Abstract
LTα-deficient (LTα−/−) mice show altered splenic microarchitecture. This includes loss of normal B cell–T cell compartmentalization, of follicular dendritic cell (FDC) clusters, and of ability to form germinal centers (GC). LTα−/− mice immunized with sheep red blood cells (SRBC) produced high levels of antigen-specific IgM but no IgG in either primary or secondary responses, demonstrating failure of Ig class switching. This inability to switch to IgG could have been due to the altered splenic microarchitecture in these mice. Alternatively, it could have been due directly to a requirement for LTα expression by lymphocytes cooperating in the antibody response. To investigate this, we performed reciprocal spleen cell transfers. When irradiated LTα−/− mice were reconstituted with wild-type splenocytes and immunized immediately with SRBC, splenic microarchitecture remained disturbed and there was no IgG response. In contrast, when irradiated wild-type animals received splenocytes from LTα−/− mice, follicle structure and a strong IgG response were retained. These data indicate that LTα-deficient B cells and T cells have no intrinsic defect in ability to generate an IgG response. Rather, the altered microenvironment characteristic of LTα−/− mice appears to result in impaired ability to switch to a productive IgG response. To investigate whether prolonged expression of LTα could alter the structure and function of spleen follicles, reciprocal bone marrow (BM) transplantation was performed. Six weeks after reconstitution of LTα−/− mice with wild-type BM, spleen follicle structure was partially restored, with return of FDC clusters and GC. B cell/T cell compartmentalization remained abnormal and white pulp zones were small. This was accompanied by restoration of IgG response to SRBC. Reconstitution of wild-type mice with LTα−/− BM resulted in loss of FDC clusters and GC, and loss of the IgG response, although compartmentalized B cell and T cell zones were largely retained. Thus, defective IgG production is not absolutely associated with abnormal B cell and T cell compartmentalization. Rather, expression of LTα supports the maturation of spleen follicle structure, including the development and maintenance of FDC clusters, which supports Ig class switching and an effective IgG response.
Lymphotoxin-α (LTα)1 shares structural features with the related cytokine, TNFα. Both LTα and TNFα exist in solution as homotrimeric proteins. In these forms, they share biological activities by virtue of their similar binding to the two defined TNF receptors, TNFR-I, and TNFR-II. Signaling via these two receptors modulates a wide variety of immune and inflammatory responses (1, 2). LTα also exists in a heteromeric form with the type II membrane protein LTβ, which in its most prevalent form on the membrane has the stoichiometry LTα1LTβ2. The LTα1LTβ2 heteromer has no measurable affinity for TNFR-I or TNFR-II, but does interact with high affinity with the TNFR-related protein (also designated the LTβ receptor, or LTβR) (3–5).
LTα−/− mice are born with defective development of LN and Peyer's patches (PP) (6). In LTα−/− mice, spleen structure is also disturbed, with small white pulp follicles that fail to segregate T cell and B cell zones, and fail to generate clusters of FDC or GC (6–8). Proper spleen microarchitecture, including the presence of primary and secondary lymphoid follicles that contain FDC, is thought to be required for all features of a mature T cell–dependent B cell response, including Ig class switching, affinity maturation, and development of antibody secreting cells (9–11). Consequently, it was striking that LTα−/− mice were able to generate high affinity anti-NP IgG antibody after immunization with high doses of the T cell–dependent antigen 4-hydroxy3-nitrophenyl-ovalbumin (NP-OVA) adsorbed to alum (8). In contrast, there was impaired production of high affinity anti-NP IgG when LTα−/− mice were immunized with low doses of NP-OVA absorbed to alum. Banks et al. (12) using an independently derived LTα−/− mouse strain, have shown impaired IgG responses in LTα−/− mice after subcutaneous immunization with KLH absorbed to alum or immunization with viral antigens. Further studies examining mice deficient in both TNFα and LTα demonstrated variable IgG responses, with deficient IgG responses after intraperitoneal immunization with SRBC but retained responses against vesicular stomatitis virus after an infectious challenge (13). Recently prepared TNFα−/− mice (14) are reported also to have an impaired IgG anti-SRBC response but a strong response to DNP-KLH adsorbed to alum. Thus, the role of LTα in supporting an effective IgG response to SRBC remains incompletely defined.
LTα−/− mice manifest a complex phenotype that includes an absence of LTα expression and also established abnormalities of lymphoid tissue development and structure (6–8). The present study was undertaken to investigate the requirements for LTα expression in lymphoid cells and for intact lymphoid tissue structure to support production of an IgG responses to the T cell–dependent antigen SRBC. We report that LTα−/− mice responded with high levels of IgM but very low levels of IgG after immunization with SRBC in the absence of adjuvant. Experiments in which suspensions of mature spleen cells or of T cell–depleted bone marrow were transferred to wild-type or LTα−/− mice demonstrated that certain elements of spleen follicle structure are plastic and are determined by the presence of LTα-expressing cells. These experiments also demonstrated that LTα−/− B cells and T cells in a structurally intact lymphoid tissue environment are competent to perform Ig isotype switching. In contrast, disturbed lymphoid tissue structure caused by absence of LTα and manifested by absence of clusters of FDC is associated with an inability to form an effective IgG response. Thus, LTα produced by bone marrow (BM)–derived cells establishes a permissive environment for an effective IgG response.
