Abstract
Interleukin (IL)-10 is a pleiotropic cytokine which inhibits a broad array of immune parameters including T helper cell type 1 (Th1) cytokine production, antigen presentation, and antigenspecific T cell proliferation. To understand the consequences of altered expression of IL-10 in immune models of autoimmune disease, the response to infectious agents, and the response to tumors, we developed transgenic mice expressing IL-10 under the control of the IL-2 promoter. Upon in vitro stimulation, spleen cells from unimmunized transgenic mice secrete higher levels of IL-10 and lower amounts of IFN-γ than do controls, although no gross abnormalities were detected in lymphocyte populations or serum Ig levels. Transfer of normally pathogenic CD4+ CD45RBhigh splenic T cells from IL-10 transgenic mice did not cause colitis in recipient severe combined immunodeficiency mice. Furthermore, co-transfer of these transgenic cells with CD4+ CD45RBhigh T cells from control mice prevented disease. Transgenic mice retained their resistance to Leishmania major infection, indicating that their cell-mediated immune responses were not globally suppressed. Lastly, in comparison to controls, IL-10 transgenic mice were unable to limit the growth of immunogenic tumors. Administration of blocking IL-10 mAbs restored in vivo antitumor responses in the transgenic mice. These results demonstrate that a single alteration in the T cell cytokine profile can lead to dramatic changes in immune responses in a manner that is stimulus dependent. These mice will be useful in defining differences in inflammatory conditions and cellular immunity mediated by IL-10.
Cytokines are secreted upon activation of immune cells and are important in regulating immune responses. Mouse CD4+ T cells can be divided into at least two subsets. Although Th1 lymphocytes promote cellular immunity by producing IL-2 and IFN-γ, Th2 lymphocytes produce IL-4, -5, and -10, leading to augmented humoral immunity and suppression of cellular immune responses (1). IL-10 was first characterized as a product of Th2 clones that could inhibit the synthesis of cytokines, such as IFN-γ, by Th1 clones (2). This inhibition of Th1 activity can lead to an alteration in the Th1–Th2 balance, although IL-10 does not directly promote Th2 development (3, 4). Most studies indicate that IL-10 acts on T cells indirectly by reducing the expression of costimulatory molecules and cytokines by APC, including macrophages and dendritic cells (5–8). By this mechanism, IL-10 can inhibit IL-12 production during a primary antigen stimulus, which in turn inhibits IFN-γ secretion and subsequently prevents the development of Th1 cells and a cell-mediated immune response. IL-10 is not expressed exclusively by Th2 cells, but also by other T cells, B lymphomas, Ly-1 B cells, keratinocytes, activated macrophages, and mast cell lines (3, 9). Consistent with this broader expression pattern, IL-10 was found to have additional activities including costimulation of thymocyte and mast cell proliferation (3, 9).
Most research focused on investigating the role of IL-10 in cell-mediated and humoral immunity has involved administering neutralizing antibodies or large amounts of IL-10 to experimental animals or cultured cells (3, 9). While these studies are helpful in broadly defining the function of IL-10, it is difficult to regulate cytokine dose and timing by these means. To determine the effects of an altered pattern and increased IL-10 synthesis in vivo, we generated IL-10 transgenic mice in which mouse IL-10 is driven by the T cell– specific human IL-2 promoter. The T lymphocytes that synthesize transgene-derived mouse IL-10 in this model should include the precursors of polarized Th1 and Th2 cells, as well as any IL-2–producing Th1 cells that can develop in these mice. The transgenic mice were used to characterize the role of IL-10 in vivo in an autoimmune disease model, in the response to an infectious agent, and in the rejection of tumor cells.
Materials and Methods
Mice.
The human IL-2 promoter–enhancer region from −567 to +54, relative to the transcriptional start site, was cloned upstream of the mouse IL-10 genomic sequence. The mouse IL-10 sequence used in the construct corresponds to positions 1,568– 6,879 of a 7.2-kb BglII fragment, in which position 1,568 represents nucleotide 16 of the cDNA (10). Transgenic mice were made by standard methods at the University of California at Los Angeles Transgenic Mouse Core Facility (Los Angeles, CA). The construct was injected into eggs from (C57BL/6 × C3H)F1 females mated to C57BL/6 males, and transgenic founders were backcrossed onto the C57BL/6 background >5 times. Four transgenic lines were initially characterized and two were selected for this study, lines 9 and 14. Presence of the transgene was confirmed using Southern blot analysis with the Genius nonradioactive detection kit, which uses random-primed probes labeled with digoxigenin-dUTP (Boehringer Mannheim, Indianapolis, IN). C57BL/6 mice, from stock obtained from Simonsen Laboratories (Gilroy, CA), and the transgenic mice lines were bred at our facility in the University of California at Los Angeles vivarium. The mice are kept in specific pathogen-free conditions and housed in microisolator cages. C57BL/6 scid/scid (SCID) mice are from Jackson Laboratory (Bar Harbor, ME) and they are kept in a separate pathogen-free facility with filtered air flow and autoclaved food and water.
Cell Culture.
Spleen cells were depleted of red blood cells by hypotonic lysis, and suspensions of splenocytes and thymocytes were cultured in RPMI supplemented with 10% FCS (JRH Biosciences, Lenexa, KS), nonessential amino acids (Irvine Scientific, Santa Ana, CA), sodium pyruvate (Irvine Scientific), penicillin, streptomycin, l-glutamine (Irvine Scientific), and 2-mercaptoethanol. For cytokine detection, cells were plated out on 24-well plates that were coated with 10–15 μg/ml anti-CD3ε (2C11) at a concentration of 106 cells/well. Supernatants from the cultures were harvested at 72 h, pooled, and used in ELISA.
Cytokine Detection.
Cytokine levels in culture supernatants were detected by a standard two-site sandwich ELISA for cytokines as described in the PharMingen (San Diego, CA) manual, except for a few alterations. High binding enzyme-linked immunoassay/radioimmunoassay Costar (Cambridge, MA) plates were coated overnight with the following antibodies from PharMingen: anti–mouse IL-10 (JES-2A5, 4 μg/ml) and IFN-γ (R4-6A2, 2 μg/ ml). Bound cytokine was detected with the following biotinylated antibodies: anti–mouse IL-10 (SXC-1, 1 μg/ml) and IFN-γ (XMG1.2, 1 μg/ml). Horseradish peroxidase (CALTAG Labs., South San Francisco, CA) and 2,2-azino-di(3-ethylbenzthiazoline sulfonate) substrate reagents (Kirkegaarde & Perry Labs., Gaithersburg, MD) were used for visualization in a spectrophotometer. Samples were assayed in triplicate in most cases, and were quantitated by comparison with standard curves of purified recombinant cytokines (PharMingen).
Serum Ig Analysis.
