Abstract
NK recognition is regulated by a delicate balance between positive signals initiating their effector functions, and inhibitory signals preventing them from proceeding to cytolysis. Knowledge of the molecules responsible for positive signaling in NK cells is currently limited. We demonstrate that IL-2–activated human NK cells can express CD40 ligand (CD40L) and that recognition of CD40 on target cells can provide an activation pathway for such human NK cells. CD40-transfected P815 cells were killed by NK cell lines expressing CD40L, clones and PBLderived NK cells cultured for 18 h in the presence of IL-2, but not by CD40L-negative fresh NK cells. Cross-linking of CD40L on IL-2–activated NK cells induced redirected cytolysis of CD40-negative but Fc receptor-expressing P815 cells. The sensitivity of human TAP-deficient T2 cells could be blocked by anti-CD40 antibodies as well as by reconstitution of TAP/MHC class I expression, indicating that the CD40-dependent pathway for NK activation can be downregulated, at least in part, by MHC class I molecules on the target cells. NK cell recognition of CD40 may be important in immunoregulation as well as in immune responses against B cell malignancies.
NK cells represent a distinct lineage of lymphocytes that are able to kill a variety of tumor (1), virus-infected (2), bone marrow transplanted (3), and allogeneic target cells (4). NK cells do not express T cell receptors or immunoglobulins and are apparently normal in mice with defects in the recombinase machinery (5, 6).
Our knowledge about NK cell specificity has increased considerably in the last years. NK cells can probably interact with target cells by a variety of different cell surface molecules, some involved in cell adhesion, some activating the NK cytolytic program (7, 8), and other ones able to inhibit this activation by negative signaling (as reviewed in reference 9).
A common feature of several inhibitory NK receptors is the capability to bind MHC class I molecules (10, 11), as predicted by the effector inhibition model within the missing self hypothesis of recognition by NK cells (12–14). Interestingly, the MHC class I receptors identified so far belong to different gene families in mouse and man; these are the p58/p70/NKAT or killer cell inhibitory receptors (KIR)1 of the immunoglobulin superfamily in man and the Ly49 receptors of the C-type lectin family in the mouse. There is also evidence that MHC class I molecules can be recognized as triggering signals in NK cells of humans, rats as well as mice (13). The inhibitory receptors allow NK cells to kill tumor or normal cell targets with deficient MHC class I expression (12, 14). This does not exclude that other activating pathways can override inhibition by MHC class I molecules (15) and, even in their absence, there must be some activating target molecules that initiate the cytolytic program. Several surface molecules are able to mediate positive signals in NK cells. Some of these structures, like NKRP1 (16), CD69 (17), and NKG2 (18) map to the NK complex region (NKC) of chromosome 6 in mice and of chromosome 12 in humans (13). CD2 (19) and CD16 (20) molecules can also play a role in the activation pathway.
NK cells resemble T cells in many respects, both may arise from an immediate common progenitor (21, 22), and share the expression of several surface molecules (23). NK cells produce cytokines resembling those secreted by some helper T cell subsets (24) and contain CD3 components in the cytoplasm (21). The expression of some surface structures, involved in TCR-dependent T cell costimulation, like CD28 in human (25), has been described on NK cells, but the functional relevance of these molecules for NK activation processes has not been fully established.
Another T cell molecule of interest is CD40L, which interacts with CD40, a 50-kD membrane glycoprotein expressed on B cells (26), dendritic cells (27), and monocytes (28). CD40 is a member of the tumor necrosis factor/nerve growth factor receptor family (29) which includes CD27 (30), CD30 (31), and FAS antigen (32). Murine and human forms of CD40L had been cloned and found to be membrane glycoproteins with a molecular mass of ∼39 kD induced on T cells after activation (33). Also mast cells (34), eosinophils (35), and B cells (36) can be induced to express a functional CD40L. The CD40L–CD40 interaction has been demonstrated to be necessary for T cell–dependent B cell activation (33, 37). Mutations in the CD40L molecule cause a hyper-IgM immunodeficiency condition in man (38, 39, 40). On the other hand, CD40–CD40L interactions also orchestrate the response of regulatory T cells during both their development (41, 42) and their encounter with antigen (43, 44).
NK cells have also been suggested to play a role in B cell differentiation and immunoglobulin production (45). Therefore, it was of interest to investigate whether NK cells could use a CD40-dependent pathway in their interactions with other cells. Therefore, we have investigated the ability of target cells expressing CD40 to induce activation of NK cytotoxicity.
Materials and Methods
Cell Lines.
K562, an MHC class I–negative human erythroleukemia cell line, T2 and T2/TAP1+2, transfectants of the T2 T-B lymphoblast hybrid line (46), were cultured in RPMI 1640 (Biochrom K.G., Berlin, Germany) supplemented with 5% heatinactivated FCS and 2 mM glutamine (Biochrom) at 37°C in 5% CO2/95% air. In the T2/TAP1+2 culture the medium was supplemented weekly with 600 μg/ml G-418 (Sigma Chemical Co., St. Louis, MO). Murine mastocytoma P815 cells stably transfected with human CD40 cDNA containing plasmid expression vectors (43, 47), were a generous gift of Dr. L. Lanier (DNAX, Palo Alto, CA). These cell lines are referred to as CD40-P815 throughout.
