Abstract
T cell–dependent humoral immune responses are initiated by the activation of naive B cells in the T cell areas of the secondary lymphoid tissues. This primary B cell activation leads to migration of germinal center (GC) cell precursors into B cell follicles where they engage follicular dendritic cells (FDC) and T cells, and differentiate into memory B cells or plasma cells. Both B cell migration and interaction with FDC critically depend on integrin-mediated adhesion. To date, the physiological regulators of this adhesion were unkown. In the present report, we have identified the c-met–encoded receptor tyrosine kinase and its ligand, the growth and motility factor hepatocyte growth factor/scatter factor (HGF/SF), as a novel paracrine signaling pathway regulating B cell adhesion. We observed that c-Met is predominantly expressed on CD38+CD77+ tonsillar B cells localized in the dark zone of the GC (centroblasts). On tonsil B cells, ligation of CD40 by CD40-ligand, induces a transient strong upregulation of expression of the c-Met tyrosine kinase. Stimulation of c-Met with HGF/SF leads to receptor phosphorylation and, in addition, to enhanced integrin-mediated adhesion of B cells to both VCAM-1 and fibronectin. Importantly, the c-Met ligand HGF/SF is produced at high levels by tonsillar stromal cells thus providing signals for the regulation of adhesion and migration within the lymphoid microenvironment.
Antigen-specific B cell differentiation, the process by which naive B cells develop into memory cells or plasma cells, requires multiple interactions of B cells with other cells, such as T cells and follicular dendritic cells (FDC)1, and with the extracellular matrix (ECM), that take place within distinct microenvironmental compartments of the lymphoid tissues (1–6). After their initial activation in the extrafollicular T cell (paracortical) area, germinal center (GC) founder cells migrate into B cell follicles where they initiate the formation of GCs (7, 8). Once in the germinal center (GC), the B cells first pass the dark zone where they undergo rapid clonal expansion and somatic hypermutation in their IgV genes (9–13). Mutated B cells then progress to centrocytes and move to the basal light zone of the GC. Here they reencounter antigen, presented as low levels of immune complexes on FDC, and undergo affinity selection (14–16). Whereas low-affinity mutants and autoreactive mutants die by apoptosis, high-affinity mutants internalize antigen and process it on their migration pathway to the apical light and outer zones of the GC. In these areas, the affinityselected B cells present antigen to antigen-specific GC T cells (17–19). Cognate T–B interaction results in expansion and Ig isotype switching of high-affinity B cells (20, 21), that mature into memory B cells or plasma cells and receive signals mediating their export from the lymphoid organ (1). Adhesion regulation, particularly regulation of lymphocyte integrin function, is believed to be fundamental to the control of cell migration and microenvironmental homing during this B cell differentiation process (22, 23).
Integrins are a widespread family of heterodimeric (αβ) transmembrane glycoproteins that can function as cell– ECM and cell–cell adhesion receptors (for review see reference 24). In the immune system they are involved at multiple levels, including interaction of lymphoid precursors with stromal cells during lymphopoiesis, lymphocyte homing, and antigen presentation. Importantly, adhesion receptors of the integrin family have recently been implicated in B cell differentiation. Integrins, specifically α4β1, were shown to be involved in adhesion and terminal differentiation of precursors during B-lymphopoiesis in the bone marrow in vitro (25, 26), and in B cell adhesion to FDC during GC reactions (27–29). In vivo experiments with α4null chimeric mice, confirmed a key role for this integrin in early B cell development (30). Together, these studies indicate that integrin mediated adhesion plays an important role in the control of several steps of B cell development, including migration and adhesion during antigen-specific differentiation. However, the physiological regulators of lymphocyte integrin activity during B cell differentiation remain unknown.
In a survey of the molecular pathways that might regulate B cell adhesion, we explored the possible role of the c-met-encoded receptor tyrosine kinase and its ligand hepatocyte growth factor/scatter factor (HGF/SF). The HGF/ SF–c-Met pathway has been shown to regulate growth, motility and morphogenesis of epithelial and endothelial cells (31–36), which requires tight regulation of adhesion and de-adhesion. Furthermore, this pathway mediates invasion and migration of tumor cells (37–39), a process reminiscent of lymphocyte migration. Here, we identify the HGF/SF–c-Met pathway as a novel molecular pathway in antigen-specific B cell differentiation, which is involved in the regulation of integrin-mediated B cell adhesion.
Materials and Methods
Antibodies.
Mouse monoclonal antibodies used were anti– c-Met, DO24 (IgG2a) (Upstate Biotechnology, Lake Placid, NY); anti-CD38, OKT-10 (IgG1) (American Type Culture Collection [ATCC], Rockville, MD); FITC-conjugated anti-CD38, HIT2 (IgG1) (Caltag Laboratories, Burlingame, CA); biotin-conjugated anti-CD38, HIT2 (IgG1) (Caltag); anti-β1 integrin (CD29), 4B4 (IgG1) (Coulter Immunology, Hialeah, FL); anti-α4 integrin (CD49d), HP2/1 (IgG1) (Immunotech, Marseille, France); anti-α5 integrin (CD49e), SAM-1 (IgG2b) (40) (a gift from A. Sonnenberg, NKI, Amsterdam, The Netherlands); anti-α4β7, Act-1 (IgG1) (41) (a gift from A. Lazarovits, University of Western Ontario, London, Canada); anti-ICAM-1 (CD54), RR1/1 (IgG1) (42) (a gift from T. Springer, Harvard University, Boston, MA); anti-HGF/SF, 24612.111 (IgG1) (R&D Systems, Abingdon, UK); anti-CD3, OKT-3 (IgG2a) (ATCC); anti-DRC-1, R4/23 (IgM) (DAKO, Glostrup, Denmark); anti-CD19, HD37 (IgG1) (DAKO); and anti-phosphotyrosine, PY-20 (IgG2b) (Affiniti, Nottingham, UK). Polyclonal antibodies used were rabbit anti–c-Met, C-12 (IgG) (Santa Cruz Biotechnology, Santa Cruz, CA); goat anti-HGF/ SF (R&D Systems); FITC-conjugated rabbit anti-IgD (DAKO); RPE-conjugated goat anti–mouse (Southern Biotechnology, Birmingham, AL); AP-conjugated goat anti–mouse (total, IgG1, or IgG2a) (Southern Biotechnology); biotin-conjugated rabbit anti– mouse (DAKO); biotin-conjugated goat anti–mouse (DAKO); biotin-conjugated rabbit anti–goat (Vector Laboratories, Burlingame, CA); HRP-conjugated goat anti–rabbit (DAKO); and HRPconjugated goat anti–rabbit (DAKO). In addition we used a rat monoclonal anti-CD77, 38.13 (IgM) (43) (provided by J. Wiels, Institute Gustave-Roussy, Villejuif, France); and RPE-Cy5-conjugated streptavidin (DAKO).
Cell Lines.
The epidermoid carcinoma cell line A431, the lung fibroblast cell line MRC-5, and the B cell lines Raji, Namalwa, Daudi, Ramos, JY, and Nalm-6 were obtained from ATCC and cultured in RPMI 1640 (Gibco BRL/Life Technologies, Paisley, UK) supplemented with 10% FCS (Integro, Zaandam, The Netherlands). The Burkitt's lymphoma line EB4B (44) was provided by R. Jefferis (University of Birmingham, Edgbaston, UK) and cultured in 10% FCS/RPMI 1640.
B Cell Isolation and Culturing.
B cells were isolated as described previously (29). Total B cell fractions were >97% pure as determined by FACS® analysis.
B cells were cultured in Iscove's medium (Gibco BRL/Life Technologies) containing 10% FCS, 0.5% BSA, 50 μg/ml human transferrin (Sigma, Bornem, Belgium) and 5 μg/ml bovine pancreas insulin (Sigma). Some media were supplemented with 50 ng/ml phorbol-12-myristate-13-acetate (PMA; Sigma).
