Abstract
Proteins encoded by members of the Ly-49 gene family are predominantly expressed on murine natural killer (NK) cells. Several members of this gene family have been demonstrated to inhibit NK cell lysis upon recognizing their class I ligands on target cells. In this report, we present data supporting that not all Ly-49 proteins inhibit NK cell function. Our laboratory has generated and characterized a monoclonal antibody (mAb) (12A8) that can be used to recognize the Ly-49D subset of murine NK cells. Transfection of Cos-7 cells with known members of the Ly-49 gene family revealed that 12A8 recognizes Ly-49D, but also cross-reacts with the Ly-49A protein on B6 NK cells. In addition, 12A8 demonstrates reactivity by both immunoprecipitation and two-color flow cytometry analysis with an NK cell subset that is distinct from those expressing Ly-49A, C, or G2. An Ly-49D+ subset of NK cells that did not express Ly49A, C, and G2 was isolated and examined for their functional capabilities. Tumor targets and concanovalin A (ConA) lymphoblasts from a variety of H2 haplotypes were examined for their susceptibility to lysis by Ly-49D+ NK cells. None of the major histocompatibility complex class I–bearing targets inhibited lysis of Ly-49D+ NK cells. More importantly, we demonstrate that the addition of mAb 12A8 to Ly-49D+ NK cells can augment lysis of FcγR+ target cells in a reverse antibody-dependent cellular cytotoxicity–type assay and induces apoptosis in Ly49D+ NK cells. Furthermore, the cytoplasmic domain of Ly-49D does not contain the V/IxYxxL immunoreceptor tyrosine-based inhibitory motif found in Ly-49A, C, or G2 that has been characterized in the human p58 killer inhibitory receptors. Therefore, Ly-49D is the first member of the Ly-49 family characterized as transmitting positive signals to NK cells, rather than inhibiting NK cell function.
Members of the Ly-49 gene family encode type II integral transmembrane proteins and are primarily expressed on the surface of murine NK cells. Several members of the Ly-49 family of proteins can bind to class I MHC and transmit inhibitory signals to NK cells. When expressed on target cells, selected class I proteins can prevent NK cells from delivering their lethal hit. Recognition of class I molecules by Ly-49+ NK cells has been proposed as a regulatory mechanism to prevent lysis of normal host cells. However, NK cell lysis can proceed upon downregulation of host class I after transformation or viral infection (1). Recent studies have identified eight Ly-49 gene family members in NK cells from B6 mice (2, 3). The prototypic member of the Ly-49 family, Ly-49A, has been shown to recognize the class I molecules H-2Dd and H-2Dk (4, 5). The interaction of Ly-49A with H-2Dd has been postulated to transmit a negative signal to the NK cell. This hypothesis has been formulated because Ly-49A+ NK cells are apparently not capable of mediating antibody-dependent cellular cytotoxicity or lectin-induced cytotoxicity against H-2Dd–expressing target cells. Upon addition of mAb A1, which recognizes Ly-49A+ NK cells (6), enhanced lysis of target cells that is not FcR dependent is observed (4). Studies have also shown that Ly-49A can recognize carbohydrate expressed on the surface of target cells, which may contribute to the interaction of Ly-49A and class I proteins (7).
The Ly-49G2 subset of NK cells also has been shown to be inhibited by target cells expressing H-2Dd and/or H-2Ld (8). Studies with Ly-49G2+ NK cells have relied primarily on the reversal of target cell inhibition by mAb 4D11 (anti–Ly-49G2) and mAb specific for H-2Dd and H-2Ld. The Ly-49C+ subset of NK cells has been shown to bind the class I molecules of the H-2b, H-2d, H-2k, and H-2s haplotypes (9). Recent data from Yu et al. (10) demonstrates that Ly-49C+ NK cells from BALB/c and BALB.B mice are inhibited by H-2d and H-2Kb class I antigens. The authors in this study concluded, however, that not all Ly49C+ NK cells function the same way in all mouse strains, and suggest that allelic differences may regulate class I recognition by these cells (10). Previous data by members of this group have shown that 5E6+ NK cells can reject bone marrow grafts expressing H-2d but not H-2b (11). These results suggest that Ly-49C binding to its H-2d ligand may not be inhibitory in the strains studied. In H-2d strain mice, Ly- 49C+ NK cells may be responsible for promoting hematopoiesis through the upregulation of GM-CSF, as demonstrated by Murphy et al. (12), implying further that some Ly-49 family members may upregulate NK cell function.
