymocytes were stained with CHE-FcD followed by phycoerythrin-conjugated goat anti–human IgG and FITC-conjugated PNA. Quadrant boundaries were determined based on background staining profiles seen with phycoerythrin-conjugated goat anti–human IgG alone or FITCconjugated PNA in the presence of 0.2 M galactose.
9-O-Acetylation of Sias in the Thymus Is Expressed Predominantly on a Subset of Mature CD4 Cells.
These results show that cell surface 9-O-acetylation is predominantly on the more mature population of murine thymocytes. While the degree of 9-O-acetylation on thymocytes ranges over 3 log scales of fluorescence intensity (Fig. 1 A, middle), we noticed that the total percentage of CHE-FcD+ thymocytes tended to correlate best with the percentage of CD4 single positive (SP) cells in the same preparation. Indeed, upon three-color analysis of the 9-O-acetyl+ cells, a large fraction of these were found to be CD4+ SP cells (Fig. 2). In contrast to a high intensity of staining in CD4+ SP cells, the degree of 9-O-acetylation was low in the other cell types, except for some enrichment in the CD4−CD8− double negative (DN) cells. Further analyses showed that 9-O-acetylation is present on ∼75% of all the CD4+ SP cells, 40% of the DN cells and only 8% of CD8+ SP cells (Fig. 3). The density of 9-O-acetylation on the 9-Oacetyl+ DN cells is relatively lower than that on the CD4+ SP cells (Fig. 3). Given the heterogeneity of DN thymocytes with respect to maturity (24), we cannot be certain which subpopulation of these cells are 9-O-acetylated. Regardless, the lowest amount of 9-O-acetylation was seen in the CD4+CD8+ double positive (DP) thymocytes (1–2%). Thereafter, there is a progressive increase in 9-O-acetylation as the cells mature from the DP stage through the CD4+ SP stage. In contrast, the cells progressing towards the CD8+ lineage show minimal increase. Cells in the CD4+ SP subset varied in levels of 9-O-acetylation; these levels correlated inversely with CD8 levels (Fig. 3). Thus, increasing cell surface 9-O-acetylation is correlated with the progression of maturation of CD4+ thymocytes.
Figure 2.
CD4 and CD8 distribution on total thymocytes and on 9-Oacetyl+ cells. Three-color flow cytometry was done after staining with CHE-FcD, anti-CD4, and anti-CD8. The 9-O-acetyl positive cells were gated to obtain a selective CD4/CD8 profile. Since CD8 SP and CD4− CD8− DN cells constitute a low percentage of total thymocytes, 100,000 total events were acquired to analyze the small percentage of total 9-Oacetyl+ thymocytes.
Figure 3.
Progressive increase in 9-O-acetylation during maturation of CD4+ cells. Threecolor flow cytometry was done after staining with CHE-FcD, anti-CD4, and anti-CD8, as in Fig. 2. Individual gates (R1-R8) delineate cells with progressing maturity from the CD4−CD8− DN to CD4+CD8+ DP stage to the CD4+CD8− and CD4− CD8+ SP stages. 100,000 total events were acquired so that significant number of cells within the indicated regions could be gated. The percentage of 9-Oacetyl+ cells within each region was determined based on the background staining observed with phycoerythrin-conjugated goat anti–human IgG.
Association of the 9-O-Acetyl+ Phenotype with other Thymocyte Markers in the CD4+ Lineage.
CD4+ thymocytes were selectively gated to study the relationship of 9-O-acetylation with other well defined traits that mark the progression of cells from DP to CD4+CD8− stage (Fig. 4). Increasing 9-O-acetylation was correlated with increasing expression of TCR and decreasing levels of HSA, indicating that these CD4 cells have been committed for further maturation after the DP stage. Thymocyte maturation to a CD4+ SP stage occurs with upregulation of CD5 and CD69, both of which are responsive to signals delivered through the TCR during positive selection (25–27). While the increase in CD5 expression remains stable, CD69 is an early T cell activation marker, being initially high during the CD4hi CD8lo stage and decreasing to lower levels in the CD4+ SP cells. Most transitional cells between the DP and the CD4 SP stages and almost all the CD4+ SP cells have a CD5hi phenotype, which fits our observation that most of the 9-O- acetyl+ cells are also CD5hi (Fig. 4). The 9-O-acetylloCD5lo cells are most probably the transitional cells. Although more of the 9- O-acetylhi cells are CD69−, the 9-O-acetyl+ cells as a whole appear to be equally divided with respect to CD69 expression, implying that 9-O-acetylation does not identify cells purely based on their activation state. Together, these results show that CD4+ thymocytes are the major carriers of 9-O-acetylation, and that expression progressively increases with maturation in the thymus.
Figure 4.
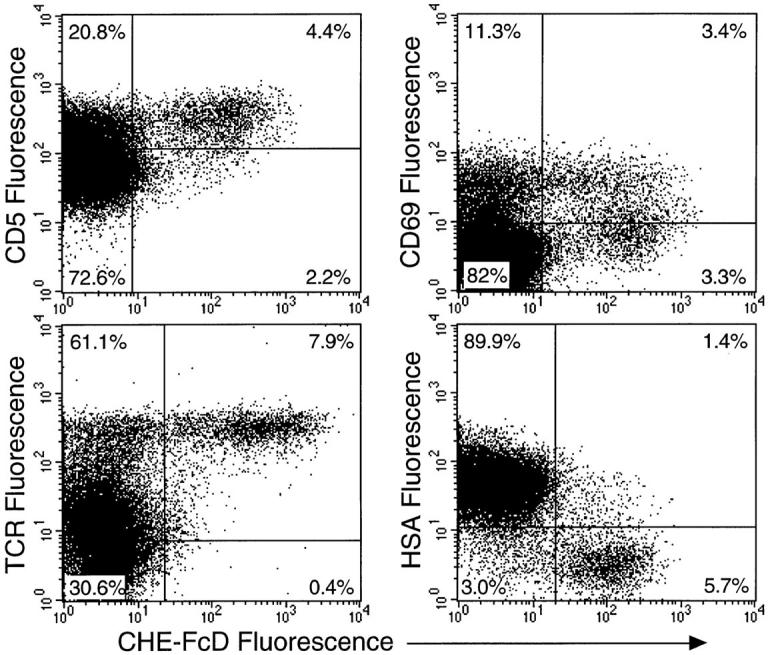
Relationship of 9-O-acetylation to expression of TCR, HSA, CD69 and CD5 on CD4+ thymocytes. Three-color staining was done with anti-CD4 and CHE-FcD along with either anti-CD3, J11D (anti-HSA), anti-CD69 or anti-CD5 as the third color. Binding of antiCD3 mAb 2C11 was detected using phycoerythrin-conjugated goat anti– hamster IgG and mAb J11D was detected by FITC conjugated donkey anti–rat IgG. Anti-CD69 and anti-CD5 were directly conjugated to FITC and anti-CD4 to tricolor reagent. Only CD4+ thymocytes were gated to show the dot plots relating 9-O-acetylation with other thymocyte markers. Quadrant boundaries were determined based on the background staining profiles obtained by the secondary antibodies for each axis.
9-O-Acetylation Is Also Enriched on CD4 T Cells in Peripheral Lymphoid Organs.
Varying levels of 9-O-acetylation were found on lymphocytes from spleen and lymph nodes. In different preparations from spleen and lymph node, the percentage of 9-O-acetylated CD4 T cells varied from 85–90%. Although 25–30% of CD8+ T lymphocytes, and 5–10% of CD4−CD8− lymphocytes also had this phenotype, the degree of 9-O-acetylation on these cells was markedly lower than on CD4 T cells. Correlation of CHE-FcD binding on CD4 T cells with other markers shows that in both spleen and lymph nodes the 9-O-acetylated cells are Mel14hi, Pgp1 (CD44)lo and CD45R(exonB)hi (Fig. 6). Indeed, CD4 cells with the phenotype Mel14+, CD45RB+ and CD44− are almost all 9-O-acetylated, indicating that high 9-O-acetylation is a marker of naive T cell phenotype (28–30).
Figure 6.

Association of other markers with 9-O-acetylation among CD4 peripheral lymphocytes. Flow cytometry was done after three color staining using anti-CD4 and CHE-FcD along with anti-Mel14, anti-Pgp1 or anti-CD45R(exonB). All peripheral T cell markers were used as biotinylated antibodies and their binding was detected using streptavidin conjugated FITC. The data displayed here represents only CD4+ gated cells. Staining on each axis of the dot plot was determined after using the secondary fluorescent conjugated reagent alone without the primary antibody or probe.