Materials and Methods
Mice.
C57BL/6J and 129Sv mice were obtained from The Jackson Laboratory (Bar Harbor, ME). LTα−/− mice (6) were maintained on a mixed 129Sv × C57BL/6 background and were bred under specific pathogen-free conditions.
Measurement of Antigen-specific Ig.
Specific antibodies were measured and analyzed as previously described (15). In brief, Immulon 4 plates (Dynatech Laboratories, Inc., Chantilly, VA) were coated with SRBC (150 μl at 5 × 107/ml) suspended in 0.25% glutaraldehyde in PBS. Diluted mouse sera were then added and incubated at 4°C for 1 h. Alkaline phosphatase-conjugated goat anti–mouse isotype-specific antisera (Southern Biotechnology, Birmingham, AL) were diluted 1:500 for IgM and 1:2,000 for total IgG or IgG subclasses, and 100 μl were added and incubated at 4°C for 1 h, followed by washing and addition of the alkaline phosphatase substrate p-nitrophenyl phosphate (Sigma Chem. Co., St. Louis, MO) at 1 mg/ml. The mean OD at 405 nm from triplicate wells was compared to a standard curve of titrated serum to calculate the relative units (RU) using linear regression analysis (15). The results represent mean ± SEM.
Transfer of Splenocytes.
Whole spleen cell suspensions were prepared from single mouse donors by mincing the spleen with scissors and teasing the tissue fragments between two frosted microscope slides. Recipients were prepared by irradiation with 750 rads 3 h before cell transfer. As indicated, SRBC were mixed together with the spleen cell suspensions before i.v. injection. Each recipient received all of the cells derived from a single donor spleen.
BM Transplantation.
BM was harvested and recipients were prepared as described previously (16). Recipient mice were lethally irradiated with 1,050 rad (10.5 Gy) and reconstituted with 5 × 106 donor BM cells. 6 wk after transplantation, recipients were immunized i.p. with 108 SRBC and serum samples were collected 10 d after primary or secondary immunization. 6 mo after transfer, wild-type mice reconstituted with LTα−/− BM showed retention of LN and PP, while LTα−/− mice reconstituted with wild-type BM showed no evidence of de novo LN or PP development.
Evaluation of Spleen Follicle Structure.
Spleens were harvested, embedded in O.C.T. compound (Miles, Elkhart, IN), and frozen in liquid nitrogen. Frozen sections (6–10 μm thick) were fixed in cold acetone. Endogenous peroxidase was quenched with 0.2% H2O2 in methanol. After washing, the sections were stained by first incubating with FITC-conjugated B220 (PharMingen, San Diego, CA), and biotinylated anti-CR1 8C12 or Thy1.2 (PharMingen) or PNA (Vector, Burlingame, CA), all at 1:100 dilution. Horseradish peroxidase (HRP)-conjugated rabbit anti-FITC (Dako, Glostrup, Denmark; diluted 1:10) was added 1 h later. Sections were then incubated for 1 h with one drop of alkaline phosphatase (AP)–conjugated streptavidin (Zymed, South San Francisco, CA) and color development for bound AP and HRP was with an AP reaction kit (Vector) and with diaminobenzidine.
Results and Discussion
LTα−/− Mice Immunized with SRBC Produce High Levels of Antigen-specific IgM but Low Levels of IgG.
Previous studies of LTα−/− mice (8, 12) have showed variable IgG responses after immunization with T cell–dependent antigens. It has been suggested that this variability was due to difference in the immunization protocols, in the routes of immunization, and in the nature of the antigens used. SRBC have been commonly used for studies of antibody responsiveness because they are T cell–dependent antigens, do not require adjuvant to elicit a strong response, and can elicit IgG antibodies following immunization by either the intradermal, subcutaneous, i.p., or i.v. routes. Studies by Eugster et al. (13) using mice deficient in both LTα and TNFα demonstrated that isotype switching in these mice was defective after immunization i.p. with SRBC. This indicated that either TNFα or LTα or both play an important role in the immune response against this antigen. These investigators subsequently showed that transfer of wild-type BM to these LTα−/−/TNFα−/− mice restored IgG responsiveness to this antigen (17). This indicated that LTα and/or TNFα- expressing cells transferred in BM determined IgG responsiveness; however, it did not define the mechanisms of action of the cytokines. Furthermore, the recent report that TNFα is required for effective isotype switching to SRBC (14) underscores the fact that the selective role of LTα in IgG responses is not defined. The following studies were performed to define the role of LTα in IgG production and to dissect the mechanisms of LTα's effects in response to SRBC.
When wild-type mice were immunized by injection of 107 SRBC into the footpad, they generated a robust IgG anti-SRBC response (Fig. 1 a). In contrast, when LTα−/− mice were similarly immunized, they showed no detectable anti-SRBC IgG after either the primary immunization or secondary boost. This clearly indicated that, in addition to TNFα, LTα is required for an intact IgG response against SRBC.
Figure 1.