The Ly-49 gene family now consists of eight distinct molecules in a single inbred stain. The original Ly-49 gene has been renamed Ly-49A, and the others have been designated Ly-49B-H (2, 3, 9). mAb specific for the Ly-49A, C, and G2 molecules have helped provide significant information on their functional attributes. Functional characterization of other Ly-49 family members has been hampered by the lack of antibodies that specifically recognize each molecule. Ly-49D is of particular interest because it has a cytoplasmic domain that is significantly different from other Ly49 family members. In this report, we describe mAb 12A8, which reacts with the Ly-49D subset of murine B6 NK cells. Although transfection studies with the known members of the Ly-49 cDNA family of genes indicates that it reacts with both Ly-49D and Ly-49A, the Ly-49D+ subset of NK cells can be isolated. Here, we characterize the functional characteristics of Ly-49D+ NK cells and suggest that the Ly-49D receptor on NK cells is an activating rather than an inhibitory receptor.
Materials and Methods
Mice and Rats.
All mice and rats were obtained from the Animal Production Facility, FCRDC. Mice were between 8 and 20 wk old when killed.
Generation of mAb 12A8.
IL-2–cultured B6 NK cells were used to immunize Fischer 344 rats. Approximately 5–10 × 106 cells were injected subcutaneously into rats and boosted every 2 wk for a total of four boosts. After the final boost, the rat spleen was harvested and spleen cells were fused with the SP2/0 hybridoma by PEG. Supernatants were first screened for rat IgG by ELISA, followed by a live cell ELISA assay against IL-2–propagated B6 NK cells, as previously described (13). mAb 12A8 was isolated and the hybridoma was cloned by limiting dilution. mAb 12A8 is of the rat IgG2a isotype.
Flow Cytometry Analysis.
NK cells were stained as previously described and were analyzed on either a FACScan® or FACSort® (Becton Dickinson & Co., Mountain View, CA). Cell sorting was performed on either an Epics 750 (Coulter Electronics, Hialeah, FL) or a FACStar® (Becton Dickinson). NK cells were either stained with the primary antibody followed by an isotypespecific, FITC-conjugated secondary reagent, or directly with a FITC-labeled primary antibody. Cells sorted for mAb 12A8 were stained with biotinylated 12A8, followed by Streptavidin PE (Becton Dickinson). Antibodies used in these experiments included 2.4G2 (FcγRII/III), PK136 (NK-1.1), A1 (Ly-49A), SW5E6 (Ly-49C), and 4D11 (Ly-49G2). All antibodies were semipurified by salt fractionation (2×) of ascites fluid. RM21 antibody against murine CD2 was affinity purified before use.
NK Cell Isolation.
Murine splenic NK cells were isolated as described previously (13). Essentially, nylon wool–nonadherent (NWNAD)1 cells were obtained and subjected to mAb plus complement (mAb + c′) depletion of T cells using anti-CD4 and CD8 mAbs. Partial depletion of Ly-49A, C, and G2+ NK cells was obtained by including mAb SW5E6 and 4D11 to the cocktail of anti-CD4 and CD8 mAbs. Lysis of NK cells with mAb 5E6 and 4D11 accomplished two goals: the first was to reduce the number of NK cells expressing known Ly-49 receptors such as C and G2 (and coincidentally, those cells coexpressing Ly-49A), and the second was to enrich the NK cell population for cells expressing 12A8 (Ly-49D) to make sorting these cells less time consuming. Treatment of NWNAD cells with this cocktail resulted in an NK cell population that was enriched for 12A8+ (Ly-49D+) NK cells, but generally <10% Ly-49A, C, and G2+. The remaining B cells were further removed by immunoadsorbance to anti–mouse IgG- coated petri plates. After culture for 6–12 d in 1,000 U/ml IL-2, the resulting cell populations were >95% NK-1.1+.
