Abstract
The role of the spleen and of other organized secondary lymphoid organs for the induction of protective antiviral immune responses was evaluated in orphan homeobox gene 11 knockout mice (Hox11−/−) lacking the spleen, and in homozygous alymphoplastic mutant mice (aly/aly) possessing a structurally altered spleen but lacking lymph nodes and Peyer's patches. Absence of the spleen had no major effects on the immune response, other than delaying the antibody response by 1–2 d. In aly/aly mice, the thymus-independent IgM response against vesicular stomatitis virus (VSV) was delayed and reduced, whereas the T-dependent switch to the protective IgG was absent. Therefore, aly/aly mice were highly susceptible to VSV infection. Since aly/aly spleen cells yielded neutralizing IgM and IgG after adoptive transfer into recipients with normally structured secondary lymphoid organs, these data suggest that the structural defect was mainly responsible for inefficient T–B cooperation. Although aly/aly mice generated detectable, but reduced, CTL responses after infection with vaccinia virus (VV) and lymphocytic choriomeningitis virus (LCMV), the elimination of these viruses was either delayed (VV) or virtually impossible (LCMV); irrespective of the dose or the route of infection, aly/aly mice developed life-long LCMV persistence. These results document the critical role of organized secondary lymphoid organs in the induction of naive T and B cells. These structures also provide the basis for cooperative interactions between antigen-presenting cells, T cells, and B cells, which are a prerequisite for recovery from primary virus infections via skin or via blood.
The immune system is functionally compartmentalized into primary lymphoid organs responsible for the generation and differentiation of mature naive T and B cells and into secondary lymphoid organs where immune responses are initiated. Only after activation do T and B cells emigrate from secondary lymphoid organs to seek antigen in the periphery (1–3). Secondary lymphoid organs include the spleen, LN, and organized lymphoid tissues associated with mucosal membranes such as the tonsils, the appendix, and the Peyer's patches (PP)1. These highly organized secondary lymphoid organs provide the structures where antigen is efficiently retained and presented and where ordered cellular interactions between APCs, T cells, and B cells take place to initiate and promote efficient immune responses (1). Although it has been postulated that T or B cells get anergized during possible initial peripheral encounter with antigen (4, 5), there is good experimental evidence indicating that antigen encounter by naive T cells does not occur, in general, outside of organized lymphoid tissues and does not lead to activation (1–3, 6–9).
Classical experiments using isolated skin flaps connected to the host via blood vessels with or without afferent lymphatic vessels showed that antigen applied to the skin flap induced a specific immune response only when both the afferent lymphatic vessel and the draining LN were intact (7, 10). These studies document, in our modern understanding, that emigrating APCs, in particular, dendritic cells (DC) reaching local LN, are essential for the induction of a specific immune response (11).
The role of the spleen in immune responses has been studied in splenectomized patients and mice. The function of the spleen is mainly to filter particulate and soluble antigens from the blood (1, 12). Absence of the spleen caused an increased susceptibility to generalized infections with encapsulated bacteria (13–15) because the thymus independent type 2 (TI-2) Ab response to polysaccharide components of bacterial cell walls was markedly reduced (16–18).
Recently, two mouse strains with interesting changes of secondary lymphoid organs have been developed or discovered. First, a mutant mouse lacking a functional form of the orphan homeobox gene 11 (Hox11) has been generated by gene targeting. Hox11 −/− knockout mice (Hox11−/−) lack the spleen, but otherwise appear normal. In particular, no defects in other secondary lymphoid organs, in the thymus, or the bone marrow have been detected (19). Second, a mouse strain with a spontaneous autosomal recessive single gene mutation, termed alymphoplasia (aly) has been described. The homozygous aly/aly mutant mice lack all secondary lymphoid organs (especially LN and PP) except the spleen, which, however, exhibited a disturbed structure. The bone marrrow appeared to be normal, whereas the morphological distinction between thymic cortex and medulla was unclear (20, 21). These mutant mice that lack either the spleen but not other secondary lymphoid organs, or lack LN, PP etc. but not the spleen, now offer a new and unique possibility to assess the role of these secondary lymphoid organs in the induction and maintenance of immune responses, particularly against viral infections.
Our study revealed that absence of the spleen had only minimal effects on antiviral immune responses, whereas absence of LN and other organized secondary lymphoid tissues caused a drastic change in the kinetics of virus replication and distribution relative to the kinetics of the protective immune response, particularly against the noncytopathic lymphocytic choriomeningitis virus (LCMV). Lack of LN led to overwhelming viral spread in vivo and, as a consequence, to specific T cell exhaustion and virus persistence.
Materials and Methods
Mice and Animal Experiments.
aly/aly mutant mice lacking LN and PP were purchased from CLEA Inc. (Tokyo, Japan) and bred locally under specific pathogen-free conditions by mating heterozygous (aly/+) females and homozygous (aly/aly) males. aly/ aly mutant mice were distinguished from aly/+ littermates by a virtual absence of IgA in their sera measured with an anti-IgA ELISA as described (20). Heterozygous littermates, which are known to be fully immunocompetent (20), or C57BL/6 (B6) mice and/or 129Sv(ev) were used as controls. In all experiments presented in this study, no significant differences between these different mouse strains used as normal controls were detected; therefore, data from one control strain (usually B6) are presented. Hox11−/− mice were generated by gene targeting on a 129Sv background and bred locally under specific pathogen-free conditions. Both mutant mice have been described in detail (19, 20). B6 and 129Sv were purchased from the breeding colony of the Institut f ür Labortierkunde (University of Zürich, Zürich, Switzerland). All animal experiments were performed with age- and sex-matched mice of 8–12 wk of age with permission of the veterinary office according to cantonal and federal law requiring the use of minimal numbers of animals.
Viruses.
The LCMV isolate WE (LCMV-WE) was originally provided by Dr. F. Lehmann-Grube (Heinrich Herre Institute, Hamburg, Germany) and the LCMV isolate Armstrong (LCMVArm) by Dr. M.B.A. Oldstone (Scripps Clinic, La Jolla, CA). LCMV-WE was grown on L929 cells and LCMV-Arm on BHK21 cells with a low multiplicity of infection (MOI). Vesicular stomatitis virus, serotype Indiana (VSV IND; Mudd-Summers isolate), originally obtained from Dr. D. Kolakofsky (University of Geneva, Geneva, Switzerland) was grown on BHK-21 cells infected at low multiplicity and plaqued on Vero cells (22). Vaccinia virus (VV, strain Lancy) was obtained from the Schweizerisches Serum und Impfinstitut (Bern, Switzerland). VV (strain WR) and recombinant VV expressing the glycoprotein of VSV IND (VaccINDG) were generous gifts of Dr. B. Moss (Laboratory of Viral Diseases, National Institute of Health, Bethesda, MD). (23). These viruses were both grown at a low MOI and plaqued on BSC 40 cells. The recombinant baculovirus expressing the glycoprotein of VSV IND (IND-G) was a gift of Dr. D.H.L. Bishop (Institute of Virology, Oxford, U.K.). The recombinant baculovirus was derived from nuclear polyhedrosis virus and was grown at 28°C in Spodoptera frugiperda cells in spinner cultures using TC-100 medium. Recombinant protein was produced as described (23).
Virus Titration.
LCMV titers of blood, centrifuged tissue homogenates, and virus stock solutions were determined with an immunological focus assay as described (24). VV was quantified by growing dilutions of tissue homogenates for 24–36 h on confluent monolayers of BSC 40 cells. Plaques were visualized by staining with crystal violet. All virus titers were expressed as log10 (PFU of virus per organ or per gram organ) or as log10 (PFU of virus per milliliter blood). The detection limits of the assays are indicated in the figures.
Statistical comparisons were made using the two-tailed unpaired Student's t test. A P value of <0.01 was regarded as significant.
Immunohistology.
Freshly removed organs were immersed in Hank's balanced salt solution and snap frozen in liquid nitrogen. Tissue sections of 5-μm thickness were cut in a cryostat, placed on siliconized glass slides, air dried, fixed with acetone for 10 min, and stored at −70°C. Secondary affinity-purified polyclonal anti-Ig antisera were diluted in Tris-buffered saline (TBS; pH 7.4) containing 5% normal mouse serum. All other dilutions were made in TBS alone. Incubations were done at room temperature for 30 min; TBS was used for all washing steps. Alkaline phosphatase was visualized using naphthol AS-BI phosphate and new fuchsin as substrate. Endogenous alkaline phosphatase was blocked by levamisole. All color reactions were performed at room temperature for 15 min with reagents from Sigma Chemical Co. (St. Louis, MO). Sections were counterstained with hemalum. Coverslips were mounted with glycerol and gelatin.
For staining for LCMV and cell differentiation markers, rehydrated tissue sections were incubated with the following rat primary mAbs: anti–MHC class I (anti–H-2Kbdk; M1/42; TIB-126; American Type Culture Collection, Rockville, MD), anti–MHC class II (anti-Iabdq; M5/114; TIB-120; American Type Culture Collection), anti-CD4 (YTS 191; reference 25), anti-CD8 (YTS 169; reference 25), anti-CD45R/B220 (RA3-6B2; PharMingen, San Diego, CA), anti-LCMV (VL-4; reference 26), anti–red pulp macrophages (RPM; F4/80; HB-198; American Type Culture Collection), anti–marginal metallophils (MM; MOMA-1; Biomedicals, Augst, Switzerland), anti–marginal zone macrophages (MZM; ERTR-9; reference 27), anti–follicular dendritic cells (FDC; 4C11; reference 28), anti–mucosal addressin cell adhesion molecule 1 (MAdCAM-1; MECA-367; PharMingen), anti–interdigitating dendritic cells (IDC; NLDC-145; Biomedicals). Primary rat mAb were revealed by a twofold sequential incubation with rabbit anti– rat Ig and rat alkaline phosphatase anti–alkaline phosphatase complex (DAKO, Glostrup, Denmark). CD11c on dendritic cells was stained with the hamster mAb N418 (HB-224; American Type Culture Collection). Primary hamster Ig were detected by alkaline phosphatase–labeled rabbit anti–hamster Ig followed by alkaline phosphatase labeled goat anti–rabbit Ig. Germinal centers (GC) were stained with peanut agglutinin (12). To stain for Ig, sections were incubated with biotinylated monoclonal rat anti–mouse IgM (R6-60.2) or rat anti–mouse IgDb (217-170) (PharMingen), followed by alkaline phosphatase–labeled avidin–biotin complexes (DAKO).