Anti-SRBC IgG responses after subcutaneous or intraperitoneal immunization. Groups of 3–5 mice (8-wk-old) (wild-type, filled symbols; LTα−/−, open symbols) were immunized at day 0 in the footpad (A, 107 SRBC ) or by intraperitoneal injection (B, 108 SRBC) and given a boost with the same dose on day 21. SRBC-specific IgG was measured using an ELISA. Data shown represent the means ± SEM of triplicate determinations from 3–5 mice. RU, relative units. One representative experiment of three is shown.
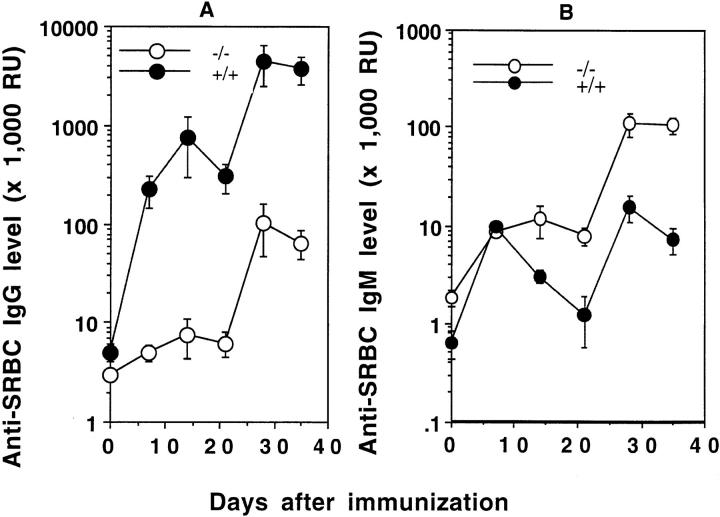
LTα−/− mice are born with defective development of LN and PP and with altered splenic microarchitecture. LN are considered to be important structures for collecting and concentrating antigens. We considered that the lack of LN in LTα−/− mice might account for their impaired IgG response after peripheral immunization; however, immunization of LTα−/− mice with higher doses of SRBC (1 × 108 or 6 × 108) i.p. still result in no primary antigen-specific IgG response and a markedly reduced response after a secondary boost (Fig. 1 b). Even when mice were immunized with a high dose of SRBC (1 × 108) i.v. to bypass the LN and to deliver antigen directly to the spleen, the primary IgG response of LTα−/− mice was only at the lowest limit of detection in the assay (Fig. 2 a), <1% that of wild-type animals. After an i.v. boost, a small IgG anti-SRBC response was detected, but again at a level <3% that seen in wild-type mice.
Figure 2.
Anti-SRBC IgM and IgG responses in LTα−/− mice after intravenous immunization. Groups of 3–5 mice (8-wk-old) were immunized i.v. with 108 SRBC in PBS and boosted with the same dose 21 d later. Serum anti-SRBC IgM (A) and IgG (B) were measured by ELISA. Symbols and RU are as described in Fig. 1.
The reduced IgG response in LTα−/− mice did not simply represent a delay, as anti-SRBC IgG remained low 2–3 wk after both the initial immunization and after the secondary boost. Measurement of the levels of anti-SRBC IgM, however, showed the presence of a brisk anti-SRBC B cell response (Fig. 2 b). In fact, compared to wild-type mice, LTα−/− mice showed prolonged persistence of the IgM response and a dramatically increased IgM response after a secondary boost. Thus, the reduced IgG production in i.v. immunized LTα−/− mice did not represent failure to deliver sufficient antigen to activate the responding B cell pool, or failure to activate these antigen-specific cells, but rather it represented a failure of effective isotype class switch or of affinity maturation to produce anti-SRBC IgG with sufficient affinity for efficient detection.
LTα-expressing Splenocytes Are not Sufficient to Direct IgG Production in LTα−/− Mice.
LTα−/− mice show altered splenic microarchitecture without distinct B cell and T cell zones and with absence of FDC and GC (6–8). Any of these structural changes could interfere with the development of a successful IgG response. Alternatively, LTα itself expressed by mature B and T lymphocytes could be an essential factor for the lymphocyte activation which is required for an effective isotype switched response against SRBC. To test whether intact splenic microarchitecture or LTα expressed by lymphocytes are required to activate Ig isotype switching, we performed reciprocal transfers of splenocytes. In these experiments, we either introduced wildtype LTα expressing B cells and T cells into the abnormal structural environment of LTα−/− mice, or we introduced LTα-deficient cells into the normal structural environment of wild-type mice.