Serum samples were assayed for Ig concentration by a two-site sandwich ELISA as described, with some minor alterations (PharMingen). For coating, unconjugated antiIgG1 (G1-6.5, 8 μg/ml) and anti-IgG2a (R19-15, 2 μg/ml) were used. Detection for IgG1 and IgG2a was done using a κ light chain antibody conjugated to biotin (R8140, 2 μg/ml, 1 μg/ml, respectively) and visualized as described for cytokine ELISAs. IgG and IgM ELISAs are described in detail elsewhere (11). Samples were assayed in triplicate and were quantitated by comparison with the recommended Ig standards.
Flow Cytometry.
For analysis, purified cells from the spleen and thymus were stained with monoclonal antibodies to CD4 (L3T4) conjugated to PE or biotin, biotin-CD8α (Ly-2), PECD45RB/220 (RA3-6B2), biotin-IgM (R6-60.2) (all from PharMingen), and Streptavidin-Tri-Color™ (CALTAG). Flow cytometric analysis was performed on a FACScan® flow cytometer (Becton Dickinson, San Jose, CA) in the University of California at Los Angeles Jonsson Cancer Center Flow Cytometry Core Facility. Cells were identified as lymphocytes by gating based on forward and side scatter profiles. Between 3,000 and 5,000 gated events were collected and analyzed using CellQuest software (Becton Dickinson). Sorting for CD4+ CD45RBhigh populations used spleen cells stained with PE-CD45RB (16A) and FITCCD4. Cells were sorted using a FACstar Plus® cell sorter in the Jonsson Cancer Center Flow Cytometry Core Facility and Lysis II software (Becton Dickinson). Cells were selected for being positive for CD4 and high for CD45RB, the brightest staining 40% of the CD4+, CD45RB positive cells. Sorted populations were >98% pure upon postsort analysis.
Colitis Induction.
200 μl of 1× PBS containing 4–5 × 105 sorted CD45RBhigh CD4+ cells from IL-10 transgenic or control mice were injected intraperitoneally into three to four C57BL/6 SCID mice aged 6–12 wk. Weight gain/loss, as a percentage of original starting weight, was scored weekly for 8–12 wk. For statistical significance, regression analysis was performed comparing data from recipients of either IL-10 transgenic or nontransgenic T cells. For co-transfer studies, 2 × 105 CD4+ CD45RBhigh T cells from IL-10 transgenic mice were mixed with 4 × 105 cells from the corresponding population from nontransgenic littermates, injected into SCID recipients, and the recipient mice were evaluated for colitis as described above. For preparation of tissue for histopathologic analysis, ∼3-mm segments of the distal large intestine were removed and fixed in a solution of 10% formalin. Fixed tissues were embedded in paraffin and sections were prepared and stained with hematoxylin and eosin. Sections were graded in a blinded fashion by the same pathologist (S. Binder). A scoring system, described previously (12), was established to compare the histopathologic changes that occurred in the intestine as a result of transferring the CD45RBhigh CD4+ lymphocytes. The following parameters weare used: (a) degree of inflammatory infiltrate in the lamina propria was scored 0–3, (b) mucin depletion 0–3, (c) reactive epithelial hyperplasia/atypia, 0–3, (d) number of intraepithelial lymphocytes in the epithelial crypts 0–3, and (e) number of inflammatory foci/10 high-powered fields examined 1–3. Severity of the inflammatory changes was based on the sum of the scores of these parameters with a higher score indicating a greater inflammatory change.
Leishmania Infection.
4–6 mice/group were infected in the hind footpads with 2 × 105 metacyclic promastigotes of Leishmania major (strain WHOM/IR/-/173), and the size of the local lesion monitored using a metric caliper. Parasite burden in the footpad was determined at the end of the experiment by limiting dilution (13). In brief, serial 10-fold dilutions of homogenates from infected footpads were cultured in triplicate and examined after 2 wk for the presence of motile promastigotes using inverted microscopy. Data were expressed as the mean titer (log10) of the last positive dilution of footpad homogenate. Cytokine production by individual lymphocytes after L. major infection was assessed using an ELISPOT assay as described previously (14). Single-cell suspensions were prepared from popliteal LN and distributed in duplicate aliquots to 96-well plates coated with the appropriate anticytokine antibody. Cytokine-secreting cells were detected using biotinylated anticytokine antibodies and streptavidin alkaline phosphatase. Color was developed using 5-bromo-4-chloro-3-indolyl phosphate in 0.1 M 2-amino-2-methyl-1-propanol buffer suspended in agarose. After solidification, individual blue spots were counted using inverted microscopy.
Tumor Challenge.
Lewis lung (3LL) carcinoma cells were cultured in RPMI supplemented as described in cell culture methods. Before injection, cells were trypsinized and washed twice in 1× PBS. To establish the tumors in vivo, groups of 9–14 mice were injected subcutaneously with 5 × 105 3LL cells. Cross-sectional diameter measurements were taken with calipers and tumor growth recorded daily. For antibody blocking studies, groups of 3–4 mice were injected with 5 × 105 3LL cells. 1.5 mg of anti– IL-10 antibody JES-2A5 or control isotype matched antibody GL113-5E7 were administered intraperitoneally 24 h before and 4 d after tumor inoculation.
Results
Lymphocytes from IL-10 Transgenic Mice Secrete Increased Levels of IL-10.
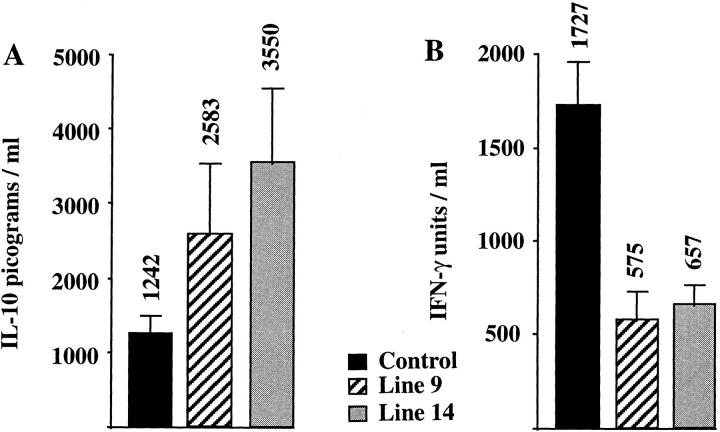
Because Thp and Th0 precursor cells synthesize IL-2 upon activation, the IL-2 promoter in the transgenic construct should permit T cell–specific expression of IL-10 from the earliest stages of T cell stimulation (15). IL-2 promoter fragments have been used previously to direct gene expression by activated T cells in transgenic mice (16–19). The promoter fragment we used is almost identical to that used in another transgenic mouse model, in which T cell–specific expression of lacZ was obtained (19). Furthermore, transient transfectants of Jurkat cells bearing this construct could be induced to synthesize IL-10 after stimulation with phorbol ester and calcium ionophore (Wei, S.H.-Y., and K.W. Moore, unpublished data). To assess the effect of the transgene on cytokine production by lymphocytes, IL-10 transgenic mice were analyzed for cytokine levels. IL-10 was not detectable in the serum of unimmunized transgenic mice by ELISA. Splenocytes from line 9 or line 14 IL-10 transgenic and nontransgenic littermates were stimulated with polyvalent anti-CD3ε mAb, and the culture supernatants were used in ELISAs to measure IFN-γ and IL-10. As seen in Fig. 1, A and B, spleen cells from IL-10 transgenic mice produce up to three times more IL-10 and approximately threefold reduced levels of IFN-γ, when compared to control littermates. Two other lines of IL-10 transgenic mice gave similar increases in IL-10 and decreases in IFN-γ levels (Hagenbaugh, A., data not shown). IL-4 could not be detected from the cultures of stimulated splenocytes from either transgenic mice or nontransgenic littermates, suggesting that the IL-10 transgene did not cause a great increase in Th2 activity. IL-4 could be detected from thymocytes cultured in parallel, providing a positive control for the T cell stimulation and IL-4 ELISA (Hagenbaugh, A., data not shown). These data indicate that IL-10 secretion by cells from the transgenic mice leads to a decrease of the prototypical Th1 cytokine IFN-γ.