Monoclonal Antibodies, Immunofluorescence, and Flow Cytometry.
mAb W6/32, (an IgG2a anti-HLA mAb recognizing a class I monomorphic determinant) was purchased from DAKO (Milan, Italy); mAb OKT3 (IgG2a anti-CD3) and TIB-200 (IgM antiCD57) were obtained from hybridomas provided by the American Type Culture Collection (Rockville, MD); mAb 14G7 (IgM anti-CD40) was a gift of Dr. R. van Lear (Central Laboratory of the Blood Transfusion Service, Amsterdam, The Netherlands); TRAP-1 (IgG1 anti-CD40L) was purchased from PharMigen (San Diego, CA); OX27 (IgG1, anti-rat MHC class I) was purchased from Serotec (Oxford, England). FITC- and PE-labeled mAbs against CD3, CD4, CD8, CD14, CD19, CD56, CD2, CD16, and isotype-matched labeled controls were purchased from Becton Dickinson (Mountain View, CA) and used to characterize the cell phenotype by immunofluorescence. Immunofluorescence, flow cytometry and data analysis were performed as described (48). To detect the CD40L expression on polyclonal IL-2–activated lymphocytes, PBMC, depleted of adherent cells, were incubated for the indicated periods in round-bottomed 96-well microtiter plates (Falcon; Becton Dickinson). At the indicated times, cells were washed in PBS and incubated for 30 min at 4°C with saturating concentrations of PE-labeled TRAP-1 mAb in the presence of FITC-labeled anti-CD3 or anti-CD56 or isotypic controls in a standard double-staining technique.
NK Polyclonal Populations, NK Lines, and NK Clones.
PBMC were isolated by centrifugation on Ficoll Hypaque (Biochrom) gradients from normal donor buffy coats obtained from the Blood Bank of the Medical School of the Federico II University of Naples. After isolation, the PBMC were washed and incubated in complete medium, in a horizontally placed plastic flask, for 2 h at 37°C to remove adherent cells. The recovered cells were used without any pretreatment or activated with rIL-2 as indicated in the results section. In some experiments, IL-2–activated effectors were depleted of CD3-positive cells by magnetic beads (Dynal, Oslo, Norway) coated with anti-CD3 mAb and a samarium cobalt magnet. The depletion procedure was repeated twice. 98% of the remaining cells were CD56+CD3−, as assessed by FACS® analysis. CD56−CD3+ effector populations were generated using anti-CD56 mAb-coated beads and the same depletion procedure. The NK3.3 human NK cell line was obtained from a healthy donor as described (49) and cultured in the presence of 1,000 IU/ml of human recombinant IL-2 (rIL-2). The JA2 NK clone was generated and characterized as described (50).
Cytotoxicity Assay.
Cytotoxicity was measured in a conventional 4-h 51Cr-release assay. Target cells were labeled with Na251CrO4 (100 μCi/2 × 106 cells) and the percent of specific lysis was calculated as ([experimental release − spontaneous release] / [maximum release − spontaneous release]) × 100. The spontaneous release never exceeded 20%. Experimental details and mAb concentrations used in the reverse ADCC test as well as in the treatment of NK effectors with plastic immobilized antibodies are indicated in Results.
Results
A CD40-dependent Activation Pathway Is Involved in Cytotoxicity Mediated by NK Clones and IL-2–activated NK Cells.
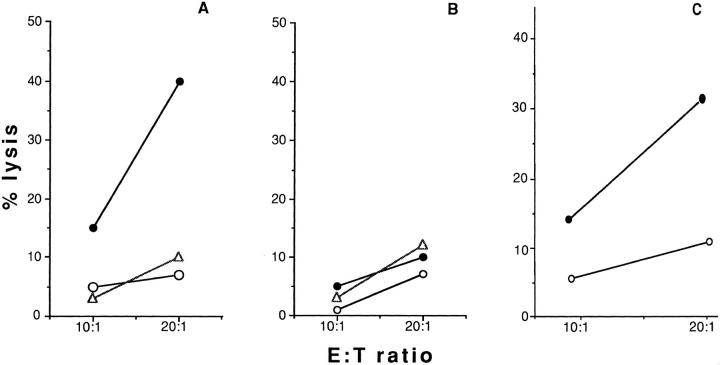
In an effort to assess the role of costimulatory molecules in NK activation, we studied the ability of the NK line NK3.3 and clone JA2 to recognize and kill P815 mastocytoma cells and their CD40 transfectants. Fig. 1 shows that the transfection of CD40 molecule is able, alone, to induce recognition and lysis of P815 targets by NK3.3 effectors (Fig. 1 A). Similar results were obtained with the NK clone JA2 (Fig. 1 B). Five NK clones were tested using the same target systems, giving comparable results (data not shown). Any interference due to possible clonal differences in the target susceptibility could be excluded by blocking experiments in which a chromium release assay was performed in the presence of targets pretreated with anti-CD40 IgM mAb 14G7 or with the isotypic control TIB-200 mAb (Fig. 1, C). These data indicated that a CD40-dependent pathway is functionally involved in the induction of cytotoxicity of human NK cell lines and clones. In contrast, fresh polyclonal NK cells that had not been exposed to IL-2 were not able to recognize the CD40-transfected targets (Fig. 2 A). However, PBMC depleted of plastic-adherent cells and activated with rIL-2 (1,000 IU/ml) were able to recognize and kill CD40 transfectants. P815 parental cells were not killed. The effect was detectable after 18 h of culture with IL-2, reached its maximum after 48 h and was still evident after 72 h of IL-2 treatment (Fig. 2 C). Depletion by magnetic beads of CD56+ but not CD3+ lymphocytes completely inhibited the killing of CD40-transfected P815 (Figs. 3, A and B). We conclude that a CD40-dependent cytotoxic pathway is functional, not only in human NK lines and clones, but also in polyclonal rIL-2–activated NK effectors, isolated from peripheral blood of normal individuals.
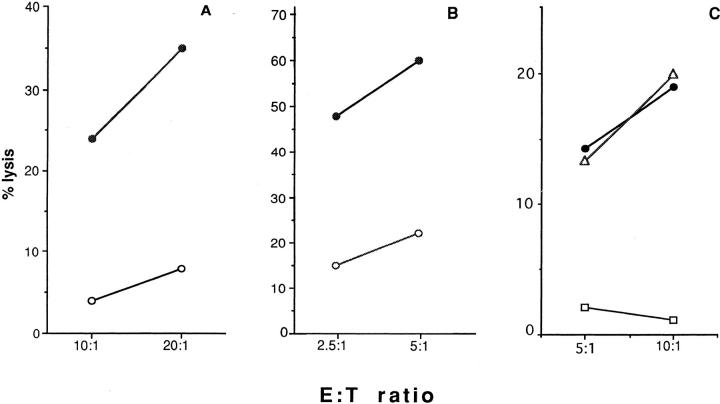
Figure 1.