For CD40 ligation, B cells were cultured on irradiated (7,000 rad) CD40L-transfected or, as a control, wild-type L cells (45) (provided by J. Banchereau, Schering Plough, Dardilly, France). In specific experiments, culture media were supplemented with either pansorbin cells of Staphylococcus aureus strain Cowan I (0.002%; Calbiochem Novabiochem, La Jolla, CA), rabbit anti–human Igcoated beads (2 μg/ml) (BioRad Laboratories, Hercules, CA), recombinant human IL-2 (100 U/ml) (Eurocetus, Amsterdam, The Netherlands), recombinant human Il-4 (100 U/ml) (Genzyme Diagnostics, Cambridge, MA), or recombinant human IL-6 (1,000 U/ml) (CLB, Amsterdam, The Netherlands).
T Cell Isolation and Culturing.
Tonsillar T cells were isolated as described for the B cell isolation, except that after the second Ficoll-Isopaque density gradient centrifugation the pellet was collected, washed, and resuspended in shock medium. The remaining B cells were removed by using a MACS magnetic cell separator (Miltenyi Biotec, Bergisch Gladbach, Germany) using anti-CD19. The T cell fraction was >98% pure as determined by FACS® analysis.
Stromal Cell Isolation and Culturing.
Tonsillar stromal cells were isolated as described (46). The cells were cultured in 100-mm petri dishes (Costar, Cambridge, MA) containing 10% FCS/RPMI 1640. After 4 d nonadherend cells were removed.
FDC Isolation and Culturing.
FDC were isolated as described (29). The cells were cultured in Iscove's medium containing 10% Fetal Clone I serum (HyClone Laboratories, Logan, UT). These FDC-enriched cell cultures contained 10–15% DRC-1–positive cells.
Transfections.
c-Met transfected Namalwa cells (Nammet) were obtained by electroporating Namalwa cells with the eukaryotic expression plasmid pA71d containing full-length c-met cDNA (a gift from G. Hartmann and E. Gherardi, University of Cambridge, Cambridge, UK). After 2 d in culture, transfectants were selected in culture medium containing 250 μg/ml hygromicin (Sigma). c-Met positive cells were sub-cloned by using a FACStarplus® flow cytometer (Becton Dickinson, Mountain View, CA).
Immunoprecipitation and Western Blot Analysis.
For analysis of tyrosine phosphorylation of the c-Met protein, cells were incubated overnight in serum-free RPMI 1640. Nammet or EB4B cells were incubated in serum-free RPMI 1640 in the presence or absence of 200 ng/ml HGF/SF (R&D Systems). After 5 min at 37°C the cells were solubilized in ice-cold 2× lysis buffer containing 20 mM Tris-HCl (pH 8), 250 mM NaCl, 20% glycerol, 2% NP-40, 20 μg/ml aprotinin (Sigma), 20 μg/ml leupeptin (Sigma), 4 mM sodium orthovandate (Sigma), 10 mM EDTA, and 10 mM NaF. After 1 h at 4°C the insoluble nuclear material was removed by centrifugation at 1 × 104 g at 4°C for 20 min after which the supernatant was precleared with protein A–Sepharose CL-4B (Pharmacia Biotech) for 45 min at 4°C. c-Met was precipitated with rabbit anti–c-Met coupled to protein A–Sepharose at 4°C for at least 2 h. The immune complexes were washed with lysis buffer and diluted in Laemmli sample buffer containing final concentrations of 62.5 mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 100 mM 2-mercaptoethanol (BioRad Laboratories), and 0.001% bromophenol blue. After boiling for 5 min, the samples were subjected to 8% SDS-PAGE. Western blotting was performed as described previously (48).
For analysis of c-Met in total cell lysates, cells were lysed in 50 mM Tris-HCl (pH 8), 150 mM NaCl, 1% NP-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM sodium orthovandate, 2 mM EDTA, and 5 mM NaF for 1 h at 4°C. After centrifugation at 1 × 104 g and 4°C for 20 min, the supernatant was diluted in Laemmli sample buffer, boiled for 5 min and subjected to 8% SDS-PAGE. Western blotting was performed as described previously (48).
FACS® Analysis.
Expression of c-Met on tonsillar B cell subpopulations was studied using a triple staining technique (47). Staining was measured by using a FACSCalibur® flow cytometer (Becton Dickinson).
Immunohistochemistry.
Expression of c-Met in tonsillar tissue was analysed by single and double staining. For single staining cryostat tonsil sections were fixed in acetone for 10 min, washed in PBS and preincubated with 10% normal goat serum (Sera Lab, Sussex, UK) in PBS for 15 min. After incubating with the primary antibody for 1 h, endogenous peroxidases were blocked with 0.1% NaN3, 0.3% H2O2, PBS for 10 min. Subsequently, the sections were stained with biotin-conjugated rabbit anti–mouse for 30 min, followed by an incubation with HRP-conjugated avidin–biotin complex for 30 min. Substrate was developed with 3,3-amino-9-ethylcarbazole (Sigma) for ∼10 min. Tissue sections were counterstained with Haematoxylin (Merck, Darmstadt, Germany).
Double staining was performed as described for the single staining, except that a cocktail of primary antibodies was used, which was detected by either a cocktail of AP-conjugated goat anti–mouse and HRP-conjugated goat anti–rabbit, or a cocktail of AP-conjugated goat anti–mouse IgG2a and HRP-conjugated goat anti–mouse IgG1. The second color was developed with Fast Blue BB (Sigma) for ∼10 min.
Adhesion Assays.
96-well flat-bottom plates (Costar) were coated overnight with 5 μg/ml human fibronectin (CLB) or 0.2 μg/ml recombinant human sVCAM-1 (R&D Systems) at 4°C. After blocking the plates with 4% BSA, RPMI 1640 (2 h at 37°C), B cells that had been pre-incubated with HGF/SF (R&D Systems), in the presence or absence of monoclonal antibodies, for 30 min at 37°C, were added. Then, the plates were centrifuged (3 min 800 rpm, no brake) and incubated at 37°C for 25 min. After washing the wells, the bound cells were fixed with 10% neutral buffered formalin solution (Sigma) and stained with Giemsa (Merck). Bound cells were quantified by using a color CCD camera (Sony) and NIH Image 1.60 software on an Apple Quadra 840AV.
c-Met ELISA.
96-well EIA/RIA plates (Costar) were coated overnight with mouse anti- HGF/SF immunoglobulins at 4°C. Then, the plates were washed and blocked with 4% BSA, PBS for 1 h at 37°C. Next, the wells were incubated with culture supernatants or with a HGF/SF concentration series for 2 h at 37°C, followed by an incubation with goat anti–c-Met immunoglobulins for 1 h at 37°C. Subsequently, the wells were incubated with biotin-conjugated rabbit anti–goat immunoglobulins for 60 min at 37°C followed by HRP-conjugated avidin–biotin complex (DAKO) for 1 h at 37°C. Substrate was developed with 1,2-phenylenediamine (Fluka Chemica, Buchs, Switzerland) in 50 mM KH2PO4, 50 mM Na2HPO4·2H2O (pH 5.4) containing H2O2. The reaction was stopped with 1 N H2SO4 and the results were analysed at 492 nm using a microplate reader (BioRad Laboratories).
RNA Isolation and RT-PCR.