Figure 1.
Altered cytokine secretion in IL-10 transgenic mice. Spleen cells from two lines of IL-10 transgenic mice and control littermates were isolated and stimulated on anti-CD3 coated plates. Supernatants were tested for the presence of IL-10 (A) and IFN-γ (B) by ELISA. Results are represented as the mean ± SE, with the mean indicated above the error bar. 3–5 mice were tested in each group.
IL-10 Transgenic Mice Have Normal T and B Lymphocyte Development.
To determine if altered IL-10 secretion leads to abnormal lymphocyte development or activity in transgenic mice, we analyzed lymphoid organs for cell number and phenotype. As summarized in Table 1, flow cytometry profiles from the spleen and thymus of line 9 IL-10 transgenic mice show no changes in the percentages of CD4+, CD8+, or double-positive T cell subsets. Cell numbers for spleen and thymus preparations were normal. In addition, B cell numbers were not altered, as B220 plus surface IgM profiles show no difference between IL-10 transgenic mice and littermate controls. Similar data were obtained from line 14 IL-10 transgenic mice (Hagenbaugh, A., data not shown).
Table 1.
>T and B Lymphocyte Differentiation Is Normal in IL-10 Transgenic Mice
| Mouse type | Cell no. | CD4+ | CD8+ | CD4+/CD8+ | B220/IgM+ | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thymus | % | % | % | % | ||||||
| Control No. 1 | 1 × 108 | 9.8 | 2.6 | 83.7 | – | |||||
| Control No. 2 | 1.2 × 108 | 10.9 | 2.6 | 82.7 | – | |||||
| IL-10 Tg No. 1 | 9.8 × 107 | 10.4 | 3.2 | 82.1 | – | |||||
| IL-10 Tg No. 2 | 1.6 × 108 | 15.3 | 3.4 | 77.4 | – | |||||
| Spleen | ||||||||||
| Control No. 1 | 4.8 × 107 | 26.6 | 17.5 | <1 | 60.8 | |||||
| Control No. 3 | 5.5 × 107 | 22.8 | 9.7 | <1 | 61.8 | |||||
| Control No. 4 | 5.4 × 107 | 19.5 | 14.4 | <1 | 60.3 | |||||
| IL-10 Tg No. 1 | 3.0 × 107 | 22.8 | 13.2 | <1 | 62.8 | |||||
| IL-10 Tg No. 2 | 3.0 × 107 | 24.9 | 13.2 | <1 | 58.4 | |||||
| IL-10 Tg No. 3 | 6.5 × 107 | 16.5 | 10.3 | <1 | 63.7 | |||||
| IL-10 Tg No. 4 | 6.5 × 107 | 23.4 | 14.6 | <1 | 50.5 |
Cells from spleen and thymus of line 9 IL-10 transgenic (Tg) mice were prepared, counted, and stained with the various cell-surface markers. Stained cells were analyzed by flow cytometry as described in Materials and Methods. Values are given as percentages of total gated lymphocyte populations, based on light scatter. –, not done.
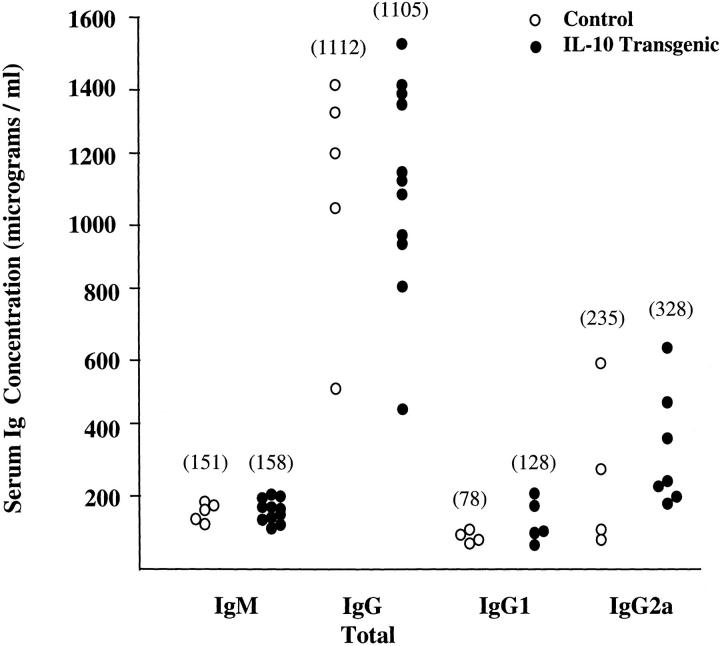
Because Th2 cytokines are involved in class switching of antibodies to producte of the ε and γ1 heavy chains, IL-10 transgenic mice were analyzed for serum Ig isotypes. Serum samples from IL-10 transgenic and littermate control mice were used in ELISAs for total IgG, IgM, IgG1, IgG2a, and IgE. The levels of total IgG and IgM were not significantly different between IL-10 transgenic and control mice, with the averages for both IgG and IgM virtually identical between the two groups (Fig. 2). There was a slight increase in the serum levels of both IgG1 and IgG2a isotypes in the IL-10 transgenic mice, which was not significant. IgE could not be detected by ELISA in the serum of either sets of mice (Hagenbaugh, A., data not shown). Thus, altered IL-10 expression in the IL-10 transgenic mice does not detectably effect Th2-specific class switching.
Figure 2.
Ig levels are not altered in IL-10 transgenic mice. Serum samples from line 9 IL-10 transgenic (filled circles) and control littermate (open circles) mice were assayed for the indicated Ig isotypes by ELISA. The group of mice analyzed for IgM and total IgG was separate from the group used for IgG1 and IgG2a analysis. The average concentration in micrograms per milliliter for each isotype is indicated in parentheses. Each symbol represents data from one individual mouse.