CD40 molecules are able to activate NK-dependent killing. P815 (open circles) and CD40-P815 (closed circles) cells were used as targets in a classical chromium release assay. A and B indicate % lysis obtained with NK cell line NK3.3 and NK clone JA2, respectively. C shows a representative experiment in which NK3.3 NK cell line was tested in a classical chromium release assay in the presence of CD40-transfected P815 target preincubated with medium (closed circles), with the IgM antiCD40 mAb 14G7 (open squares), or with the control anti-CD57 mAb TIB-200 (open triangles). Both mAb were used as 1:1,000 ascites dilution.
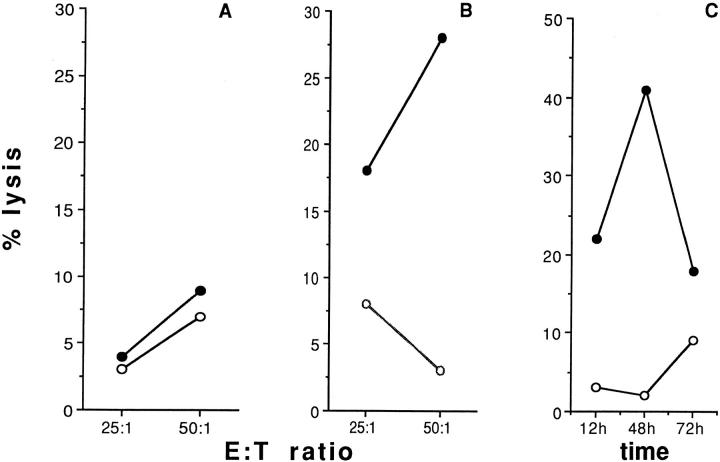
Figure 2.
Effect of IL-2 incubation on the cytotoxic activity of NK polyclonal effectors against CD40 expressing targets, and time titration curves. A and B indicate % lysis obtained by using freshly isolated or o.n. IL-2– activated polyclonal NK cells, respectively, in the presence of CD40-P815 (closed circles), P815 parental cells (open circles). Data obtained from 18 independent donors were analyzed by twotailed paired Student's t test; the killing differences between P815 and CD40-P815 were found statistically significant (P <0.002). C indicates results obtained by using polyclonal NK effectors incubated with rIL-2 for the indicated time in the presence of CD40-P815 (closed circles) or P815 (open circles); E/T ratio was always 50:1. Results refer to one of five independent experiments.
Figure 3.
CD40-transfected P815 are killed by IL-2–activated NK cells. (A) Percent lysis obtained by using CD3-depleted, IL-2–activated effectors, prepared as described in Materials and Methods, in the presence of CD40-P815 (closed circles), or P815 parental cells (open circles). (B) CD3depleted (closed symbols) or CD56-depleted (open symbols) effectors, obtained by two unrelated donors, were tested for their ability to lyse CD40-P815 transfectants in a classical chromium release assay. Results refer to one of three independent experiments.
Expression of CD40L by NK Clones and IL-2–activated CD3−CD56+ Lymphocytes.
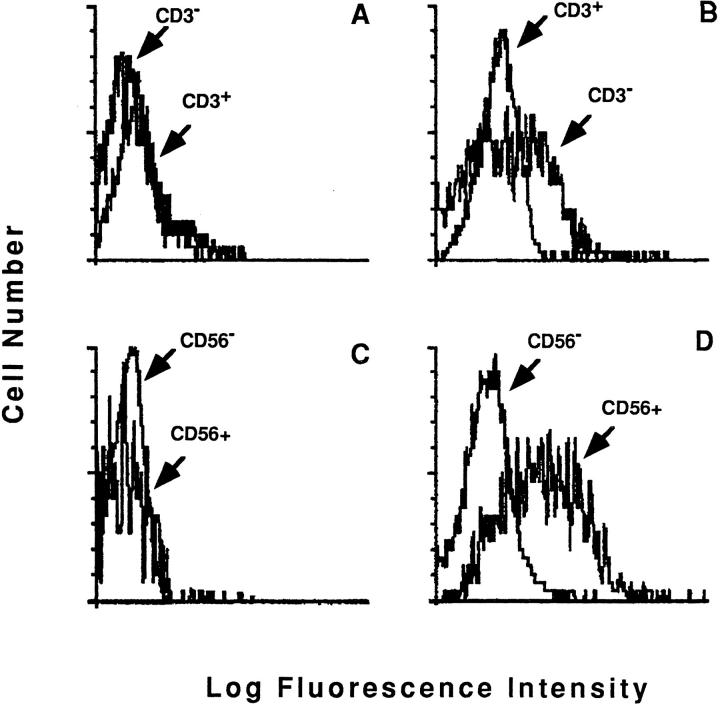
To better characterize the induction of the CD40-dependent cytotoxic pathway in the human NK lines and clones, we studied their expression of surface CD40L. Fig. 4 shows profiles obtained by staining the NK3.3 cell line (A) and JA2 NK clone (B) with the anti-CD40L mAb TRAP-1. Both NK populations expressed CD40L molecule. We also assessed the expression of CD40L on polyclonal NK effectors derived from peripheral blood of normal donors, by double staining with anti-CD40L mAb TRAP-1 and anti-CD3 or anti-CD56 mAb. A clear TRAP-1 binding on the CD3−CD56+ population was demonstrated after overnight (o.n.) incubation in the presence of rIL-2, but not with medium alone (Fig. 5). No binding was observed on fresh cells (data not shown). These data indicate that CD3−CD56+ NK effectors, activated with rIL-2, can express the CD40L molecule; this is likely to account for their ability to specifically recognize and kill CD40-expressing targets.