Total RNA was isolated with RNAsol (Cinna/Biotex Laboratories, Houston, TX) according to manufacturers description. First-strand cDNA synthesis was performed on total RNA by a standard reverse transcription reaction, using Moloney leukemia virus reverse transcriptase (Gibco BRL/Life Technologies) and p(dN)6 random hexamers (Pharmacia Biotech). PCR was performed with Taq DNA Polymerase (Gibco BRL/Life Technologies), 200 μM dNTPs (Pharmacia Biotech) and 1.5 mM MgCl2 in 1× PCR Buffer (both Gibco BRL/ Life Technologies). Primers used were HGF-1 (5′-CGACAGTGTTTCCCTTCTCG-3′) in combination with HGF-3 (5′-GGTGGGTGCAGACACAC-3′), or 5′β2M (5′-ATCCAG CGTACTCCAAAGATT-3′) in combination with 3′β2M (5′-CATGTCTCGATCCCACTTAAC-3′). PCR was started with a 5 min denaturation step at 95°C, after which amplification was performed in 35 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 1 min and elongation at 72°C for 2 min. After a final elongation step for 10 min at 72°C, samples were cooled on ice and analysed by electrophoresis in a 1.5% agarose TBE gel containing ethidium bromide.
Results
The c-Met Receptor Tyrosine Kinase Is Expressed by Activated Human Tonsillar B Cells as well as by Several B Cell Lines.
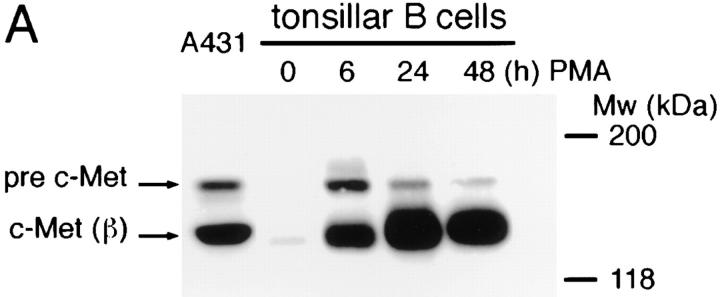
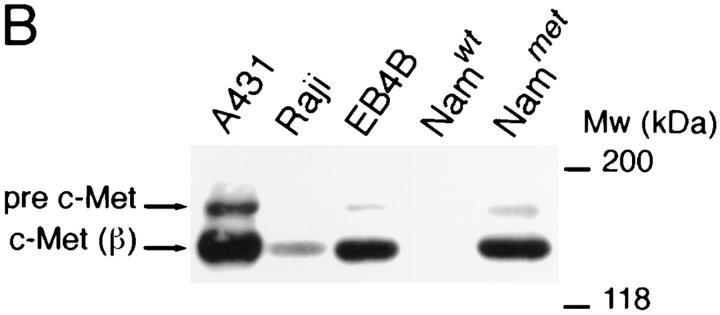
Expression of c-Met by human tonsillar B cells and by a panel of B cell lines was assessed by Western blotting and by FACS® analysis. On Western blot, c-Met expression was hardly detectable in freshly isolated tonsillar B cells, but we observed a strong induction of c-Met (and the c-Met precursor [pre c-Met]) upon stimulation with the phorbolester PMA (Fig. 1 A). Furthermore, constitutive expression of c-Met was found in the Burkitt's lymphoma cell lines Raji and EB4B, but not in the Burkitt's lymphoma cell line Namalwa (Fig. 1 B) nor in the B cell lines Daudi, Ramos, JY, or Nalm-6 (data not shown). As positive controls, c-Met expression of the epidermoid carcinoma cell line A431 and of Namalwa cells stably transfected with c-Met (Nammet) are shown (Fig. 1 B).
Figure 1.
c-Met expression on human B cells. (A) PMA induces c-Met expression on tonsillar B cells. (B) Several B cell lines constitutively express c-Met. A431 (positive control) is an epidermoid carcinoma cell line. Raji, EB4B and Namalwa (Namwt) are Burkitt's lymphoma cell lines. Nammet are c-Met–transfected Namalwa cells. In both A and B the Western blot of the cell lysates was stained with anti–c-Met. The c-Met precursor (pre–c-Met) and c-Met β chain (c-Met (β)) are indicated.
The c-Met Receptor on B Cells Is Functional.
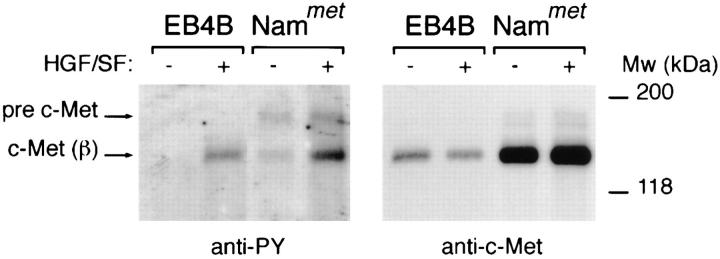
The above findings clearly show that B cells can express c-Met and, hence, might potentially be triggered via the HGF/SF–c-Met pathway. To demonstrate that the c-Met receptor on B cells can indeed be functionally activated by HGF/SF, we studied c-Met receptor phosphorylation on tyrosine residues in response to HGF/SF. As is shown in Fig. 2, HGF/ SF stimulation of EB4B cells as well as of Nammet B cells resulted in an enhanced tyrosine phosphorylation of c-Met. This indicates that the HGF/SF–c-Met pathway on B cells is capable of signaling.
Figure 2.
Tyrosine phosphorylation of c-Met on B cells in response to HGF/SF. c-Met on EB4B and Nammet B cells becomes phosphorylated on tyrosine residues upon triggering with HGF/SF. c-Met was precipitated with anti–c-Met antibodies and the Western blot was consecutively stained with anti-phosphotyrosine- or with anti–c-Met antibodies. The c-Met precursor (pre–c-Met) and c-Met β chain are indicated.
c-Met Receptor Expression on Human Tonsillar B Cell Subsets.
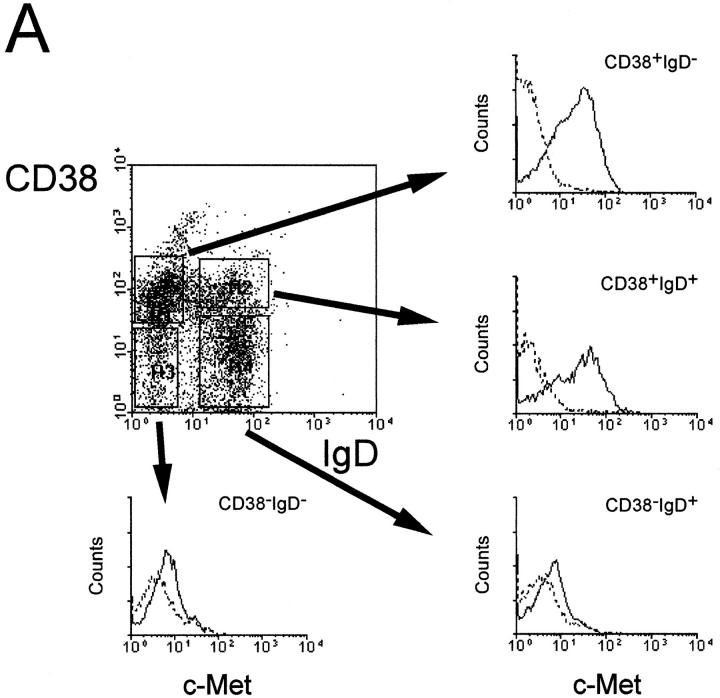
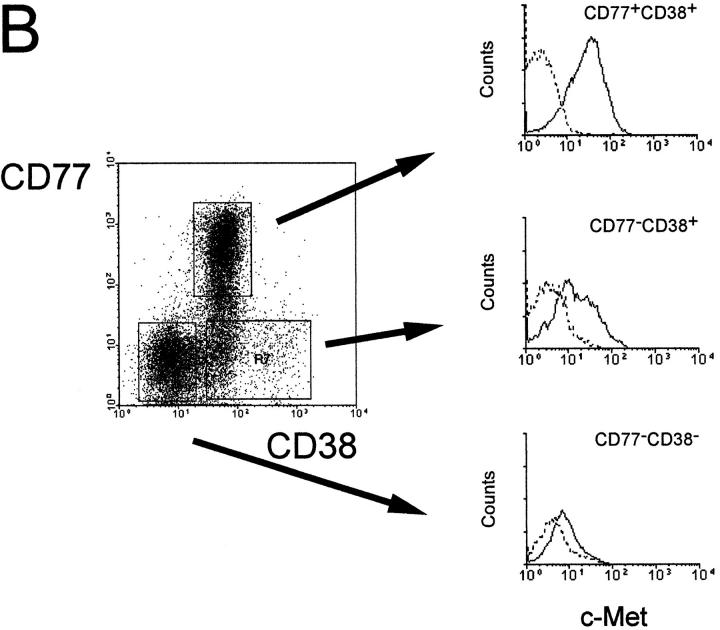
To investigate whether c-Met induction is a physiological phenomenon, that occurs also during antigen-specific B cell differentiation in vivo, we assessed the expression of c-Met on human tonsillar B cell subsets using FACS® triple staining. The subsets studied, recently defined by Pascual et al. (13), were: the naive B cell subset, IgD+CD38− (Bm1-2); two GC B cell subsets, IgD−CD38+CD77+ centroblasts (Bm3), and IgD−CD38+CD77− centrocytes (Bm4); and an IgD−CD38− memory B cell subset (Bm5). Fig. 3 shows that c-Met is expressed by CD38+CD77+ centroblasts (Bm3) and by a part of the CD38+CD77− subset. This finding is supported by immunohistochemical studies on frozen sections of human tonsillar tissue: as is shown in Fig. 4, c-Met is predominantly expressed by lymphocytes within the dark zone of the GC, which contains rapidly dividing centroblasts and low numbers of FDC. These results mean that c-Met induction in vivo, occurs in GC-cells at a pre-selection stage, i.e., cells that have recently been recruited by antigen plus antigen-specific T lymphocytes in the T cell– rich extrafollicular microenvironment.