IL-10 Transgenic Spleen Cells Prevent Colitis Induction.
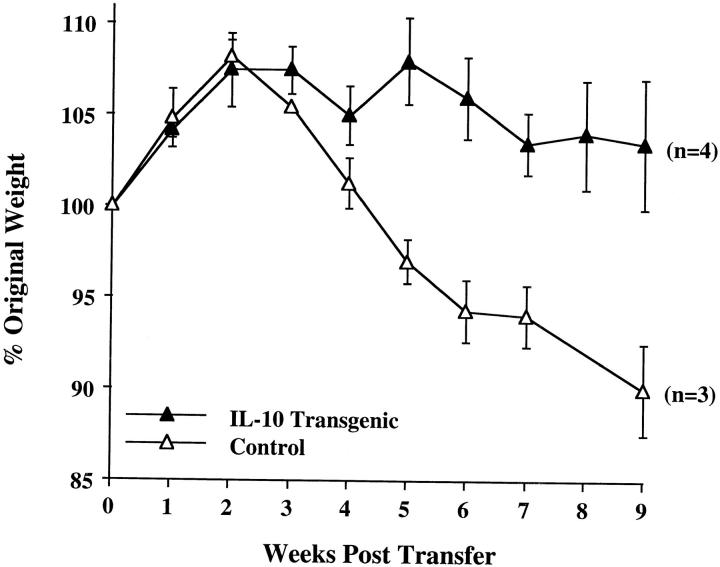
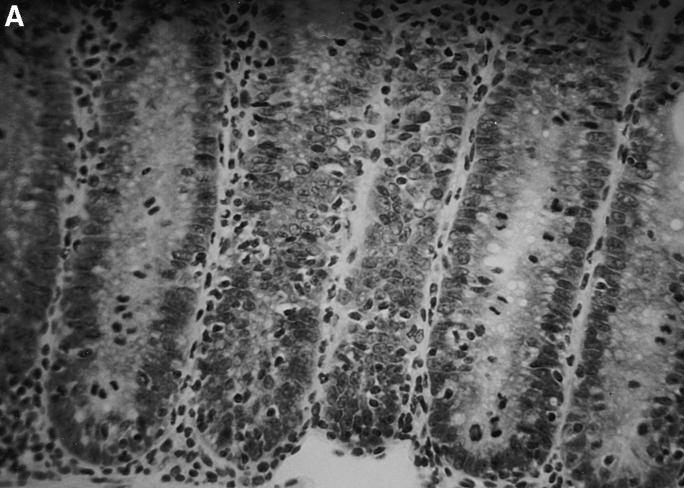
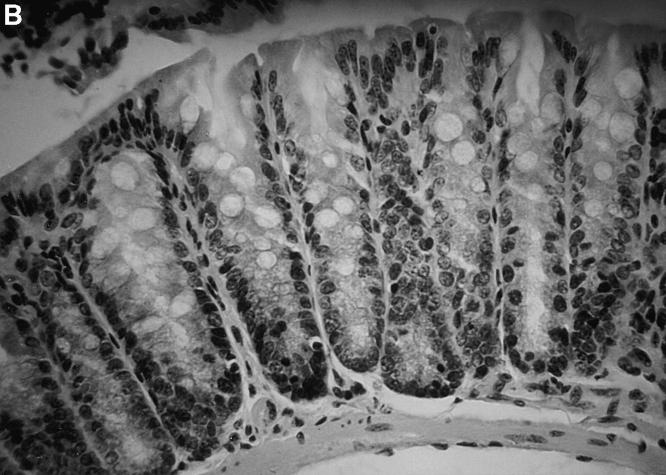
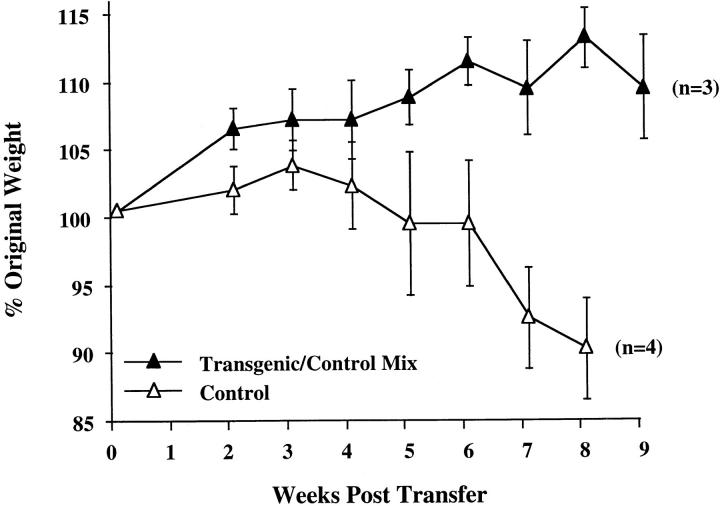
To determine the effect of altered IL-10 expression in the transgenic mice on Th1 cytokine synthesis during an in vivo immune response, we investigated a colitis model dependent upon the secretion of IFN-γ and TNF-α for pathogenesis. Transfer of CD4+ CD45RBhigh splenic T cells to SCID or RAG-2−/− immune-deficient recipients leads to a wasting disease that is characterized by Th1 cytokine release and inflammation of the large intestine, which might be similar to inflammatory bowel disease in humans (20–23). Recipients of control CD4+ CD45RBhigh splenic T cells followed a typical course of disease, losing weight at about 4 wk and remaining sick for the duration of the study (Fig. 3). These mice required killing by 9 wk after transfer, as they developed a hunched appearance and piloerection of the coat. By contrast, the recipients of CD4+ CD45RBhigh T cells from the IL-10 transgenic mice gained weight early on, and were able to maintain their weight for the entire experiment (Fig. 3). No other signs of illness were seen in recipients of T cells from the transgenic mice throughout the course of the study. Histological analysis indicates that the large intestines of recipients of CD4+ CD45RBhigh T cells from nontransgenic littermates have the typical hallmarks of inflammation of this model, consisting of lymphocyte infiltration, epithelial hyperplasia, and loss of goblet cells (Fig. 4 A). The recipients of IL-10 transgenic T lymphocytes have virtually normal large intestines (Fig. 4 B). The average histological scores were evaluated as described previously (12) and in Materials and Methods. For the distal colon of recipients of nontransgenic T cells, the score was 13.5 out of a possible 15 (n = 4). The score was 4.5 for the recipients of IL-10 transgenic T cells (n = 3), which is similar to that of normal mouse distal colon (12). Preparations of the small and large intestinal lamina propria lymphocytes and intraepithelial lymphocyte populations of the SCID recipients revealed numbers of IL-10 transgenic derived lymphocytes comparable to that after transfer of nontransgenic T cells (Hagenbaugh, A., data not shown). These data indicated that the CD4+ CD45RBhigh T cells from IL-10 transgenic mice were not impaired in their ability to colonize the SCID intestine.