Tumor Targets and Con A Blasts.
Tumor targets were maintained in culture as previously described (13). Splenic Con A lymphoblasts were essentially prepared by the method of Chadwick and Miller (14). Blasts were prepared and frozen at −70°C before labeling.
Cytotoxicity Assays.
Tumor targets and Con A lymphoblasts were labeled with 51Cr and used in 4-h cytotoxicity assays, as described previously (13). Assays involving mAb included the specific antibodies at a concentration of ∼2 μg/well for the duration of the cytotoxicity assay. Data are either presented as lytic units per 107 cells or the percent of specific lysis.
Immunoprecipitation.
IL-2–cultured NK cells were 125I-surface labeled with lactoperoxidase and lysed in 0.5% Triton X-100 containing protease inhibitors, as described previously (13). A single lysate was subjected to sequential immunoprecipitation using an mAb cross-linked to protein G–Sepharose. The order of immunoprecipitations was control rat IgG2a, A1, 4D11, SW5E6, and 12A8. Approximately equal amounts of radioactivity (except for the control antibody in which the entire eluate was used) was applied to each well of a 10% SDS-PAGE gel under nonreducing and reducing conditions. Gels were fixed, dried, and autoradiography was performed.
Transfection of Cos-7.
cDNA plasmids encoding Ly-49A, D, E, F, G1, and G2 were initially transfected into Cos-7 cells using the DEAE-dextran method (15). Cells were harvested with versene (GIBCO BRL, Gaithersburg, MD) 3 d after transfection and were analyzed by flow cytometry analysis (FCA) for binding of mAb 12A8. Reactivity was confirmed as shown in Fig. 2 by using Cos-7 cells transfected with Lipofectamine (Life Technologies, Gaithersburg, MD). A 1:60 dilution of Lipofectamine and 5 μg of Ly-49 plasmid cDNA was used according to the manufacturer's instructions. Transfection was performed for 6 h at 37 °C, followed by washing, trypsinization, and replating cells in a T-150 flask. After 72 h of culture at 37°C, the cells were removed using HBSS without Ca++ and Mg++ and with 1 mM EDTA. FCA was performed as described previously.
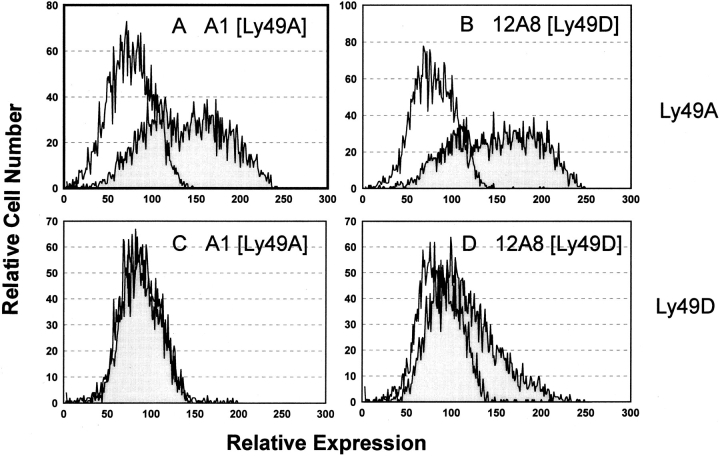
Figure 2.