Distribution of Cell Surface 9-O-Acetylation among Glycoproteins and Glycolipids.
Trypsinization of thymocytes caused partial loss of 9-O-acetylation in most of the positive cells (Fig. 7 A, bottom). Likewise, when FACS®-sorted CD4+ SP cells were trypsin-treated, there was a significant decrease in 9-O-acetylation (nearly a fivefold decrease in mean fluorescence intensity). Most glycoproteins are expected to be trypsin sensitive, while the trypsin-resistant 9-O-acetylated glycoconjugates are likely to be sialylated glycolipids (gangliosides). One important subset of cell surface glycoproteins are mucins with clustered sialylated O-linked oligosaccharides (which includes CD43 and some isoforms of CD45) (31, 32). These can be selectively eliminated by the enzyme OSGPase (19), an endopeptidase that will only cleave mucins (20, 21). Upon treatment of thymocytes with OSGPase (Fig. 7 A, middle), there was a marked decrease in 9-Oacetylation (nearly a fivefold decrease in mean fluorescence intensity) indicating that many of the 9-O-acetyl groups are on Sias attached to O-linked oligosaccharides of mucin-type glycoproteins (murine erythroleukemia cells (15) served as positive controls for this enzyme, data not shown). The substantial loss of the high 9-O-acetylated CD4+ SP cells with OSGPase treatment indicates that mucin-type glycoproteins are the major carriers for 9-O-acetylation in these cells.
Figure 7.
Changes in CD4 cell surface 9-O-acetylation after trypsinization, OSGPase treatment or activation. (A) Thymocytes were treated with trypsin or OSGPase and then washed before staining. Following OSGPase treatment, CD4+ SP cells were gated on a CD4/CD8 dot plot to examine the CHE-FcD staining profile (staining of CD4 and CD8 was unchanged by the OSGPase treatment, data not shown). To study the effect of trypsin on CHE-FcD binding to CD4+ SP cells, CD4+ SP thymocytes were first FACSorted® after two-color staining with anti-CD4 and anti-CD8 antibodies, prior to CHE-FcD staining. (B) CD4 cells from peripheral lymph nodes were purified by FACSorting® and treated with trypsin or OSGPase and then washed before staining (left). One aliquot of these cells were activated by cross-linking with anti-CD3 on a 96-well plate; the profiles displayed in the right panels represent gated CD4 cells from the third day after activation; similar patterns were seen thereafter for 10 d (data not shown). In each case, the percentage positivity of the cells is shown within the histogram display, and background level of staining is represented by the dashed line.
Similar trypsin and OSGPase treatments done on purified peripheral CD4 T cells from the spleen and lymph nodes also indicates that most of the 9-O-acetylated glycoproteins are of the O-linked mucin-type (Fig. 7 B, left, showing a three- to fivefold decrease in the mean fluorescence intensity of staining after enzyme treatment). However, there is also a significant amount of trypsin- and OSGPaseresistant 9-O-acetylation (Fig. 7 B, left). Indeed, the shift in the profile upon protease digestion indicates that almost all CD4 cells that have 9-O-acetylated glycoproteins may also have 9-O-acetylated gangliosides. Notably, the CD8 molecule was preserved after treatment with OSGPase, despite the fact that it carries several scattered O-linked oligosaccharides (data not shown). This provides an internal control for the selectivity of the enzyme in cleaving only mucintype proteins with many clustered sialylated O-linked chains.
To confirm that the residual staining after protease digestion was due to 9-O-acetylated gangliosides, an ELISA assay with the CHE-FcD probe was done on the lipid extracts obtained from CD4 cells. The binding of the probe to the lipids on the plate was abrogated by base treatment, indicating the presence of 9-O-acetylated gangliosides in the extracts (Fig 8). One of the better studied 9-O-acetylated gangliosides is 9-O-acetylated GD3, which has recently been shown to occur on human peripheral T cells as one of the epitopes in the CD60 cluster (33). This molecule was not detectable on mouse thymocytes or on mouse peripheral CD4 T cells using both JONES and 27A antibodies (data not shown). However, these antibodies are highly specific for 9-O-acetylated GD3, and would not detect many other 9-O-acetylated gangliosides. We therefore tried 8A2, a mAb that recognizes certain other larger 9-O-acetylated gangliosides in human melanoma cells (34). This antibody was also unable to bind to mouse thymocytes and peripheral CD4 T cells (data not shown). There is also no correlation between 9-O-acetylation and the presence or absence of epitopes recognized by antibody SM3G11 (35, 36), which is known to detect the mouse T cell ganglioside GD1c (data not shown). These data suggest that while certain gangliosides on murine CD4 cells constitute a significant proportion of total 9-O-acetylated molecules, they must be structurally distinct from those reported as CD60 markers on human T cells.
Figure 8.
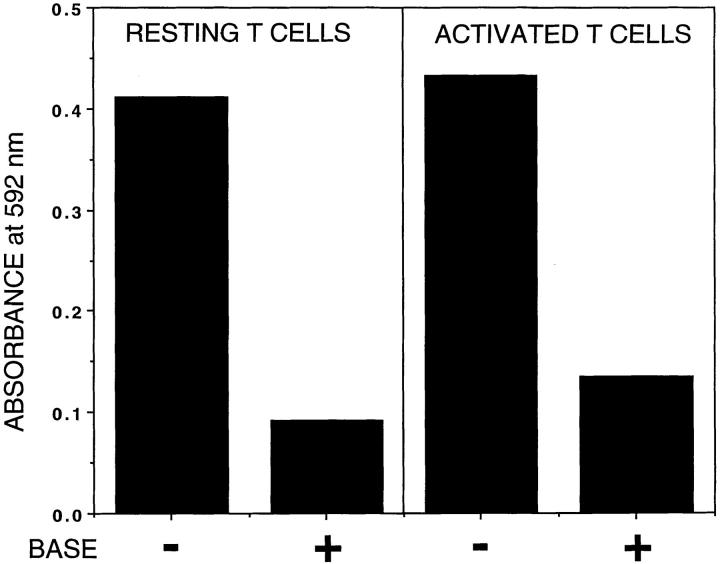
9-O-acetylated gangliosides are present in purified lipid extracts from CD4 T cells. Lipid extracts containing gangliosides from resting or activated CD4 cells were dried on to 96-well flat-bottomed plates and CHE-FcD was allowed to bind with or without base treatment (0.1 N sodium hydroxide for 15 min) to remove 9-O-acetyl groups. Binding was detected with HRPO-conjugated second antibody and substrate. Results shown are mean values from triplicates.
Effect of CD4 Cell Activation on Cell Surface 9-O-Acetylation.
Mature CD4 T cells from lymph nodes were purified and activated by anti-CD3 or by PMA/Ionomycin. The activation phenotype was confirmed by looking for cell surface activation markers such as CD25 on various days after activation ranging from day 2 up to day 13. CD4 cell surface 9-O-acetylation showed nearly a two- to threefold decrease by day 3 (Fig. 7 B, right). The residual 9-O-acetylation following activation is almost entirely refractory to treatment with trypsin and OSGPase (Fig. 7 B, right), indicating 9-O-acetylation is lost on mucin-type proteins, but not on gangliosides. This is confirmed by direct analysis of gangliosides from the activated cells (Fig. 8). There was no correlation of 9-O-acetylation with expression of CD69, CD25, CD45R(exonB), Mel14 or Pgp1 on any of the days after activation (data not shown). Thus, 9-O-acetylation of sialic acids on mucin-type glycoproteins appears to be a distinctive marker on CD4 T cells that is down-regulated upon activation. In contrast, 9-O-acetylation of gangliosides persists after activation.
Ligation of 9-O-Acetyl Groups on CD4 Cells Does Not Cause Activation.
CD60 antibodies are reported to be weakly mitogenic to human peripheral blood mononuclear cells, and are comitogenic with the phorbol ester PMA for purified resting T cells (37–39). Although murine T lymphocytes are not CD60+, the 9-O-acetyl groups can be ligated by CHEFcD. Lymphocytes from murine lymph nodes were cultured on plates precoated with protein A–Sepharose and then coated with increasing amounts of CHE-FcD. This crosslinking of 9-O-acetylated molecules on the cell surface did not result in any proliferation of cells, when followed over the next few days (data not shown, there was also no proliferation with equivalent amounts of CHE-Fc or with an irrelevant chimera). Likewise, there was also no proliferative response from purified CD4 T cells when the CHE-FcD was used in a soluble form, in the presence of mitomycin treated costimulatory cells (data not shown). Fig. 9 (top and bottom) shows that soluble CHE-FcD in concentrations up to 50 μg/ml neither potentiates nor inhibits T cell activation mediated either by PMA or by anti-CD3. The experiment was done over a range showing a typical dose response relationship to the stimulating agent to see if there are any noticeable differences at submaximal doses of PMA or anti-CD3. Taken together, the data indicate that ligating the 9-O-acetyl groups accessible to the CHE-FcD chimera does not result in cellular activation.
