Abstract
Immunization of mice with myelin components results in experimental autoimmune encephalomyelitis (EAE), which is mediated by myelin-specific CD4+ T cells and anti-myelin antibodies. Tumor necrosis factor α (TNF-α) and lymphotoxin α (LT-α) are thought to be involved in the events leading to inflammatory demyelination in the central nervous system. To ascertain this hypothesis 129 × C57BL/6 mice with an inactivation of the tnf and lta genes (129 × C57BL/6−/−) and SJL/J mice derived from backcrosses of the above mentioned mutant mice (SJL−/−) were immunized with mouse spinal cord homogenate (MSCH) or proteolipid protein. Both 129 × C57BL/6−/− mice and SJL−/− mice developed EAE. In SJL−/− mice immunized with MSCH, a very severe form of EAE with weight loss, paralysis of all four limbs, and lethal outcome was observed. The histologic hallmark was an intense perivascular and parenchymal infiltration with predominantly CD4+ T cells and some CD8+ T cells associated with demyelination in both brain and spinal cord. These results indicate that TNF-α and LT-α are not essential for the development of EAE.
Experimental autoimmune encephalomyelitis (EAE) is a T cell–mediated inflammatory disease of the central nervous system (CNS). It is induced in experimental animals by either active immunization with CNS homogenate, myelin proteins, or the adoptive transfer of myelin basic protein (MBP)-specific CD4+ T cell lines. In SJL mice immunized with myelin components, the histologic hallmarks of the disease comprise a perivascular and parenchymal inflammation with infiltration of CD4+ T cells, activation of macrophages and microglial cells, and various degrees of demyelination. Recent work has suggested that TNF- and lymphotoxin (LT)-α, also known as TNF-β, may play a pivotal role in EAE, since both TNF- and LT-α messenger RNA and protein have been identified in the CNS in the acute phase of the disease (1–5). Furthermore, the administration of soluble type I receptor of TNF, as well as antibodies to TNF- and LT-α, prevent the development of EAE in rodents immunized with MBP or injected with encephalitogenic T cells (6–9). The ability of MBP-specific T cells to transfer the disease was found to be positively correlated with the amount of TNF- and/or LT-α produced by the T cells (10). TNF-α is produced in high amounts by astrocytes from EAE-susceptible Lewis rats, but not from EAE-resistant Brown-Norway rats (11). Demyelination has been suggested to be due to the action of TNF- and LT-α because both cytokines mediate oligodendrocyte and myelin damage in vitro (12, 13). Likewise, upon overexpression in the CNS of transgenic mice, TNF-α causes demyelination (14). Furthermore, injections of TNF-α lead to prolongation of EAE or can induce relapses in SJL mice that have been partially or completely recovered from acute EAE (15, 16).
In this study we have investigated the influence of TNF- and LT-α on the course of EAE using mice having a simultaneous deletion of the tnf and lta genes (17). Despite previous evidence for a pathogenetic role of both TNF- and LT-α in EAE, we find TNF- and LT-α double-deficient mice to be highly susceptible to acute EAE.
Materials and Methods
Mice.
Female SJL/J (H-2s) mice were purchased from Biological Research Laboratories Ltd. (Füllinsdorf, Switzerland). 129 SV/EV × C57BL/6 with inactivated tnf and lta genes (called 129 × B6−/−, H-2b) have been described recently (17) and were bred under specific pathogen-free (SPF) conditions. These mice were backcrossed onto the SJL/J (H-2s) background for six generations and are called SJL−/− throughout this paper. Mice homozygous for inactivation of both the tnf and lta genes were obtained by intercrossing heterozygous animals. Homozygous animals were identified by a diagnostic PCR on genomic DNA described for the 129 × B6−/− mice (17) and a negative PCR result using the following primer pair: 5′-TCT CAT CAG TTC TAT GGC CC-3′ and 5′-GGG AGT AGA CAA GGT ACA AC-3′, proving the lack of the deleted genes. The knockout phenotype was also ascertained by the lack of lymph nodes and altered splenic microarchitecture (17). Flow cytometric analysis of splenocytes showed SJL−/− mice to be predominantly of the H-2b haplotype (FITC anti-mouse H-2Db, clone KH95; PharMingen, San Diego, CA), and not H-2s (FITC anti–mouse H-2Ds, clone KH43; PharMingen). Animals were housed under SPF conditions up to 8 wk of age and then transferred to conventional housing where they were immunized at 10–20 wk of age. Paralyzed animals were given easy access to food and water.