mAb 12A8 stains Cos-7 cells transfected with Ly-49A and Ly-49D. Cos-7 cells were transfected with either Ly-49A or Ly-49D using Lipofectamine and were harvested after a 72-h culture at 37°C for protein expression. Cells transfected with Ly-49A (A and B) and Ly-49D (C and D) were stained with mAb A1 (A and C) or mAb 12A8 (B and D). mAb 12A8 staining was observed on both Ly-49A and Ly-49D transfectants, whereas mAb A1 stained only Cos-7 cells tranfected with Ly-49A. The unshaded histogram represents staining with FITC-GAM (mAb A1) or FITC-GART (mAb 12A8). Shaded histograms represent staining observed with a primary antibody (A1 or 12A8) plus FITC-GAM or FITC-GART.
Apoptosis.
Highly enriched populations of NK cells were obtained from C57BL/6 splenocytes as described previously. The methods used to measure apoptosis have been developed by Ortaldo et al. (15a). 106 NK cells were cultured for 24 h in RPMI 1640 + 10% FBS + 103 U/ml IL-2 in 24-well plates. NK cells were collected, and viable cells were obtained after passage over Lympholyte-M (Cedarlane Labs., Ontario, Canada). Cells were then cultured in 48-well plates at 106 cells/well, to which a variety of mAb were added, including A1, 12A8, 4D11, 5E6, and PK136 at a concentration of 40 μg/well in a total volume of 100 μl. After a 15-min incubation at room temperature, 40 μg of a secondary goat anti–mouse or anti–rat antibody was added to each well. Plates were incubated at 37°C for 6 and 12 h. NK cells were harvested and stained with propidium iodide for FCA. Flow cytometry was performed on a FACSort® using Lysis II software (Becton Dickinson), which analyzed the percentage of cells incorporating propidium iodide vs. forward scatter.
Results
mAb 12A8 Identifies a Unique Subset of NK Cells.
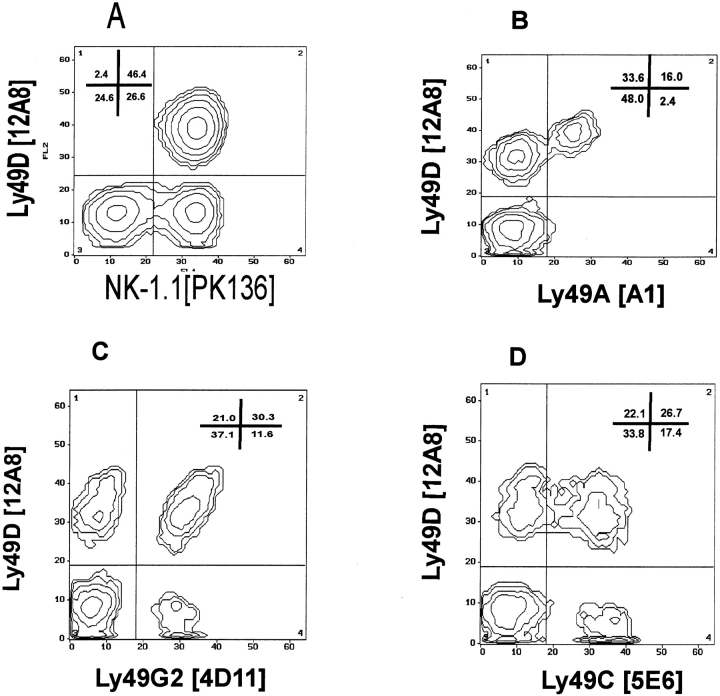
mAb 12A8 was generated by fusing spleen cells from Fischer 344 rats that had been immunized with C57BL/6–IL-2–cultured NK cells, with the SP2/0 myeloma. Screening of lymphoid cells by FCA revealed that mAb 12A8 did not react with thymocytes, T cells, or B cells from B6 mice (data not shown). mAb 12A8 did react, however, with a unique subset of B6 NK cells. Fig. 1 A shows the staining profile of mAb 12A8 on highly enriched populations of freshly isolated NK cells. Two-color FCA of 12A8 vs. NK-1.1 demonstrated that virtually all 12A8+ cells were NK-1.1+, and that 12A8+ cells represented ∼63% of the NK-1.1+ cells. The staining profiles and reactivity of mAb 12A8 on freshly isolated and IL-2–cultured NK cells indicated that this antibody may be similar to the previously characterized mAbs, 4D11 (13) and 5E6 (11). Two-color FCA was used to examine this possibility. Fig. 1 presents the staining profiles of mAb 12A8 vs mAb A1 (Ly-49A; Fig. 1 B), mAb 4D11 (Ly-49G2; Fig. 1 C), and mAb 5E6 (Ly-49C; Fig. 1 D). These staining profiles revealed that mAb 12A8 reacted with a unique subset of B6 NK cells, and that it did not demonstrate reactivity similar to mAb 5E6 or 4D11. However, an interesting staining profile was observed on NK cells when costained with mAb 12A8 vs. A1 (Fig. 1 B). It was apparent that all cells that stained with mAb A1 were also 12A8+. One explanation for the expression of mAb 12A8 on all A1+ cells could simply be that all A1+ cells express both Ly-49A and another molecule recognized by mAb 12A8. An alternative explanation would be that mAb 12A8 cross-reacts with both Ly-49A and another highly homologous member of the Ly-49 gene family. Blocking studies were performed with mAbs 12A8 and A1 to determine if binding of one mAb could block the reactivity of the alternate mAb. Binding of mAb 12A8 to NK cells does reduce the staining intensity (mean channel fluorescence) of FITC A1; however, mAb A1 does not block binding of FITC 12A8 (data not shown). Control blocking studies revealed that mAb 12A8 did not affect staining by mAb 4D11 or 5E6. The results of our FCA data suggested that mAb 12A8 identified a unique molecule on the surface of NK cells with possible cross-reactivity to Ly-49A.
Figure 1.
mAb 12A8 identifies a distinct subset of C57BL/6 NK cells. Freshly isolated C57BL/6 splenic NK cells were enriched by passage over nylon wool columns, depleted of T and B cells, and prepared for FCA as follows: (A) PK136 plus biotinylated 12A8 followed by FITC– goat anti–mouse IgG2a and Steptavidin-PE, (B) FITC-A1 plus biotinylated 12A8 followed by Streptavidin PE, (C) FITC4D11 plus biotinylated 12A8 followed by Streptavidin PE, and (D) FITC-5E6 plus biotinylated 12A8 followed by Streptavidin PE.
mAb 12A8 Recognizes Ly-49D and Ly-49A on Transfected Cos-7 Cells.
Cos-7 cells were transfected with cDNA for Ly-49A or Ly-49D, and stained for reactivity with mAb A1 (Ly-49A) or mAb 12A8. Fig. 2 presents the profiles of these staining reactivities. These results clearly reveal that cells transfected with Ly-49A react with both mAb A1 and 12A8 (Fig. 2, A and B), whereas cells transfected with Ly49D only react with mAb 12A8 (Fig. 2 D). Cos-7 cells transfected with the cDNAs for Ly-49E, F, G1, and G2 did not react with mAb 12A8 (data not shown). Furthermore, transfection of Cos-7 cells with Ly-49B, C, and H did not reveal any cells staining positive with mAb 12A8 (data not shown). These transfection studies explain our FCA results (Fig. 1 B), in which all A1+ cells were also 12A8+, and they conclusively demonstrated 12A8 binding to Ly-49D. Therefore, mAb 12A8 is the first reported antibody to bind to Ly49D and should be a useful reagent to help understand the biological role of Ly-49D+ NK cells.
mAb 12A8 Immunoprecipitates a 50-kD Homodimer on NK Cells.