Figure 9.
CHE-FcD does not affect T cell activation mediated by PMA or by cross-linking with anti-CD3 antibodies. Total cells from mouse lymph nodes were activated with varying amounts of PMA, or by cross-linking with increasing amounts of anti-CD3 antibody. [3H]thymidine incorporation into the cells measured the proliferative response of the T cells. The effect of adding CHE-FcD or CHE-Fc in solution (final 50 μg/ml in all the wells) on the dose-response curve was plotted. Values represent the mean of triplicate values. Background values for sham treated cells were consistently lower than the smallest stimulating dose (not shown).
Sialomucins on CD8 T Cells Are Not 9-O-Acetylated, but They Mask Recognition of 9-O-Acetylated Gangliosides by CHE-FcD.
The level of 9-O-acetylation detected on mature CD8 T cells was considerably lower than on CD4 T cells. We considered the possibility that this might be due to differential expression of sialylated substrates for 9-O-acetylation on the respective cell types. Given the preponderance of 9-O-acetylated sialomucins on CD4 T cells we looked for their presence on CD8 T cells. As shown in Fig. 10 A, removal of the sialomucins by OSGPase actually gives an increase in CHE-FcD binding to CD8 T cells, i.e., the opposite of the result with CD4 T cells. The same result was confirmed by treating cells with trypsin (Fig. 10 A) or by a combination of trypsin followed by OSGPase (data not shown) indicating that detection of protease resistant 9-O-acetylated gangliosides can be masked by the presence of sialomucins on CD8 cells. This pattern of increased CHE-FcD binding after trypsin or OSGPase treatment was also reproduced on CD8 SP thymocytes (Fig. 10 B). These results demonstrate the presence of sialomucins on CD8 T cells, and indicate that unlike in CD4 cells, these molecules are not substrates for 9-O-acetylation.
Figure 10.

Sialomucins on CD8 T cells can mask the recognition of 9-O-acetylated gangliosides by CHE-FcD probe. (A) Mouse spleen cells were stained with CHE-FcD before or after OSGPase or trypsin treatment and staining profiles were traced from gated CD4 and CD8 subpopulations. Trypsin treatment was done on CD4 and CD8 T cells that were pre-sorted on a FACSorter®. Loss of binding of anti-Mel14 was a positive control for trypsinization (data not shown). (B) Mouse thymocytes were treated with OSGPase as described earlier, washed and stained with anti-CD4, CD8, and CHE-FcD. Sham treated cells were incubated with an equal volume of buffer under identical conditions. 100,000 live events were acquired and CD4 and CD8 SP cells were gated from a CD4/CD8 dot plot. CHE-FcD binding within those populations was plotted in the histograms. The dotted line shows background staining with the second antibody.
CD43 and CD45R(exonB) Isoforms Represent Some of the 9-O-Acetylated Mucins.
CD43 (leukosialin) and CD45 are two major, well-characterized T cell surface glycoproteins known to carry large amounts of O-linked oligosaccharides with terminal sialic acids (31, 32, 40). Assuming that these might be some of the mucin-like molecules carrying 9-Oacetylation and/or masking CHE-FcD binding to gangliosides, we looked for any difference in their levels on CD4 and CD8 cells. Both thymic and peripheral T cells express high molecular weight isoforms of CD45, and the exon 5 (coding for RB) specified isoforms are relatively predominant as compared to RA and RC specified isoforms (41, 42). Amongst various antibodies recognizing the CD45RB isoforms, the 23G2 clone had shown better binding on splenic CD4 T cells (41). Since these were the cells we were focusing on, we chose this antibody for all FACS® and Western blot studies, conceding the fact that this antibody would be specific to only a major subset of all CD45 isoforms. Fig. 11 shows that CD43 and CD45(exon B) coded isoforms are sialomucins, since OSGPase treatment of both CD4 and CD8 T cell surfaces almost completely destroys their binding to the respective antibodies. This was further confirmed by Western blot analysis of T cell membrane protein extracts treated with or without OSGPase, using antiCD43 and anti-CD45RB antibodies (data not shown). These results are in agreement with earlier reports using methods such as Western blots and/or FACS® analysis that describe loss of CD43 recognition with OSGPase treatment (21) and CD45RB epitopes by O-glycanase (40) or OSGPase digestions (21). As evaluated by mean fluorescence intensity of staining at varying dilutions of the antibodies, expression of these cell surface glycoproteins actually showed higher levels of both CD43 and CD45R(exonB) containing isoforms on CD8 T cells than on CD4 cells (data not shown). This observation is consistent with earlier results using the same anti-CD43 S7 clone (43) and anti-CD45RB (41). The data in Fig. 11 shows the relative abundance of these glycoproteins on CD8 T cells in comparison to CD4 T cells at saturating levels of antibodies. Thus, the differences in sialomucin 9-O-acetylation is not due to differences in the levels of expression of the parent molecules between CD4 and CD8 cells.
Figure 11.
CD43 and CD45RB are sialomucins expressed on both CD8 cells and on CD4 cells. Freshly isolated mouse splenic lymphocytes were incubated with OSGPase or with buffer alone, under identical conditions. This was followed by three-color staining with anti-CD4, CD8, and either anti-CD43 (S7) or CD45RB (23G2) at dilutions of 5 μg/ml. The mean fluorescence intensity of staining on gated populations of CD4 and CD8 T cells was determined on a FACScan®.
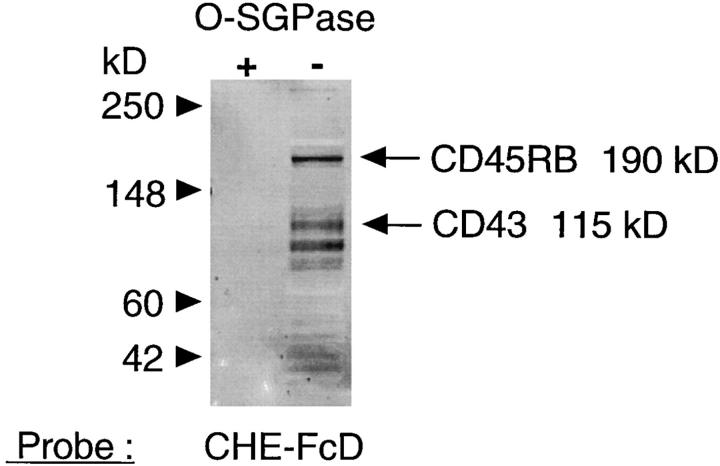
Western blot analyses of membrane proteins isolated from total splenic mononuclear cells with the CHE-FcD probe reveals several high molecular mass proteins that are 9-O-acetylated (Fig. 12). The specificity of recognition is confirmed by the absence of any binding with the CHE-Fc at similar concentration (data not shown). Pre-incubation of the proteins with OSGPase destroys all the positive bands, confirming that sialomucins are the major 9-O-acetylated glycoproteins in these cells. Two of the major bands coincided in molecular mass with that expected for CD45RB and CD43. To evaluate this matter further, 9-O-acetylated glycoproteins were precipitated from the total membrane protein extracts using CHE-FcD bound to protein A–Sepharose via its human IgG Fc tail. The CHE-Fc protein was used as a control for any nonspecific precipitation. The bound proteins were separated on a reducing SDS-PAGE gel, transferred to nitrocellulose membranes and probed with monoclonal antibodies recognising CD43 and CD45R (exon B) isoforms. Fig. 12 demonstrates that the proteins precipitated by CHE-FcD include molecules recognized by anti-CD43 and by anti-CD45RB, that comigrate at the same position with bands from total membrane proteins recognized by the same antibodies. The weaker intensity of the bands reflects the lower amounts of CD43 and CD45 present in the immunoprecipitates (the precipitation was not complete, data not shown). While the anti-CD45RB gives the expected band of 190 kD (and some weaker bands above), anti-CD43 detects a band migrating slightly below the bulk population of molecules. This might be due to differential mobilities of different CD43 glycoforms (43). The bright band seen at ∼120 kD with the CD43 probe is nonspecific, since it was present on blots probed with alkalinephosphatase conjugated neutravidin alone, and also with the CD45RB probe (data not shown). Upon probing the material precipitated with CHE-FcD, and comparing the profile with the CHE-FcD Western blot from the untreated total protein extracts, it was clear that not all 9-O-acetylated glycoproteins were precipitated (data not shown); this may be due to the fact that only a single round of depletion was done or be due to the differential affinity of CHE-FcD for various 9-O-acetylated proteins. Regardless, it is evident from these data that CD43 and CD45(exonB) are among the cell surface mucins that are 9-O-acetylated.