Induction of EAE.
Mice were immunized either with mouse spinal cord homogenate (MSCH; 18) or proteolipid protein (PLP) peptide in CFA according to a modified method of Brown and McFarlin (19) and Al-Sabbagh et al. (20), respectively. On day 0, each mouse received 0.3 ml of a mixture of 5.0 mg MSCH in 0.15 ml PBS (pH 7.4) or 0.2 mg PLP peptide 139-151 (HSLGKWLGHPDKF, synthesized by Chiron Technologies, Clayton, Victoria, Australia) dissolved in 0.15 ml PBS and 0.75 mg of Mycobacterium tuberculosis, (H37Ra; Difco, Detroit, MI) in 0.15 ml incomplete Freund's adjuvant (Difco). The inoculum of 0.3 ml was injected at 50 μl in each hind footpad and in each of four spots on the back (two anterior, two posterior, on each side). On the day of immunization and 2 d later, 1010 heat-inactivated Bordetella pertussis (Calbiochem Corp., La Jolla, CA) were injected intraperitoneally in 0.4 ml pertussis diluent (0.015 M Tris, 0.5 M NaCl, and 0.017% (vol/vol) Triton X-100 in distilled water; reference 21).
Clinical Evaluation of EAE.
After the encephalitogenic challenge, mice were monitored, weighed daily, and disease severity was scored on a scale of 0 to 5, with 0.5 points for intermediate clinical findings. The score was designated as follows: 0, no detectable signs of EAE; 1, weakness of the tail; 2, definite tail and partial hind limb paralysis; 3, complete paralysis of the hind limbs often associated with incontinence; 4, total paralysis of hind and forelimbs (mice at grade 4 for >1 d were euthanized); 5, moribund or death.
Histological Analysis.
Animals were perfused under anesthesia through the heart either with Ringer's solution (for cryosections, flow cytometry, and reverse transcriptase (RT)-PCR) or with 4% paraformaldehyde in buffered PBS (for paraffin sections) and spleen, brain, and spinal cord were dissected out. Paraffin-embedded tissue sections were stained with hematoxylin and eosin and for the degree of demyelination with Luxol fast blue. Tissue-Tek–embedded sections were stained with anti-CD4 mAb (GK1.5; PharMingen), anti-CD8 mAb (5H10-1; PharMingen), anti-F4/80 mAb (C1:A3-1; Serotec Ltd., Oxford, U.K.) and corresponding isotype-matched primary mAbs. The labeled streptavidin–biotin technique (DAKO Diagnostics AG, Zug, Switzerland) was chosen for visualization.
RT-PCR Analysis for TNF-α, LT-α, IFN-γ, and GAPDH.
Total RNA was isolated from frozen tissue according to Chomczynski and Sacchi (22). 10 μg of total RNA was mixed with 2 μg oligo dT primer (Boehringer Mannheim, Rotkreuz, Switzerland) in a volume of 10 μl RNase free water and denatured for 5 min at 70°C. Cooling on ice allowed annealing of RT primers. Reverse transcription was performed in a total volume of 30 μl containing 6 nmol dNTPs, 0.3 nmol dithiothreitol, 25 units RNAguard (Pharmacia, Dübendorf, Switzerland) and 100 U Moloney murine leukemia virus RT (GIBCO, Basel, Switzerland) for 4 h at 37°C. The reaction was terminated by incubation at 95°C for 5 min followed by 5 min at 4°C. The PCR reaction mix consisted of 2 μl RT reaction mix, 2.5 μl PCR buffer, 2.5 μl 12 mM MgCl2, 2 μl 10 μM primer mix, 2.5 μl 2 mM dNTP mix, and 0.5 units Taq polymerase (0.5 U in 11.4 μl; Appligene, Oncor, Basel, Switzerland) were added at 55°C after an initial denaturation at 95°C for 1 min. A two-step PCR with denaturation for 15 s at 94°C and annealing for 30 s at 55°C was done for 30 or 35 cycles with a final extension at 72°C for 5 min. PCR products were visualized in 2% agarose gels containing ethidium bromide. The following mouse specific primers were used for PCR: TNF-α sense primer, 5′-TCTCATCAGTTCTATGGCCC-3′; TNF-α antisense primer, 5′-GGGAGTAGACAAGGTACAAC-3′; LT-α sense primer, 5′-CCCATCCACTCCCTCAGAAG-3′; LT-α antisense primer, 5′-CGCACTGAGGAGAGGCACAT-3′; IFN-γ sense primer, 5′-GCTCTGAGACAATGAACGCT-3′; IFN-γ antisense primer, 5′-AAAGAGATAATCTGGCTCTGC-3′; GAPDH sense primer, 5′-CCACCACCCTGTTGCTGTAG3′; GAPDH antisense primer, 5′-CCATCAACGACCCCTTCATT-3′.