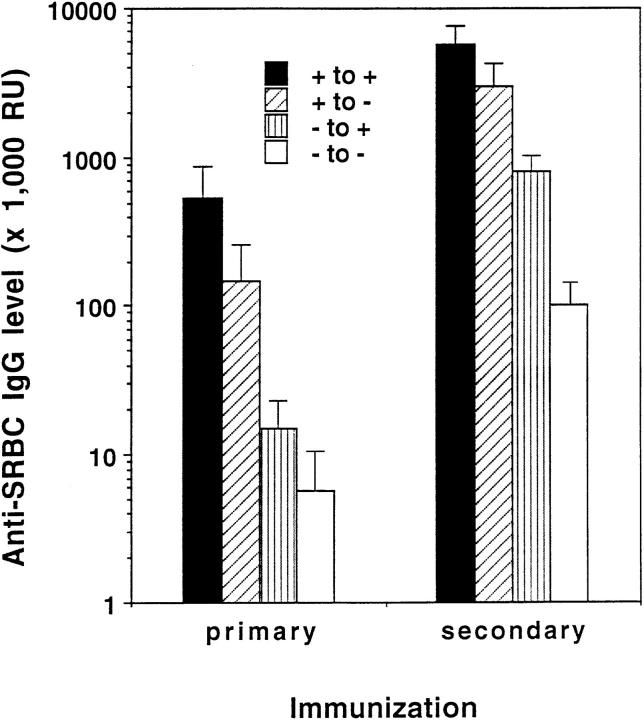
Wild-type mice that were irradiated and immunized with SRBC without reconstitution with splenocytes showed no detectable B220+ or Thy-1+ cells in their spleens and produced no detectable anti-SRBC IgM or IgG at day 10 (data not shown), indicating that development of an antibody response could not be supported directly by any radioresistant cells in the recipient animals under these experimental conditions. When irradiated wild-type mice were treated with LTα−/− splenocytes and were immunized i.v. with SRBC, they produced high levels of IgG anti-SRBC similar to wild-type mice that had received wild-type splenocytes (Fig. 3). This indicated that LTα-deficient B cells and T cells have no intrinsic defect in Ig isotype switching, and that LT-expressing lymphocytes are not required for effective anti-SRBC IgG production in the context of an LT wild-type environment. In contrast, when LTα−/− mice were irradiated and reconstituted with wild-type splenocytes, they failed to mount a detectable IgG response to i.v. SRBC (Fig. 3). Thus, mature LTα-expressing lymphocytes are not sufficient for IgG isotype switching. Rather, underlying wild-type spleen microarchitecture or spleen stromal and non-lymphoid cellular elements which rely on LTα for their proper formation may be necessary for the IgG response against SRBC.
Figure 3.
IgG production after spleen cell transfer. Wild-type or LTα−/− mice were irradiated with 750 rad, then treated with an infusion of spleen cells from wild-type or LTα−/− donors together with 108 SRBC. Ten days later, serum was collected and anti-SRBC IgG was measured by ELISA. The results represent mean ± SEM of 3–5 mice per group. This experiment was repeated five times with similar results.
Interestingly, although the structure of the spleen white pulp nodule and the ability to mount an antigen-specific IgG response are disturbed in LTα−/− mice, several other features of normal immune responsiveness appear to be fully retained. First, splenocytes harvested from either wildtype or LTα−/− mice that had been immunized i.p. with 108 SRBC showed indistinguishable proliferative responses when challenged in vitro with SRBC (data not shown). Similarly, when wild-type or LTα−/− mice were sensitized with trimethlyamine in a hapten-specific delayed type hypersensitivity response and were challenged 7 d later by injection of hapten into the hind footpad, similar amounts of footpad swelling were observed (data not shown). Finally, LTα−/− and wild-type mice rejected H-2 disparate skin allografts in similar fashion, with mean graft survival of 11 ± 1.3 d in wild-type recipients and 13 ± 1.3 d in LTα−/− recipients. All of these data suggest that T cell function is retained in the LTα−/− mice. Furthermore, since dendritic cells are thought to act importantly during the activation of these T cell–dependent responses, they suggest that dendritic cell function is retained in spite of the disturbance in spleen structure.
Disturbed Splenic Lymphoid Structure Correlates with an Impaired IgG Response to SRBC.
It is thought that establishment of proper B cell–T cell interactions in lymphoid follicles is essential for the initiation and maturation of humoral responses to T cell–dependent antigens. Within follicles, clustered follicular dendritic cells (FDC) with complement receptors 1 and 2 and FcR are known to trap immune complexes and are thought to support development of GC in which the development of effective IgG responses occurs (9–11, 15). In a previous study, we demonstrated that LTα−/− mice show altered B cell–T cell zones, loss of FDC clusters and absence of immune complex trapping (6, 8).
To study the relationship between altered splenic microarchitecture and the ability to generate an anti-SRBC IgG response, mice that had been irradiated and treated with infusions of spleen cells were analyzed histologically. Wild-type mice reconstituted with either wild-type or LTα−/− splenocytes showed similarly segregated B cell and T cell zones (Fig. 4, A and C) and clusters of FDC (Fig. 4, E and G). This was associated with competence for antiSRBC IgG responses (see Fig. 3). In contrast, when irradiated LTα−/− mice were reconstituted with either wild-type or LTα−/− cells, B cell and T cell zones were disorganized (Fig. 4, B and D) without detectable FDC clusters (Fig. 4, F and H). This was associated with absence of anti-SRBC IgG responses (see Fig. 3). Production of antigen-specific IgM, however, was retained by all mice that received either wild-type or LTα−/− splenocytes, even in the context of disturbed splenic microarchitecture (data not shown).
Figure 4.