Figure 12.
CD43 and CD45R (exonB) are among the 9-Oacetylated membrane mucins in splenic cells. The left panel shows a Western blot of splenic lymphocyte membrane proteins (15 μg each lane) probed with CHE-FcD (at 4°C) with (lane 1) or without (lane 2) prior treatment of the proteins with OSGPase. The right panel shows a Western blot of total membrane proteins, proteins precipitated by CHE-FcD, and CHE-Fc controls, each probed with antiCD43 and anti-CD45RB monoclonal antibodies as indicated. The expected positions of CD45RB (∼190 kD) and CD43 (∼115 kD) are indicated in each panel. The relatively bright band just above 115 kD in the right lanes of the right panel is nonspecific, since it was also seen on probing with the alkaline phosphatase conjugated secondary reagent alone.
9-O-Acetylation on CD4 Cells Does Not Block CD22 Ligands.
Earlier studies in our lab showed that 9-O-acetylation of sialic acids could mask the binding of recombinant soluble CD22 (CD22Rg) to its ligands on some cells in murine lymphoid organs (16). We found high amounts of CD22Rg ligands on peripheral T cells and on CD4+ and CD8+ SP thymocytes, and lower amounts on immature thymocytes by flow cytometry (data not shown). Treatment with CHE-Fc did not significantly increase the binding of CD22Rg to either mature peripheral CD4 cells or to CD4+ SP thymocytes (data not shown). This result is consistent with the fact that most of the 9-O-acetylated glycoproteins on CD4 cells have mucin type O-linked structures which are unlikely to carry the Sia α2-6Galβ1-4 GlcNAc sequence typical of CD22 ligands (44).
Discussion
Each type of cell has its own unique surface signature determined by its glycosylated molecules. While sialic acids with different α-ketosidic linkages from the 2- position to the underlying glycoconjugate can confer a fair degree of diversity, modifications of the sialic acids enhance this diversity further. Until recently, 9-O-acetylated sialic acids were considered as rare oddities among the plethora of glycosylated structures found in mammalian systems. It is now known that they occur in several tissues of neuroectodermal and mesenchymal descent (2). Studies such as in situ histochemistry using specific probes indicate a temporal and spatial pattern of regulation of 9-O-acetylation in developing tissues (3, 8, 9, 45–48). On a given cell type, 9-O-acetylation tends to be non-randomly distributed with respect to both the type of cell surface glycoconjugate (49), as well with regard to the type of sialic acid linkage on a given glycoconjugate (50). Such data suggest specific roles for these modifications in cell–cell recognition events inherent to tissue organization and/or development.
Most descriptions of 9-O-acetylation have been based on chemical analyses or on the binding of certain highly specific monoclonal antibodies that also recognize other details of the underlying molecules. Earlier studies showed by indirect methods that mouse thymocytes might have 9-Oacetylated sialic acids (51). It was also reported that T cells from patients with various malignancies had O-acetylated sialic acids (52, 53) and that 9-O-acetylation was present on pig lymphocytes (54). However, the extraction methods used could not specifically distinguish cell surface 9-O-acetylation. Other assays involved the use of intact influenza C virions that specifically recognize 9-O-acetylated sialic acids (18, 55). This approach is limited by the purity and stability of the virus preparations and a relatively high background. The availability of a soluble recombinant chimera (CHEFcD) derived from influenza C and its versatility in a variety of in vitro assays (3) makes it possible to look for 9-Oacetylation on cell surfaces, irrespective of the underlying sugar structure. We have studied here the cell surface expression of 9-O-acetylated sialic acids on intact fresh lymphocytes isolated from normal mice. While B lymphocytes and CD8 T lymphocytes were heterogenous in their expression of low levels of 9-O-acetylated sialic acids, the singular pattern of high density expression on CD4 T cells led to the current set of investigations.
In earlier work from our lab (16), CHE-FcD staining was noted on medullary regions in histological sections from the thymus. In keeping with this, we show that the acquisition of the 9-O-acetylation phenotype occurs almost entirely within a subset of PNA− mature thymocytes. A low percentage of thymocytes are positive yet highly heterogenous in their cell surface density of 9-O-acetylation, with a disproportionately higher percentage of CD4+ SP cells and CD4−CD8− DN cells and a lower percentage of CD4+ CD8+ DP cells. Thus, it seems that 9-O-acetylation of Sia on mucins must be very specifically regulated during T cell ontogeny (Fig. 13). In this regard, it is notable that other changes in glycosylation during maturation of thymocytes have been demonstrated, including varying patterns of expression of four different glycosyltransferases on cells with significant phenotypic and functional differences (56, 57).
Figure 13.
Expression of 9-Oacetylated sialic acids during T cell ontogeny in the adult thymus and on peripheral T cells. This figure summarizes the distribution of 9-O-acetylated sialic acids detected by CHEFcD on developing and on peripheral resting and activated T cells. Varying intensities of CHE-FcD binding are shown as low (lo), intermediate (int) and high (hi) along with a representative percentage of positive cells. Note that while ∼40% of CD4−CD8− DN cells are 9-Oacetyllo+, the phenotype of the cells that actually progress to a DP stage is not defined by our current data. Also note that the probe may not detect all ganglioside-bound 9-O-acetyl Sias (see Fig. 14 and Discussion).
Among the CD4−CD8− DN thymocytes, 35–40% have low level expression of surface 9-O-acetylation. These constitute only 2–3% of total thymocytes (58), and are heterogenous in the expression of several markers such as HSA, CD44 (Pgp-1), CD25 (interleukin 2 receptor α chain), Mel14, CD5, and c-kit. During this stage, the TCR-β locus undergoes rearrangement and cells that undergo productive rearrangement are likely selected for further progress to the DP stage characterized by increasing surface expression of TCR. During the transition from DN to DP stage, thymocytes undergo several rounds of proliferation, upregulate TCR-α rearrangement, stop TCR-β rearrangement, and begin to turn on CD4 and CD8 expression. Since we did not do a full phenotypic characterization of the 9-O-acetyl+ DN cells, it is unclear whether these represent the precursors entering the thymus or a relatively more mature subset.
The conspicuous upregulation of cell surface mucin 9-O-acetylation as thymocytes mature to CD4+CD8− stage from the DP stage establishes this as a novel marker identifying cells committed to the CD4 lineage (Figs. 13 and 14). In contrast, there is only a small increase in 9-O-acetylation on the CD8+ SP cells, and this is not found on mucins. During lineage commitment, DP thymocytes undergo several sequential changes in expression of CD69, HSA, and CD5. CD4+ thymocytes that are CD69+CHE-FcD− represent cells that are still in the DP stage. Thymocytes with low amounts of 9-O-acetylation are either CD69+ or CD69− suggesting the 9-O-acetylation phenotype of the maturing CD4+ cells is not specifically associated with this early activation marker. A significant fraction of cells with high 9-O-acetylation that must be CD4+ SP cells seem to have lost their CD69 expression, which fits in with their expected downregulation on mature SP thymocytes. A strong correlation of CD5hiTCRhiHSA− pattern of expression with increasing surface 9-O-acetylation suggests that O-acetylation identifies cells that have successfully been selected and are on their way to becoming mature cells.
Figure 14.