Results and Discussion
Irrespective of whether the tnf and lta genes are inactivated or not, 129 × C57BL/6 mice, which had been immunized with MSCH, developed a mild form of EAE with mean clinical scores of 1 (Table 1). The disease started between days 11 and 17. None of the mice progressed to severe EAE. Histologic analysis revealed a few perivascular cuffs, predominantly in spinal cord, but no demyelination. The same clinical and histological picture emerged when studying SJL/J mice which were immunized with MSCH. However, when using PLP as the immunogen, SJL/J mice suffered from a more severe form of EAE, which progressed to a mean disease score of 2.2 ± 1.2 (Table 1). Mice with severe disease (clinical score >2) had histologic evidence for perivascular and parenchymal infiltration of CD4+ T cells and demyelination (data not shown).
Table 1.
>EAE in TNF and LT-α–deficient mice
| Genotype | Haplotype H-2 | TNF-α | LT-α | Age | Immunized with | Number sick/total* | Mean day of onset‡ | Mean max. clinical score§ | Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wk | % | |||||||||||||||||
| 129 × B6 | b | +/+ | +/+ | 11 | MSCH | 5/5 | 14.6 ± 0.9 | 1.1 ± 0.2 | 0 | |||||||||
| 129 × B6 | b | −/− | −/− | 11 | MSCH | 5/5 | 13.8 ± 1.1 | 1.0 ± 0 | 0 | |||||||||
| SJL/J | s | +/+ | +/+ | 12–15 | MSCH | 3/8 | 15.7 ± 1.5 | 1.0 ± 0 | 0 | |||||||||
| SJL/J | s | +/+ | +/+ | 10–12 | PLP | 8/8 | 12.3 ± 1.3 | 2.2 ± 1.2 | 25 | |||||||||
| SJL/J | b | −/− | −/− | 15–20 | MSCH | 8/8 | 13.0 ± 2.3 | 3.9 ± 1.6 | 100 | |||||||||
| SJL/J | b | −/− | −/− | 12–15 | PLP | 6/6 | 16.3 ± 3.7 | 1.8 ± 1.3 | 16.7 |
Number of animals with clinical signs per total in experiment.
Day of onset of clinical signs (mean ± SEM).
Based only on those animals with clinical disease (mean ± SEM).
To investigate the influence of TNF- and LT-α in the prototype mouse model of EAE, the TNF- and LT-α double-deficient (129 × B6−/−) mice were backcrossed into SJL/J. In these SJL−/− mice, the extent of disease was comparable when immunized with PLP (Table 1). However, immunization of SJL−/− mice with MSCH resulted in a dramatic acute form of EAE. Clinically, all eight mice showed the development of an ascending paralysis affecting the tail and all four limbs, which uniformly led to death of the animals. Their weights, which were monitored at daily intervals, were found to increase from 21.7 ± 2.7 g at the day of immunization to a maximum of 23.3 ± 2.1 g between days 9 and 13. Thereafter weight loss was dramatic, with a 17% decrease at the day of death of the animal (mean ± SEM: 19.4 ± 2.9 g). The development of cachexia in EAE is a well-established feature and may be attributed to excessive production of TNF, which has been named cachectin due to its capability to initiate weight loss when injected into mice (23). However, the observation of cachexia in TNF- and LT-α–deficient mice with EAE shows that loss of weight is due to other mechanisms, e.g., dysfunction of brain centers regulating food intake.