Figure 3.
Expression of c-Met on tonsillar B cell subsets. (A) Germinal center (CD38+) B cells express the c-Met tyrosine kinase, while naive (IgD+CD38−) and memory (IgD−CD38−) tonsillar B cells are c-Met negative. Tonsillar B cells were triple-stained with anti-CD38, anti-IgD, and either anti–c-Met (solid line) or control antibodies (dotted line). (B) c-Met is expressed on centroblasts (CD38+CD77+) and on a subset of CD38+CD77− GC cells. Tonsillar B cells were triple stained with anti-CD38, anti-CD77, and either anti–c-Met (solid line) or control antibodies (dotted line).
Figure 4.
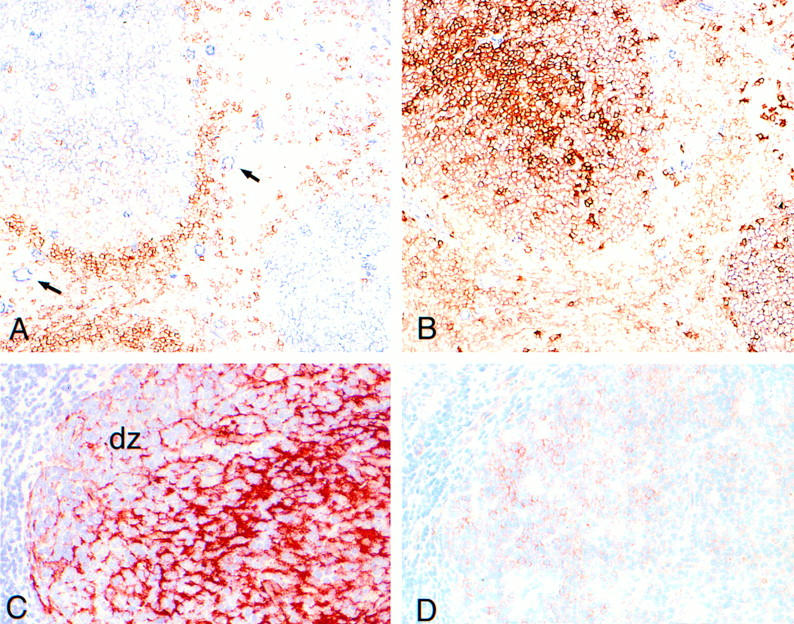
c-Met expression in the human tonsil. (A) Immunohistochemical double-staining for c-Met (blue) and IgD (red). c-Met is expressed by GC cells and by vascular endothelium (arrows). Prominant IgD expression is present on B cells of the mantle zones. (B) Serial section of A, stained for c-Met (blue) and CD38 (red). There are virtually no single c-Met–positive (blue) lymphocytes. Part of the GC cell show double staining (pink) (most clearly visible in the GC at the lower-right of the picture). A and B are not counterstained. (C) Immunohistochemical single-staining for DRC-1 (red) showing the FDC-network of the GC. The FDC poor area at the left handside represents the GC-darkzone (dz). (D) serial section of C, stained for c-Met (red). c-Met–positive cells are predominantly present in the GC-darkzone. C and D are counterstained with hematoxylin (blue).
CD40 Ligation Induces a Transient Expression of c-Met on Tonsillar B Cells.
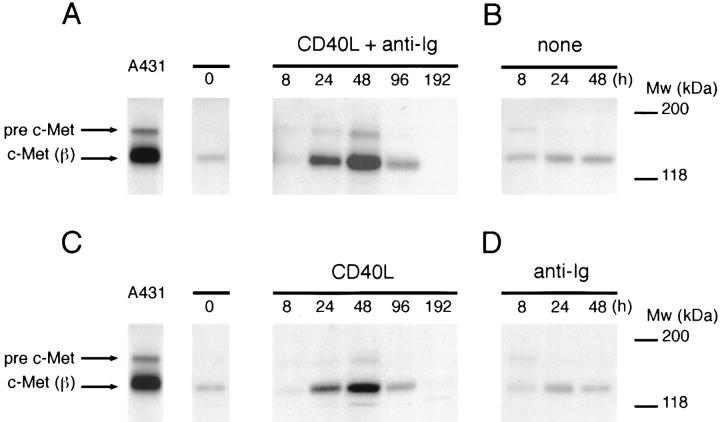
Ligation of the B cell antigen receptor (BCR) and CD40 plays a key role in the initiation of a T cell dependent B cell response and initiates the GC reaction (1, 49–51). In view of the expression of c-Met on centroblasts, i.e., on recent GC immigrants, we hypothesized that these receptors might also regulate c-Met expression. To address this hypothesis, the biological conditions for B cell activation were mimicked in vitro. Tonsillar B cells were cultured on CD40 ligand (CD40L) transfected L cells or, as a control, on wild-type L cells, in the presence or absence of BCR stimuli (anti-Ig antibodies or Staphylococcus aureus Cowans strain I [SAC]). As is shown in Fig. 5 A, concurrent ligation of CD40 and the BCR induced a strong transient induction of c-Met in human tonsillar B cells, peaking at 48 h. Single triggering of CD40 also strongly induced c-Met (Fig. 5 C) but single ligation of the BCR did not induce c-Met expression above control levels (untransfected L cells and medium alone) (Fig. 5, B and D). In approximately half of the experiments, concurrent CD40 and BCR stimulation resulted in a c-Met induction that was stronger than after CD40 ligation alone, suggesting synergy between the CD40 and BCR pathways. Stimulation by various cytokines including IL-2, IL-4, and IL-6 did not induce c-Met (data not shown).
Figure 5.