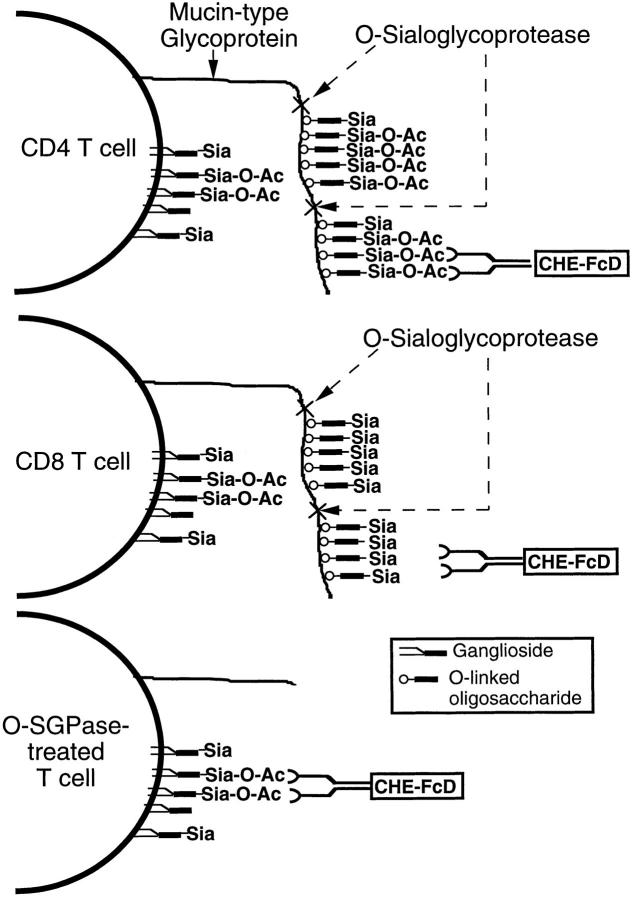
Model depicting the likely differential distribution of 9-Oacetylated sialoglycoconjugates between resting peripheral CD4 and CD8 T cells. Sialomucins are present on both CD8 and CD4 cells, but are only 9-O-acetylated in the latter. Cell surface sialomucin molecules usually have a rod like extended conformation and a high negative charge, and appears to prevent CHE-FcD access to O-acetylated glycolipids (gangliosides) that are much closer to the cell surface membrane. OSGPase recognizes sialylated clustered O-linked glycans and cleaves the adjacent polypeptide backbone, thus unmasking recognition of the enzyme-resistant O-acetylated gangliosides by the CHE-FcD probe.
Fewer CD4+ SP thymocytes are 9-O-acetylated as compared to peripheral resting CD4 T cells. It is unclear if the increase in CD4+ cell 9-O-acetylation occurs at a peripheral location after emigration from the thymus, or if 9-Oacetylhi CD4+ SP cells are selectively exported from the thymus. Functionally distinct subpopulations in CD4 SP cells have been reported. One study showed the late CD4hi thymocytes were functionally more mature than their immediate precursors in several assays. They were able to colonize and expand in the peripheral lymphoid organs while the CD4hi CD8lo subset had an obligatory requirement for thymic microenvironment for further maturation (59). T cells need to express homing molecules to enter the thymic lymphatics or venules and exit at the corticomedullary junction. Such molecules must be expressed at the SP stage after migration from the cortex through the corticomedullary junction into the medulla where they would undergo negative selection. Surviving cells would, in addition to being activated to divide, express homing receptors probably influenced by the local microenvironment. Assuming that a critical threshold of homing receptors is required for a mature SP cell to emigrate, the progressive increase in cell surface 9-O-acetylation in the CD4 SP lineage is a candidate for subserving such a function.
Correlation of 9-O-acetylation on the peripheral CD4 T cells with the expression of Mel14, Pgp-1, and CD45R(exonB) indicates that this modification may be a marker for naive T cells (28, 30). More than 95% of the naive CD4 T cells and only ∼60% of the cells with a memory/activated phenotype are 9-O-acetylated. This could be relevant either to the specific recirculation patterns of these cells in the mouse and/or recognition of some costimulatory molecule involved in T cell activation. Thus, 9-O-acetylated sialic acids could recognize a specific ligand in the milieu the T cells expose themselves to, or this modification could mask recognition of the underlying carbohydrate structure on T cells by an unknown receptor.
Fig. 13 summarizes the differential distribution of total 9-O-acetylated sialic acids on peripheral resting and activated CD4 T cells as detected by CHE-FcD. We know of no other cell surface marker (other than CD4 itself) described to date that specifically identifies the murine CD4 lineage. There have been studies showing higher expression of some specific markers in the CD8 lineage, including CD27 (60), CD45RB and RC isoforms (41), an epitope dependent upon a post translational modification of CD45RB and recognized by a monoclonal antibody CZ-1 (61), and the 130-kD isoform of CD43 (43). The susceptibility of the 9-O-acetylated glycoconjugates to the various enzyme treatments suggests that most 9-O-acetylated thymocytes have both 9-O-acetylated glycoproteins and gangliosides. In contrast to the naive population where mucins are the major carriers of 9-O-acetylation, in vitro activated CD4 T cells appear to carry them primarily on protease-resistant molecules (presumably gangliosides). Although there is a substantial decrease in the mean density of 9-O-acetylation on the activated cells, there is no significant difference in the percentage of cells that are 9-O-acetylated. The presence of 9-O-acetylated gangliosides in the resting and activated CD4 T cells was confirmed in an ELISA using the CHE-FcD. Further studies will be needed to define their precise structure.
In addition to differences in the levels of 9-O-acetylation, CD4 and CD8 T cells also differ in their distribution of O-acetylation on glycoconjugate classes. Although sialomucins are the predominant 9-O-acetylated molecules on CD4 T cells, these do not seem to be O-acetylated in the case of CD8 T cells. This is not due to a quantitative difference in the amount of cell surface mucins, if anything, the cell surface densities of two prominent sialomucins CD43 and CD45 (exon B isoforms) on CD8 cells are actually higher than on CD4 T cells. Because of their rod-like rigid structure, mucin molecules on both CD4 and CD8 T cells could physically restrict the binding of CHE-FcD to the O-acetylated gangliosides that are closer to the cell membrane. Indeed, such an unmasking effect of OSGPase and trypsin on CHE-FcD binding is particularly obvious on the CD8 T cells. It is unclear how two developmentally closely related cell types differ so radically in this sialic acid modification. It might be due to differences in the regulation of O-acetyltransferase gene expression or in the underlying sialic acid linkages on the same mucin molecules between the cell types.
It must be re-emphasized that not all O-linked sialoglycoproteins get indiscriminately digested by OSGPase. Rather, this enzyme specifically recognizes clustered sialic acid residues on O-linked glycans and cleaves the peptide bonds close to the O-linked structures. Thus, while the CD8 molecule itself is modified by O-linked glycosylation, it is resistant to this protease, because the sugar chains are not clustered. Fig. 14 summarizes the concept evolving from our observations. Recognition of T cell surface gangliosides by a soluble or a neighboring cell surface receptor might be modulated by expression of mucin-like structures on T cells. These mucins may or may not themselves bear a similar carbohydrate epitope to that carried on the gangliosides. With respect to 9-O-acetylation, this attribute is so well demarcated between CD4 and CD8 cells that it might be of some relevance to their biology. Although no well-defined receptors for 9-O-acetylated glycoconjugates have been documented, such a masking phenomenon in vivo could, in principle, prevent the recognition of 9-O-acetylated gangliosides on resting T cells. Furthermore, it is clear from our data from activated CD4 T cells this masking can be modulated by the state of T cell differentiation. Assuming there might be differences in physiological consequence mediated by receptors recognizing 9-O-acetylated sialic acids on sialomucins and gangliosides, such a masking on CD4 cells could be biologically relevant. Regardless of their biological significance, these results underscore the possibility that macromolecular probes for cell surface glycolipids may fail to detect their ligands because of shielding by the larger sialomucins on the same surface (62, 63).
Our results also demonstrate 9-O-acetylation on two well characterized sialomucins of T cells, CD43 and CD45. CD43 (leukosialin) is a major cell surface glycoprotein on T cells and is also present on cells of erythroid and myeloid lineages. It is heavily glycosylated and the O-glycans attached to it can vary with both the cell type and within T cells based on their state of differentiation (32, 64). Differences in the expression of its two major glycoforms 115 and the 130-kD species, have been shown between CD4 and CD8 T cells (43, 65). Our results suggest that 9-Oacetylation of CD43 occurs differently on CD4 and CD8 T cells. In this regard, it is interesting to note that the expression of the 130-kD isoform of CD43 shows an inverse correlation with 9-O-acetylation; it is preferentially upregulated on thymocytes committed to CD8 SP cells while being low on CD4 SP thymocytes, and then prevails at high levels on most peripheral CD8 T and lower levels on about 20% of CD4 T cells (43). Furthermore the 130-kD isoform is markedly upregulated on human CD4 T cells upon activation. A thorough glycosylation analysis of the two murine CD43 isoforms needs to be done before one can speculate on how this could affect 9-O-acetylation on sialic acids.