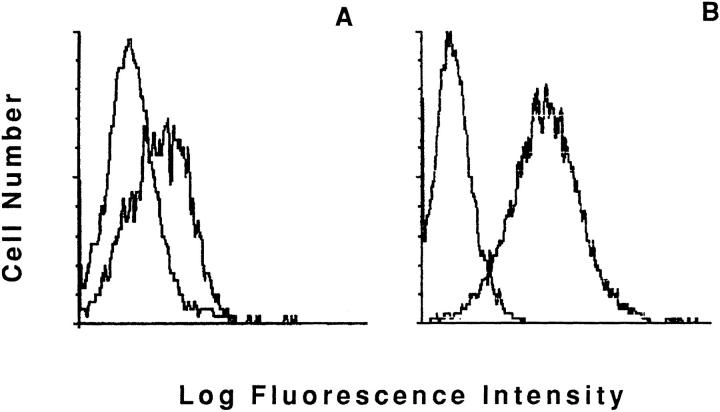
Figure 4.
CD40 Ligand expression on NK3.3 cell line and JA2 NK clone. Staining profiles obtained by using anti-CD40L TRAP-1 mAb or isotypic controls, as indicated. A and B show the NK3.3 NK cell line and JA2 NK clone staining data, respectively.
Figure 5.
CD56+CD3− lymphocytes express CD40L after incubation with IL-2. Staining profiles of o.n. cultured lymphocytes, obtained as indicated in the Materials and Methods section, by using PE-labeled antiCD40L TRAP-1, FITC-labeled anti-CD3, or anti-CD56 mAbs in a classical double fluorescence assay. Results were evaluated as histograms referring to TRAP-1 binding on CD3+ or CD56+ gated cell. The comparison with the binding obtained in the counterpart population (CD3−/ CD56−) was performed by histogram overlapping, as indicated. The analysis was performed on the same population of lymphocytes cultured o.n. in the presence of medium (A and C) or with rIL-2 (B and D), respectively.
Killing of Resistant Targets Induced by Cross-linking of CD40L on Human Cell Lines.
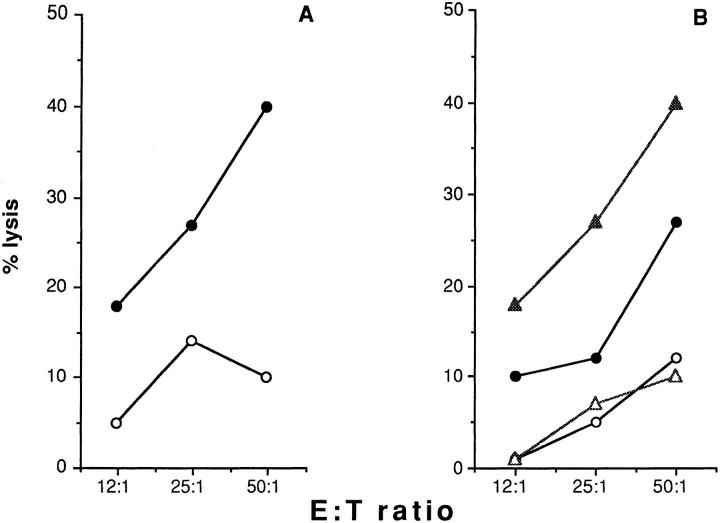
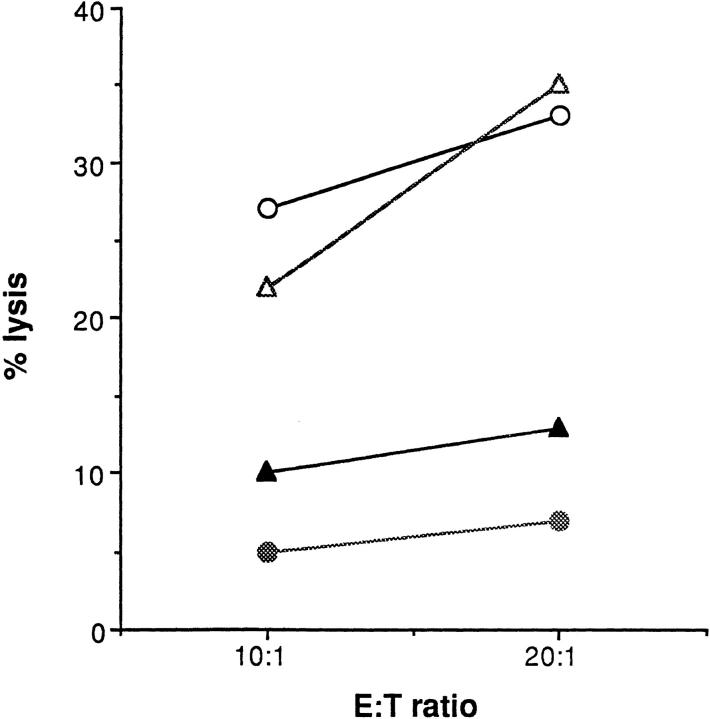
We investigated next whether crosslinking of CD40L can induce signals able to activate NK killing. We took advantage of the observation that the P815 parental line is completely resistant to lysis by the NK3.3 cell line (Fig. 1 A), and in addition expresses Fc receptors. It should thus be possible to induce CD40L+ NK3.3 cells to lyse the Fc receptor-expressing P815 by crosslinking CD40L with TRAP-1 in a reverse ADCC experiment. Fig. 6 shows one of five independent experiments in which such an effect was demonstrated. Pretreatment of P815 cells with TRAP-1 mAb, but not with the isotypic control OX27 recognizing the rat MHC class I antigens, was able to induce killing by NK3.3 effectors. Target treatment with 200 ng of anti-CD40L mAb TRAP-1 or the isotypic control OX27 was performed as previously described (51). We observed a similar phenomenon using two other IL-2–activated NK cell lines (data not shown). NK3.3 cells starved from IL-2 could not be activated by CD40L cross-linking (Fig. 6 B), although they efficiently kill K562 NK-susceptible control cell line (data not shown). These data indicate that CD40L triggering induces a cytotoxic pathway in the 4-h period required for a standard 51Cr-release assay. In an effort to better investigate the role of CD40L engagement in the induction of a cytotoxic phenotype in human NK effectors, we performed experiments in which NK cells were incubated for 4 h at 37°C with plastic-immobilized anti-CD40L mAb TRAP-1 or the isotypic control OX27 and then used in a classical chromium release assay in the presence of the CD40-negative resistant target P815. Fig. 6 C shows the results obtained in a representative experiment. As shown, preincubation of NK3.3 cell line effectors for 4 h in the presence of coated TRAP-1 mAb, but not the isotypic control OX27 enables NK cells to significantly kill P815 targets. Therefore, CD40L engagement clearly induces human NK effectors to recognize and kill CD40-negative resistant targets.