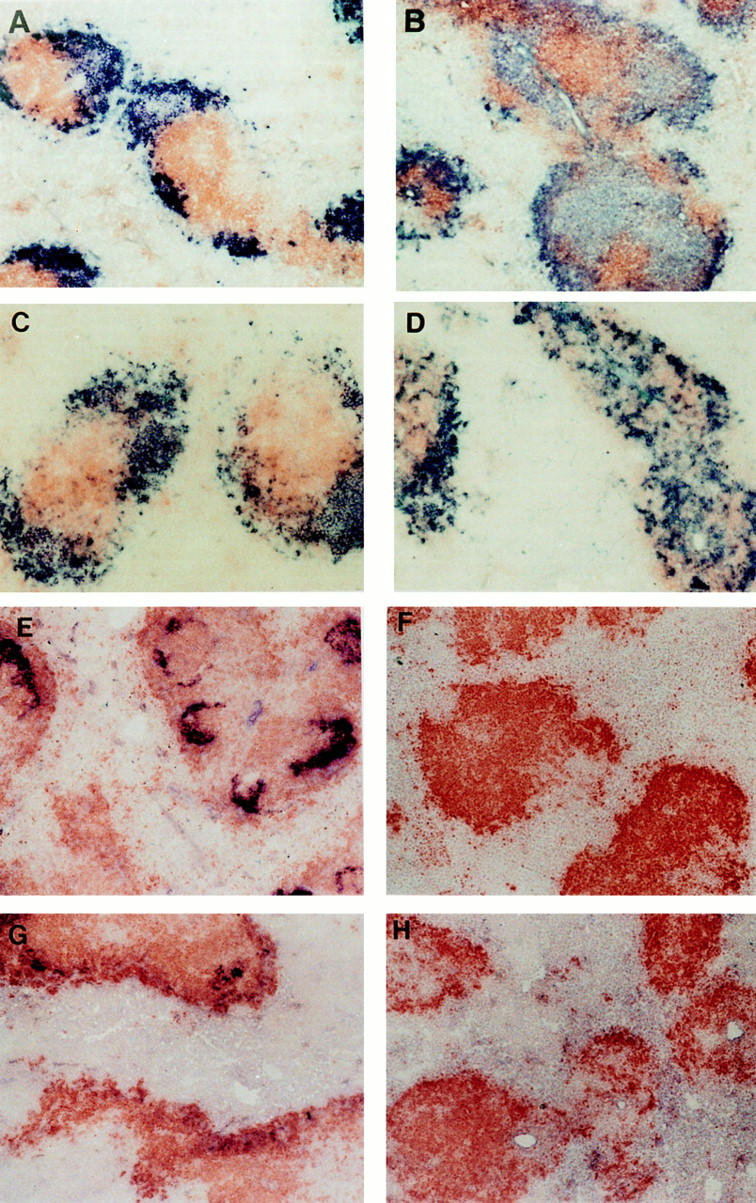
Structure of spleen follicles in irradiated mice reconstituted with wild-type or LTα−/− splenocytes. After serum was collected from mice shown in Fig. 3, the spleens were harvested and frozen sections were stained with anti-B220 (brown) and anti-Thy1.2 (blue) to visualize the B cell and T cell zones (A–D). Distinct B cell and T cell zones were present in wild-type mice that received splenocytes from either normal (A) or LTα−/− mice (C), whereas there was disturbed segregation of B cells and T cells in LTα−/− mice that received splenocytes from either normal (B) or LTα−/− mice (D). FDC clusters were observed by staining with the anti-CR1 monoclonal antibody 8C12 (blue) (E–H). FDC clusters were retained in the spleen follicles of wild-type mice that received splenocytes from either wildtype (E) or LTα−/− mice (G), whereas FDC clusters were absent in the spleens of LTα−/− mice that received splenocytes from either wild-type (F) or LTα−/− mice (H).
In this short-term spleen cell reconstitution model, LTαexpressing cells are unable to reprogram discrete B cell and T cell zones or clusters of FDC in irradiated LTα−/− mice. Similarly, established clusters of FDC are not in the short term dependent on the presence of LTα-expressing cells (Fig. 4 G). The ability to introduce LTα-expressing cells into a disturbed microenvironment and LTα-deficient cells into a wild-type microenvironment allows us to examine the role of intact follicle structure on antibody production. As manifested by the presence of clustered FDC and/or normal B cell–T cell compartmentalization, wild-type follicle structure determines the ability to develop a productive isotype switched antibody response. LTα-expressing lymphocytes are not per se required for switching.
Bone Marrow-derived LTα-expressing Cells Reprogram the Structure of the LTα−/− Spleen to Support the Development of an IgG Response.
BM transfer provides an alternate model to evaluate the role of LTα in determination of spleen microarchitecture and antibody responsiveness permitting long term reconstitution. 6 wk after lethally irradiated LTα−/− mice were given wild-type BM, they showed restoration of 8C12-staining FDC clusters (Fig. 5 B) and of the ability to form morphologically intact germinal centers (Fig. 5 F); however, the follicles remained small and segregation of B cell and T cell zones was incomplete (Fig. 6 B). In contrast, when lethally irradiated wild-type mice were reconstituted with LTα−/− BM, follicle size was maintained and segregation of B cell and T cell zones was at least partially retained (Fig. 6 C), but FDC clusters and GC formation were lost (Fig. 5, C and G). As expected, reconstitution of wild-type mice with wild-type BM resulted in the re-establishment of morphologically normal follicles and reconstitution of LTα−/− mice with LTα−/− BM yielded small follicles with globally disturbed structure (Figs. 5 and 6). These data underscore the difference between shortterm and long-term reconstitution in this system. When LTα−/− mice are reconstituted with LTα-expressing splenocytes and examined 10 d later, they still have no FDC clusters and are unable to generate GC. In contrast, when LTα−/− mice are reconstituted with LTα-expressing BM cells and examined 6 wk later, FDC clusters have developed and GC can form in response to immunization. It is currently unclear whether the differences we observed between BM and spleen cell transfers represent differences in the functionality of BM and spleen cells (with BM containing a cell population capable of supporting development of spleen FDC clusters and with splenocyte suspensions being devoid of such cells) or more likely represents differences in the time course of the experiment (with LTα-expression in a transferred cell being required for more than 10 d in order for FDC clusters to form).