Detection of Virus-specific Cytotoxic T Cells In Vitro.
CTL activity of spleen or LN cells was determined by a 51Cr–release assay as described previously (29). In brief, mice were infected with VV, VSV, or LCMV at the indicated doses. 6 d (VV, VSV) or 8 d (LCMV) later, spleen or LN cells were suspended at 9 × 106/ml in MEM supplemented with 2% FCS. MC57G (H-2b) fibroblast target cells were infected with LCMV (MOI = 0.01) 48 h, or with VV (MOI = 5) 3 h, or with VSV (MOI = 15) 3 h before they were used. Target cells were labeled with 0.4 mCi of [51Cr]sodium chromate for 2 h at 37°C and suspended at 105/ml. Threefold dilutions of spleen or LN cells (100 μl) were incubated with 100 μl of targets for 5 h (LCMV, VSV) or 6 h (VV) in 96-well round-bottom plates. 70 μl of supernatant was assayed for released 51Cr. The percent specific 51Cr-release was calculated as: [(experimental release − spontaneous release) × 100/(total release − spontaneous release)].
Alternatively, spleen cells were restimulated in vitro before testing; 4 × 106 spleen cells from infected mice were cultivated in the presence of 2 × 106 irradiated (25 gray) B6 stimulator spleen cells in 2 ml of IMDM supplemented with 10% FCS, 10−5 2-mercaptoethanol and 5% Con A supernatant as a source of interleukin-2 in 24-well plates. Stimulators were infected with LCMV (MOI = 0.1) or UV-inactivated VSV (MOI = 15) for 2 h at 37°C. 5 d later, restimulated spleen cells of one culture well were resuspended in 1 ml of MEM supplemented with 2% FCS and serial threefold dilutions of effectors were prepared (referred to as dilution of standard culture) and tested as described above.
Serum Neutralization Test.
Neutralizing titers of sera were determined as described (30). In brief, sera were prediluted 40-fold in supplemented MEM and then heat-inactivated for 30 min at 56°C. Serial twofold dilutions were mixed with equal volumes of virus diluted to contain 500 PFU/ml. After incubation for 90 min at 37°C in an atmosphere containing 5% CO2, 100 μl of the serum–virus mixture was transferred onto Vero cell monolayers in 96-well plates and incubated for 1 h at 37°C. The monolayers were then overlaid with 100 μl DMEM containing 1% methyl cellulose. After incubation for 24 h at 37°C, the overlay was removed and the monolayer was fixed and stained with 0.5% crystal violet. The highest dilution of the serum that reduced the number of plaques by 50% was taken as the neutralizing titer. To determine IgG titers, undiluted serum was first pretreated with an equal volume of 0.1 M 2-mercaptoethanol in saline. Titers represent log2 steps of 40-fold prediluted sera.
Assessment of Footpad Swelling.
Mice were inoculated with the indicated LCMV doses in 30 μl MEM in both hind footpads, and footpad swelling was assessed daily with a spring-loaded caliper (31).
In Vivo Depletion of CD4+ T Cells and Splenectomy.
T helper cells were depleted as described (25). Efficiency of depletion was verified by FACS® (Becton Dickinson, Mountain View, CA) analysis of blood at the day of immunization, and again 8–12 d later. After anesthesia of the mice, the spleen was prepared and two ligatures were set with unresorbable silk (USP 4-0; B. Braun, SSC AG, Emmenbrücke, Switzerland) before removal of the spleen. The peritoneal cavity was closed with a silk suture and the skin was stapled.
Adoptive Transfer Experiments.
Recipient mice were sublethally irradiated with 5.0 gray 1 d before indicated numbers of spleen cells were transferred adoptively. Immunization with 2 × 106 PFU live VSV IND or VaccINDG was performed 1–3 d after transfer (see Fig. 8). For the detection of LCMV-specific effector functions, 5 × 107 spleen cells of unprimed or of day-8 LCMVWE immunized mice (200 PFU LCMV-WE intravenously) were adoptively transferred into aly/aly or aly/+ recipients which had been infected with 2 × 105 LCMV-WE inoculated into the footpads 3 h before transfer. Thereafter, the resulting footpad swelling reaction was monitored as described.
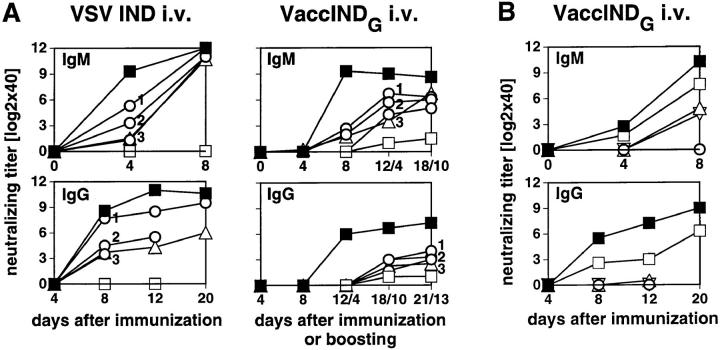
Figure 8.
VSV-neutralizing Ab responses of irradiated recipients after AT of naive spleen cells. Recipients were irradiated with 5.0 gray on day −2 and adoptively transfused with various numbers of spleen cells on day −1 and then immunized with 2 × 106 PFU live VSV IND or VaccINDG intravenously on day 0 and boosted on day 8 with the same antigen (8 A, right). Blood was taken from the mice at the indicated time points after priming or boosting and the neutralizing IgM (top) or IgG titers (bottom) were measured. (A) ▵, AT of 108 aly/aly spleen cells into irradiated B6; ○, AT of 108, 3 × 107 or 107 aly/+ spleen cells into irradiated B6; □, irradiated B6 not receiving cells; ▪, untreated B6. no Spl, no spleen cells transferred. (B) ▿, AT of 108 B6 spleen cells into irradiated aly/aly; ▵, untreated aly/aly; ○, irradiated aly/aly not receiving cells; □, AT of 108 B6 spleen cells into irradiated aly/+; ▪, untreated aly/+ mice. no Spl, no spleen cells transferred; irr., irradiated. Each line represents the mean of four mice, variations of individual mice were below two 1:2 dilution steps.
Flow Cytometry.
50 μl of blood was prepared in PBS containing 2% FCS, 0.2% NaN3, and 10 mmol EDTA. Cell surface markers were quantified by staining with mAb conjugated with fluorochromes. The following Abs were used: anti-CD4 PE (clone H129.19), anti-CD8 FITC (clone 53-6.7) from GIBCO BRL (Uxbridge, U.K.), anti-CD45R/B220 PE (clone RA3-6B2) from Sigma Chemical Co. Multiparameter analysis was performed with a FACScan® using logarithmic scales. Viable cells were gated by forward and side scatter of light.
Results
Immunohistochemistry of aly/aly and aly/+ Spleen
The histological structures of the spleen of unimmunized aly/aly mice and of heterozygous littermates were evaluated with different mAb by immunohistochemistry (Fig. 1). The absence of the distinct follicular structure of the white pulp with an ill-defined boundary between white and red pulp was obvious at low magnifications. This is clearly demonstrated by the stainings for B cells (B220, IgM, IgD) and for certain macrophage populations such as RPM, MM, or MZM.
Figure 1.
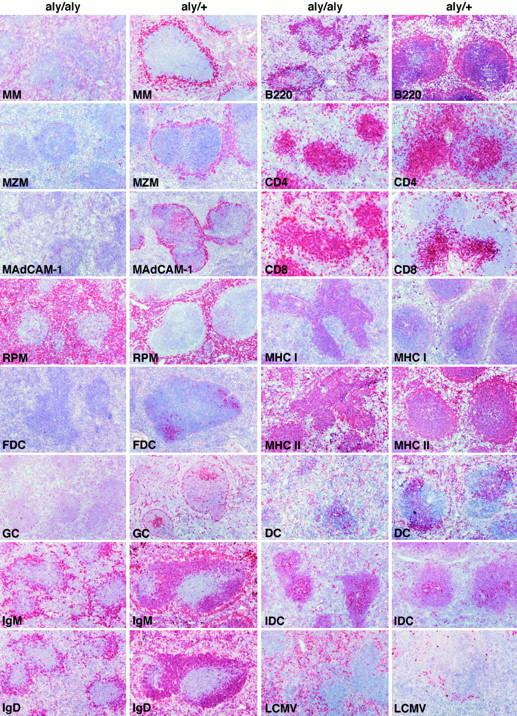
Immunohistochemical analysis of aly/aly and aly/+ spleens. Spleen sections of unimmunized (exception: LCMV staining) aly/aly and aly/+ mice were stained with Abs of the indicated specificity. MM, MOMA-1; MZM, ERTR-9; MAdCAM-1 on endothelial cells of the marginal sinus, MECA-367; RPM, F4/80; FDC, 4C11; GC, PNA; IgM, surface IgM+ B cells, R6-60.2; IgD, surface IgD+ B cells, 217-170; B220, CD45R+ B cells, RA3-6B2; CD4, CD4+ T cells, YTS-191; CD8, CD8+ T cells, YTS-169; MHC I, H-2Kbdk, M1/42; MHC II, Iabdq on macrophages, DC, and B cells, M5/114; IDC, NLDC-145; DC, CD11c on dendritic cells N418; LCMV, LCMV-infected cells 8 d after infection with 200 PFU LCMV-WE intravenously, VL-4. Original magnification: 100-fold for all panels.