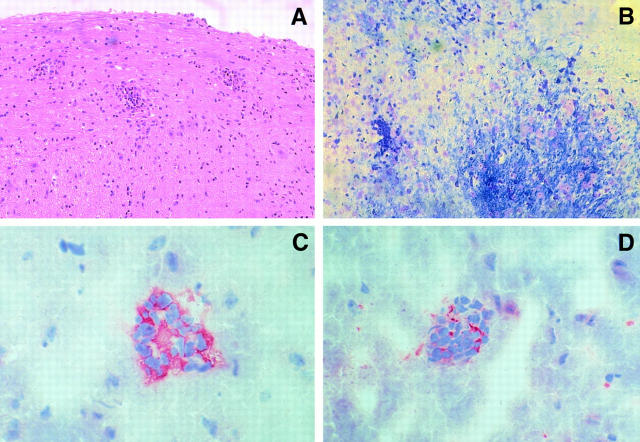
Histologic examination of SJL−/− mice immunized with MSCH and a disease score of 3 to 5 showed perivascular and parenchymal cell infiltrates and demyelination in both brain and spinal cord. Demyelination was patchy and confined to areas of cell infiltrates. In SJL−/− mice with EAE induced by immunization with PLP, there was only minimal demyelination despite impressive cellular infiltrates. By immunohistochemistry, the infiltrates were found to consist mainly of CD4+ T and some CD8+ T lymphocytes (Fig. 1).
Figure 1.
Histopathologic analysis of spinal cord and brains from TNF- and LT-α–deficient mice with acute EAE after immunization with MSCH. (A) Perivascular infiltrates in spinal cord (longitudinal section, hematoxilin and eosin stain) and (B) demyelination in areas of cell infiltrates in brain tissue (Luxol fast blue stain). Mononuclear cells in brain tissue consists mainly of CD4+ T lymphocytes (C) and CD8+ T cells (D) as shown by immunohistochemistry. Staining was performed on 1-μm paraffin sections (A and B) or 3-μm cryosections (C and D). Original magnifications of A and B, 200; C and D, 500.
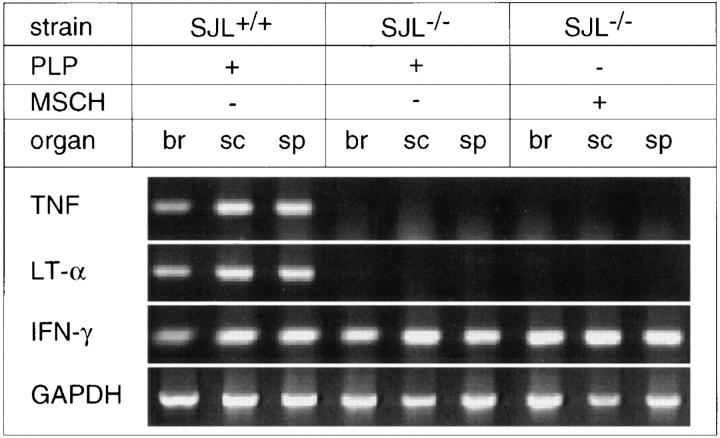
As expected, TNF- and LT-α transcripts as measured by RT-PCR were absent in brain, spinal cord, and spleen in the diseased mutant mice (Fig. 2). In agreement with other reports, the acute phase of EAE in the wild-type controls is paralleled by expression of both cytokine genes in the CNS (1–5). No difference was noted when assessing the expression of the IFN-γ gene, which was also detected in the CNS in TNF- and LT-α knockout mice (Fig. 2). The detection of expression of IFN-γ in mice with severe EAE is of note since evidence is accumulating that IFN-γ has a rather beneficial influence on the disease course. Genetic inactivation of the IFN-γ receptor gene rendered EAEresistant mice susceptible (24). Likewise, a more aggressive disease is observed in IFN-γ knockout mice (25, 26).
Figure 2.
RT-PCR cytokine transcripts from wild type and TNF- and LT-α–deficient mice during acute EAE. Transcripts from different tissues (br, brain; sc, spinal cord; sp, spleen) from acute EAE induced with PLP or MSCH from representative mice are shown.