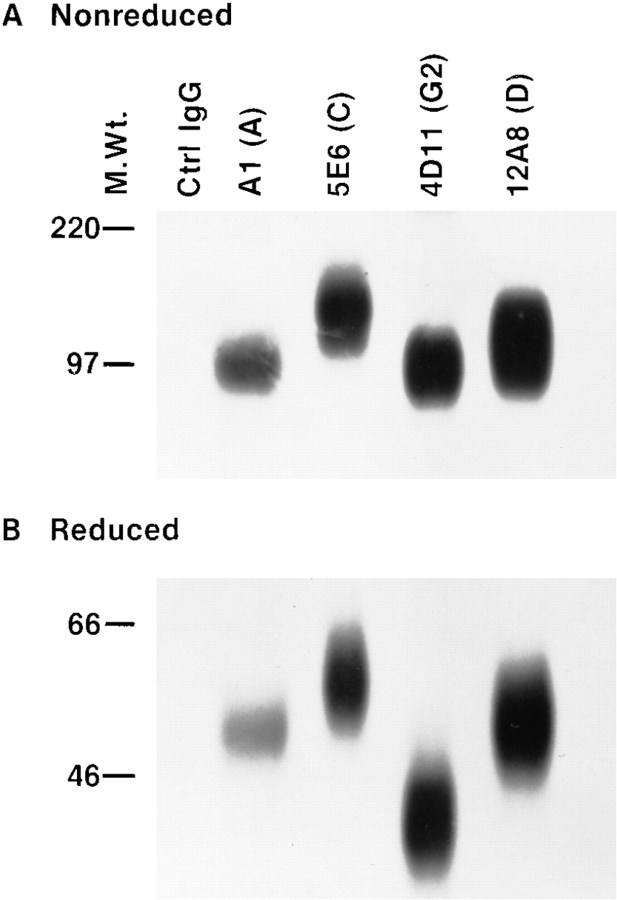
Considering the unique phenotypic characterization of the 12A8+ subset of NK cells and its reactivity with Ly-49D, we decided to examine the biochemical characteristics of the antigen recognized by this antibody. IL-2–cultured NK cells from B6 mice were surface labeled with 125I, lysed, and subjected to immunoprecipitation with an mAb linked to protein G–Sepharose. Preliminary experiments demonstrated that mAb 12A8 immunoprecipitated a glycoprotein of ∼100 kD (nonreduced) and ∼50 kD (reduced). To confirm our transfection data and to determine if mAb 12A8 could immunoprecipitate a unique Ly-49 glycoprotein, we performed sequential immunoprecipitation (IP). A single lysate from 125I-labeled NK cells was subjected to IP, first with an isotype control mAb, followed by mAbs A1, 5E6, 4D11, and 12A8, respectively. IP with mAb A1 was performed with an excess of protein G–Sepharose– linked antibody, and was allowed to react at 4°C overnight to remove cross-reactive Ly-49A+ glycoproteins. Figs. 3, A and B, presents the results of these IP experiments. Despite the highly glycosylated nature of the Ly-49 proteins that result in diffuse bands during SDS-PAGE analysis, clear differences between the IPs were observed. IP with mAb A1 (16), 5E6 (11), and 4D11 (13) resulted in bands under both nonreducing and reducing conditions that were consistent with previously published reports. mAb 12A8 immunoprecipitation yielded proteins with noticeably higher molecular weight bands than those seen with mAb 4D11 and slightly higher than those seen with mAb A1. 5E6 IP, however, resulted in proteins that were slightly larger than those seen with mAb 12A8. These results support our FCA data on both NK cells and transfected Cos-7 cells, that mAb 12A8 recognizes a unique glycoprotein on B6 NK cells.
Figure 3.
Immunoprecipitation using mAb 12A8 reveals a unique disulfide-linked homodimer. B6 NK cells were cultured for 7 d in high dose IL-2. Cells were radiolabeled with 125I and lysed in 0.5% Triton X-100. Sequential IP was performed on a single lysate by the following mAb crosslinked to protein G–Sepharose: Control rat IgG2A, A1, 5E6, 4D11, and 12A8, respectively. Approximately equal CPMs were applied to a 10% SDSPAGE gel and electrophoresed under both nonreduced (A) and reduced (B) conditions.