The T cell areas stained with anti-CD4 or anti-CD8 mAbs seemed to be largely intact in the spleen of aly/aly mice. The total number of CD4+ and CD8+ T cells and their ratios were normal in spleen and peripheral blood (FACS® analyses not shown). In addition, the expression of class I and II MHC molecules did not markedly differ. IDC and DC seemed to be present in aly/aly mice in numbers and distribution that were comparable to that of their heterozygous littermates. In contrast, numbers of B cells were about threefold reduced in spleen and peripheral blood (FACS® analyses not shown) of aly/aly mice, and no primary or secondary follicles or GC were detectable in the spleen, as previously reported (32). The lack of GC was accompanied by a complete lack of FDC. MM and MZM were absent in aly/aly mice. In addition, MAdCAM-1, an adhesion molecule normally expressed in the splenic marginal zone (MZ) (33), was undetectable in aly/aly spleens (34). These data confirm and extend findings from Koike et al. that showed the lack of the MZ in the spleen of aly/aly mice (34). In summary, aly/aly mice showed, besides the total lack of LN and PP, a prominent structural and cellular defect of the splenic MZ accompanied by a reduction of B cells and an absence of primary and secondary B cell follicles together with a lack of differentiated FDC and of GC.
LCMV-specific CTL Responses, Immunopathological Footpad Swelling Reaction, and Perforin-dependent Virus Clearance in aly/aly and Hox11− /− Mice
To assess CD8+ T cells and perforin-dependent antiviral immune responses against a noncytopathic virus, mice were immunized with 200 PFU LCMV (WE or Armstrong isolate) intravenously or subcutaneously into the hind footpads, and 8 or 9 d later, spleen and/or LN cells were directly tested in a 51Cr–release assay. The two LCMV strains differ in their tissue tropism and replication kinetics (35). Control B6 and Hox11−/− mice yielded high LCMV-specific CTL responses 8 d after infection, whereas aly/aly mice showed no detectable LCMV-specific CTL activity (Fig. 2 A).
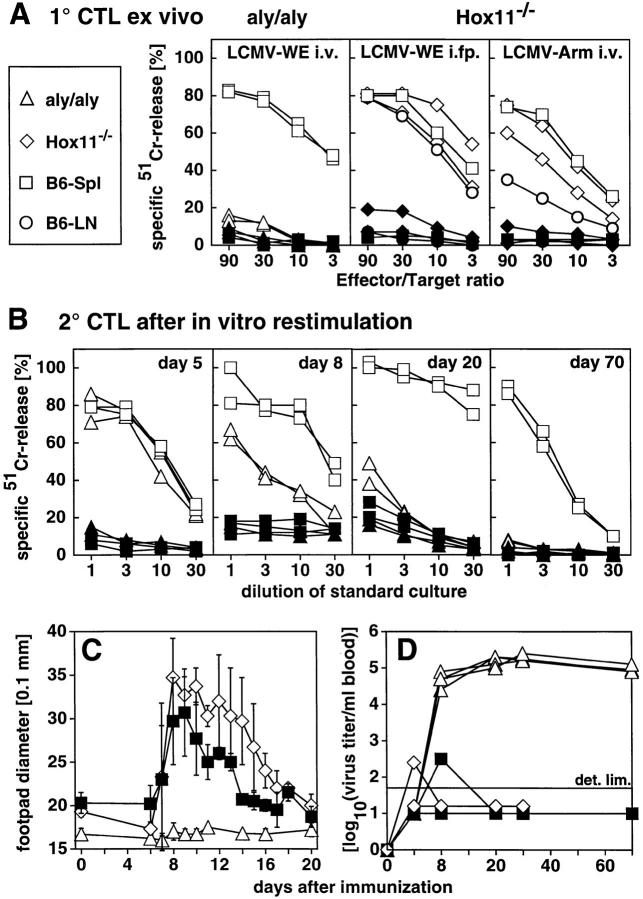
Figure 2.
CTL response, footpad swelling reaction, and blood virus titer after LCMV infection of aly/aly and Hox11−/− mice. Mice were immunized with 200 PFU LCMV-WE intravenously (A, left, B and D), LCMV-Arm intravenously (A, right), or 100 PFU LCMV-WE into both hind footpads (i.fp., A, middle, and C). After 8 d, spleens were removed and single cell suspensions were tested directly ex vivo in a conventional 51Cr–release assay (primary [1°] CTL ex vivo) with LCMV-infected (open symbols) or untreated (closed symbols) MC57G as target cells. ▵ ▴, aly/aly spleen cells; ⋄ ♦, Hox11−/− LN cells; □ ▪, spleen cells of control B6 mice; ○ •, LN cells of control B6. Each line represents an individual mouse with the exception of the B6 mice in A, middle and right (squares and circles) representing the mean of three mice. (B) spleens were removed at the indicated time points after infection with 200 PFU LCMV-WE intravenously and restimulated in vitro for 5 d (secondary [2°] CTL after in vitro restimulation) with irradiated LCMV-infected spleen cells. Cultures were then tested on LCMV-infected (open symbols) or untreated (closed symbols) MC57G cells radioactively labeled with 51Cr. ▵ ▴, aly/aly mice; □ ▪, aly/+ control mice. Each line represents one individual mouse. (C) mice were infected with 100 PFU LCMV-WE into both hind footpads. The footpad thickness was measured daily with a spring-loaded caliper. ▵, aly/aly mice; ⋄, Hox11−/− mice; ▪, B6 controls. Each line represents the mean of both hind footpads of three mice. Error bars indicate the SD within the experimental group. Results of one (out of three) similar experiment are shown. (D) Mice were infected with 200 PFU LCMV-WE intravenously. Blood was taken at the indicated time points and virus titers were determined as described (24). LCMV titers are given as log10 (virus titer/ml blood). ▵, aly/aly mice; ⋄, Hox11−/− mice; ▪, control B6 mice. Each line represents one mouse. Data of 4 mice/group are shown. The detection limit of the assay is indicated (det. lim.). One out of two similar experiments is shown.
To evaluate the kinetics of LCMV-specific CTL responses in aly/aly mice with a very sensitive assay, spleen cells were restimulated at different time points after LCMV infection (day 5, 8, 20, and 70) for 5 d in vitro with LCMV-infected, irradiated spleen cells (Fig. 2 B). The LCMV-specific CTL activity of aly/aly mice on day 5 after infection was identical to control mice, demonstrating the induction of virus-specific CTLs in aly/aly mice. However, already on day 8 after infection, the CTL activity of aly/aly mice was ∼10-fold reduced compared to heterozygous littermates. By day 20, virtually no LCMV-specific CTLs were detectable in aly/aly mice. The mice did not recover from this state of unresponsiveness until day 70.
To assess the in vivo consequences of the in vitro–measured CTL activities, the CD8+ T cell–dependent immunopathological footpad swelling reaction was measured daily after local infection with 100 PFU LCMV-WE into both hind footpads (Fig. 2 C). Control and Hox11−/− mice exhibited a similar CD8+ T cell–mediated swelling reaction from day 6 until day 10. The swelling reaction was prolonged in Hox11−/− mice, possibly reflecting a more pronounced CD4+ T cell response (31). In contrast, aly/aly mice showed no footpad swelling reaction. After intracerebral (i.c.) infection with 3, 30, or 300 PFU of the neurotropic LCMV-Arm, aly/aly mice did not succumb to the usually observed lethal immunopathological choriomenigitis mediated by CD8+ CTLs; nine of nine aly/aly mice (three mice per virus dose) survived for >30 d without clearance of the virus, whereas nine of nine control B6 and six of six Hox11−/− mice died between 7–9 d after infection.
To evaluate the kinetics of virus clearance in vivo, the elimination of LCMV after peripheral infection was monitored. As shown in Fig. 2 D, aly/aly mutant mice were not able to clear the virus from their blood or any other organ tested (spleen, see Fig. 1, LCMV staining). Virus persisted for life after infection with any of the tested doses (3– 10,000 PFU) or routes of infection (intravenous, intraperitoneal, intracerebral, or subcutaneous) of both LCMV strains (WE or Arm) used in this study. It has been shown that >106 PFU of intravenous LCMV-WE are needed to provoke CTL exhaustion and virus persistence in adult immunocompetent B6 mice (36). Thus, aly/aly mice were at least 106-fold more susceptible to become LCMV carriers than normal B6 mice. In contrast to aly/aly mutant mice, blood virus titers of control and Hox11−/− mice reached barely detectable levels (Fig. 2 D); the latter mice cleared LCMV from the tested organs at the latest by day 15 (not shown).
Careful analysis of LCMV titers in different organs of aly/aly and aly/+ mice during the first 4 d after intravenous infection revealed that virus titers of the spleen were ∼100 times lower in aly/aly mice, 12, 24, and 48 h after infection, whereas in peripheral solid organs (liver and lung) the titers were generally higher (Table 1). This suggests that LCMV particles were less efficiently removed from the blood by the spleen of aly/aly mice. Therefore, probably more infectious viral particles reached peripheral solid organs.
Table 1.