Our results are remarkable for two reasons. First, it is interesting that TNF- and LT-α deficient mice are able to generate an autoimmune response to CNS myelin. This finding was not per se to be expected since TNF- and LT-α–deficient mice not only have an abnormal spleen microarchitecture, blood lymphocytosis, and absence of lymph nodes, but also functional alterations (17). These include defects in T cell–dependent and –independent immune responses against sheep red blood cells and dinitrophenol-alanyl-glycyl-glycyl-Ficoll, respectively, and a corresponding lack of germinal center formation (27, 28). In addition the CTL response to vaccinia virus and lymphocytic choriomeningitis virus was found to be reduced (17). Since after in vivo priming the secondary CTL response to lymphocytic choriomeningitis virus in vitro was much more pronounced, it has been suggested that in vivo preformed virus-specific precursor CTL require a prolonged activation period in vitro to become competent effector cells. On the humoral site, the SRBC response in TNF- and LT-α–deficient mice was reduced for IgM, IgG1, and IgG2b class Igs. These may be of importance since antimyelin antibodies led to demyelination in T cell adoptive transfer EAE (29). However, recent experiments with genetically B cell–deficient mice that were immunized with an MBP encephalitogenic peptide have shown that B cells are not required for the activation of encephalitogenic T cells, and hence the induction of EAE (30).
The data presented here provide evidence that autoimmunity to myelin can be induced in the absence of TNF- and LT-α and in the context of the above mentioned structural and functional deficiencies of the immune system present in these mutant mice. The second feature of interest from our data is the observation that the autoimmune response to CNS myelin leads to EAE with CNS inflammation and demyelination. This is in contrast to the expectations which are based on (a) detection of TNF- and LT-α at the lesion site in EAE, (b) the induction of relapses of EAE by injection of TNF-α, and (c) prevention of EAE by anti-TNF antibodies or soluble TNF type I receptors (1– 16). The demonstration of CD4+ T cells in CNS lesions of TNF- and LT-α knockout mice with EAE indicates the generation of sufficient numbers of autoimmune CD4+ T cells, which are able to adhere to CNS endothelium and to penetrate into the tissue. Thus, these steps, which are required for induction of EAE, are independent of the action of TNF- and LT-α. Alternatively, in their absence, other cytokines may compensate for the defect. It is of note that three other studies did not come to the conclusion that TNF- and LT-α function as key mediators in EAE. In SJL and B10.PL mice immunized with synthetic MBP peptides, spinal cord lesions were negative for cells expressing TNF (31). Furthermore, TNF delivered by using a recombinant vaccinia virus system did not enhance, but rather inhibited the development of EAE (32). In the same experimental paradigm, anti-TNF antibodies either enhanced, had no effect or inhibited EAE, depending on the antibody investigated (21, 32). Thus, the antibodies used in different studies may show variable cross-reactivity with other immunomodulating molecules of the TNF superfamily. In mice with Theiler's virus encephalomyelitis, the administration of rTNF dramatically reduced the extent of demyelination without influencing the CNS virus titers (33). Rather than causing demyelination, TNF-α and IFN-γ seem to affect remyelination by inhibition of growth and differentiation of oligodendrocytes (34).
With the generation of gene knockout mice, a new area has been opened to study the involvement of distinct gene products in experimental murine disease models. Studies using mice carrying an inactivated IFN-γ (25, 26) or its receptor (24) gene have generated results contradicting the current view on EAE. The same holds true for the TNF- and LT-α knockout mice presented here. One explanation for these contradicting results might be that the immune system of knockout mice becomes biased in a way to favor compensatory mechanisms leading to the observed outcome. It is therefore tempting to speculate that conditional knockout mice, allowing for the temporal and spatial inactivation of a gene (35), might lead to more accurate results in regard of cytokine-mediated pathogenesis, not only in EAE, but many other pathologies.
EAE is used as an animal model to study the inflammatory process in multiple sclerosis (MS). TNF- and LT-α have been identified in acute and chronic active MS lesions, but were absent from chronic silent lesions (36). Furthermore, an increase in production of TNF by peripheral blood mononuclear cells in vitro often precedes acute relapses in patients with MS (37). The data presented here may question the immunopathogenic role attributed to TNF- and LT-α in this human demyelinating disease.
Acknowledgments
We thank Mrs. R. Muljana for performing the histology, Mrs. J. von Arx for expert breeding of the SJL−/− mice and Dr. M. Hoechli (Laboratory for Electron Microscopy, University of Zürich, Zürich, Switzerland) for his excellent assistance in digital image processing.
This work was supported by the Swiss National Science Foundation (grants 32-33966.92 to H.-P. Eugster and 31-42900.95 to A. Fontana). C.S. Constantinescu is supported by the National Institutes of Health (grant K11HD-01049).