Induction of c-Met expression on tonsillar B cells by CD40 and BCR ligation. c-Met expression by tonsillar B cells cultured on (A) CD40L-transfected L cells plus anti-Ig antibodies; (B) wild-type L cells; (C) CD40L-transfected L cells; or (D) wild-type L cells plus anti-Ig antibodies. Western blots of the cell lysates were stained with anti–c-Met antibodies. The c-Met precursor (pre–c-Met) and the c-Met β chain (c-Met (β)) are indicated. In the absence of CD40 stimulation (B and D), no viable B cells were recovered at 96 and 192 h.
These data clearly identify CD40-CD40L as a major pathway for induction of c-Met in B cells.
HGF/SF Induces Integrin-mediated Adhesion of c-Met–positive B Cells to VCAM-1 and Fibronectin.
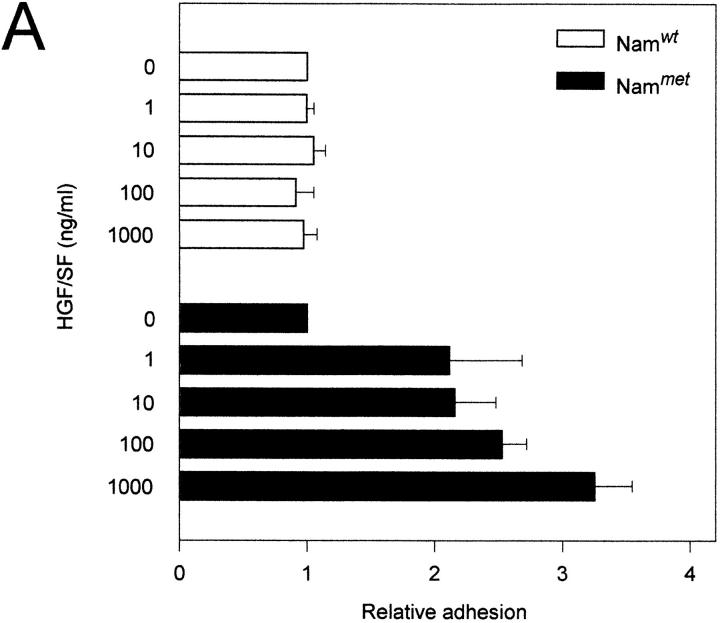
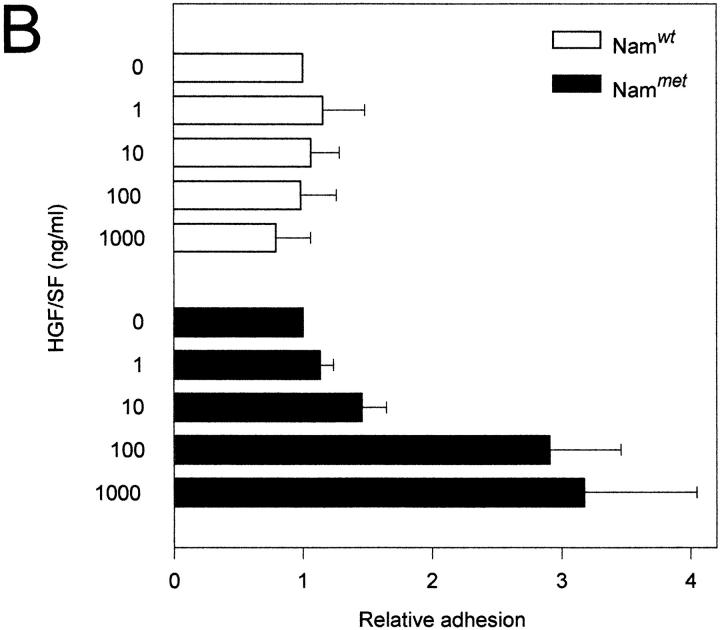
Cell motility and morphogenesis, major functions of the c-Met–HGF/SF pathway, are dependent on tightly controlled cell adhesion. This prompted us to study whether the c-Met–HGF/SF pathway might regulate B cell adhesion. Since c-Met–positive B cells represent a subset of tonsillar B cells that cannot be readily purified by negative selection procedures, we addressed this question by using Namalwa cells transfected with c-met cDNA (Nammet). The expression of c-Met in this B cell lymphoma line and the wild-type control (Namwt) are shown in Fig. 1 B. We observed that HGF/SF induces a strongly augmented adhesion to both vascular cell adhesion molecule 1 (VCAM-1) and fibronectin of c-met transfected Namalwa B cells (Nammet) (Fig. 6, A and B). This effect of HGF/SF on B cell adhesion was dose dependent and was not observed upon stimulation of wild-type Namalwa cells (Namwt). An increased adhesion in response to HGF/SF to both VCAM-1 and fibronectin was also observed with the Burkitt's lymphoma cell lines Raji and EB4B (data not shown).
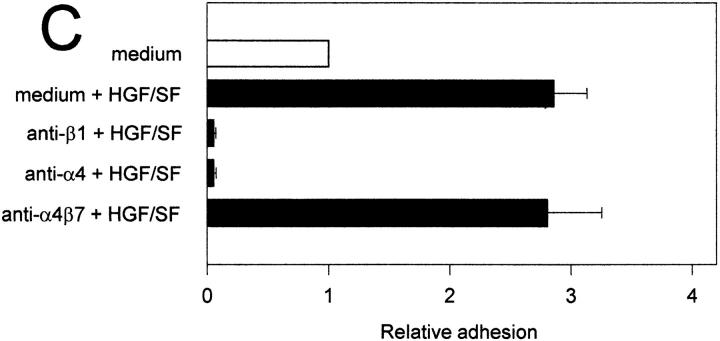
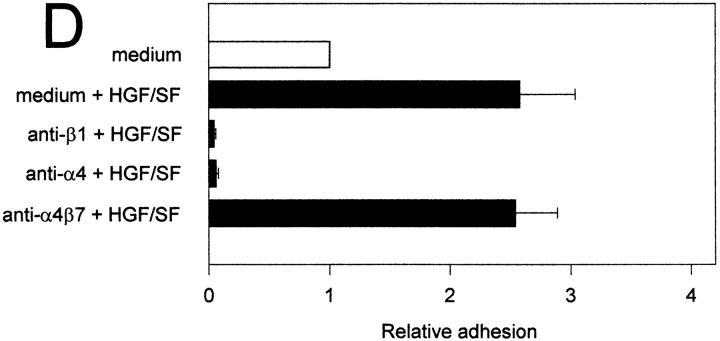
Figure 6.
The HGF/SF-c-Met pathway regulates α4β1 integrin-mediated adhesion to sVCAM-1 and fibronectin. (A) Effect of HGF/SF on the binding of c-Met transfected (Nammet) and control (Namwt) B cells to sVCAM-1. (B) Effect of HGF/SF on the binding of Nammet and Namwt B cells to fibronectin. (C) Effect of anti-β1 (4B4), anti-α4 (HP2/1), and anti-α4β7 (Act-1) integrin antibodies on the binding of Nammet B cells to sVCAM-1. (D) Effect of anti-β1 (4B4), anti-α4 (HP2/1), and anti-α4β7 (Act-1) integrin antibodies on the binding of Nammet B cells to fibronectin. Cells were preincubated with HGF/SF in the presence or absence of anti-integrin monoclonal antibodies. The results are expressed as relative (compared with the control cells not incubated with HGF/SF) adhesion. Error bars represent the standard deviation of triplicate wells.
To identify the adhesion receptors on Nammet responsible for enhanced VCAM-1 and fibronectin binding, antibody blocking experiments were performed. Nammet expresses α4β1 and α4β7, which both are receptors for VCAM-1 and for an alternatively spliced segment (CS-1) of fibronectin, but expresses no detectable level of the fibronectin receptor α5β1 (data not shown). We observed that adhesion to VCAM-1 and fibronectin was completely blocked by mAbs against both the α4 and β1 integrin chain (Fig. 6, C and D). Since c-Met stimulation by HGF/SF did not lead to increased α4β1 expression (and also did not upregulate or induce α4β7 or α5β1) (data not shown), these results indicate that the c-Met–HGF/SF pathway enhances B cell adhesiveness through activation of the α4β1 integrin.