Figure 3.
CD4+ CD45RBhigh cells from IL-10 transgenic mice fail to induce colitis in SCID recipients. 4–5 × 105 FACS® sorted CD4+ CD45RBhigh cells from IL-10 transgenic or control littermate mice were injected into SCID mice. Weight gain/loss was scored weekly for 9 wk. Values are shown as a percentage of original weight at day 0, the time of cell injection. Number of mice in each group is indicated in parentheses. Regression analysis of the two curves shown indicated the data to be highly significant, P = 0.0012. (Similar results were obtained using Rag-2−/− mice as T cell recipients.)
Figure 4.
Lack of intestinal inflammation in SCID recipients of CD4+ CD45RBhigh T cells from IL-10 transgenic mice. Tissues from the distal colon were stained with hematoxylin and eosin and scored as described in Materials and Methods. (A) Section of distal colon from a SCID recipient injected with control CD4+ CD45RBhigh T cells. Note the loss of goblet cells and disorganization of the epithelial cells. (B) Section of distal colon from a SCID recipient injected with IL-10 transgenic, CD4+ CD45RBhigh T cells. Original magnification, ×78 for both sections.
Previous studies have shown that co-transfer of CD4+ CD45RBlow T cells with the corresponding CD45RBhigh pathogenic population is protective, and these CD45RBlow T cells may prevent disease by production of cytokines that inhibit the development or function of Th1 and inflammatory cells (20, 22). To determine if CD4+ CD45RBhigh T cells from the IL-10 transgenic mice are merely incapable of causing disease, or if altered IL-10 secretion converts them to a suppressive population similar to CD45RBlow T lymphocytes, co-transfers of IL-10 transgenic and nontransgenic CD4+ CD45RBhigh splenic T cells were carried out. As can be seen from Fig. 5, there was no weight loss in cotransfer recipients, demonstrating that CD4+ CD45RBhigh T cells from the IL-10 transgenic mice can actively prevent colitis induction by pathogenic Th1 lymphocytes.
Figure 5.
Co-transfer of CD4+ CD45RBhigh T cells from IL-10 transgenic mice prevents colitis pathogenesis. 2 × 105 CD4+ CD45RBhigh T cells from IL-10 transgenic mice were co-injected with 4 × 105 cells of the same type from control mice into SCID recipients. The recipients were monitored weekly for weight loss as described in Fig. 3.
IL-10 Transgenic Mice are Resistant to L. Major Infection.
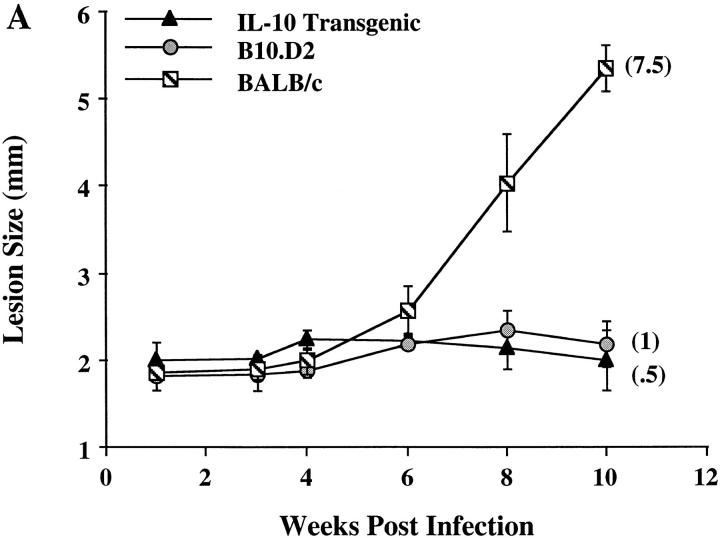
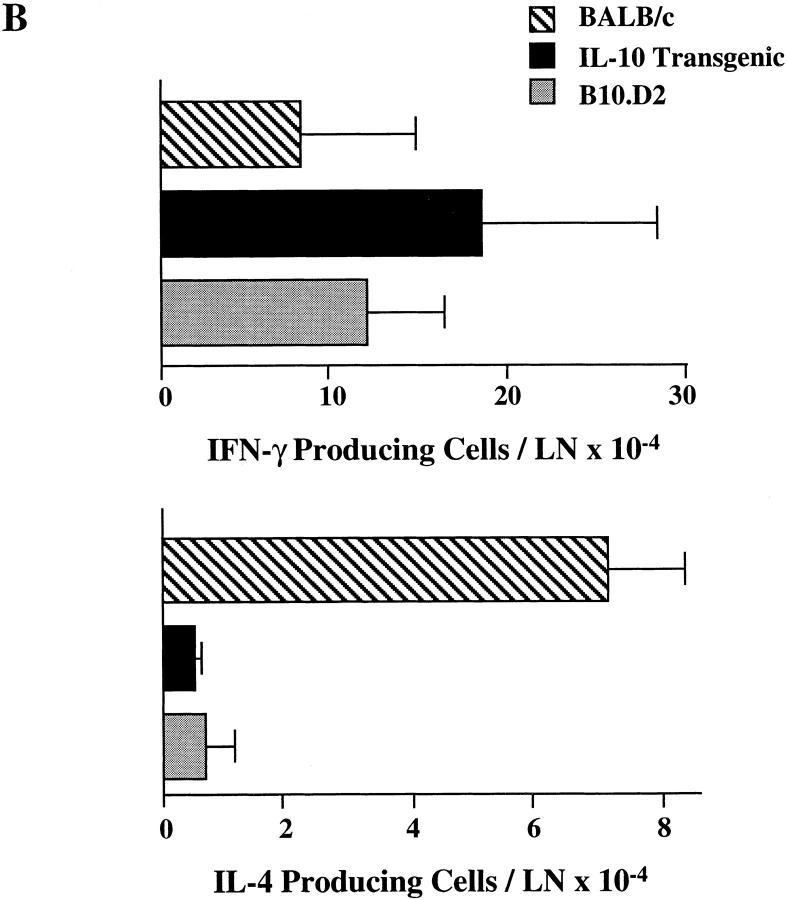
L. major infection of inbred mouse strains remains a wellcharacterized model for control of Th1 and Th2 differentiation in vivo. Mouse strains including C57BL/6 and B10.D2 mount a healing Th1 response to the parasite, whereas BALB/c mice mount a nonhealing Th2 response (24). IL-10 transgenic mice are on the healing C57BL/6 background. To determine if altered expression of IL-10 by T cells can affect this ability to mount a protective Th1 response, IL-10 transgenic, along with control B10.D2 and BALB/c mice, were infected with L. major. As assessed by lesion size and parasite burden after 10 wk, IL-10 transgenic mice controlled infection in a manner comparable to resistant B10.D2 mice (Fig. 6 A). As described previously, BALB/c mice were unable to clear the parasite. ELISPOT analysis of cytokines from peripheral lymph node cells from infected IL-10 transgenic mice were consistent with the differentiation of Th1 effector cells, in contrast with the differentiation of Th2 effector cells that occurred in the susceptible BALB/c mice (Fig. 6 B).