Figure 6.
CD40 ligand crosslinking induces redirected cytotoxicity. P815 cells were incubated with 200 ng of anti-CD40L mAb TRAP-1 (closed circles), the isotypic control OX27 (open triangles), or medium (open circles) and then used as target in an in vitro cytotoxicity assay. The results refer to one of five independent experiments. A and B show data obtained by using as effectors NK3.3 cells cultured in the presence of rIL-2 or starved from IL-2 for 24 h, respectively. C shows data obtained by using NK3.3 effectors pre-incubated for 4 h at 37°C in the presence of plastic immobilized TRAP-1 mAb, or the control OX27 mAb. The treatment was performed in a 96-well microtiter plate precoated with 50 μl of the antibody solution, at the concentration of 10 μg/ml. NK effectors were collected and used in a classical chromium release assay. Closed and open circles indicate preincubation of effectors in the presence of plastic immobilized TRAP-1 or OX27 control mAb, respectively. The results refer to one of four independent experiments.
Regulation of CD40-dependent NK Cytotoxicity by Target Cell Class I Expression.
NK cell recognition is regulated by a delicate balance between positive signals, that initiate their effector function, and inhibitory signals that prevent cytolysis. Negative signals can be mediated by MHC class I molecules. Therefore, we tested the ability of NK3.3 NK effectors to kill the T2 cell line, in which a defect in TAP1– TAP2 peptide transporter genes leads to reduced MHC class I surface expression. TAP1+2 transfectants of T2 (T2/ TAP1+2), in which the defect was completely reconstituted, were used as controls. These cell lines constitutively express similar levels of the CD40 costimulatory molecules, as assessed by indirect immunofluorescence (data not shown). Fig. 7 shows one representative experiment, in which the pretreatment of T2 cells with anti-CD40, but not with anti-CD57 mAb, significantly inhibited NK3.3-dependent cytotoxicity. If we used T2/TAP1+2 cells as targets in the same experimental model, the cytotoxic effect was completely inhibited (Fig. 7). The activating signals, mediated by costimulatory molecules, may therefore be modulated by the presence of optimal levels of surface HLA class I antigens. However, data obtained by using a panel of more potent NK effectors revealed a residual susceptibility to NK lysis of target cells expressing MHC class I and CD40. Therefore, we cannot exclude that the triggering effect of CD40 can override MHC class I–dependent negative signals (data not shown) under other conditions.
Figure 7.
The reexpression of MHC class I on NK targets expressing CD40 is able to inhibit NK3.3-mediated cytotoxicity. Percent lysis obtained by using effector NK3.3 cell line in the presence of untreated T2 cells (open circles), T2 preincubated with the anti-CD57 IgM mAb TIB200 (open triangles), with the anti-CD40 IgM mAb 14G7 (closed triangles). Closed circles indicate cytotoxicity obtained with T2/TAP1+2 targets. The results refer to one representative experiment out of four performed.
Discussion
There is an emerging consensus that NK cell recognition is regulated by a balance between positive signals, which initiate their effector functions, and inhibitory signals preventing cytolysis. At present, there is no evidence for a unique NK cell–specific receptor, responsible for positive signaling in NK cells; rather, it is likely that different receptors may be used, depending on the activation state of the NK cell and the availability of the relevant ligands on the target cell.
This study demonstrates that a CD40-mediated activation pathway is functional in the induction of cytotoxicity mediated by IL-2–activated NK cells in humans. This effect is likely to be mediated by surface-expressed CD40L on human IL-2–activated CD3−CD56+ lymphocytes. To the best of our knowledge, this receptor has not been described earlier on NK cells. We found that fresh NK cells from normal donors did not express CD40L and were unable to kill the CD40 transfectants. Activation with IL-2 induced the CD40L as well as the capability to kill CD40 expressing targets. CD40L is normally expressed by T cells 6–8 h after activation, then it is quickly downmodulated (52). It is possible that a CD40-dependent pathway is functional also in NK effectors in vivo after activation with relevant stimuli. Our findings thus add the CD40–CD40L interaction to other triggering pathways for NK cells.