References
- 1.Issazadeh S, Ljungdahl Å, Höjeberg B, Mustafa M, Olsson T. Cytokine production in the central nervous system of Lewis rats with experimental autoimmune encephalomyelitis: dynamics of mRNA expression for interleukin10, interleukin-12, cytolysin, tumor necrosis factor α and tumor necrosis factor β. J Neuroimmunol. 1995;61:205–212. doi: 10.1016/0165-5728(95)00100-g. [DOI] [PubMed] [Google Scholar]
- 2.Renno T, Krakowski M, Piccirillo C, Lin J, Owens T. TNF-a expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 3.Baker D, O'Neill JK, Turk JL. Cytokines in the central nervous system of mice during chronic relapsing experimental allergic encephalomyelitis. Cell Immunol. 1991;134:505–510. doi: 10.1016/0008-8749(91)90321-2. [DOI] [PubMed] [Google Scholar]
- 4.Held W, Meyermann R, Qin Y, Mueller C. Perforin and tumor necrosis factor α in the pathogenesis of experimental allergic encephalomyelitis: comparison of autoantigen induced and transferred disease in Lewis rats. J Autoimmun. 1993;6:311–322. doi: 10.1006/jaut.1993.1027. [DOI] [PubMed] [Google Scholar]
- 5.Okuda Y, Nakatsuji Y, Fujimura H, Esumi H, Ogura T, Yanagihara T, Sakoda S. Expression of the inducible isoform of nitric oxide synthase in the central nervous system of mice correlates with the severity of actively induced experimental allergic encephalomyelitis. J Neuroimmunol. 1995;62:103–112. doi: 10.1016/0165-5728(95)00114-h. [DOI] [PubMed] [Google Scholar]
- 6.Ruddle NH, Bergman CM, McGrath KM, Lingenheld EG, Grunnet ML, Padula SJ, Clark RB. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–1200. doi: 10.1084/jem.172.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selmaj K, Raine CS, Cross AH. Anti–tumor necrosis factor therapy abrogates autoimmune demyelination. Ann Neurol. 1991;30:694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- 8.Selmaj K, Papierz W, Glabinski A, Kohno T. Prevention of chronic relapsing experimental autoimmune encephalomyelitis by soluble tumor necrosis factor receptor I. J Neuroimmunol. 1995;56:135–141. doi: 10.1016/0165-5728(94)00139-f. [DOI] [PubMed] [Google Scholar]
- 9.Martin D, Near SL, Bendele A, Russell DA. Inhibition of tumor necrosis factor is protective against neurologic dysfunction after active immunization of Lewis rats with myelin basic protein. Exp Neurol. 1995;131:221–228. doi: 10.1016/0014-4886(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 10.Powell MB, Mitchell D, Lederman J, Buckmeier J, Zamvil SS, Graham M, Ruddle NH, Steinman L. Lymphotoxin and tumor necrosis factor–alpha production by myelin basic protein-specific T cell clones correlates with encephalitogenicity. Int Immunol. 1990;2:539–544. doi: 10.1093/intimm/2.6.539. [DOI] [PubMed] [Google Scholar]
- 11.Chung IY, Norris JG, Benveniste EN. Differential tumor necrosis factor α expression by astrocytes from experimental allergic encephalomyelitis–susceptible and –resistant rat strains. J Exp Med. 1991;173:801–811. doi: 10.1084/jem.173.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins DS, Shirazi Y, Drysdale B-E, Lieberman A, Shin HS, Shin ML. Production of cytotoxic factor for oligodendrocytes by stimulated astrocytes. J Immunol. 1987;139:2593–2597. [PubMed] [Google Scholar]
- 13.Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- 14.Probert L, Akassoglou K, Pasparakis M, Kontogeorgos G, Kollias G. Spontaneous inflammatory demyelinating disease in transgenic mice showing central nervous system–specific expression of tumor necrosis factor-α. Proc Natl Acad Sci USA. 1995;92:11294–11298. doi: 10.1073/pnas.92.24.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda Y, Shimamoto Y. Human tumor necrosis factor–α augments experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1991;34:159–164. doi: 10.1016/0165-5728(91)90125-q. [DOI] [PubMed] [Google Scholar]
- 16.Crisi GM, Santambrogio L, Hochwald GM, Smith SR, Carlino JA, Thorbecke GJ. Staphylococcal enterotoxin B and tumor necrosis factor–α–induced relapses of experimental allergic encephalomyelitis: protection by transforming growth factor-β and interleukin-10. Eur J Immunol. 1995;25:3035–3040. doi: 10.1002/eji.1830251108. [DOI] [PubMed] [Google Scholar]
- 17.Eugster H-P, Müller M, Karrer U, Car BD, Schnyder B, Eng VM, Woerly G, Le Hir M, di Padova F, Aguet M, et al. Multiple immune abnormalities in tumor necrosis factor and lymphotoxin-α double deficient mice. Int Immunol. 1996;8:23–36. doi: 10.1093/intimm/8.1.23. [DOI] [PubMed] [Google Scholar]
- 18.Brocke, S., K. Gijbels, and L. Steinman. 1994. Experimental autoimmune encephalomyelitis in the mouse. In Autoimmune Disease Models. A Guide Book. I.R. Cohen and A. Miller, editors. Academic Press, San Diego. 1–14.