Figure 6.
Resistance to L. major infection in IL-10 transgenic mice. Groups of four to six IL-10 transgenic, B10.D2, and BALB/c mice were inoculated in the rear footpads with promastigotes of L. major. (A) Data represent the mean lesion size ± SE for the indicated groups of mice throughout infection. Parasite burden, representing the log10 of viable Leishmania in cultured footpad homogenates after 10 wk, is indicated in parentheses (see Materials and Methods). (B) Popliteal LN cells draining the site of infection were used in ELISPOT assays for the detection of IFN-γ and IL-4. Data represent the number of cytokine-producing cells per LN ± SE for designated mice after 10 wk of infection.
Antitumor Reactivity is Decreased in IL-10 Transgenic Mice.
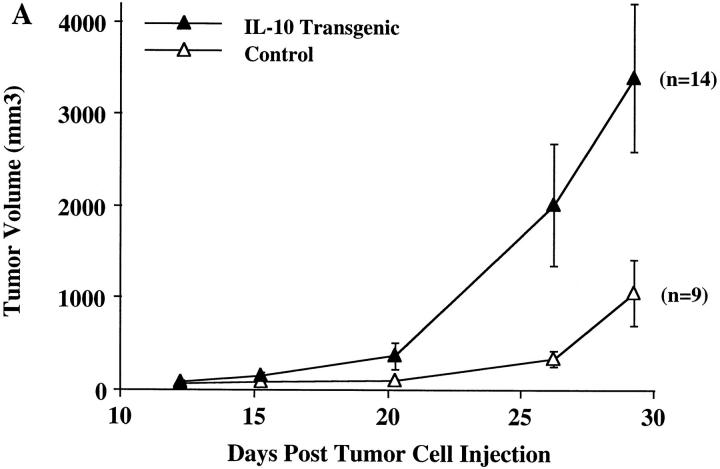
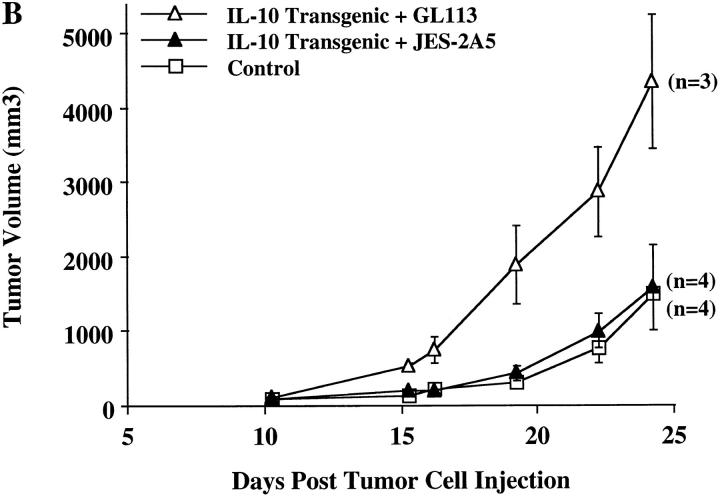
It has been demonstrated that IL-10 is present in the local tumor environment in nonsmall cell lung carcinomas (NSCLCs)1 and other tumors, where this cytokine might provide a mechanism for evasion of the local cell-mediated immune response (25, 26). The mouse NSCLC 3LL has a well-defined peptide antigen (27) which can be recognized by CD8+ T cells which are an important component of the antitumor response. To further elucidate the role of local IL-10 production in tumor rejection, IL-10 transgenic mice were injected with 3LL cells and monitored for tumor formation. In comparison to control littermates, IL-10 transgenic mice developed relatively large tumors (Fig. 7 A). To assure that the effect observed was due to direct action by IL-10, we used a monoclonal antibody known to block IL-10 action. Injection of the JES-2A5 anti–IL-10 antibody at 24 h before and 4 d after tumor cell injection enabled the IL-10 transgenic mice to control the growth of transplanted tumors comparable to their nontransgenic littermates (Fig. 7 B). Injection of the control isotype-matched GL113 antibody had no effect on the susceptibility of the IL-10 transgenic mice to 3LL tumor cells (Fig. 7 B). This indicates that the production of IL-10 by infiltrating lymphocytes prevents the development of an effective immune response against this tumor.
Figure 7.
Uncontrolled tumor growth in IL-10 transgenic mice. (A) Groups of 9–14 IL-10 transgenic and control littermate mice were injected subcutaneously with 3LL carcinoma cells. Tumor growth was recorded on a daily basis using metric calipers. Data are shown as the mean tumor volume for each group of mice ± SE at each indicated time point. This is representative of one of three experiments, which all yielded similar results. (B) 1.5 mg of IL-10 neutralizing antibody, JES-2A5, or control isotype antibody, GL113-5E7, were administered intraperitoneally at 24 h before and 4 d after 3LL cell injection to IL-10 transgenic mice. Control littermates were injected with 3LL cells as a negative control for tumor growth. Groups of three to four mice were analyzed. Data is shown as mean tumor volume ± SE.
Discussion
We have described an in vivo model to assess the role of IL-10 in the regulation of an immune response leading to autoimmunity, as well as protective immune responses. By using the human IL-2 promoter to drive mouse IL-10 cytokine expression, transgene-derived IL-10 produced only transiently in response to T cell activation. At the same time, these transgenic mice have an increased population of T cells that make IL-10 and should produce IL-10 earlier in the immune response. We predicted that activated Th1 type T cells in the IL-10 transgenic mice would downregulate their own activity, including IL-10 secretion from the transgene. Consistent with our expectations, polyclonally activated splenic T cells from transgenic mice secreted two- to fourfold increased levels of IL-10, as well as decreased levels of IFN-γ. The IL-10 transgenic mice were otherwise macroscopically normal, and did not exhibit any defects in growth and development. The relatively subtle alterations in cytokine production observed after polyclonal T cell activation in the transgenic mice may reflect the activity of naive T cells, which normally are capable of making only low amounts of IL-2 (15). These subtle alterations, however, led to a dramatic decrease in the ability to mount certain immune responses under several circumstances described below.
Consistent with their overall good health and development, the immune system of the IL-10 transgenic mice is by most measures not significantly different from the control littermates. T and B cell numbers and phenotype were normal. Although IL-10 is a potent downregulator of Th1 cytokine production, IFN-γ synthesis is not eliminated in the IL-10 transgenic mice. No major differences were apparent in the serum Ig profiles either. We therefore conclude that the amount of IL-10 synthesized in these mice is not sufficient to cause either a complete suppression of Th1 cytokines or immune dysfunction in unimmunized mice.