>LCMV Titers of aly/aly and aly/+ Mice Early after Infection with 104 PFU LCMV-WE Intravenously
| Time after infection | Spleen | Liver | Lung | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aly/aly | aly/+ | aly/aly | aly/+ | aly/aly | aly/+ | |||||||
| h | ||||||||||||
| 12 | <DL* ‡ | 3.7 ± 0.6* | 3.2 ± 0.2 | 3.3 ± 0.5 | <DL | <DL | ||||||
| 24 | 3.7 ± 0.7* | 6.6 ± 0.1* | 3.5 ± 0.4 | 3.9 ± 0.4 | <DL | <DL | ||||||
| 48 | 6.0 ± 0.3* | 7.8 ± 0.2* | 4.3 ± 0.3 | 3.4 ± 0.7 | 4.3 ± 0.3* | 3.3 ± 0.2* | ||||||
| 96 | 6.6 ± 0.1 | 6.4 ± 0.1 | 5.4 ± 0.3* | 4.6 ± 0.3* | 5.6 ± 0.2* | 4.6 ± 0.3* | ||||||
Mice were infected with 104 PFU LCMV-WE intravenously. At the indicated time points after infection mice were killed and virus titers were determined in different organs. Values are given as log10(PFU LCMV/g organ) ± SD of 3–4 mice/experimental group.
P <0.01, significant difference of LCMV titers between aly/aly and aly/+ mice (Student's t test).
DL, detection limit of the assay (spleen, 2.0; liver, 3.0; lung, 2.5). Representative data of one out of three comparable experiments are shown.
To test whether only the induction of LCMV-specific CTL responses was less efficient or whether effector functions of primed CD8+ T cells were affected by the structural defect of aly/aly mice also, the following adoptive transfer experiments were performed. Naive aly/aly or aly/+ recipient mice were infected with 2 × 105 LCMV-WE into the footpads. 3 h later, 5 × 107 spleen cells of either naive or day-8 LCMV-primed B6 mice (200 PFU LCMVWE intravenous) were adoptively transferred. Thereafter, the immunopathological footpad swelling reaction was monitored. aly/aly and aly/+ recipients of LCMV-primed spleen cells showed a similar early swelling peak already 18–24 h after transfer, reflecting the local LCMV-specific effector phase of the transferred LCMV-primed cells. In contrast, recipients of naive spleen cells showed no early swelling reaction. The usual primary footpad swelling reaction peaking around day 8 after LCMV infection was only detectable in aly/+ recipients of naive spleen cells (Fig. 3 A). aly/aly recipients of naive B6 spleen cells did not mount a detectable swelling reaction. On day 9 after infection, LCMV titers in different organs and the CTL response of 5 d in vitro restimulated splenocytes was measured. aly/+ recipients of naive and primed spleen cells showed a potent CTL response and complete clearance of the virus from the tested organs. In contrast, aly/aly recipients exhibited clear differences in virus titers and CTL activity dependent upon whether the transferred cells were primed or naive. Viral titers were about 100 times lower and the CTL activity 10–30-fold higher in the aly/aly recipients of primed spleen cells, demonstrating that the effector function of the transferred primed T cells was unimpaired in aly/aly mice. (Fig. 3, B and C).
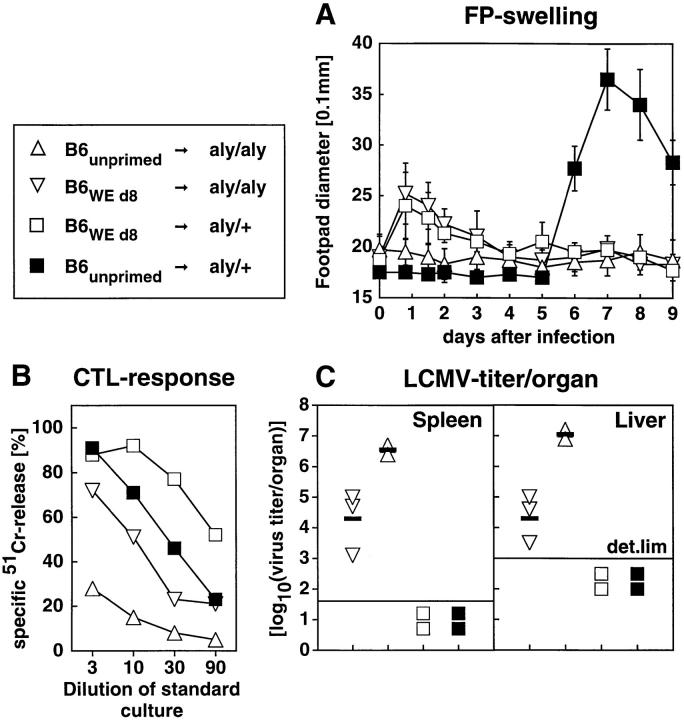
Figure 3.
LCMV-specific immune response of aly/aly mice after adoptive transfer (AT) of naive or LCMV-primed B6 spleen cells. Recipient mice were infected with 2 × 105 LCMV-WE into the footpads. 3 h later, 5 × 107 naive or day 8 LCMV-WE–primed B6 spleen cells were adoptively transferred. ▵, aly/aly recipients, adoptively transfused with naive B6 spleen cells; ▿, aly/aly recipients transfused with day-8 LCMVprimed B6 spleen cells; □ aly/+ recipients transfused with day-8 LCMVprimed B6 spleen cells; ▪ aly/+ recipients transfused with naive B6 spleen cells. The footpad swelling reaction was monitored daily (A). Means and SD are given for both hind footpads of two to three mice per group in a 0.1-mm scale. The CTL response was measured on day 9 after infection (B). The spleen cells were restimulated with irradiated LCMV-infected spleen cells for 5 d. Cultures were then tested on LCMV-infected 51Cr-labeled MC57G cells. Unspecific lysis of uninfected targets was <10%. Each line represents the mean of two to three mice. LCMV titers in spleen and liver on day 9 after infection (C). Single values of individual mice, means (horizontal bars) and the detection limit (det. lim.) of the assay are indicated. Values are given as log10(PFU per organ). The data represent one out of two comparable experiments.
VV-specific CTL Responses and Virus Elimination Kinetics of aly/aly and Hox11− /− Mice
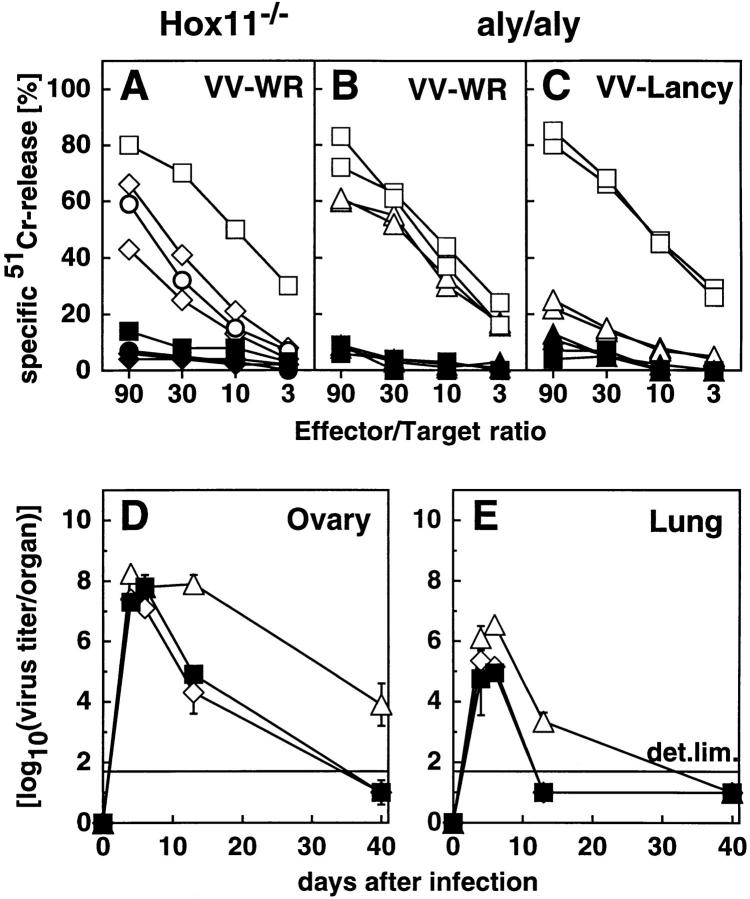
To test the CTL response of these mutant mice against a cytopathic virus, mice were immunized with 2 × 106 PFU vaccinia virus (VV) strain WR or Lancy. 6 d later, spleens and/or LN were removed and tested directly in a VV-specific 51Cr–release assay with VV-infected MC57G fibroblasts as target cells. LN cells of Hox11−/− mice exhibited comparable CTL activity to LN cells of B6 and 129Sv controls (Fig. 4 A).
Figure 4.
VV-specific CTL response and VV clearance. Mice were infected with 2 × 106 VV-WR (A, B, D, and E) or VV-Lancy (C). On day 6, spleens and/or LN were removed and directly tested in a standard 51Cr–release assay (A–C). (Open symbols) VV-infected 51Cr-labeled MC57G cells (A–C) as target cells. (Closed symbols) Uninfected target cells. ▵ ▴, aly/aly spleen cells; ⋄ ♦, Hox11−/− LN cells; □ ▪, control B6 spleen cells; ○ •, control B6 LN cells. Each line represents one individual mouse with the exception of the controls in A (squares and circles) where the mean of three mice is shown. D and E show VV titer in ovary and lung given as log10(PFU per organ) at different time points after infection with 2 × 106 PFU VV-WR intravenously. Each line represents the mean and the SD of three mice per group. The detection limit of the assay (det. lim.) is indicated. ▵, aly/aly mice; ⋄, Hox11−/− mice; ▪, B6 control mice. Data of one of two comparable experiments is shown.
The VV-WR–specific CTL response of aly/aly mice was only ∼3–10-fold reduced compared to control B6 mice (Fig. 4 B). If mice were infected with VV-Lancy, an attenuated VV strain that does not measurably replicate in immunocompetent mice (37), the CTL response was reduced by at least a factor of 30 (Fig. 4 C). The elimination of VVWR from the tested organs was clearly delayed in aly/aly mice compared to Hox11−/− or control B6 mice (Fig. 4, D and E).