Tonsillar Stromal Cells Produce High Levels of HGF/SF.
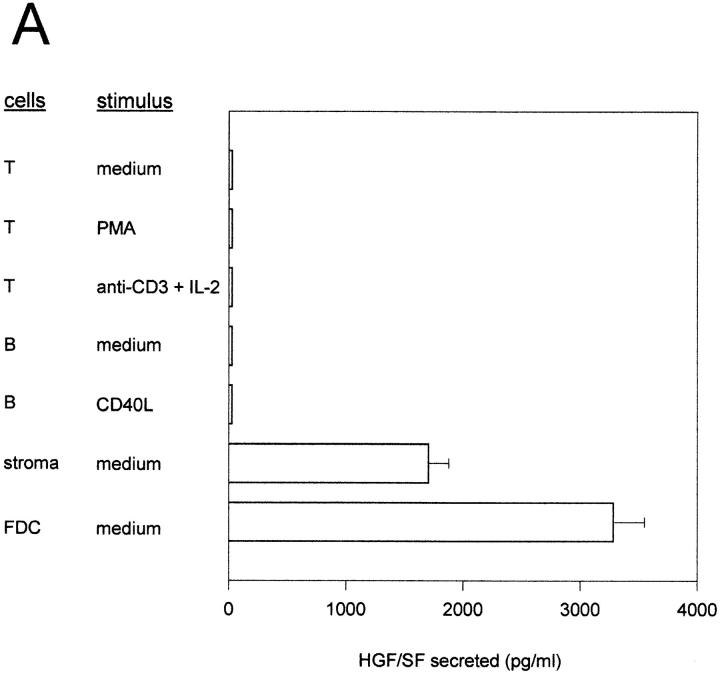
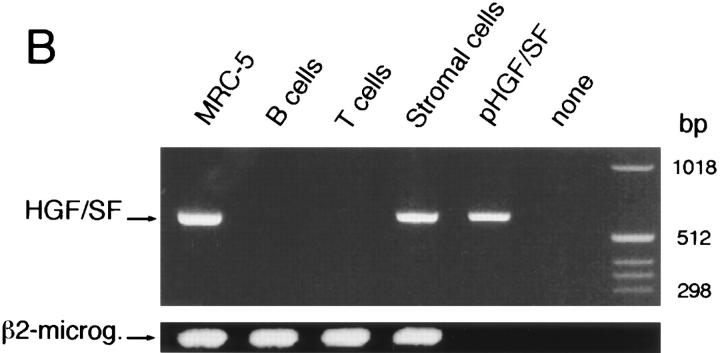
All together, the above data strongly favor a functional role of the c-Met–HGF/SF pathway in B cell differentiation, namely in the regulation of B cell adhesiveness. However, obviously, an in vivo biological role in this process would require the availability of HGF/SF within the lymphoid tissue microenvironment. This prompted us to (a) assay the production of HGF/SF by primary cultures of various tonsillar cell populations, and (b) study the expression of HGF/SF mRNA within these cell populations by RTPCR. Determinations of HGF/SF production by ELISA demonstrate that high levels of HGF/SF are produced by primary cultures of tonsillar stromal cells, including cultures of FDC-enriched tonsillar cell subfractions (Fig. 7 A). By contrast, tonsillar T- or B-lymphocytes, cultured in the presence or absence of various mitogenic stimuli, did not produce detectable levels of HGF/SF. Consistent with these results, in RT-PCR studies HGF/SF mRNA was exclusively detectable in tonsillar stromal cells (Fig. 7 B).
Figure 7.
Expression of HGF/SF protein and mRNA by tonsillar stromal cells and lymphocytes. (A) HGF/SF secretion by cultured T cell, B cells, stromal cells, and FDC enriched tonsillar cells. ELISAs were performed to determine HGF/SF concentrations in the culture media (lower limit of detection 400 pg/ml). T and B cells were stimulated as indicated. (B) Expression of HGF/SF mRNA in T cells, B cells, tonsillar stromal cells, and, as a positive control, in the lung fibroblast cell line MRC-5. The RT-PCR was performed on total RNA, a plasmid containing full-length human HGF/SF cDNA (pHGF/SF), or on water. Primers used were HGF/SF-specific or, as a control, β2-microglobulin specific.
Discussion
The products of proto-oncogenes are important regulatory molecules that exert a wide range of effects on basic cellular functions such as the control of cell growth and differentiation. The c-met proto-oncogene product is a receptor tyrosine kinase (52, 53) that binds HGF/SF, a mesenchymally derived cytokine with pleiotropic biological effects on proliferation, cell motility and morphogenesis of epithelial, endothelial, and myogenic cells (31–36, 54). More recently, the c-Met–HGF/SF pathway has also been implicated in the proliferation and differentiation of early hematopoietic progenitor cells (55–58) and in monocyte-macrophage differentiation (59). Here, we demonstrate that the c-Met–HGF/SF pathway is also operative during T cell– dependent B cell differentiation, where it is involved in the control of lymphocyte integrin function on B cells, a process regulating adhesion and homing of B cells within the lymphoid microenvironment.
We observed that stimulation of tonsillar B cells with phorbol ester PMA leads to a rapid c-Met induction (Fig. 1 A). Induction of c-Met expression upon protein kinase C (PKC) stimulation by phorbol ester has previously also been reported in epithelial cell lines (60). In addition, we observed constitutive expression of c-Met on several B cell lymphoma lines (Fig. 1 B). The c-Met receptor on these B cell lines is signaling competent, as triggering by HGF/SF resulted in enhanced tyrosine phosphorylation of c-Met (Fig. 2). These findings present the first direct evidence for expression of a functional c-Met receptor on B lymphocytes. Indirect evidence for a role of c-Met in B cells has previously been provided by Delaney et al. (61), who demonstrated that HGF/SF enhances immunoglobulin production by murine B cells. However, as c-Met expression was not studied and whole splenocyte cultures were used, indirect effects of HGF/SF were not ruled out. For T cells, Shaw and colleagues reported that HGF/SF stimulated the adhesion and migration of the memory subset. However, these target cells appeared not to express c-Met (62).
One of the key findings of our study is that concurrent CD40 and BCR ligation induces a strong transient expression of c-Met on B cells in vitro (Fig. 5). Presumably, BCR and CD40 mediated signals are also instrumental in the physiological induction of c-Met. This is suggested by the fact that c-Met is expressed in vivo on a subset of tonsillar centroblasts (CD38+CD77+) (Figs. 3 and 4). Centroblasts are the offspring of B cells that have recently been activated at extrafollicular sites by antigen plus accessory signals provided by antigen-specific T cells (1). These signals critically involve CD40/CD40L interactions: patients with x-linked hyper-IgM syndrome (due to mutated and consequently defective CD40L) do not develop GC and blocking of the CD40/CD40L pathway in mice leads to complete inhibition of GC reactions (49–51, 63, 64). Our results strongly suggest that c-Met induction is directly linked to the initiation of the B cell immune response. Indeed, we observed that dual ligation of CD40 and the BCR also induces c-Met on naive (IgD+CD38−) B cells (our own unpublished observation).