It is well documented that transfer of the CD4+ CD45RBhigh T cell population of normal mice into SCID or RAG-2−/− mice causes severe inflammation in the large intestine, mediated in part by secretion of TNF-α and IFN-γ (21, 23). We have shown that transfer of this same population of T cells from the IL-10 transgenic mice does not cause the wasting condition that accompanies this disease. Histological analysis confirmed that the recipients of IL-10 transgenic T cells have few of the hallmarks of intestinal inflammation which are typical of this model. We hypothesize that production of IL-10 alters cytokine secretion from the CD4+ CD45RBhigh T cells, and thereby inhibits their ability to cause disease. When CD4+ CD45RBhigh T cells from the IL-10 transgenic mice were co-transferred with the corresponding pathogenic population from control animals, colitis pathogenesis was prevented. These data suggest that the altered IL-10 secretion by the transgenic T cells can control the production of inflammatory cytokines by other cells. In other studies, IL-10−/− mice spontaneously developed colitis (28), and transfer of unfractionated lamina propria lymphocytes from IL-10−/− mice into RAG-2−/− recipients gave rise to severe inflammation of the colon (29). Together, these data indicate that T cell– derived IL-10 is critical for regulating the cytokine balance in the intestine that may lead to or prevent colitis pathogenesis.
Our data are consistent with results from a previous report in which transfer of CD4+ CD45RBhigh cells into SCID mice was accompanied by daily administration of recombinant IL-10 (21). This treatment prevented the development of colitis and reduced the amount of IFN-γ in the colon. However, the effects were short lived since these mice developed colitis indistinguishable from controls 4 wk after the last injection of rIL-10. The transient nature of the effect of rIL-10 treatment could be due to the systemic distribution and rapid metabolism of the cytokine. These problems would not have been expected in the transgenic mouse model we describe here, and consistent with this, disease was not observed in SCID recipients of IL-10 transgenic T cells up to 3 mo after transfer. In contrast to these results, it was shown that co-transfer of CD4+ CD45RBhigh and CD45RBlow cells into SCID mice, along with anti–IL-10 blocking antibody, did not abolish the protective effect of CD4+ CD45RBlow T cells (22). Because, in our model, Th1-produced IL-10 was sufficient to convert pathogenic T cells to active inhibitors of colitis in co-transfer, we conclude that although the CD4+ CD45RBlow T cells may use IL-10 to suppress colitis, other IL-10–independent pathways exist in CD4+ CD45RBlow T cells that may be capable of preventing disease.
Control of L. major infection in mice is mediated by IFN-γ–producing Th1 cells that promote a cell-mediated immune response. IL-10 transgenic mice on the healing C57BL/6 genetic background developed Th1 responses to L. major that were comparable to those in resistant B10.D2 mice. Thus, IL-10 secretion in these transgenic mice neither skewed cytokine production from T cells, nor inhibited cell-mediated immune responses to the parasite. It is not known why Th1 type cell-mediated responses are diminished in the colitis model but not in response to L. major infection. The intracellular forms of the parasite induce high levels of IL-12 (30), and the amount of transgene-derived IL-10 produced under the control of the IL-2 promoter may not be sufficient to overcome the activation of Th1 cells by the pathogen. Reconstitution of SCID mice with L. major-specific CD4+ CD45RBlow T cells conferred susceptibility to disease that could be reversed by anti–IL-4, but not anti–IL-10 antibodies (22), consistent with studies in both wild-type (31) and IL-4–deficient mice (32). Although IL-10 can inhibit Th1 development and proliferation, IL-4 is critical for the differentiation of Th2 cells (33– 36). The lack of elevated IL-4 levels in the IL-10 transgenic mice after infection presumably underlies the inability of the transgenic mice to develop progressive disease. In summary, the data are consistent with previous information indicating that IL-10 alone is not a key regulator of host responses to L. major.
Although tumors may express antigens, they often escape the host's immune surveillance. It is well known that tumor cells have the capacity to mediate immunosuppression, in part, through the elaboration of immune inhibitory cytokines (25, 26). Not only may tumors directly suppress immune responses, but they may also induce host immune cells to release immunosuppressive cytokines. For example, human NSCLC cells have been shown to cause a 10–100fold induction of lymphocyte-derived IL-10 (37). Thus, because the IL-10 transgenic mice used in these studies may parallel the apparent defect in response to lung cancer in human patients, we hypothesized that these mice would have a limited capacity to control tumor growth. For these studies we used an immunogenic murine lung cancer, 3LL. This tumor has a mutant connexin 37 gap junction protein, and a peptide containing the mutated sequence is recognized by anti-3LL CTL (27). Antitumor CTL are protective against 3LL tumor challenge, suggesting that the cytotoxic responses of CD8+ T lymphocytes are important in limiting tumor growth. To determine if lymphocyte-derived IL-10 interfered with the host antitumor response, IL-10 transgenic and control mice were challenged with 3LL tumor cells. IL-10 transgenic mice were unable to control tumor growth, whereas control recipients showed tumor progression at a significantly slower rate. The inability of the IL-10 transgenic mice to limit 3LL tumor growth is due to the secretion of IL-10, as administration of anti–IL-10–blocking antibody reduced tumor growth in the IL-10 transgenic mice to that of control mice. These data demonstrate that the effect of the IL-10 transgene is due directly to IL-10 action. Previously, only viral, but not mouse, IL-10 was shown to be effective in preventing immune responses to melanomas and sarcomas (38). There are several ways in which altered expression of mouse IL-10 may have inhibited an effective antitumor immune response. These include the reduction of MHC class I expression (39) or costimulatory molecules (6), immune deviation (40), and induction of anergy in responding T cells (40, 41). Further experiments are required to characterize the mechanism for tumor unresponsiveness in IL-10 transgenic mice, and to determine if other Th1 or CD8+ T cell responses are likewise suppressed in these transgenic mice.
IL-10 previously has been implicated in the regulation of a wide variety of inflammatory and other immune responses (3, 9, 42), but many of the studies have relied upon the unphysiologic administration of exogenous IL-10. The transgenic mice described here provide an important new model for studying the role of T cell–derived IL-10 synthesis in vivo. These mice have been backcrossed extensively onto the C57BL/6 background, so that the IL-10 transgenic mice and their littermates are close to being isogenic strains. Furthermore, IL-10 synthesis is transient and related to T cell activation, thereby avoiding any potential toxic effects of either constitutive expression of IL-10, or very high levels of IL-10 over expression. Finally, evidence from two models, a pathogenic immune response leading to colitis and a beneficial immune response leading to tumor rejection, demonstrates that some, but not all, cell-mediated immune responses are profoundly altered in these transgenic animals. Recently, IL-10 transgenic mice that express cytokine under the control of the CD2 promoter have been described (43). Consistent with the results described here, these mice show some evidence of a suppressed Th1 type response, as they are more susceptible to infection with Mycobacterium tuberculosis. Additional experiments now underway are designed to determine the mechanism for decreased colitis pathogenesis and tumor cell rejection in the IL-10 transgenic mice.