Immune Responses against VSV
VSV-specific CTL Response.
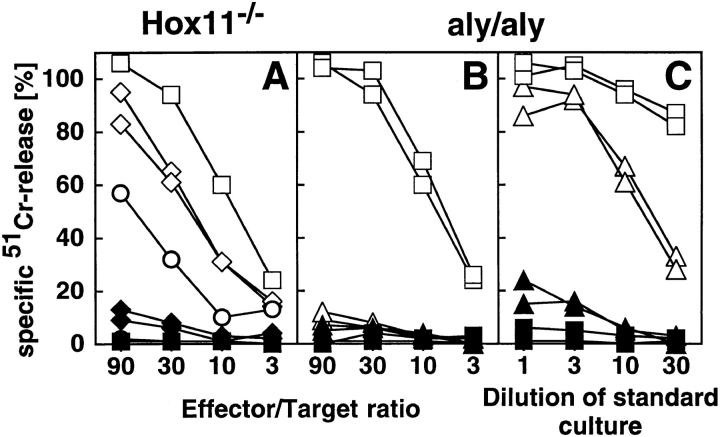
The dispensable but usually induced VSV-specific primary CTL response on day 6 after immunization with 2 × 106 live VSV IND of Hox11−/− and control B6 mice was comparable (Fig. 5 A). No primary ex vivo CTL activity could be detected in aly/ aly mice (Fig. 5 B). Upon in vitro restimulation for 5 d with VSV-infected, irradiated spleen cells, VSV-specific CTLs were also detectable in aly/aly mice, but still 10–30-fold reduced compared to B6 mice (Fig. 5 C).
Figure 5.
VSV-specific CTL response. Mice were infected with 2 × 106 live VSV IND intravenously. On day 6, spleens and LN were removed and directly tested in a standard 51Cr–release assay (A and B). Alternatively, spleen cells were restimulated in vitro for 5 d with VSV-infected and irradiated spleen cells (C) before cultures were tested. (Open symbols) VSV infected MC57G cells as target cells. (Closed symbols) Uninfected target cells. ▵ ▴, aly/aly spleen cells; ⋄ ♦, Hox11−/− LN cells; □ ▪, control B6 spleen cells; ○ •, control B6 LN cells. Each line represents one individual mouse, with the exception of the controls in A (squares and circles) where the average of three mice is shown. Data of one out of three representative experiments is shown.
VSV-specific Neutralizing Ab Response.
The VSV-neutralizing Ab response of aly/aly, Hox11−/−, and control mice was assessed using different antigenic preparations derived from VSV IND, e.g., live and UV-inactivated VSV IND particles, a recombinant VV expressing the glycoprotein of VSV IND (VaccINDG), and IND-G produced and extracted from a baculovirus expression system. Earlier studies showed that the neutralizing IgM response to VSV particles peaks around day 8 after immunization, is largely T helper cell–independent, and reflects B cell responsiveness directly (38–40). Live and UV-inactivated VSV IND particles have been defined to be TI-1 antigens without stimulating B cells polyclonally, whereas VaccINDG and IND-G were shown to be TI-2 antigens (40, 41). The neutralizing IgG response peaks around day 12 and is T helper cell–dependent.
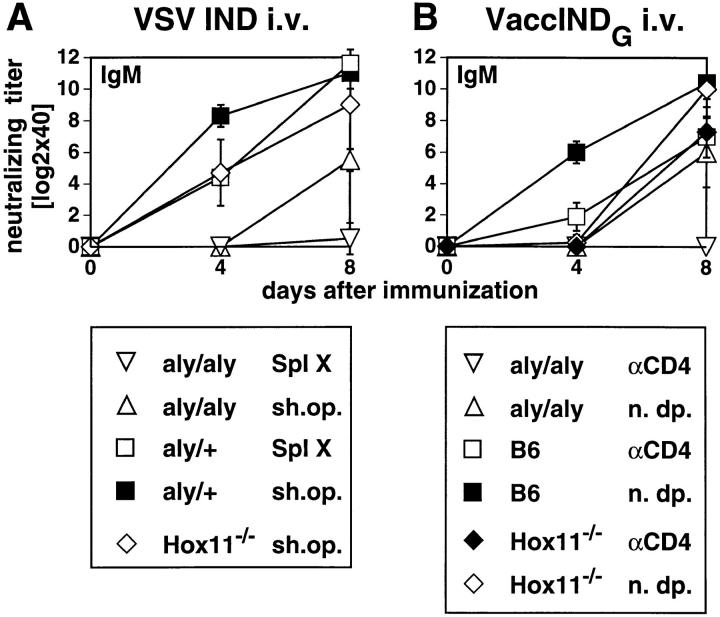
Upon intravenous immunization with 2 × 106 live VSV IND, aly/aly mice showed a delayed and overall reduced production of neutralizing IgM. By day 8, they reached titers comparable to controls. Most importantly, aly/aly mice failed to produce neutralizing IgG at any time point tested (Fig. 6 A). The protection from lethal encephalitis caused by VSV mostly depends on the production of neutralizing IgG by day 6–8 (42). Therefore, all aly/aly mice died after infection with live VSV IND between day 6 and day 10. Spleenless Hox11−/− mice showed a delay in the IgM response upon intravenous infection and, to a smaller degree, in the production of IgG, but they were protected from encephalitis.
Figure 6.
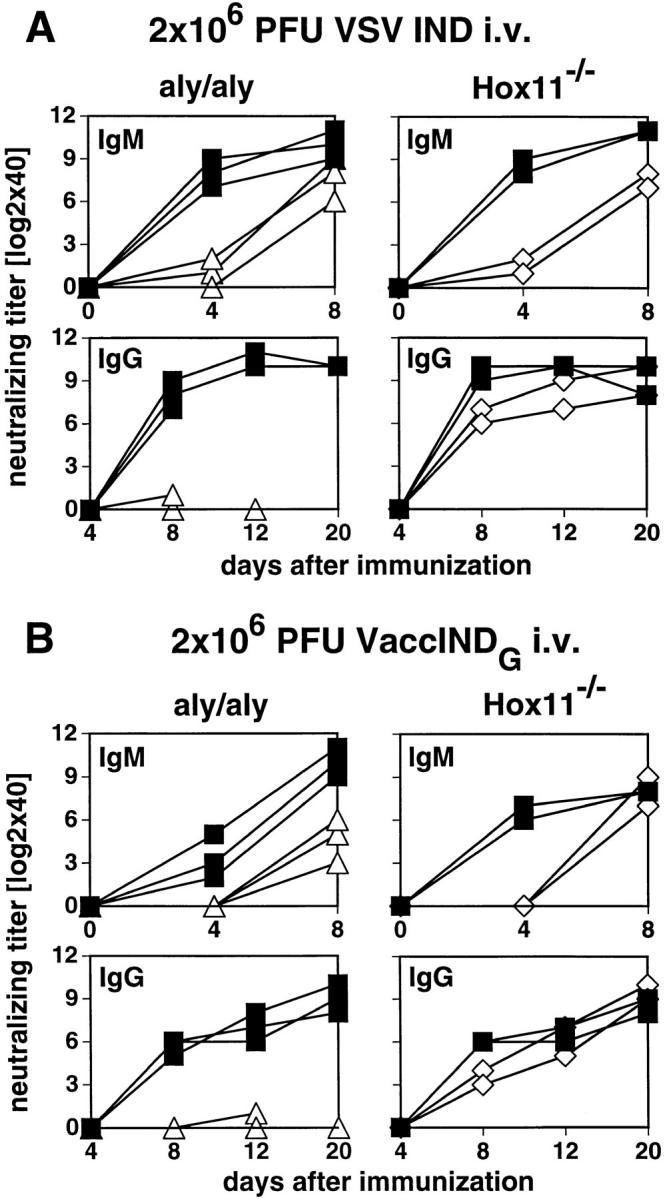
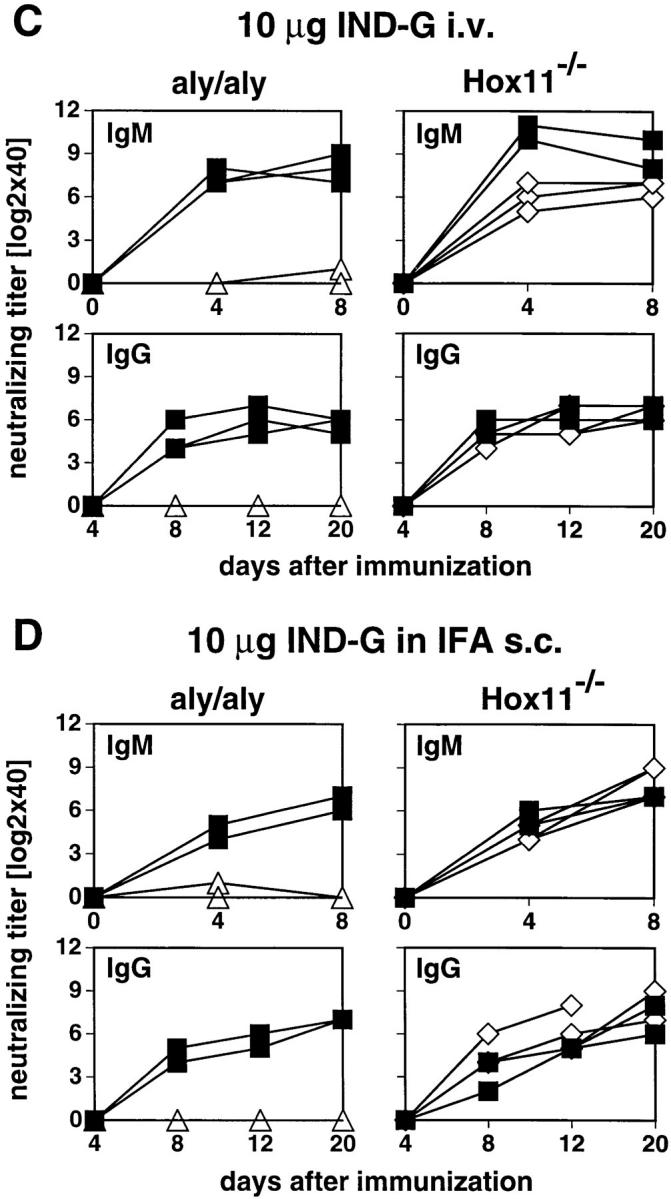
VSV-neutralizing IgM and IgG responses. Mice were immunized with 2 × 106 PFU live VSV IND intravenously (A), 2 × 106 PFU VaccINDG intravenously (B), or with 10 μg IND-G in balanced salt solution intravenously (C), or emulsified in IFA and injected subcutaneously at the base of tail (D). Blood was taken at the indicated time points and the neutralizing IgM (upper panels) or IgG titers (lower panels) were determined in the sera. Titers represent log2 dilutions of 40-fold prediluted sera. ▵, aly/aly mice; ⋄, Hox11−/− mice; ▪, B6 control mice. Each line represents one individual mouse. One out of three similar experiments is shown.