- 19.Brown AM, Mc DE, Farlin Relapsing experimental allergic encephalomyelitis in the SJL/J mouse. Lab Invest. 1981;45:278–284. [PubMed] [Google Scholar]
- 20.Al-Sabbagh A, Miller A, Santos LMB, Weiner HL. Antigen-driven tissue-specific suppression following oral tolerance: orally administered myelin basic protein suppresses proteolipid protein-induced experimental autoimmune encephalomyelitis in the SJL mouse. Eur J Immunol. 1994;24:2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 21.Teuscher C, Hickey WF, Korngold R. An analysis of the role of tumor necrosis factor in the phenotypic expression of actively induced experimental allergic orchitis and experimental allergic encephalomyelitis. Clin Immunol Immunopathol. 1990;54:442–453. doi: 10.1016/0090-1229(90)90057-w. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenolchloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Beutler B, Cerami A. Cachectin and tumor necrosis factor as two sides of the same biological coin. Nature (Lond) 1986;320:584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- 24.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible for the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 25.Krakowski M, Owens T. Interferon-γ confers resistance to experimental allergic encephalomyelitis. Eur J Immunol. 1996;26:1641–1646. doi: 10.1002/eji.1830260735. [DOI] [PubMed] [Google Scholar]
- 26.Willenborg DO, Fordham S, Bernard CCA, Cowden WB, Ramshaw IA. IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 27.Le Hir M, Bluethmann H, Kosco-Vilbois M, Müller M, Di Padova F, Moore M, Ryffel B, Eugster HP. Differentiation of follicular dendritic cells (FDC) and full antibody responses require TNF receptor-1 (TNFR-1) signaling. J Exp Med. 1996;183:2367–2372. doi: 10.1084/jem.183.5.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryffel B, Le Hir M, di Padova F, Schreier MH, Mueller M, Eugster HP, Quesniaux VFJ. Defective Ig isotype switch to both T-cell dependent and independent antigens in TNF/LT-α deficient mice. J Immunol. 1997;158:2126–2133. [PubMed] [Google Scholar]
- 29.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf SD, Dittel BN, Hardardottir F, Janeway CA. Experimental autoimmune encephalomyelitis induction in genetically B cell–deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merrill JE, Kono DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci USA. 1992;89:574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willenborg DO, Fordham SA, Cowden WB, Ramshaw IA. Cytokines and murine autoimmune encephalomyelitis: inhibition or enhancement of disease with antibodies to select cytokines, or by delivery of exogenous cytokines using a recombinant vaccinia virus system. Scand J Immunol. 1995;41:31–41. doi: 10.1111/j.1365-3083.1995.tb03530.x. [DOI] [PubMed] [Google Scholar]
- 33.Paya CV, Leibson PJ, Patick AK, Rodriguez M. Inhibition of Theiler's virus–induced demyelination in vivo by tumor necrosis factor alpha. Int Immunol. 1990;2:909–913. doi: 10.1093/intimm/2.9.909. [DOI] [PubMed] [Google Scholar]
- 34.Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-γ and tumor necrosis factor-α on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996;8:1106–1116. doi: 10.1111/j.1460-9568.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MA, Tonegawa S. Synaptic plasticity, place cells and spatial memory: study with second generation knockouts. Trends Neurosci. 1997;20:102–106. doi: 10.1016/s0166-2236(96)01023-5. [DOI] [PubMed] [Google Scholar]
- 36.Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest. 1991;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? . Acta Neurol Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]