CD45 is a family of high molecular weight glycoproteins with various isoforms resulting from differential splicing of exons 4, 5, and 6. All three exons code for regions rich in O-glycosylated linkages (31, 40). While these isoforms can differentially regulate T cell receptor signalling (66, 67), it is not clear what role the carbohydrates play; neither is it certain if there are any specific glycosylation differences on CD45 molecules between naive CD4 and CD8 T cells. Although we demonstrate 9-O-acetylation on the exon B specific isoforms it is possible this may be differentially glycosylated between CD4 and CD8 T cells.
9-O-acetylation of sialic acids has been studied by others in human lymphoid tissue. Using intact influenza C virus, uncharacterized 9-O-acetylated glycoproteins have been demonstrated by Western blots derived from human T cells (55). Human peripheral blood T lymphocytes are also positive for the marker CD60, which represents GD3-related gangliosides with 9-O-acetylation (33, 37). This antibody binds to ∼25% of normal circulating human T lymphocytes and to most T cells infiltrating psoriatic skin lesions and the synovial fluid space in rheumatoid arthritis (68, 69). In patients with cutaneous T cell lymphoma, a majority of circulating T cells and in dermal lesions were also positive for this marker (70). Staining of mouse T cells with specific antibodies for 9-O-acetylated GD3 did not give any positive results. Thus, while 9-O-acetylation of Sia appears common to human and murine lymphocytes, it must be carried on distinct glycolipids. Interestingly, most reported gangliosides in mouse T cells such as GD1a and GD1α arise from the a or α series biosynthetic pathways (71, 72) and there is no evidence for GD3, or any other member from the b series pathway. Recently, the presence of an unique ganglioside GD1c sharing the same terminal trisaccharide with GD3 has been identified by mAbs 3G11 (36) and YK-3 (73) on mouse T cells with a preference for cells with a Th1 phenotype (73). It is possible that this structure is the functional homologue of GD3 seen on human peripheral T cells. Unfortunately, there is no antibody currently available against 9-O-acetylated GD1c. It must also be remembered that most sialic acids in the mouse have a N-glycolyl group attached to the fifth carbon atom instead of the N-acetyl group found in humans (1); this change can also influence recognition by antibodies.
Several reports suggest that 9-O-acetylated CD60 epitopes on human T cells might serve in an alternate functional activation pathway for T cells compartmentalized in lesions of psoriasis and arthritis (37, 39, 68, 69). A mAb U5 preferentially recognizing 7-O-acetylated-GD3 on human CD4 and CD8 T cells could elicit a even stronger proliferative response from these cells (74). Since mouse peripheral T cells showed no evidence of 9-O-acetyl-GD3, we studied CD4 T cells activation with bound or soluble forms of CHE-FcD that would cross-link any 9-O-acetylated cell surface molecule. We also looked for any potentiation of PMA induced mitogenicity of CD4 T cells as reported earlier with CD60 antibodies on human T cells (37–39). While we did not see activation, this could be because CHE-FcD binding to sialomucins is blocking access to glycolipids. There was also no effect in potentiating antiCD3–mediated T cell activation (Fig. 9). Since CHE-FcD binds to any cell surface glycoconjugate bearing 9-O-acetylated sialic acids it might be hard to assess the effect of simultaneous binding to a variety of different molecules on T cell activation. A specific mAb directed against the 9-OAc-gangliosides in murine T cells would be needed to make a valid comparison with CD60 activation in human T cells.
The finding of 9-O-acetylated sialic acids on mouse T cells underscores the widespread yet selective expression of this unique modification on sialic acids. Several factors could regulate the extent of 9-O-acetylation of sialic acids. An earlier study demonstrated 9-O-acetylation on murine erythrocyte sialic acids could be regulated by an autosomal dominant gene and its expression can thus depend on the genetic background of the animal being studied (14). Our group recently reported 9-O-acetylation on Chinese hamster ovary cells was dependent on the availability of the correct linkage of the sialic acid to the underlying sugar chain (50). Such observations coupled with the present findings are strong predictors for a functional role of 9-Oacetylated sialic acids. It remains to be seen how the remarkably different 9-O-acetylation of sialomucins on mouse CD4 and CD8 cells might influence the in vivo biology of those cells.
Figure 5.
Association of 9-Oacetylation with CD4 cells among peripheral lymphocytes. Cells from peripheral lymph nodes or spleen of a 8-wk-old B10.A mouse were three color stained with anti-CD4, antiCD8 and CHE-FcD and subjected to flow cytometry. 9-Oacetylation profiles on CD4 and CD8 positive cells were obtained by gating on the respective cells on a CD4/CD8 dot plot. The background (dashed line) represents staining seen with the CHE-Fc. The percentage positivity of the cell groupings displayed in the histograms is indicated.
Acknowledgments
The authors thank Hui-Ling Han for assistance in some experiments, Steve Hedrick and Jamey Marth for critical reading of the manuscript, and the UCSD Flow Cytometry Core Facility for cell sorting.
This work was supported by National Institutes of Health grant RO1-GM32373 to Ajit Varki.
Footnotes
1 Abbreviations used in this paper: BM, bone marrow; CHE, influenza C virus hemagglutinin-esterase protein with the fusion peptide eliminated by mutation; CHE-Fc, chimeric protein made of CHE and the Fc portion of human IgG1; CHE-FcD, DFP treated CHE-Fc (esterase activity irreversibly inactivated); DFP, diisopropylfluorophosphate; DN, double negative; DP, double positive; HSA, heat stable antigen; OSGPase, O-sialoglycoprotease; PNA, peanut agglutinin; Sia, sialic acids; SP, single positive; TBS, Tris-buffered saline.
References
- 1.Schauer, R. 1982. Sialic Acids: Chemistry, Metabolism and Function, Cell Biology Monographs, Vol. 10. Springer-Verlag, New York.