After infection with 2 × 106 PFU of the TI-2 Ag VaccINDG intravenously, aly/aly mice mounted a delayed and reduced neutralizing IgM response, but completely failed to switch to IgG. Hox11−/− mice again exhibited a delay in the neutralizing IgM and IgG response (Fig. 6 B).
To examine the influence of the route of Ag administration on the neutralizing Ab response against VSV, we immunized aly/aly and Hox11−/− mice with 10 μg of the nonreplicating TI-2 Ag IND-G solubilized in balanced salt solution intravenously or emulsified in IFA subcutaneously (Fig. 6, C and D). Both immunization protocols failed to induce detectable neutralizing IgM or IgG in aly/aly mice, even after a booster injection. After hyperimmunization by four injections of 10 μg IND-G or 108 PFU UV-inactivated VSV IND in IFA intraperitoneally in 10-d intervals, a substantial neutralizing IgM titer, but no IgG, was induced in aly/aly mice (not shown). Hox11−/− mice promptly responded to IND-G by both routes after a single immunization; a delay of 2–3 d in the IgM response was noticed only after intravenous immunization.
Effects of Splenectomy or Depletion of CD4+ T Cells of aly/aly, Hox11− /−, and B6 Control Mice on the Neutralizing IgM Response after Immunization with VSV IND or VaccINDG
The role of secondary lymphoid organs in TI-1 B cell responses was further assessed in mice lacking all secondary lymphoid organs, i.e., splenectomized aly/aly mice. aly/aly and control aly/+ mice were splenectomized or sham operated and 3 wk later they were immunized with 2 × 106 PFU live VSV IND intravenously. The IgM response was completely absent in three out of four splenectomized aly/ aly mice. Only one mouse still produced a low neutralizing IgM titer on day 8, whereas the IgM response of sham operated aly/aly mice was similar to untreated ones. All aly/ aly mice, splenectomized or not, died from VSV-induced encephalitis within 9 d. The IgM response of splenectomized aly/+ (or 129Sv, not shown) controls was very similar to the response of Hox11−/− mice showing a delay after intravenous immunization compared to sham-operated control mice (Fig. 7 A).
Figure 7.
VSV-neutralizing Ab response of aly/aly, Hox11−/−, and B6 control mice after splenectomy and immunization with VSV IND (A) or after depletion of CD4+ T cells and immunization with VaccINDG (B). (A) Mice were splenectomized or sham operated and 3 wk later they were immunized with 2 × 106 PFU live VSV IND intravenously. Blood was taken at the indicated time points and the neutralizing IgM titer was determined. Each line represents the mean of four mice; error bars indicate the SD. ▵, aly/aly mice, sham operated; ▿, splenectomized aly/aly mice; □, splenectomized aly/+ controls; ▪, aly/+ controls, sham operated; ⋄, Hox11−/−, sham operated. Spl X, splenectomized; sh.op., sham operated. Data from one of two independent experiments are shown. (B) Mice were depleted of CD4+ T cells and then immunized with 2 × 106 PFU VaccINDG intravenously. Blood was taken at the indicated time points and the neutralizing IgM titer was determined. Efficiency of depletion was confirmed by FACS® analysis at the day of immunization and at day 8. Each line represents the mean of three to four mice, error bars indicate the SD. ▿, aly/aly mice, CD4 depleted; ▵, aly/aly mice, not depleted; □, B6 controls, CD4 depleted; ▪, B6 controls, not depleted; ♦, Hox11−/−, CD4 depleted; ⋄, Hox11−/−, not depleted. αCD4, depleted of CD4+ T cells; n.dp., not depleted. Data from one of three independent experiments are shown.
To further analyze whether the IgM response to the TI B cell Ag VaccINDG or VSV IND was still independent of T help in these mutant mice, aly/aly, Hox11−/−, and control B6 mice were depleted of CD4+ T cells before immunization. The neutralizing IgM response after immunization with the TI-2 Ag VaccINDG was T help dependent in aly/aly mice, but it was largely T help independent in Hox11−/− and in B6 controls (Fig. 7 B). This indicates that a low level of cooperation between T and B cells still occurs in aly/aly mice, which is sufficient to support the IgM response to VaccINDG, but not sufficient to induce the switch to IgG. As expected, aly/aly, Hox11−/−, and B6 control mice treated identically with anti-CD4 and immunized with the TI-1 Ag live VSV IND generated a T help independent IgM response (data not shown).
Effect of Reciprocal Adoptive Transfer of Naive Spleen Cells Between aly/aly and Control Mice on the VSV-specific Ab Response
To evaluate in vivo whether the defect of cognate interaction between B and T cells in aly/aly mice seen in the previous experiments is mainly due to a cellular defect of the B or T cell compartment, or of both together, or is due to the structural defect of secondary lymphoid organs in aly/aly mice, reciprocal adoptive transfer experiments between aly/aly and control mice were performed (Fig. 8).
On day −2 recipients were irradiated with 5.0 gray, on day −1 indicated numbers of spleen cells were transferred, and on day 0 mice were immunized with 2 × 106 PFU live VSV IND or VaccINDG. 108 aly/aly spleen cells or a titrated number of aly/+ spleen cells (108, 3 × 107, or 107) were transferred into irradiated B6 recipients (Fig. 8 A). Irradiated B6 recipients without adoptive cell transfer did not respond to both immunization protocols, demonstrating the effectiveness of the irradiation. After transfer of graded numbers of aly/+ spleen cells, the recipients responded with correspondingly graded neutralizing Ab responses; the mice receiving the highest cell number responded the best. Recipients of 108 aly/aly spleen cells exhibited a neutralizing IgM and IgG response against VSV IND and VaccINDG that was comparable to that of recipients transfused with 107 − 3 × 107 aly/+ spleen cells. This result indicates that aly/aly spleen cells exhibit the capacity to respond to thymus-dependent and -independent Ag after transfer into a recipient with an intact structure of secondary lymphoid organs. After compensation for the threefold reduced number of B cells (FACS® analysis not shown), the response was almost comparable to recipients of aly/+ spleen cells.
A second adoptive transfer experiment evaluated whether naive spleen cells of B6 mice adoptively transferred into irradiated aly/aly or aly/+ recipients were able to respond to immunization with VaccINDG (Fig. 8 B). The transfer of 108 B6 spleen cells into aly/aly recipient mice failed to increase the neutralizing IgM or IgG response compared to untreated aly/aly mice. Irradiation prevented aly/aly and aly/+ mice from responding to VaccINDG. In control experiments, 108 B6 spleen cells were transferred into aly/+ recipients and neutralizing IgM and IgG were readily induced.
These adoptive transfer experiments indicate that the T and B cells of aly/aly mice can respond to viral antigens if transferred into normal mice possessing organized secondary lymphoid organs. In contrast, spleen cells of normal B6 mice transferred into aly/aly mice did not function properly.
Discussion
In this study we investigated the effects of an altered morphology of secondary lymphoid organs on antiviral immune responses using Hox11−/− and aly/aly mutant mice. Absence of the spleen in Hox11−/− mice resulted in a certain delay of the systemic Ab response without measurable consequences for T cell–dependent immune functions. Absence of LN and PP, together with structural alterations of the splenic white pulp, resulted in a complete shift of the balance between virus replication and the host's antiviral immune defense, with, as a consequence, establishment of persistent infection with a noncytopathic virus (LCMV) and increased susceptibility to cytopathic viruses such as VSV and VV.
The immunohistological analysis of aly/aly spleens shown in this study confirms and extends previous reports on the absence of MM and MZM and of FDC in aly/aly mice (32, 34). The genetic defect leading to the aly/aly phenotype is not yet defined (43). Since mice deficient for lymphotoxin α or for both lymphotoxin α and TNF-α also lack LN and PP and show similar structural changes in the splenic white pulp, there might be a common mechanism during organogenesis, even though the genomic localizations of the affected genes are different (43–47).