Previous reports (45, 53) demonstrated that NK cells can induce B cell maturation as well as immunoglobulin secretion and isotype switching. Our data open the possibility that these phenomena might in part be mediated by a CD40–CD40L interaction. CD40L+ T cells were recently described to induce IL-12 secretion by human peripheral blood monocytes, favoring Th1 priming in vitro (54, 55). mAb directed against CD40L molecules were able to prevent Th1-mediated IFN-γ secretion in the same experimental model. Therefore, one may speculate as to whether NK cells expressing CD40L can mediate immunoregulatory functions. Our results suggest that negative signals mediated by MHC class I molecules on target cells can downregulate NK killing also when CD40 costimulatory molecules are involved in the triggering of cytotoxicity. These data suggest the possibility that the NK effector encounter with an APC could play a role in the regulation of some immune responses. The final outcome of this interaction could depend on the balance between activating molecules, like costimulatory structures, and MHC class I antigens expressed on the surface of the APC. In this context, inhibitory receptors, recognizing self MHC class I molecules and expressed on NK effectors, may serve as a fail-safe mechanism to prevent inappropriate responses and destruction of normal cells expressing CD40. Under some circumstances, soluble mediators, like inflammatory cytokines, may alter the balance between MHC class I inhibitory signals and costimulatory positive stimuli, activating the NK effector function. This could imply elimination of infected cells or old APCs no longer needed and potentially harmful by giving a persistent stimulation of a response.
NK cells can express distinct receptor sets able to control NK cytotoxicity. Inhibitory receptors were described to recognize MHC class I antigens expressed on targets cell surface, however some isoforms of such kind of receptors were also able to trigger NK cytotoxicity (13). Here we propose CD40L as a new NK activating molecule. Our observations suggest that the functional pathways involved in the induction of NK cytotoxicity rather than redundant, might be able to complement each other. In this context, the presence on the surface of NK effectors of activating receptors for MHC class I molecules, could be involved in the induction of cytotoxic mechanisms during allogeneic bone marrow rejection, or be able to sense modification in the structure or peptide loading of self MHC class I molecules expressed on the surface of infected cells. At variance, a role for CD40L in mechanisms of NK-mediated immune regulation, likely involving CD40 expressing targets, as monocytes and dendritic cells, could also be proposed. In addition, high CD40 expression levels might override MHC class I–mediated inhibition of NK cytotoxicity.
Recent observations, obtained in CD40–CD40L knockout murine models, have demonstrated a critical role for CD40–CD40L interaction in the activation of T cells in vivo (44, 56). Two possible mechanisms are believed to likely account for such a role. First, CD40L might be a receptor for T cell costimulation by CD40 expressing APC (42, 43); second, CD40L may be an inducer of costimulatory activity and the induced costimulatory molecules are essential for T cell activation (57, 58). Our data indicate that CD40L triggering enables human NK effectors to recognize and kill resistant targets. On the other hand, we are not able to discriminate if CD40L engagement on NK cells can directly affect the cytotoxicity machinery or mediates the induction of distinct molecules in order to activate the NK lysis programs. Similar findings were already referred to in CD40 knockout mice, in which in vivo priming in the presence of soluble CD40 was shown to partially overcome the need for CD40 expressing B cells in order to obtain a mature antibody response and to generate memory cell populations (56).
The described CD40–CD40L NK activation pathway is disrupted in the hyper-IgM immunodeficiency syndrome. These patients have a defective CD40L expression, leading to an impairment of the Ig switch and persistency of IgM production. Many of the infections in these patients can be attributed to the resulting lack of the humoral response. Some are more typical of defective cell-mediated response. The results presented here suggest that these pathologies might be due to a defective CD40L expression on NK cells.
Acknowledgments
We wish to thank H.-G. Ljunggren's group for the useful discussion and suggestions, Professor E. Simpson for critically reading the manuscript, and Drs. L. Lanier and R. van Lear for the generous gift of transfectants and monoclonal antibodies, respectively.
This work was supported by grants from the Scuola Superiore di Immunologia “Ruggero Ceppellini” (Naples), the Associazione Italiana per la Ricerca sul Cancro (AIRC, Milan), and the Italian Health Ministry Project “Tubercolosi”. G. Terrazzano was supported by a fellowship from Istituto Italiano per gli Studi Filosofici, Naples. E. Carbone was supported, during his stay at Karolinska Institutet (Stockholm), by a fellowship from “Progetto di Scambi Internazionali”, University of Naples “Federico II”.
Footnotes
1 Abbreviations used in this paper: CD40L, CD40 ligand; KIR, killer cell inhibitory receptors; o.n., overnight.
References
- 1.Herberman, R.B. 1982. NK Cells and Other Natural Effector Cells. Academic Press, New York. 912 pp.