- 2.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2:25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein A, Krishna M, Varki NM, Varki A. 9-Oacetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutinin-esterase. Proc Natl Acad Sci USA. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manzi AE, Dell A, Azadi P, Varki A. Studies of naturally occurring modifications of sialic acids by fastatom bombardment-mass spectrometry. Analysis of positional isomers by periodate cleavage. J Biol Chem. 1990;265:8094–8107. [PubMed] [Google Scholar]
- 5.Varki A, Diaz S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Anal Biochem. 1984;137:236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- 6.Kamerling JP, Schauer R, Shukla AK, Stoll S, van Halbeek H, Vliegenthart JFG. Migration of O-acetyl groups in N,O-acetylneuraminic acids. Eur J Biochem. 1987;162:601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 7.Butor C, Diaz S, Varki A. High level O-acetylation of sialic acids on N-linked oligosaccharides of rat liver membranes. Differential subcellular distribution of 7- and 9-Oacetyl groups and of enzymes involved in their regulation. J Biol Chem. 1993;268:10197–10206. [PubMed] [Google Scholar]
- 8.Blum AS, Barnstable CJ. O-acetylation of a cell-surface carbohydrate creates discrete molecular patterns during neural development. Proc Natl Acad Sci USA. 1987;84:8716–8720. doi: 10.1073/pnas.84.23.8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constantine-Paton M, Blum AS, Mendez-Otero R, Barnstable CJ. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature (Lond) 1986;324:459–462. doi: 10.1038/324459a0. [DOI] [PubMed] [Google Scholar]
- 10.Varki A, Hooshmand F, Diaz S, Varki NM, Hedrick SM. Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell. 1991;65:65–74. doi: 10.1016/0092-8674(91)90408-q. [DOI] [PubMed] [Google Scholar]
- 11.Herrler G, Rott R, Klenk HD, Muller HP, Shukla AK, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. EMBO (Eur Mol Biol Organ) J. 1985;4:1503–1506. doi: 10.1002/j.1460-2075.1985.tb03809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultze B, Herrler G. Bovine coronavirus uses N-acetyl-9-O-acetylneuraminic acid as a receptor determinant to initiate the infection of cultured cells. J Gen Virol. 1992;73:901–906. doi: 10.1099/0022-1317-73-4-901. [DOI] [PubMed] [Google Scholar]
- 13.Vlasak R, Krystal M, Nacht M, Palese P. The influenza C virus glycoprotein (HE) exhibits receptor-binding (hemagglutinin) and receptor-destroying (esterase) activities. Virology. 1987;160:419–425. doi: 10.1016/0042-6822(87)90013-4. [DOI] [PubMed] [Google Scholar]
- 14.Varki A, Kornfeld S. An autosomal dominant gene regulates the extent of 9-O-acetylation of murine erythrocyte sialic acids. A probable explanation for the variation in capacity to activate the human alternate complement pathway. J Exp Med. 1980;152:532–544. doi: 10.1084/jem.152.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 16.Sjoberg ER, Powell LD, Klein A, Varki A. Natural ligands of the B cell adhesion molecule CD22β can be masked by 9-O-acetylation of sialic acids. J Cell Biol. 1994;126:549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelm S, Schauer R, Manuguerra J-C, Gross H-J, Crocker PR. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconjugate J. 1994;11:576–585. doi: 10.1007/BF00731309. [DOI] [PubMed] [Google Scholar]
- 18.Muchmore E, Varki A. Inactivation of influenza C esterase decreases infectivity without loss of binding; a probe for 9-O-acetylated sialic acids. Science (Wash DC) 1987;236:1293–1295. doi: 10.1126/science.3589663. [DOI] [PubMed] [Google Scholar]
- 19.Abdullah KM, Udoh EA, Shewen PE, Mellors A. A neutral glycoprotease of Pasteurella haemolyticaA1 specifically cleaves O-sialoglycoproteins. Infect Immun. 1992;60:56–62. doi: 10.1128/iai.60.1.56-62.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norgard KE, Moore KL, Diaz S, Stults NL, Ushiyama S, McEver RP, Cummings RD, Varki A. Characterization of a specific ligand for P-selectin on myeloid cells. A minor glycoprotein with sialylated O-linked oligosaccharides. J Biol Chem. 1993;268:12764–12774. [PubMed] [Google Scholar]
- 21.Sutherland DR, Abdullah KM, Cyopick P, Mellors A. Cleavage of the cell-surface O-sialoglycoproteins CD34, CD43, CD44, and CD45 by a novel glycoprotease from Pasteurella haemolytica. . J Immunol. 1992;148:1458–1464. [PubMed] [Google Scholar]
- 22.Reivinen J, Holthöfer H, Miettinen A. A celltype specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992;42:624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- 23.Sjoberg ER, Varki A. Kinetic and spatial interrelationships between ganglioside glycosyltransferases and O-acetyltransferase(s) in human melanoma cells. J Biol Chem. 1993;268:10185–10196. [PubMed] [Google Scholar]
- 24.Crispe IN. CD4/CD8-negative T cells with alpha beta antigen receptors. Curr Opin Immunol. 1994;6:438–441. doi: 10.1016/0952-7915(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 25.Dutz JP, Ong CJ, Marth J, Teh HS. Distinct differentiative stages of CD4+CD8+ thymocyte development defined by the lack of coreceptor binding in positive selection. J Immunol. 1995;154:2588–2599. [PubMed] [Google Scholar]
- 26.Swat W, Dessing M, von Boehmer H, Kisielow P. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur J Immunol. 1993;23:739–746. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 27.Bendelac A, Matzinger P, Seder RA, Paul WE, Schwartz RH. Activation events during thymic selection. J Exp Med. 1992;175:731–742. doi: 10.1084/jem.175.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bradley LM, Atkins GG, Swain SL. Longterm CD4+memory T cells from the spleen lack MEL-14, the lymph node homing receptor. J Immunol. 1992;148:324–331. [PubMed] [Google Scholar]
- 29.Butterfield K, Fathman CG, Budd RC. A subset of memory CD4+ helper T lymphocytes identified by expression of Pgp-1. J Exp Med. 1989;169:1461–1466. doi: 10.1084/jem.169.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swain SL, Croft M, Dubey C, Haynes L, Rogers P, Zhang X, Bradley LM. From naive to memory T cells. Immunol Rev. 1996;150:143–167. doi: 10.1111/j.1600-065x.1996.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 31.Trowbridge IS. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991;266:23517–23520. [PubMed] [Google Scholar]
- 32.Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991;1:347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- 33.Kniep B, Flegel WA, Northoff H, Rieber EP. CDw60 glycolipid antigens of human leukocytes: Structural characterization and cellular distribution. Blood. 1993;82:1776–1786. [PubMed] [Google Scholar]
- 34.Drazba J, Pierce M, Lemmon V. Studies of the developing chick retina using monoclonal antibody 8A2 that recognizes a novel set of gangliosides. Dev Biol. 1991;145:154–163. doi: 10.1016/0012-1606(91)90221-n. [DOI] [PubMed] [Google Scholar]
- 35.Greer JM, Koerner TAW, Hayakawa K, Hardy RR, Kemp JD. The 3G11+ antigen, a marker for murine CD4+TH1 lymphocytes, is a ganglioside. Glycobiology. 1993;3:391–401. doi: 10.1093/glycob/3.4.391. [DOI] [PubMed] [Google Scholar]
- 36.Dittrich F, Hayakawa K, Nimtz M, Ebel F, Mühlradt PF. Identification of the mouse helper T lymphocyte differentiation antigen 3G11 as the ganglioside IV3(NeuAc)2-GgOse4Cer. Biochem Biophys Res Commun. 1994;200:1557–1563. doi: 10.1006/bbrc.1994.1628. [DOI] [PubMed] [Google Scholar]
- 37.Reivinen J, Holthofer H, Miettinen A. O-acetyl GD3 ganglioside in human peripheral blood T lymphocytes. Int Immunol. 1994;6:1409–1416. doi: 10.1093/intimm/6.9.1409. [DOI] [PubMed] [Google Scholar]
- 38.Higgs JB, Zeldes W, Kozarsky K, Schteingart M, Kan L, Bohlke P, Krieger K, Davis W, Fox DA. A novel pathway of human T lymphocyte activation. Identification by a monoclonal antibody generated against a rheumatoid synovial T cell line. J Immunol. 1988;140:3758–3765. [PubMed] [Google Scholar]
- 39.Fox DA, Davis W, Zeldes W, Kan L, Higgs J, Duby AD, Holoshitz J. Activation of human T cell clones through the UM4D4/CDw60 surface antigen. Cell Immunol. 1990;128:480–489. doi: 10.1016/0008-8749(90)90042-p. [DOI] [PubMed] [Google Scholar]
- 40.Pulido R, Sánchez-Madrid F. Glycosylation of CD45: carbohydrate composition and its role in acquisition of CD45R0 and CD45RB T cell maturation-related antigen specificities during biosynthesis. Eur J Immunol. 1990;20:2667–2671. doi: 10.1002/eji.1830201221. [DOI] [PubMed] [Google Scholar]