Acknowledgments
We thank Drs. G.R. Crabtree and N. Arai for providing plasmids containing the human IL-2 promoter region, and Dr. J. Abrams for neutralizing anti–mouse IL-10 and isotype control monoclonal antibodies. We also thank Dr. Robert Modlin for critical reading of the manuscript, Katherine Williams for excellent assistance with the generation of the IL-10 transgenic mice, Quynh Le for technical support, and Dr. Hui Ying Yang for assistance with statistical analysis of the cell transfer studies.
This work is supported by National Institutes of Health grants DK 46763, AI 26918, and CA 71818 a grant from the Drown Foundation University of California at Los Angeles Frontiers in Science Program, Veteran's Administration Merit Research Funds, and the American Lung Association. A. Hagenbaugh is supported by National Institutes of Health training grant GM 07185. S. Sharma is supported by the Parker B. Francis Fellowship Program. S. Dubinett is a Career Investigator of the American Lung Association. D. Fowell is funded by the Juvenile Diabetes Foundation. R.M. Locksley is a Burroughs Wellcome Fund Scholar in Molecular Parasitology. DNAX Research Institute is supported by Schering-Plough Corporation.
Footnotes
1 Abbreviation used in this paper: NSCLC, nonsmall cell lung carcinoma.
References
- 1.Mosmann TR, Schumacher JH, Street NF, Budd R, O'Garra A, Fong TA, Bond MW, Moore KW, Sher A, Fiorentino DF. Diversity of cytokine synthesis and function of mouse CD4+T cells. Immunol Rev. 1991;123:209–229. doi: 10.1111/j.1600-065x.1991.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 2.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho AS, Moore KW. Interleukin-10 and its receptor. Ther Immunol. 1994;1:173–185. [PubMed] [Google Scholar]
- 4.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cellreceptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding L, Shevach EM. IL-10 inhibits mitogeninduced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 6.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 7.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–3822. [PubMed] [Google Scholar]
- 8.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 9.Moore KW, O'Garra A, de Waal R, Malefyt, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 10.Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992;148:3618–3623. [PubMed] [Google Scholar]
- 11.Tsao BP, Ohnishi K, Cheroutre H, Mitchell B, Teitell M, Mixter P, Kronenberg M, Hahn BH. Failed self-tolerance and autoimmunity in IgG anti-DNA transgenic mice. J Immunol. 1992;149:350–358. [PubMed] [Google Scholar]
- 12.Aranda R, Sydora BC, McAllister PL, Binder SW, Yang HY, Targan SR, Kronenberg M. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhighT cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 13.Corry DB, Reiner SL, Linsley PS, Locksley RM. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J Immunol. 1994;153:4142–4148. [PubMed] [Google Scholar]
- 14.Wang ZE, Reiner SL, Zheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon γ-deficient mice infected with Leishmania major. . J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 16.Minasi LE, Kamogawa Y, Carding S, Bottomly K, Flavell RA. The selective ablation of interleukin 2–producing cells isolated from transgenic mice. J Exp Med. 1993;177:1451–1459. doi: 10.1084/jem.177.5.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emmel EA, Verweij CL, Durand DB, Higgins KM, Lacy E, Crabtree GR. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science (Wash DC) 1989;246:1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- 18.Verweij CL, Guidos C, Crabtree GR. Cell type specificity and activation requirements for NFAT-1 (nuclear factor of activated T-cells) transcriptional activity determined by a new method using transgenic mice to assay transcriptional activity of an individual nuclear factor. J Biol Chem. 1990;265:15788–15795. [PubMed] [Google Scholar]
- 19.Brombacher F, Schafer T, Weissenstein U, Tschopp C, Andersen E, Burki K, Baumann G. IL-2 promoterdriven lacZ expression as a monitoring tool for IL-2 expression in primary T cells of transgenic mice. Int Immunol. 1994;6:189–197. doi: 10.1093/intimm/6.2.189. [DOI] [PubMed] [Google Scholar]
- 20.Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scidmice. Int Immunol. 1993;5:1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- 21.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 22.Powrie F, Correa-Oliveira R, Mauze S, Coffman RL. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med. 1994;179:589–600. doi: 10.1084/jem.179.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+T cells. J Exp Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 25.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–3853. [PubMed] [Google Scholar]
- 26.Kim J, Modlin RL, Moy RL, Dubinett SM, McHugh T, Nickoloff BJ, Uyemura K. IL-10 production in cutaneous basal and squamous cell carcinomas. A mechanism for evading the local T cell immune response. J Immunol. 1995;155:2240–2247. [PubMed] [Google Scholar]
- 27.Mandelboim O, Berke G, Fridkin M, Feldman M, Eisenstein M, Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature (Lond) 1994;369:67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- 28.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10–deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 29.Davidson NJ, Leach MW, Fort MM, ThompsonSnipes L, Kuhn R, Muller W, Berg DJ, Rennick DM. T helper cell 1–type CD4+T cells, but not B cells, mediate colitis in interleukin 10–deficient mice. J Exp Med. 1996;184:241–251. doi: 10.1084/jem.184.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti–interleukin 4 monoclonal antibody. Evidence for a T cell–dependent, interferon γ-independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K-H, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4–deficient Balb/c mice resist infection with Leishmania major. . J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopf M, Le Gros G, Bachmann M, Lamers MC, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature (Lond) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 34.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature (Lond) 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 35.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4–induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature (Lond) 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 37.Huang M, Sharma S, Mao JT, Dubinett SM. Non-small cell lung cancer-derived soluble mediators and prostaglandin E2 enhance peripheral blood lymphocyte IL-10 transcription and protein production. J Immunol. 1996;157:5512–5520. [PubMed] [Google Scholar]
- 38.Suzuki T, Tahara H, Narula S, Moore KW, Robbins PD, Lotze MT. Viral interleukin 10 (IL-10), the human herpes virus 4 cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J Exp Med. 1995;182:477–486. doi: 10.1084/jem.182.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda M, Salazar F, Petersson M, Masucci G, Hansson J, Pisa P, Zhang QJ, Masucci MG, Kiessling R. Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med. 1994;180:2371–2376. doi: 10.1084/jem.180.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L, Goillot E, Tepper RI. IL-10 inhibits alloreactive cytotoxic T lymphocyte generation in vivo. Cell Immunol. 1994;159:152–169. doi: 10.1006/cimm.1994.1304. [DOI] [PubMed] [Google Scholar]
- 41.Rohrer JW, Coggin JH. CD8 T cell clones inhibit antitumor T cell function by secreting IL-10. J Immunol. 1995;155:5719–5727. [PubMed] [Google Scholar]
- 42.Mosmann TR, Moore KW. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991;12:A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- 43.Murray PJ, Wang L, Onufryk C, Tepper RI, Young RA. T cell–derived IL-10 antagonizes macrophage function in mycobacterial infection. J Immunol. 1997;158:315–321. [PubMed] [Google Scholar]