Although LCMV infection led to the induction of virusspecific CTLs in the spleen of aly/aly mice, virus replicated throughout the whole body and life-long virus persistence was established. The absence of organized secondary lymphoid organs led to rapid exhaustion of CTLs after infection with very low doses of a rather slow replicating strain of LCMV, even if administered locally into the footpads. Previous experiments had shown that exhaustion by activation and deletion of LCMV-specific CTLs in adult immunocompetent mice was only possible with high doses (>106 PFU) of fast replicating LCMV strains after systemic intravenous infection (36). Exhaustion was not established after subcutaneous infection apparently because staggered induction of LCMV-specific CTL precursors, first in the draining LN, and subsequently in other secondary lymphoid organs, reduced their concerted end differentiation (48). Absence of LN in aly/aly mice correlated with the dramatically increased susceptibility to exhaustion after subcutaneous infection; due to this absence, the distribution of virus and the kinetics of CTL activation and exhaustion were apparently similar after local subcutaneous and systemic intravenous infection in aly/aly mice. Our results offer at least two possible explanations for the tremendously increased susceptibility to exhaustion of LCMV-specific CTLs in aly/ aly mice. Due to the poor organization of the white pulp with the lack of the MZ Ag trapping and probably Ag presentation was less efficient in spleens of aly/aly mice due to the lack of the MZ and due to the poor organization of the splenic white pulp. This could first lead to a delay in CTL induction. Second, the initial virus distribution was different in aly/aly mice; less viral particles were retained in the spleen and therefore more virus reached peripheral solid organs where replication is probably less restricted (Table 1) and where induced CTL in addition may die because of interleukin deprivation.
Although we were not able to demonstrate a less efficient CTL induction early after LCMV infection (Fig. 2 B), the 3–30-fold reduced VV- and VSV-specific CTL responses suggested that CTL induction was affected in these mice. This impairment was modulated by the amount of inflammation and bystander activation correlating with the virulence of the infecting virus (49, 50).
aly/aly mice produce delayed and reduced T help–independent neutralizing IgM against live VSV. Since early IgM responses are usually associated with antigen in MM and MZM (12, 26, 51, 52), this result probably also reflects the insufficiency of aly/aly spleens to filter out antigen due to the absence of an organized MZ. The defect of aly/aly mice also prevented cooperative interactions of B and T cells to induce isotype switch. Adoptive transfer experiments indicated that the B and T cells of aly/aly mice were able to cooperate and to mount an immune response upon transfer into a recipient with a normal structure of secondary lymphoid organs. In contrast, transfer of spleen cells of B6 origin into aly/aly recipients did not reconstitute the immune response. These data are compatible with in vitro findings showing normal activities of aly/aly spleen cells when used in mitogen-induced in vitro proliferation of B and T cells, and also in mixed lymphocyte reactions as responders and stimulators (20). We therefore conclude that the defect of aly/aly mice relevant for the observed immunodeficiency is mainly related to the impaired structure of secondary lymphoid organs, i.e., the lack of LN and PP and the described alterations of the spleen, and not due to a cellular defect of the T and/or B cell compartment. However, the minor effect shown in Fig. 8, where we had to transfer ∼10 times more aly/aly than aly/+ spleen cells to elicit the same neutralizing IgM response, could correlate with a possible impairment of B cells, as it has been suggested in a recent report showing a defect of aly/aly B cells in class switching and somatic hypermutation (32).
It is well established in humans and mice that the spleen plays a major role in the protection against blood borne infectious agents, particularly encapsulated bacteria (13–15). Especially the TI-2 Ab response to polysaccharide components of bacterial cell walls is known to be dependent on an intact spleen. Splenectomized patients and mice have been shown to respond very poorly to primary immunizations with TI-2 antigens (16, 17). In earlier studies, mainly MZM of the spleen were regarded as critical components in the induction of TI-2 responses (53, 54). More recent data suggested that FDC, rather than MZM, are the critical cells in the initiation of TI-2 responses (55, 56). Since aly/aly mice lack both MZM and FDC, it was not surprising that their Ab response to the TI-2 antigens VaccINDG and IND-G was severely reduced. CD4 depletion revealed that the IgM response of aly/aly mice against VaccINDG was T help dependent (Fig. 7 B). This finding, therefore, suggests that T–B collaboration in the spleen of aly/aly mice is not sufficient to induce class switch to IgG, but is sufficient to promote the TI-2 IgM response to VaccINDG.
It was surprising that Hox11−/− mice responded nearly normally to the glycoprotein of VSV IND offered as a TI-2 antigen. The IgM response to intravenous immunization with VaccINDG and IND-G was delayed by 1–2 d, but was otherwise unaltered, even after CD4 depletion (Fig. 7 B). Apparently, the spleen is not critical for these responses and/or Hox11−/− mice have developed compensational mechanisms for their absent spleen during ontogeny and the neonatal period. This is also suggested by a finding in rats that only adult, but not neonatal, splenectomy resulted in a depression of the Ab response to TI-2 antigens (57).
Thus, the importance of antigen migration to LN in the induction of immune responses to model antigens or to skin allografts is confirmed in this study for antiviral immunity (7, 9, 10). The experiments here also show that effector cells, once they were induced, functioned in aly/aly mice and that LN were not limiting for emigration and function of activated effector T cells (Fig. 3). This result is compatible with the classical experiments of Frey and Wenk and those of Barker and Billingham, who showed that primed, immune T cells were capable of inflaming or rejecting skin in the absence of afferent lymph vessel or LN connection (7, 10).
Splenectomized aly/aly mice devoid of all organized secondary lymphoid organs did not respond to immunizations with potent TI-1 B cell antigens. This finding complements those for T cells as just discussed and indicates that induction of B cells must occur in organized secondary lymphoid organs. Once B cells are induced, they can emigrate to the bone marrow and home as Ab-forming cells (12, 58).
In conclusion, this study shows that the induction of an efficient and balanced antiviral immune response is not only dependent on mature B and T cells and APCs; without critical interactions in the highly organized structure of secondary lymphoid organs, T and B cells are not properly induced. These results suggest that naive T cells do not usually encounter antigen and cannot be induced in the periphery (a) because they cannot emigrate into solid tissue, (b) because antigen transport via afferent lymph to organized secondary lymphoid organs is critical for the prompt induction of an immune response, and (c) because only upon specific activation can T cells emigrate to seek antigen in the periphery. Therefore, the balance between kinetics and distribution of antigen in the periphery versus antigen in organized lymphoid organs seems to determine whether antigen is ignored or induces or exhausts T cells.
Acknowledgments
We would like to thank L. Vek, B. Storto, and E. Horvath for excellent technical assistance, N. Wey for the microphotographs, M.F. Bachmann and P. Aichele for critically reading the manuscript, and A. Oxenius, M. van den Broek, T. Fehr, and D. Binder for helpful and encouraging discussions.
This work was supported by grants from the Swiss National Science Foundation and from the Kanton of Zürich (Zürich, Switzerland).
Footnotes
1 Abbreviations used in this paper: aly/aly, homozygous alymphoplastic mutant mice; aly/+, heterozygous normal littermates; B6, C57BL/6 mice; DC, dendritic cells; FDC, follicular DC; GC, germinal centers; Hox11−/−, orphan homeobox gene 11 knockout mice; IDC, interdigitating DC; IND, serotype Indiana; IND-G, glycoprotein of VSV IND; LCMV, lymphocytic choriomeningitis virus; MAdCAM-1, mucosal addressin cell adhesion molecule 1; MM, marginal metallophils; MOI, multiplicity of infection; MZ, marginal zone; MZM, MZ macrophages; PP, Peyer's patches; RPM, red pulp macrophages; TI, thymus independent; VaccINDG, VV recombinant for IND-G; VSV, vesicular stomatitis virus; VV, vaccinia virus.
References
- 1.Paul, W.E. 1993. Fundamental Immunology. Raven Press, New York. 1490 pp.