- 2.Borysiewicz LK, Rodgers B, Morris S, Graham S, Sissons JGP. Lysis of human cytomegalovirus infected lymphoblasts by natural killer cells: demonstration of an interferon-independent component requiring expression of early viral proteins and characterization of effector cells. J Immunol. 1985;134:2695–2705. [PubMed] [Google Scholar]
- 3.Murphy WJ, Kumar V, Bennett M. Acute rejection of murine bone marrow allograft by Natural Killer cells and T cells. J Exp Med. 1987;166:1499–1503. doi: 10.1084/jem.166.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciccone E, Viale O, Pende D, Malnati M, Biassoni R, Melioli G, Moretta A, Long EO, Moretta L. Specific lysis of allogeneic cells after activation of CD3−lymphocytes in mixed lymphocyte culture. J Exp Med. 1988;168:2403–2407. doi: 10.1084/jem.168.6.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dorshkind K, Pollack SB, Bosma MJ, Phillips RA. Natural Killer (NK) cells are present in mice with severe combined immunodeficiency (scid) J Immunol. 1985;134:3798–3801. [PubMed] [Google Scholar]
- 6.Mombaerts P, Mizoguchi N, Ljunggren H-G, Iacomini J, Ishikawa H, Wang L, Grusby MJ, Glimcher LH, Winn HJ, Bhan AK, Tonegawa S. Peripheral lymphoid development and function in TCR mutant mice. Int Immunol. 1994;134:1061–1070. doi: 10.1093/intimm/6.7.1061. [DOI] [PubMed] [Google Scholar]
- 7.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules on human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathew BA, Garni-Wagner H, Land K, Takashima A, Stoneman E, Bennett M, Kumar V. Cloning and characterization of the 2B4 gene encoding a molecule associated with non-MHC restricted killing mediated by activated NK cells and T cells. J Immunol. 1993;151:5328–5334. [PubMed] [Google Scholar]
- 9.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 10.Kane PK. Ly49 mediates EL4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J Exp Med. 1994;179:1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohring C, Colonna M. Human natural killer cell inhibitory receptors bind to HLA class I molecules. Eur J Immunol. 1996;26:365–370. doi: 10.1002/eji.1830260215. [DOI] [PubMed] [Google Scholar]
- 12.Ljunggren H-G, Kärre K. In search of the missing self: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 13.Gumperz JE, Parham P. The enigma of the natural killer cell. Nature (Lond) 1995;378:245–248. doi: 10.1038/378245a0. [DOI] [PubMed] [Google Scholar]
- 14.Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren H-G, Latour A, Koller B, Kärre K. Recognition of β2-microglobulin negative (β2m-) T-cell blast by natural killer cells from normal but not from β2m-mice. Proc Natl Acad Sci USA. 1991;88:10332–10337. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers BJ, Salcedo M, Ljunggren H-G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 16.Bezouska K, Yuen CT, O'Brien J, Childs RA, Chai W, Lawson AM, Drbal K, Fiserovà A, Pospisil M, Feizi T. Oligosaccharide ligands for NKR-P1 protein activate NK cells and cytoxicity. Nature (Lond) 1994;372:150–157. doi: 10.1038/372150a0. [DOI] [PubMed] [Google Scholar]
- 17.Moretta A, Poggi A, Pende D, Tripodi G, Orengo A, Pella N, Augugliaro R, Bottino C, Ciccone E, Moretta L. CD69 mediated pathway of lymphocytes activation: anti-CD69 monoclonal antibodies trigger the cytolytic activity of different lymphoid effector cells with the exception of cytolytic-lymphocytes expressing T cell receptor α/β. J Exp Med. 1991;174:1393–1398. doi: 10.1084/jem.174.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dücher M, Offterdinger M, Holzmüller H, Lipp J, Chu CT, Aschauer B, Bach FH, Hofer E. NKG2-C is a receptor on human natural killer cells that recognizes structures on K562 target cells. Eur J Immunol. 1995;25:2923–2931. doi: 10.1002/eji.1830251032. [DOI] [PubMed] [Google Scholar]
- 19.Siliciano RF, Pratt JC, Schmidt RE, Ritz J, Reinherz EL. Activation of cytolytic T lymphocytes and natural killer cells function through the T11 sheep eritrocyte binding protein. Nature (Lond) 1985;317:428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- 20.Chambers WH, Vujanovic NL, Deleo AB, Olszowy MW, Herberman RB, Hiserodt JC. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. J Exp Med. 1989;169:1373–1389. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanier LL, Chang C, Spits H, Phillips JH. Expression of cytoplasmic CD3ε protein in activated adult natural killer (NK) cells and CD3 γ, δ, ε complexes in fetal NK cells. J Immunol. 1992;149:1876–1880. [PubMed] [Google Scholar]
- 22.Rodewald HR, Moingeon P, Lucich JL, Dosiou C, Lopez P, Reinherz EL. A population of early fetal thymocytes expressing Fcγ RII/III contains precursors of T lymphocytes and natural killer cells. Cell. 1992;69:139–150. doi: 10.1016/0092-8674(92)90125-v. [DOI] [PubMed] [Google Scholar]
- 23.Lanier LL, Phillips JH, Hackett J, Jr, Tutt M, Kumar V. Natural killer cells: definition of a cell rather than a function. J Immunol. 1986;137:2735–2739. [PubMed] [Google Scholar]
- 24.Perussia B. Lymphokine-activated killer cells, natural killer cells and cytokines. Curr Opin Immunol. 1991;3:49–55. doi: 10.1016/0952-7915(91)90076-d. [DOI] [PubMed] [Google Scholar]
- 25.Azuma M, Cayabyab M, Buck D, Phillips JH, Lanier LL. Involvement of CD28 in MHC-unrestricted cytotoxity mediated by a human natural killer leukemia cell line. J Immunol. 1992;149:1115–1123. [PubMed] [Google Scholar]
- 26.Clark EA. CD40: a cytokine receptor in search for a ligand. Tissue Antigens. 1990;36:33–39. doi: 10.1111/j.1399-0039.1990.tb01795.x. [DOI] [PubMed] [Google Scholar]
- 27.Schiever F, Freeman AS, Freeman G, Messner E, Lee D, Daley J, Nadler LM. Isolated human dendritic cells display a unique antigenic phenotype. J Exp Med. 1989;169:2043–2058. doi: 10.1084/jem.169.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–674. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith CA, Farrah T, Goodwin GR. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 30.Camerini D, Walz G, Loenen WAM, Borst J, Seed B. The cell activation antigen CD27 is a member of the NGF/TNF receptor gene family. J Immunol. 1991;147:3165–3171. [PubMed] [Google Scholar]