Figure 5.
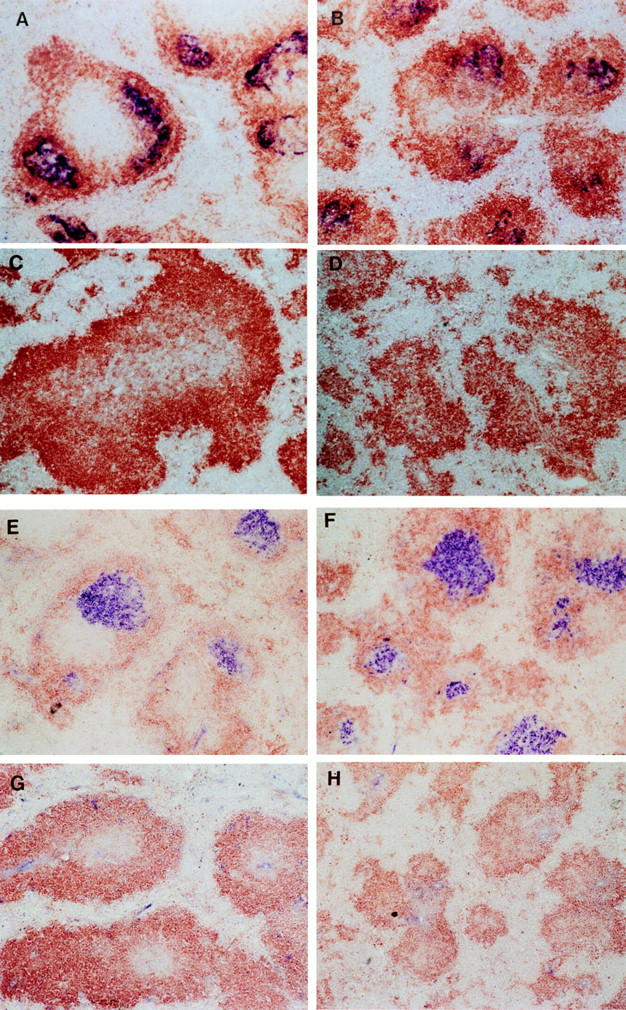
Spleen follicle structure in recipients of wild-type or LTα−/− bone marrow. 6 wk after bone marrow reconstitution, mice were immunized i.p. with SRBC and 10 d later sections of frozen spleen were stained with 8C12 (blue) to detect FDC, and with anti-B220 (brown). Clusters of FDC were detected in both wild-type mice (A) and LTα−/− mice (B) that had been reconstituted with wild-type BM. Clusters of FDC were not detected in either wild-type (C) or LTα−/− mice (D) that had received BM from LTα−/− mice. The GC reaction was assessed by staining spleen sections with PNA (blue) and anti-IgD (brown). GC were observed in both wild-type mice (E) and LTα−/− mice (F) that had received BM from wildtype donors, but were not detected in either wild-type (G) or LTα−/− mice (H) that were reconstituted with BM from LTα−/− donors.
Figure 6.

B cell/T cell organization in irradiated mice reconstituted with wild-type or LTα−/− bone marrow. Frozen sections of spleens from the recipients of BM transfer shown in Figure 5 were analyzed by staining with anti–Thy-1.2 (blue) and anti-B220 (brown). Wild-type mice that received BM from either wild-type (A) or LTα−/− mice (B) showed segregation of B cells and T cells within lymphoid follicles, whereas the follicles of LTα−/− mice reconstituted with BM from either wild-type (C) or LTα−/− mice (D) showed little or no segregation of B and T cell zones.
Our data are in contrast to those of Muller et al. (17). These investigators studying mice with functional ablation of both the LTα and the TNFα genes, demonstrated recovery of the IgG response and almost complete recovery of spleen B cell–T cell compartmentalization after these mice were irradiated and reconstituted with wild-type bone marrow. It is currently unclear whether the apparent differences in ability of LTα−/− and TNFα/LTα doubledeficient mice to manifest restored B cell–T cell segregation after reconstitution with wild-type BM reflects a modulating effect of TNFα or represents subtle differences in tissue staining or BM transfer methods. Direct comparison of the two mouse strains using identical techniques will be required to resolve this question.