- 2.Mackay CR. Homing of naive, memory and effector lymphocytes. Curr Opin Immunol. 1993;5:423–427. doi: 10.1016/0952-7915(93)90063-x. [DOI] [PubMed] [Google Scholar]
- 3.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 4.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 5.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 6.Freund J, Stern ER, Pisani TM. Isoallergic encephalomyelitis and radiculitis in guinea pigs after one injection of brain and mycobacteria in water-in-oil emulsion. J Immunol. 1947;57:179–194. [PubMed] [Google Scholar]
- 7.Frey JR, Wenk P. Experimental studies on the pathogenesis of contact eczema in the guinea-pig. Int Arch Allergy. 1957;11:81–100. doi: 10.1159/000228405. [DOI] [PubMed] [Google Scholar]
- 8.Kündig TM, Bachmann MF, DiPaolo C, Simard J, Battegay M, Lother H, Gessner A, Kühlcke K, Döhring C, Ohashi PS, et al. Fibroblasts as efficient antigen presenting cells in lymphoid organs. Science (Wash DC) 1995;268:1343–1347. doi: 10.1126/science.7761853. [DOI] [PubMed] [Google Scholar]
- 9.Zinkernagel RM. Immunology taught by viruses. Science (Wash DC) 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 10.Barker CF, Billingham RE. The role of afferent lymphatics in the rejection of skin homografts. J Exp Med. 1968;128:197–221. doi: 10.1084/jem.128.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 12.Bachmann MF, Kündig TM, Odermatt B, Hengartner H, Zinkernagel RM. Free recirculation of memory B cells versus antigen-dependent differentiation to antibody forming cells. J Immunol. 1994;153:3386–3397. [PubMed] [Google Scholar]
- 13.Singer, D.B. 1973. Postsplenectomy sepsis. In Perspectives in Pediatric Pathology. H.S. Rosenberg and R.P. Bolande, editors. Book Medical Publishers Inc., Chicago. 1:285–311. [PubMed]
- 14.Diamond L. Splenectomy in childhood and the hazard of overwhelming infection. Pediatrics. 1969;43:886–889. [PubMed] [Google Scholar]
- 15.Evans D. Postsplenectomy sepsis 10 years or more after operation. J Clin Pathol (Lond) 1985;38:309–311. doi: 10.1136/jcp.38.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amlot PL, Hayes AE. Impaired human antibody response to the thymus-independent antigen DNP-ficoll, after splenectomy. Lancet. 1985;1:1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- 17.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus independent (TI-2) antigens. Eur J Immunol. 1985;15:508–512. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 18.Mond JJ, Lees A, Snapper CM. T-cell–independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 19.Roberts CW, Shutter JR, Korsmeyer SJ. Hox11controls the genesis of the spleen. Nature (Lond) 1994;368:747–749. doi: 10.1038/368747a0. [DOI] [PubMed] [Google Scholar]
- 20.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 21.Nanno M, Matsumoto S, Koike R, Miyasaka M, Kawaguchi M, Masuda T, Miyawaki S, Cai Z, Shimamura T, Fujiura Y, et al. Development of intestinal intraepithelial T lymphocytes is independent of Peyer's patches and lymph nodes in aly mutant mice. J Immunol. 1994;153:2014–2020. [PubMed] [Google Scholar]
- 22.McCaren L, Holland JJ, Syverton JT. The mammalian cell–virus relationship. I. Attachment of poliovirus to cultivated cells of primate and non-primate origin. J Exp Med. 1959;109:475–485. doi: 10.1084/jem.109.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey MJ, McLeod DA, Kang CY, Bishop D. Glycosylation is not required for the fusion activity of the G protein of vesicular stomatitis virus in insect cells. Virology. 1989;169:323–331. doi: 10.1016/0042-6822(89)90157-8. [DOI] [PubMed] [Google Scholar]
- 24.Battegay M, Cooper S, Althage A, Baenziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24 or 96 well plates. J Virol Methods. 1991;33:191–198. doi: 10.1016/0166-0934(91)90018-u. [DOI] [PubMed] [Google Scholar]
- 25.Cobbold SP, Jayasuriya A, Nash A, Prospero TD, Waldmann H. Therapy with monoclonal antibodies by elimination of T cell subsets in vivo. Nature (Lond) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 26.Odermatt B, Eppler M, Leist TP, Hengartner H, Zinkernagel RM. Virus-triggered acquired immunodeficiency by cytotoxic T-cell dependent destruction of antigen-presenting cells and lymph follicle structure. Proc Natl Acad Sci USA. 1991;88:8252–8256. doi: 10.1073/pnas.88.18.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Vliet E, Melis M, Van Ewijk W. Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J Histochem Cytochem. 1985;33:40–44. doi: 10.1177/33.1.3880783. [DOI] [PubMed] [Google Scholar]
- 28.Gray D, Kosco M, Stockinger B. Novel pathways of antigen presentation for the maintenance of memory. Int Immunol. 1991;3:141–148. doi: 10.1093/intimm/3.2.141. [DOI] [PubMed] [Google Scholar]
- 29.Zinkernagel RM, Leist TP, Hengartner H, Althage A. Susceptibility to lymphocytic choriomeningitis virus isolates correlates directly with early and high cytotoxic T cell activity, as well as with footpad swelling reaction, and all three are regulated by H-2D. J Exp Med. 1985;162:2125–2141. doi: 10.1084/jem.162.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roost HP, Charan S, Zinkernagel RM. Analysis of the kinetics of antiviral memory T help in vivo: characterization of short lived cross-reactive T help. Eur J Immunol. 1990;20:2547–2554. doi: 10.1002/eji.1830201204. [DOI] [PubMed] [Google Scholar]
- 31.Moskophidis D, Lehmann-Grube F. Virus-induced delayed-type hypersensitivity reaction is sequentially mediated by CD8+ and CD4+T lymphocytes. Proc Natl Acad Sci USA. 1989;86:3291–3295. doi: 10.1073/pnas.86.9.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkura R, Matsuda F, Sakiyama T, Tsubata T, Hiai H, Paumen M, Miyawaki S, Honjo T. Defects of somatic hypermutation and class switching in alymphoplasia (aly)mutant mice. Int Immunol. 1996;8:1067–1075. doi: 10.1093/intimm/8.7.1067. [DOI] [PubMed] [Google Scholar]
- 33.Butcher EC. Cellular and molecular mechanisms that direct leucocyte traffic. Am J Pathol. 1990;136:3–11. [PMC free article] [PubMed] [Google Scholar]
- 34.Koike R, Nishimura T, Yasumizu R, Tanaka H, Hataba Y, Watanabe T, Miyawaki S, Miyasaka M. The splenic marginal zone is absent in alymphoplastic alymutant mice. Eur J Immunol. 1996;26:669–675. doi: 10.1002/eji.1830260324. [DOI] [PubMed] [Google Scholar]
- 35.Buchmeier MJ, Welsh RM, Dutko FJ, Oldstone MBA. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- 36.Moskophidis D, Lechner F, Pircher HP, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond) 1993;362:758–761. doi: 10.1038/362758a0. [DOI] [PubMed] [Google Scholar]
- 37.van den Broek M, Müller U, Huang S, Aguet M, Zinkernagel RM. Antiviral defense in mice lacking both α/β and γ interferon receptors. J Virol. 1995;69:4792–4796. doi: 10.1128/jvi.69.8.4792-4796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burns WH, Billups LC, Notkins AL. Thymus dependence of viral antigens. Nature (Lond) 1975;256:654–656. doi: 10.1038/256654a0. [DOI] [PubMed] [Google Scholar]
- 39.Freer G, Burkhart C, Ciernik I, Bachmann MF, Hengartner H, Zinkernagel RM. Vesicular stomatitis virus Indiana (VSV-IND) glycoprotein as a T cell-dependent and -independent antigen. J Virol. 1994;68:3650–3655. doi: 10.1128/jvi.68.6.3650-3655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell–independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? . Eur J Immunol. 1995;25:3445–3451. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- 41.Fehr T, Bachmann MF, Bluethmann H, Kikutani H, Hengartner H, Zinkernagel RM. T-independent activation of B-cells by vesicular stomatitis virus: no evidence for the need of a second signal. Cell Immunol. 1996;168:184–192. doi: 10.1006/cimm.1996.0065. [DOI] [PubMed] [Google Scholar]
- 42.Wagner, R.R. 1987. The Rhabdoviruses. Plenum Press, New York. 544 pp.
- 43.Kuramoto T, Mashimo T, Koike R, Miyawaki S, Yamada J, Miyasaka M, Serikawa T. The alymphoplasia (aly)mutation co-segregates with the intercellular adhesion molecule-2 (ICAM-2) on mouse chromosome 11. Int Immunol. 1994;6:991–1004. doi: 10.1093/intimm/6.7.991. [DOI] [PubMed] [Google Scholar]
- 44.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss SJ, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science (Wash DC) 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- 45.Banks TA, Rouse BT, Kerley MK, Blair PJ, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-α deficient mice: effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- 46.Eugster H, Müller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, Di Padova F, Aguet M, et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-α double deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto M, Mariathasan S, Nahm MH, Baranyay F, Peschon JJ, Chaplin DD. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science (Wash DC) 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 48.Moskophidis D, Battegay M, van den Broek M, Laine E, Hoffmann-Rohrer U, Zinkernagel RM. Role of virus and host variables in virus persistence or immunopathological disease caused by a noncytolytic virus. J Gen Virol. 1995;76:381–391. doi: 10.1099/0022-1317-76-2-381. [DOI] [PubMed] [Google Scholar]
- 49.Wu Y, Liu Y. Viral induction of co-stimulatory activity on antigen-presenting cells bypasses the need for CD4+ T-cell help in CD8+T-cell responses. Curr Biol. 1994;4:499–505. doi: 10.1016/s0960-9822(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 50.Kündig TM, Shahinian A, Kawai K, Mittrucker HW, Sebzda E, Bachmann MF, Mak TW, Ohashi PS. Duration of TCR-stimulation determines costimulatory requirements of T-cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 51.Claassen E, Kors N, Van Rooijen N. Influence of carriers on the development and localization of anti-2,4,6trinitrophenyl (TNP) antibody-forming cells in the murine spleen. II. Suppressed antibody response to TNP-ficoll after elimination of marginal zone cells. Eur J Immunol. 1986;16:492–497. doi: 10.1002/eji.1830160505. [DOI] [PubMed] [Google Scholar]
- 52.Kraal G, Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986;58:665–669. [PMC free article] [PubMed] [Google Scholar]
- 53.Humphrey JH. Splenic macrophages: antigen presenting cells for TI-2 antigens. Immunol Lett. 1985;11:149–152. doi: 10.1016/0165-2478(85)90161-0. [DOI] [PubMed] [Google Scholar]
- 54.Goud SN, Muthusamy N, Subbarao B. Differential responses of B-cells from the spleen and lymph node to TNP-Ficoll. J Immunol. 1988;16:492–497. [PubMed] [Google Scholar]
- 55.Van den Eertwegh AJM, Laman JD, Schellekens MM, Boersma WJA, Claassen E. Complement-mediated follicular localization of T-independent type-2 antigens: the role of marginal zone macrophages revisited. Eur J Immunol. 1992;22:719–726. doi: 10.1002/eji.1830220315. [DOI] [PubMed] [Google Scholar]
- 56.Kraal G, Ter HH, Meelhuizen C, Venneker G, Claassen E. Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur J Immunol. 1989;19:675–680. doi: 10.1002/eji.1830190416. [DOI] [PubMed] [Google Scholar]
- 57.Gray D, Chassoux D, MacLennan ICM. Selective depression of thymus independent anti-DNP antibody responses induced by adult but not neonatal splenectomy. Clin Exp Immunol. 1985;60:78–86. [PMC free article] [PubMed] [Google Scholar]
- 58.Slifka MK, Matloubian M, Ahmed R. Bone marrow is a major site of long-term antibody production after acute viral infection. J Virol. 1995;69:1895–1902. doi: 10.1128/jvi.69.3.1895-1902.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]