As both the migratory and morphogenic responses to HGF/SF are critically dependent on cell adhesion, could regulation of the c-Met–HGF/SF pathway also have a regulatory role in B cell adhesion? This idea is supported by the finding that HGF/SF augments adhesion of c-Met transfected Namalwa B cells as well as the c-Met expressing B cell lines Raji and EB4B to VCAM-1 and fibronectin (Fig. 6 and data not shown). This HGF/SF induced adhesion was mediated through activation of the integrin α4β1. Previous studies from our own and from other laboratories have shown an important role for α4β1 in GC formation (27– 29). In the GC, α4β1 mediates B cell adhesion to VCAM-1 on FDC, an interaction that regulates the formation of the microenvironment required for the affinity selection of GC B cells. Apart from establishing physical contact between B cells and FDC, α4β1 presumably contributes directly to the B cell selection process itself, as signaling through the α4β1–VCAM-1 pathway costimulates rescue of GC B cells from apoptosis (6, 29, 47). Furthermore, α4β1 also regulates cell adhesion to fibronectin (65), an important substrate for cell migration.
The above data strongly favor a functional role of the c-Met–HGF/SF pathway in B cell differentiation, namely in the regulation of B cell adhesiveness. Obviously, however, an in vivo biological role in this process requires the availability of HGF/SF within the lymphoid microenvironment. Interestingly, we indeed observed production of high levels of HGF/SF as well as expression of HGF/SF mRNA by tonsillar stromal cells. In contrast, tonsillar T- or B-lymphocytes were HGF/SF negative (Fig. 7).
Adhesion regulation is believed to be fundamental to the control of cell migration and microenvironmental homing during lymphocyte differentiation. This migration and homing within tissues, like recruitment from the blood, presumably is determined by an organized display of adhesive ligands and regulatory factors, specific for a given microenvironment (23). Thusfar, most studies on the regulation of integrin-mediated adhesion have focussed on cytokines of the chemokine family (66). Chemokines, which bind to G protein linked 7-transmembrane serpentine receptors (67, 68), have been shown to mediate chemotaxis and rapid functional activation of leukocyte integrins on myeloid cells, macrophages, and lymphocytes (69–73). HGF/SF belongs to the family of plasminogen-related growth factors (74), that also includes macrophage stimulating protein. These molecules, that are structurally unrelated to the chemokines, have the basic domain organization and mechanism of activation of the blood proteinase plasminogen, i.e., they are characterized by the presence of a kringle domain(s), an activation domain, and a serine proteinase domain. Our present data strongly support a physiological role of HGF/SF in the regulation of B cell adhesiveness, microenvironmental homing and in the morphogenesis of the GC. Local production of HGF/SF has been demonstrated surrounding blood vessels in inflammation (75, 76), and in this paper we demonstrate that HGF/SF is produced by FDC-enriched cell populations. In view of the pleiotropic effects of HGF/SF on other cell types, HGF/SF may have additional, as yet unkown, roles in antigen-specific B cell differentiation. In particular, cross-talk between integrins and c-Met–signaling pathways triggered by FDC might contribute to B cell survival (6, 29, 47). Interestingly, like the chemokines, HGF/SF has a high affinity for heparin, which is present on cell surfaces and in the ECM in the form of heparan sulphate proteoglycans. This heparan sulphate binding limits diffusion, thus allowing the development of chemotactic gradients and the localization of proadhesive activity to the appropriate lymphoid microenvironment (77).
Acknowledgments
We thank Dr. G. Koopman for helpful advise, Dr. J. Banchereau for CD40L transfected L cells, Drs. G. Hartmann and E. Gherardi for c-Met c-DNA, Mr. J.B.G. Mulder for technical assistance, and Drs. C.M. van Noesel, F.M. van den Berg, and M. Snoek for critical reading of the manuscript.
This study was supported by a grant from the University of Amsterdam.
Footnotes
1 Abbreviations used in this paper: BCR, B cell antigen receptor; CD40L, CD40 ligand; ECM, extracellular matrix; FDC, follicular dendritic cells; GC, germinal center; HGF/SF, hepatocyte growth factor/scatter factor; PKC, protein kinase C; SAC, Staphylococcus aureus Cowans strain; VCAM-1, vascular cell adhesion molecule 1.
References
- 1.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 2.Nossal GJ. Differentiation of the secondary B-lymphocyte repertoire: the germinal center reaction. Immunol Rev. 1994;137:173–183. doi: 10.1111/j.1600-065x.1994.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 3.Thorbecke GJ, Amir AR, Tsiagbe VK. Biology of germinal centers in lymphoid tissue. FASEB J. 1994;8:832–840. doi: 10.1096/fasebj.8.11.8070632. [DOI] [PubMed] [Google Scholar]
- 4.Liu YJ, Grouard G, de Bouteiller O, Banchereau J. Follicular dendritic cells and germinal centers. Int Rev Cytol. 1996;166:139–179. doi: 10.1016/s0074-7696(08)62508-5. [DOI] [PubMed] [Google Scholar]
- 5.Rajewski K. Clonal selection and learning in the antibody system. Nature (Lond) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 6.Lindhout, E., G. Koopman, S.T. Pals, and C. de Groot. 1997. Triple check for antigen specificity of B lymphocytes during germinal centre reactions. Immunol. Today. In press. [DOI] [PubMed]
- 7.Liu YJ, Zhang J, Lane PJ, Chan EY, MacLennan IC. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2961. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 8.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 10.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centers. Nature (Lond) 1991;354:389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 11.Küppers R, Zhao M, Hansmann H-L, Rajewsky K. Tracing B cell development in human germinal centers by molecular analysis of single cells picked from histological sections. EMBO (Eur Mol Biol Organ) J. 1993;12:4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHeyzer-Williams MG, McLean MJ, Lalor PA, Nossal GJV. Antigen-driven B cell differentiation, in vivo. J Exp Med. 1993;178:295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual V, Liu YJ, Magalski A, de Bouteiller O, Banchereau J, Capra JD. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994;180:329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nossal GJV, Abbot A, Mitchell J, Lummus Z. Ultrastructural features of antigen capture in primary and secondary lymphoid follicles. J Exp Med. 1968;127:277–290. doi: 10.1084/jem.127.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tew JG, Kosco MH, Burton GF, Szakal AK. Follicular dendritic cells as accessory cells. Immunol Rev. 1990;117:185–211. doi: 10.1111/j.1600-065x.1990.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 16.Hardie DL, Johnsons GD, Khan M, MacLennan ICM. Quantative analysis of molecules which distinguish functional compartments within germinal centers. Eur J Immunol. 1993;23:997–1004. doi: 10.1002/eji.1830230502. [DOI] [PubMed] [Google Scholar]
- 17.Fuller KA, Kanagawa O, Nahm MH. T cells within germinal centers are specific for the immunizing antigen. J Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- 18.Casamayor-Palleja M, Khan M, MacLennan ICM. A subset of CD4+memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J Exp Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng B, Han S, Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J Exp Med. 1996;184:1083–1091. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraal G, Weissman IC, Butcher EC. Germinal centre B cells: antigen specificity and changes in heavy chain class expression. Nature (Lond) 1982;298:377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- 21.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 22.Koopman G, Pals ST. Cellular interactions in the germinal center: role of adhesion receptors and significance for the pathogenesis of AIDS and malignant lymphoma. Immunol Rev. 1992;126:21–45. doi: 10.1111/j.1600-065x.1992.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 23.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science (Wash DC) 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 24.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 25.Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991;173:599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roldan E, Garcia-Pardo A, Brieva JA. VLA-4 fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J Exp Med. 1992;175:1739–1747. doi: 10.1084/jem.175.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman AS, Munro JM, Rice GE, Bevilaqua MP, Morimoto C, McIntyre BW, Rhynheart K, Pober JS, Nadler LM. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science (Wash DC) 1990;249:1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- 28.Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJLM, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen-1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–1304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koopman G, Keehnen RMJ, Lindhout E, Newman W, Shimizu Y, van Seventer GA, de Groot C, Pals ST. Adhesion through the LFA-1 (CD11a/CD18)-ICAM1(CD54) and the VLA-4 (CD69d)-VCAM-1(CD106) pathways prevents apoptosis of germinal center B cells. J Immunol. 1994;152:3760–3767. [PubMed] [Google Scholar]