To test the impact of BM reconstitution and its accompanying restoration of FDC clusters and GC reactivity on the character of the antibody response, we immunized mice 6 wk after BM transplantation and analyzed antigenspecific serum IgG in both the primary and the secondary responses. LTα−/− mice that received wild-type BM demonstrated an anti-SRBC IgG response similar in magnitude to that seen in wild-type mice similarly reconstituted (Fig. 7). This was associated with restoration of GC containing FDC clusters, but not with the formation of normal B cell and T cell zones (see Figs. 5 and 6). In contrast, wild-type mice that received LTα−/− BM had a severely impaired IgG response (Fig. 7). This defective IgG response was in the context of partially retained B cell–T cell compartmentalization but without detectable FDC clusters or GC (see Figs. 5 and 6). Taken together, these findings suggest that proper segregation of B cell and T cell zones is not required for productive isotype switching after i.v. immunization with SRBC. Rather, they showed a strong correlation between the ability to form an anti-SRBC IgG response and the presence of FDC clusters and GC determined by the presence of LTα-expressing BM-derived cells. Thus, BMderived LTα-expressing cells are required in order for B cells to switch to secretion of IgG, probably by virtue of the action of this cytokine to support the development and maintenance of FDC clusters and GC. Additional cellular and/or stromal elements besides FDC are no doubt required for the development of a functional GC reaction, including for example elements of the dendritic cell network. We have no data, however, to support a specific role for LTα as a regulator of development or function of these additional elements.
Figure 7.
IgG production after bone marrow transfer. Wild-type and LTα−/− mice (3–4 mice/group) were lethally irradiated and reconstituted with wild-type or LTα−/− as in Fig. 5. 6 wk later, they were immunized i.p. with SRBC (108) and boosted 21 d later. Serum was collected 10 d after both primary and secondary immunization and anti-SRBC IgG was measured by ELISA as in Fig. 3. Results represent means ± SEM. Similar results were obtained in two additional experiments.
Further support for the role of FDC clusters in the development of a robust IgG response comes from analysis of the spleen histology and anti-SRBC IgG responses over time after spleen cell transfer. In irradiated wild-type mice that had received LTα−/− splenocytes, although splenic FDC clusters were easily detected 10 d after spleen cell transfers, FDC clusters disappeared over the course of 2–3 wk. In contrast, 3 wk after transfer of wild-type splenocytes to irradiated LTα−/− mice, we began to detect clusters of FDC, and concomitant ability to form the development of an antigen-specific IgG response (data not shown).
One difficulty in interpreting the results of longer term spleen cell transfer experiments is that recipient lymphoid cells can reconstitute the animals and compete with the transferred cells. We have, therefore, performed similar experiments transferring wild-type or LTα−/− splenocytes to mildly irradiated (300 rad) or non-irradiated RAG-1–deficient mice. This allows observation of the action of the transferred lymphoid cells in the absence of any host-derived lymphocytes. These experiments gave similar results compared to those described above. LTα-expressing splenocytes elicit the formation over the course of approximately 3 wk of clusters of FDC and the potential to form GC and antigen-specific IgG (data not shown).
The data reported here demonstrate that the IgG response of LTα−/− mice immunized with high doses of SRBC is defective. This contrasts with our previous report in which immunization of LTα−/− mice i.p. with high doses of NP-OVA adsorbed to alum led to the development of a strong IgG response to NP (8). Recent studies using TNFα−/− mice (14) have shown similar variability of the IgG response depending on the details of the immunization program. When TNFα−/− mice were immunized with SRBC in PBS, they showed a severely impaired IgG response. When they were immunized with TNF-KLH in complete Freund's adjuvant, they showed productive isotype switching. We have performed preliminary experiments to investigate this phenomenon and to determine if adjuvant restores the anti-SRBC IgG response in LTα−/− mice. These studies showed that when LTα−/− mice were immunized i.p. with 108 SRBC emulsified with IFA, a brisk IgG response developed similar to that of wild-type mice.
Although these preliminary studies do not address the mechanism of the adjuvant effect, they demonstrate strong correlation between the presence of FDC clusters and GC responsiveness for effective isotype switching. The adjuvant-induced restoration of anti-SRBC in LTα−/− mice, however, was associated with neither the de novo formation of FDC clusters nor the development of GC (data not shown). This suggests that antigens administered i.p. with adjuvant can circumvent the need for proper follicle structure in the generation of a mature IgG response. This could be the consequence of the ability of adjuvant to induce local production of cytokines that substitute for signals produced in an intact follicle, or perhaps the ability of adjuvant to affect antigen presentation or interaction of antigen with responding B cells or T cells. In this regard, it is of interest that Banks et al. (12) observed that LTα−/− mice immunized twice s.c. with KLH plus incomplete Freund's adjuvant generated a weak IgG response. The failure of adjuvant to restore the IgG response in this setting might have been due to disturbed antigen trafficking without regional LN. In addition, the defect in production of IgG in mice deficient in either CD40 or CD40L was also not overcome by immunization with adjuvant (18, 19). This suggests that the mechanisms by which LTα and CD40/CD40L support Ig isotype switching may be different.
In conclusion, the studies presented here demonstrate that LTα contributes importantly to the development of normal spleen microarchitecture. LTα also provides signals that support the maturation of primary and secondary splenic follicles after antigen challenge. Our data suggest that the ability of LTα to support the development of an intact IgG response is permissive, a consequence of this cytokine's action to establish elements of intact spleen follicle structure.