- 41.Hathcock KS, Laszlo G, Dickler HB, Sharrow SO, Johnson P, Trowbridge IS, Hodes RJ. Expression of variable exon A-, B-, and C-specific CD45 determinants on peripheral and thymic T cell populations. J Immunol. 1992;148:19–28. [PubMed] [Google Scholar]
- 42.Rogers PR, Pilapil S, Hayakawa K, Romain PL, Parker DC. CD45 alternative exon expression in murine and human CD4+ T cell subsets. J Immunol. 1992;148:40544065. [PubMed] [Google Scholar]
- 43.Jones AT, Federsppiel B, Ellies LG, Williams MJ, Burgener R, Duronio V, Smith CA, Takei F, Ziltener HJ. Characterization of the activation-associated isoform of CD43 on murine T lymphocytes. J Immunol. 1994;153:3426–3439. [PubMed] [Google Scholar]
- 44.Powell LD, Varki A. The oligosaccharide binding specificities of CD22β, a sialic acid-specific lectin of B cells. J Biol Chem. 1994;269:10628–10636. [PubMed] [Google Scholar]
- 45.Muchmore E, Varki N, Fukuda M, Varki A. Developmental regulation of sialic acid modifications in rat and human colon. FASEB J. 1987;1:229–235. doi: 10.1096/fasebj.1.3.3623000. [DOI] [PubMed] [Google Scholar]
- 46.Mendez-Otero R, Ramon-Cueto A. Expression of 9-O-acetylated gangliosides during development of the rat olfactory system. Neuroreport. 1994;5:1755–1759. doi: 10.1097/00001756-199409080-00017. [DOI] [PubMed] [Google Scholar]
- 47.Reinhardt-Maelicke S, Cleeves V, Kindler-Röhrborn A, Rajewsky MF. Differential recognition of a set of O-acetylated gangliosides by monoclonal antibodies RB13-2, D1.1, and JONES during rat brain development. Dev Brain Res. 1990;51:279–282. doi: 10.1016/0165-3806(90)90286-8. [DOI] [PubMed] [Google Scholar]
- 48.Mendez-Otero R, Friedman JE. Role of acetylated gangliosides on neurite extension. Eur J Cell Biol. 1996;71:192–198. [PubMed] [Google Scholar]
- 49.Manzi AE, Sjoberg ER, Diaz S, Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. J Biol Chem. 1990;265:13091–13103. [PubMed] [Google Scholar]
- 50.Shi WX, Chammas R, Varki A. Linkage-specific action of endogenous sialic acid O-acetyltransferase in Chinese hamster ovary. J Biol Chem. 1996;271:15130–15138. doi: 10.1074/jbc.271.25.15130. [DOI] [PubMed] [Google Scholar]
- 51.Schwarting GA, Gajewski A. Glycolipids of murine lymphocyte subpopulations. Structural characterization of thymus gangliosides. J Biol Chem. 1983;258:5893–5898. [PubMed] [Google Scholar]
- 52.Holzhauser R, Faillard H, Klose W, Huber W, Stickl H, Landthaler M. Alterations of acyl-neuraminic acids on T-lymphocytes in cases of melanoma. Klin Wochenschr. 1988;66:540–544. doi: 10.1007/BF01736523. [DOI] [PubMed] [Google Scholar]
- 53.Stickl H, Huber W, Faillard H, Becker A, Holzhauser R, Graeff H. Changes of acylneuraminic acids content on T-lymphocytes in patients with mamma carcinoma. Klin Wochenschr. 1991;69:5–9. doi: 10.1007/BF01649046. [DOI] [PubMed] [Google Scholar]
- 54.Hueso P, Cabezas J A, Reglero A. O-acetylated sialic acids in gangliosides from pig spleen lymphocytes. Ital J Biochem. 1988;37:302–309. [PubMed] [Google Scholar]
- 55.Zimmer G, Suguri T, Reuter G, Yu RK, Schauer R, Herrler G. Modification of sialic acids by 9-Oacetylation is detected in human leucocytes using the lectin property of influenza C virus. Glycobiology. 1994;4:343–350. doi: 10.1093/glycob/4.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baum LG, Derbin K, Perillo NL, Wu T, Pang M, Uittenbogaart C. Characterization of terminal sialic acid linkages on human thymocytes—correlation between lectin-binding phenotype and sialyltransferase expression. J Biol Chem. 1996;271:10793–10799. doi: 10.1074/jbc.271.18.10793. [DOI] [PubMed] [Google Scholar]
- 57.Baum LG, Pang M, Perillo NL, Wu T, Delegeane A, Uittenbogaart CH, Fukuda M, Seilhamer JJ. Human thymic epithelial cells express an endogenous lectin, galectin-1, which binds to core 2 O-glycans on thymocytes and T lymphoblastoid cells. J Exp Med. 1995;181:877–887. doi: 10.1084/jem.181.3.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scollay R, Godfrey DI. Thymic emigration: conveyor belts or lucky dips? . Immunol Today. 1995;16:268–273. doi: 10.1016/0167-5699(95)80179-0. [DOI] [PubMed] [Google Scholar]
- 59.Dyall R, Nikolic-Zugic J. The majority of postselection CD4+ single-positive thymocytes requires the thymus to produce long-lived, functional T cells. J Exp Med. 1995;181:235–245. doi: 10.1084/jem.181.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vanhecke D, Leclercq G, Plum J, Vandekerckhove B. Characterization of distinct stages during the differentiation of human CD69+CD3+ thymocytes and identification of thymic emigrants. J Immunol. 1995;155:1862–1872. [PubMed] [Google Scholar]
- 61.Vargas-Cortes M, O'Donnell CL, Appel MC, Maciaszek JW, Yurkunas KS, Welsh RM. A lymphocyte differentiation and activation antigen, CZ-1, that distinguishes between CD8+ and unstimulated CD4+ T lymphocytes. Eur J Immunol. 1992;22:1043–1047. doi: 10.1002/eji.1830220425. [DOI] [PubMed] [Google Scholar]
- 62.Urdal DL, Hakomori S. Characterization of tumor-associated ganglio-N-triaosylceramide in mouse lymphoma and the dependency of its exposure and antigenicity on the sialosyl residues of a second glycoconjugate. J Biol Chem. 1983;258:6869–6874. [PubMed] [Google Scholar]
- 63.Shi WX, Chammas R, Varki A. Regulation of sialic acid 9-O-acetylation during the growth and differentiation of murine erythroleukemia cells. J Biol Chem. 1996;271:31517–31525. doi: 10.1074/jbc.271.49.31517. [DOI] [PubMed] [Google Scholar]
- 64.Carlsson SR, Sasaki H, Fukuda M. Structural variations of O-linked oligosaccharides present in leukosialin isolated from erythroid, myeloid, and T-lymphoid cell lines. J Biol Chem. 1986;261:12787–12795. [PubMed] [Google Scholar]
- 65.Ellies LG, Jones AT, Williams MJ, Ziltener HJ. Differential regulation of CD43 glycoforms on CD4+ and CD8+T lymphocytes in graft-versus-host disease. Glycobiology. 1994;4:885–893. doi: 10.1093/glycob/4.6.885. [DOI] [PubMed] [Google Scholar]
- 66.Leitenberg D, Novak TJ, Farber D, Smith BR, Bottomly K. The extracellular domain of CD45 controls association with the CD4-T cell receptor complex and the response to antigen-specific stimulation. J Exp Med. 1996;183:249–259. doi: 10.1084/jem.183.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chui D, Ong CJ, Johnson P, Teh HS, Marth JD. Specific CD45 isoforms differentially regulate T cell receptor signaling. EMBO (Eur Mol Biol Organ) J. 1994;13:798–807. doi: 10.1002/j.1460-2075.1994.tb06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baadsgaard O, Tong P, Elder JT, Hansen ER, Ho V, Hammerberg C, Lange-Vejlsgaard G, Fox DA, Fisher G, Chan LS. UM4D4+ (CDw60) T cells are compartmentalized into psoriatic skin and release lymphokines that induce a keratinocyte phenotype expressed in psoriatic lesions. J Invest Dermatol. 1990;95:275–282. doi: 10.1111/1523-1747.ep12484908. [DOI] [PubMed] [Google Scholar]
- 69.Fox DA, Millard JA, Kan L, Zeldes WS, Davis W, Higgs J, Emmrich F, Kinne RW. Activation pathways of synovial T lymphocytes. Expression and function of the UM4D4/CDw60 antigen. J Clin Invest. 1990;86:1124–1136. doi: 10.1172/JCI114817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hansen ER, Vejlsgaard GL, Cooper KD, Heidenheim M, Larsen JK, Ho VC, Ross CW, Fox DA, Thomsen K, Baadsgaard O. Leukemic T cells from patients with cutaneous T-cell lymphoma demonstrate enhanced activation through CDw60, CD2, and CD28 relative to activation through the T-cell antigen receptor complex. J Invest Dermatol. 1993;100:667–673. doi: 10.1111/1523-1747.ep12472333. [DOI] [PubMed] [Google Scholar]
- 71.Muthing J, Schwinzer B, Peter-Katalinic J, Egge H, Muhlradt PF. Gangliosides of murine T lymphocyte subpopulations. Biochemistry. 1989;28:2923–2929. doi: 10.1021/bi00433a027. [DOI] [PubMed] [Google Scholar]
- 72.Ebel F, Schmitt E, Peter-Katalinic J, Kniep B, Mühlradt PF. Gangliosides: differentiation markers for murine T helper lymphocyte subpopulations TH1 and TH2. Biochemistry. 1992;31:12190–12197. doi: 10.1021/bi00163a031. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura K, Suzuki H, Hirabayashi Y, Suzuki A. IV3α(NeuGcα2-8NeuGc)-Gg4Cer is restricted to CD4+T cells producing interleukin-2 and a small population of mature thymocytes in mice. J Biol Chem. 1995;270:3876–3881. [PubMed] [Google Scholar]
- 74.Kniep B, Claus C, Peter-Katalinic J, Monner DA, Dippold W, Nimtz M. 7-O-acetyl-GD3in human T-lymphocytes is detected by a specific T-cell-activating monoclonal antibody. J Biol Chem. 1995;270:30173–30180. doi: 10.1074/jbc.270.50.30173. [DOI] [PubMed] [Google Scholar]