- 30.Arroyo AG, Tang YT, Rayburn H, Hynes RO. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell. 1996;85:997–1008. doi: 10.1016/s0092-8674(00)81301-x. [DOI] [PubMed] [Google Scholar]
- 31.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature (Lond) 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature (Lond) 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 33.Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- 34.Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992;119:629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123:223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinkmann V, Foroutan H, Sachs M, Weidner KM, Birchmeier W. Hepatocyte growth factor/scatter factor induces a variety of tissue-specific morphogenic programs in epithelial cells. J Cell Biol. 1995;31:1573–1586. doi: 10.1083/jcb.131.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter Factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giordano S, Zhen Z, Medico E, Gaudino G, Galmi F, Comoglio PM. Transfer of motogenic and invasive response to scatter factor/hepatocyte growth factor by transfection of human MET protooncogene. Proc Natl Acad Sci USA. 1993;90:649–653. doi: 10.1073/pnas.90.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rong S, Segal S, Anver M, Resau JH, Vande GF, Woude Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci USA. 1994;91:4731–4735. doi: 10.1073/pnas.91.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keizer GD, Te AA, Velde, Schwarting R, Figdor CG, De Vries JE. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987;17:1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- 41.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984;133:1857–1862. [PubMed] [Google Scholar]
- 42.Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- 43.Nudelman E, Kannagi R, Hakomori S, Parsons M, Lipinski M, Wiels J, Fellous M, Tursz T. A glycolipid antigen associated with Burkitt Lymphoma defined by a monoclonal antibody. Science (Wash DC) 1983;220:509–511. doi: 10.1126/science.6836295. [DOI] [PubMed] [Google Scholar]
- 44.Partridge LJ, Lowe J, Hardie DL, Ling NR, Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. II. Antigenic differences between secreted and membrane IgG demonstrated using monoclonal antibodies. J Immunol. 1982;128:1–6. [PubMed] [Google Scholar]
- 45.Arpin C, Dechanet J, van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory and plasma cells in vitro. Science (Wash DC) 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 46.Clark RA, Alon R, Springer TA. CD44 and hyaluronan-dependent rolling interactions of lymphocytes on tonsillar stroma. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koopman G, Keehnen RMJ, Lindhout E, Zhou DFH, de Groot C, Pals ST. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur J Immunol. 1997;27:1–7. doi: 10.1002/eji.1830270102. [DOI] [PubMed] [Google Scholar]
- 48.Taher TEI, Smit L, Griffioen AW, Schilder-Tol EJM, Borst J, Pals ST. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lckin T lymphocytes. J Biol Chem. 1996;271:2863–2867. doi: 10.1074/jbc.271.5.2863. [DOI] [PubMed] [Google Scholar]
- 49.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Sealand S. The CD40 Ag and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 50.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claasen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han S, Hathcock K, Zheng B, Kepler RB, Hodes R, Kelsoe G. Cellular interaction in germinal centres: roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–567. [PubMed] [Google Scholar]
- 52.Cooper LS, Park M, Blair DG, Rainsky MA, Huebner K, Groce CM, Vande GF, Woude Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature (Lond) 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 53.Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande G, Woude Sequence of METproto-oncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bladt F, Riethmacher D, Isenman S, Aguzzi A, Birchmeier C. Essential role for the c–met receptor in the migration of myogenic precursor cells into the limb bud. Nature (Lond) 1995;376:768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- 55.Kmiecik TE, Keller JR, Rosen E, Vande GF, Woude Hepatocyte growth factor is a synergistic factor for the growth of hematopoietic progenitor cells. Blood. 1992;80:2454–2457. [PubMed] [Google Scholar]
- 56.Mizuno K, Higuchi O, Ihle JN, Nakamura T. Hepatocyte growth factor stimulates growth of hematopoietic progenitor cells. Biochem Biophys Res Commun. 1993;194:178–186. doi: 10.1006/bbrc.1993.1801. [DOI] [PubMed] [Google Scholar]
- 57.Galimi F, Bagnara GP, Bonsi L, Cottone E, Follenzi A, Simeone A, Comoglio PM. Hepatocyte growth factor induces proliferation and differentiation of multipotent and erythroid hemopoietic progenitors. J Cell Biol. 1994;127:1743–1754. doi: 10.1083/jcb.127.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishino T, Hisha H, Hishino N, Adachi M, Ikehara S. Hepatocyte growth factor as a hematopoietic regulator. Blood. 1995;85:3093–3100. [PubMed] [Google Scholar]
- 59.Chen Q, DeFrances MC, Zarnegar R. Induction of metproto-oncogene (hepatocyte growth factor receptor) expression during human monocyte-macrophage differentiation. Cell Growth Differ. 1996;7:821–832. [PubMed] [Google Scholar]
- 60.Boccaccio C, Gaudino G, Gambarotta G, Galimi F, Comoglio PM. Hepathocyte growth factor (HGF) expression is inducible and is part of the delayed early response to HGF. J Biol Chem. 1994;269:12846–12851. [PubMed] [Google Scholar]
- 61.Delaney, B., W.S. Koh, K.H. Yang, S.C. Strom, and N.E. Kaminsky. 1993. Hepatocyte growth factor enhances B cell activity. Life Sciences. 53:PL89–93. [DOI] [PubMed]
- 62.Adams DH, Harvath L, Bottaro DP, Interrante R, Catalano G, Tanaka Y, Strain A, Hubscher SG, Shaw S. Hepatocyte growth factor and macrophage inflammatory protein 1β: structurally distinct cytokines that induce rapid cytoskeletal changes and subset-preferential migration in T cells. Proc Natl Acad Sci USA. 1994;91:7144–7148. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematzu S, Yoshida N, Kishimoto T, Kikutani H. The immune response in CD40 deficient mice: impaired immunoglobuline class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 64.Facchetti F, Appiani C, Salvi L, Levy J, Notarangelo LD. Immunohistologic analysis of ineffective CD40CD40 ligand interaction in lymphoid tissues from patients with X-linked immunodeficiency with hyper-IgM. Abortive germinal center cell reaction and severe depletion of follicular dendritic cells. J Immunol. 1995;154:6624–6633. [PubMed] [Google Scholar]
- 65.Wayner EA, Carcia-Pardo A, Humphries MJ, McDonald JA, Carter WG. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain in plasma fibronectin. J Cell Biol. 1989;109:1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oppenheim JJ, Zachariae COC, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 67.Kelvin D, Michiel DF, Johnston JA, Lloyd AR, Sprenger H, Oppenheim JJ, Wang JM. Chemokines and serpentines: the molecular biology of chemokine receptors. J Leukocyte Biol. 1993;54:604–612. doi: 10.1002/jlb.54.6.604. [DOI] [PubMed] [Google Scholar]
- 68.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. T cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature (Lond) 1993;361:79–83. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 70.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 71.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preverential migration of activated CD4+ and CD8+T cells in response to MIP-1α and MIP-1β. Science (Wash DC) 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 72.Loyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extra-cellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- 73.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Donate LE, Gherardi E, Srinivasan N, Sowdhamini R, Aparicio S, Blundell TL. Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP) Protein Science. 1994;3:2378–2394. doi: 10.1002/pro.5560031222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P, Rosen EM. Scatter factor induces blood vessel formation in vivo. . Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koch AE, Halloran MM, Hosaka S, Shah MR, Haskell CJ, Baker SK, Panos RJ, Haines GK, Bennett GL, Pope RM, Ferrara N. Hepatocyte growth factor. A cytokine mediating endothelial migration in inflammatory arthritis. Arthritis Rheum. 1996;39:1566–1575. doi: 10.1002/art.1780390917. [DOI] [PubMed] [Google Scholar]
- 77.Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]