Additional studies of the TNF family members OX40 and OX40L have shown that interactions between this ligand–receptor pair are required for the formation of antibody forming cell foci, but not for the formation of morphologically intact GC (20). Furthermore, signaling through the nerve growth factor receptor, another member of the TNFR superfamily, provides autocrine stimulation of memory B cells (21). Thus, signaling via multiple members of the TNF ligand-receptor family supports efficient maturation of the B cell response, each probably acting at a different activation stage. It is well established that certain members of this family are capable of regulating the expression of others (22, 23). It remains to be determined whether members of this family act sequentially or via independent pathways to support the maturation of the antibody response.
Acknowledgments
We thank Jori Scripter, David Randolph, and Shuhua Han for helpful discussions.
This work was supported in part by a National Institutes of Health grant (D.D. Chaplin). D.D. Chaplin is an investigator of the Howard Hughes Medical Institute.
Footnotes
1 Abbreviations used in this paper: AP, alkaline phosphatase; BM, bone marrow; FDC, follicular dendritic cells; LN, lymph nodes; LTα, lymphotoxin-α; NP-OVA, 4-hydroxy-3-nitrophenyl-ovalbumin; PP, Peyer's patches; RU, relative units.
References
- 1.Ruddle NH. Tumor necrosis factor (TNF-α) and lymphotoxin (TNFβ) Curr Opin Immunol. 1992;4:327–332. doi: 10.1016/0952-7915(92)90084-r. [DOI] [PubMed] [Google Scholar]
- 2.Ware CF, VanArsdale TL, Crowe PD, Browning JL. The ligands and receptors of the lymphotoxin system. Curr Topics Microbiol Immunol. 1995;198:175–218. doi: 10.1007/978-3-642-79414-8_11. [DOI] [PubMed] [Google Scholar]
- 3.Browning JL, Ngam-ek A, Lawton P, DeMarinis J, Tizard R, Chow EP, Hession C, O'Brine-Greco B, Foley SF, Ware CF. Lymphotoxin β, a novel member of the TNF family that forms a heteromeric complex with lymphotoxin on the cell surface. Cell. 1993;72:847–856. doi: 10.1016/0092-8674(93)90574-a. [DOI] [PubMed] [Google Scholar]
- 4.Crowe PD, VanArsdale TL, Walter BN, Ware CF, Hession C, Ehrenfels B, Browning JL, Din WS, Goodwin RG, Smith CA. A lymphotoxin-β-specific receptor. Science (Wash DC) 1994;264:707–710. [PubMed] [Google Scholar]
- 5.Browning JL, Miatkowski K, Sizing I, Griffiths D, Zafari M, Benjamin CD, Meier W, Mackay F. Signaling through the lymphotoxin β receptor induces the death of some adenocarcinoma tumor lines. J Exp Med. 1996;183:867–878. doi: 10.1084/jem.183.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science (Wash DC) 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. The role of lymphotoxin and type I TNF receptor in the formation of germinal centers. Science (Wash DC) 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Lo SF, Carruthers CJL, Min J, Mariathasan S, Huang G, Plas DR, Martin SM, Geha RS, Nahm MH, Chaplin DD. Affinity maturation without germinal centers in lymphotoxin-α (LTα) deficient mice. Nature (Lond) 1996;382:462–466. doi: 10.1038/382462a0. [DOI] [PubMed] [Google Scholar]
- 9.Kelsoe G. Life and death in germinal centers (redux) Immunity. 1996;4:107–111. doi: 10.1016/s1074-7613(00)80675-5. [DOI] [PubMed] [Google Scholar]
- 10.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 11.Rajewsky K. Clonal selection and learning in the antibody system. Nature (Lond) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 12.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-α-deficient mice: effects on secondary lymphoid organ development and humoral immune response. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 13.Eugster H-P, Muller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-α double-deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 14.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks, and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molina H, Holers M, Li B, Fang Y-F, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr RW, Chaplin DD. Markedly impaired humoral immune response in mice deficient in complement receptors 1 and 2. Proc Natl Acad Sci USA. 1996;93:3357–3361. doi: 10.1073/pnas.93.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariathasan S, Matsumoto M, Baranyay F, Nahm MH, Kanagawa O, Chaplin DD. Absence of lymph nodes in lymphotoxin-α (LTα)-deficient mice is due to abnormal organ development, not defective lymphocyte migration. J Inflamm. 1995;45:72–78. [PubMed] [Google Scholar]
- 17.Muller M, Eugster H-P, Le Hir M, Shakhov A, Di Padova F, Maurer C, Quesniaux VFJ, Ryffel B. Correction or transfer of immunodeficiency due to TNFLTα deletion by bone marrow transplantation. Mol Med. 1996;2:247–255. [PMC free article] [PubMed] [Google Scholar]
- 18.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 19.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature (Lond) 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 20.Stuber E, Strober W. The T cell–B cell interaction via Ox40-OX40L is necessary for the T cell–dependent humoral immune response. J Exp Med. 1996;183:979–989. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torcia T, Bracci-Laudiero L, Lucibello M, Nencioni L, Labardi D, Rubarteli A, Cozzolino F, Aloe L, Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 22.Worm M, Geha RS. CD40 ligation induces lymphotoxin α gene expression in human B cells. Int Immunol. 1994;6:1883–1890. doi: 10.1093/intimm/6.12.1883. [DOI] [PubMed] [Google Scholar]
- 23.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]