- 31.Dürkop HJ, Latza U, Hummel M, Eitelbach F, Sead B, Stein H. Molecular cloning and expression of a new member of nerve growth factor receptor family that is characteristic for Hodgkin's disease. Cell. 1992;68:421–427. doi: 10.1016/0092-8674(92)90180-k. [DOI] [PubMed] [Google Scholar]
- 32.Itoh N, Yonehara A, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–245. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 33.Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand gp39. Annu Rev Immunol. 1996;14:591–617. doi: 10.1146/annurev.immunol.14.1.591. [DOI] [PubMed] [Google Scholar]
- 34.Gaushat JF, Henchoz S, Mazzei G, Aubry JP, Brunner T, Blasey H, Life P, Talabot D, Flores-Romo L. Induction of human IgE synthesis in B cells by mast cells and basophilis. Nature (Lond) 1993;365:340–443. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 35.Gauchat JF, Henchoz S, Fattah D, Mazzei G, Aubry JP, Jomotte T, Dash L, Page K, Solari R, Aldebert D, Capron M, Dahinden C, Bonnefoy JY. CD40 ligand is functionally expressed on human eosinophils. Eur J Immunol. 1995;25:863–865. doi: 10.1002/eji.1830250335. [DOI] [PubMed] [Google Scholar]
- 36.Grammer AC, Bergman MC, Miura Y, Fujita K, Davis LS, Lipsky PE. The CD40 ligand expressed by human B cells costimulates B cell responses. J Immunol. 1995;154:4996–5010. [PubMed] [Google Scholar]
- 37.Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, Van Kooten C, Liu YS, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 38.Fuleihan R, Ramesh N, Loh R, Jabara H, Rosen RS, Chatila T, Fu SM, Stamenkovic I, Geha RS. Defective expression of the CD40 ligand in X chromosomelinked immmunoglobulin deficiency with normal or elevated IgM. Proc Natl Acad Sci USA. 1993;90:2170–2173. doi: 10.1073/pnas.90.6.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farrington M, Grosmaire LS, Nonoyama S, Fisher SH, Hollenbaugh D, Ledbetter JA, Noelle RJ, Aruffo JA, Ochs HD. CD40 ligand expression is defective in a subset of patients with common variable immunodeficiency. Proc Natl Acad Sci USA. 1994;91:1099–1104. doi: 10.1073/pnas.91.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and its role in X-linked hyperIgM syndrome. Immunol Today. 1993;14:559–563. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 41.Foy TM, Page DM, Waldschimidt TJ, Schoneveld JD, Laman JD, Masters SR, Tygrett L, Ledbetter JA, Aruffo A, Claassen E, et al. An essential role for gp39, the ligand for CD40, in thymic selection. J Exp Med. 1995;182:1377–1388. doi: 10.1084/jem.182.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggiero G, Martìnez E, Cacères, Voordouw A, Noteboon E, Graf D, Kroczek RA, Spits H. CD40 expressed on thymic epithelial cells provides costimulation for proliferation but not for apoptosis of human thymocytes. J Immunol. 1996;156:3737–3746. [PubMed] [Google Scholar]
- 43.Cayabyab MH, Phillips HJ, Lanier LL. CD40 preferentially costimulates activation of CD4+T lymphocytes. J Immunol. 1994;152:1523–1532. [PubMed] [Google Scholar]
- 44.Grewal IS, Xu J, Flavell RA. Impairment of antigen specific T cell priming in mice lacking CD40 ligand. Nature (Lond) 1995;378:617–620. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 45.Gray JD, Horwitz DA. Activated human NK cells can stimulate resting B cells to secrete immunoglobulins. J Immunol. 1995;154:5656–5664. [PubMed] [Google Scholar]
- 46.Momburg F, Ortiz-Navarrete V, Neefjes J, Goulemy E, Van de Wal Y, Walden P, Hämmerling GJ. Proteasome subunits encoded by the Major Histocompatibility Complex are not essential for antigen presentation. Nature (Lond) 1992;360:174–176. doi: 10.1038/360174a0. [DOI] [PubMed] [Google Scholar]
- 47.Azuma MD, Cayabyab M, Buck D, Phillips JH, Lanier LL. CD28 interaction with B7 costimulates primary allogeneic proliferative responses and cytotoxicity mediated by small resting T cells. J Exp Med. 1992;175:353–358. doi: 10.1084/jem.175.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lanier LL, Recktenweld D. Multicolor immunofluorescence and flow cytometry. Methods (Orlando) 1991;2:192–198. [Google Scholar]
- 49.Kornblhut J, Flomberg N, Dupont B. Cell surface phenotype of cloned line of human Natural Killer cells. J Immunol. 1982;129:2831–2836. [PubMed] [Google Scholar]
- 50.Carbone E, Terrazzano G, Colonna M, Tuosto L, Piccolella E, Franksson L, Palazzolo G, Perez-Villar JJ, Fontana S, Kärre K, Zappacosta S. Natural killer clones recognize specific soluble HLA class I molecules. Eur J Immunol. 1996;26:683–689. doi: 10.1002/eji.1830260326. [DOI] [PubMed] [Google Scholar]
- 51.Moretta A, Vitale M, Bottino C, Orengo AM, Morelli L, Augugliaro R, Barbaresi M, Ciccone E, Moretta L. P58 molecules as putative receptors for major histocompatibility complex (MHC) class I molecules in human natural killer (NK) cells. Anti-p58 antibodies reconstitute lyis of MHC class I protected cells in NK clones displaying different specificities. J Exp Med. 1993;178:597–604. doi: 10.1084/jem.178.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp-39, the CD40 ligand, on normal and cloned CD4+T cells. J Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 53.Snapper CM, Yamaguchi H, Moorman MA, Sneed R, Smoot D, Mond JJ. Natural killer cells induce activated murine B cells to secrete Ig. J Immunol. 1993;151:5251–5260. [PubMed] [Google Scholar]
- 54.Stüber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin-12 secretion. J Exp Med. 1996;183:693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shu U, Kiniwa M, Wu CY, Malistewski C, Vezzio N, Hakimi J, Gately M, Delespesse G. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 56.van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature (Lond) 1995;378:620–623. doi: 10.1038/378620a0. [DOI] [PubMed] [Google Scholar]
- 57.Guo Y, Wu Y, Shinde S, Sy M, Aruffo A, Liu Y. Identification of a costimulatory molecule rapidly induced by CD40L as CD44H. J Exp Med. 1996;184:955–961. doi: 10.1084/jem.184.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanjay S, Wu Y, Guo Y, Niu Q, Xu J, Grewal IS, Flavell R, Liu Y. CD40L is important for induction of, but not response to, costimulatory activity. J Immunol. 1996;157:2764–2768. [PubMed] [Google Scholar]