Isolation of Ly-49D+ NK Cells and Lysis of Tumor Targets.
Murine NK cells from C57BL/6 mice are heterogenous in their expression of Ly-49 gene family members (Fig. 1), where individual NK cells are likely to express multiple Ly-49 gene products. To examine the functional properties of Ly-49D+ NK cells, it was necessary to isolate cells expressing this marker from those NK cells expressing other Ly-49 proteins such as Ly-49C, G2, and particularly Ly-49A, with which it cross-reacts. Splenic NWNAD cells were depleted of T and B cells along with partial depletion of the Ly-49C+ and Ly-49G2+ subsets of NK cells using the appropriate mAb + C′. Coexpression of Ly-49A on Ly-49C+ and G2+ NK cells resulted in the removal of the majority of Ly-49A+ NK cells during the lysis step. These depletion experiments resulted in a population of NK cells expressing <10% Ly-49A, C, and G2+ cells. Ly-49A+ NK cells (that cross-react with mAb 12A8) were separated from Ly-49D+ NK cells by two-color cell sorting. NK cells were stained using biotinylated mAb 12A8 vs. FITCmAbA1 and sorted. From this population, the Ly-49D+/A− and Ly-49D−/A− cells were collected and cultured in IL-2. Upon completion of cell sorting, these subsets were >98% 12A8+ or 12A8− for their respective phenotypes.
The purified NK cell subsets were examined for their cytolytic properties against various class I target cells. A panel of tumor target cells was examined for susceptibility to lysis by Ly-49D+/A− and Ly-49D−/A− NK cells. Table 1 presents the lytic data on the ability of the input population (Ly-49A, C, and G2 reduced), Ly-49D+/A−, and Ly49D−/A− NK cells to lyse tumor target cells expressing different class I phenotypes. It is apparent from this data that Ly-49D+/A− NK cells are capable of lysing tumor target cells expressing H-2a, H-2b, H-2d, and H-2k. Lysis by Ly49D+ NK cells was equivalent to, if not greater than, Ly49D−/A− NK cells and the input population. These results indicate that lytic activity of Ly-49D+ cells is not inhibited by these class I–bearing tumor targets. Previous studies in our laboratory have demonstrated that both P815 and WEHI-164 (H-2d targets) are not lysed efficiently by Ly49A+ or G2+ NK cells when compared to the Ly-49A− and G2− subsets (data not shown).
Table 1.
Ly-49D+ NK Cells Lyse Tumor Targets That Express Different Class I Phenotypes
| YAC | P815 | EL-4 | SST | WEHI-164 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2a | H2d | H2b | H2k | H2d | ||||||
| Input | 4223+ | 361 | 589 | 1239 | 140 | |||||
| Ly-49D+/A− | 4642 | 423 | 1073 | 1087 | 86 | |||||
| Ly-49D−/A− | 3278 | 125 | 523 | 876 | 92 | |||||
| NK cells were partially depleted of Ly-49C and G2+ cells (with a subsequent elimination of most Ly-49A+ cells), as described in Materials and Methods, and labeled “Input.” The input cells were sorted into Ly49D+/A− and Ly-49D−/A− subsets on day 0, and all three populations were expanded by culturing IL-2 for 7–12 d. NK cell lysis of the indicated targets was examined in a 4-h 51Cr-release assay starting at E/T ratios of 20:1. The Ly-49 phenotype of these subsets after culture in IL-2 was as follows: | ||||||||||
| Input | Ly-49D+/A− | Ly-49D−/A− | ||||
| Ly-49A | 11% | 7% | 1% | |||
| Ly-49C | 12% | 14% | 4% | |||
| Ly-49G2 | 5% | 9% | 4% | |||
| Ly-49D | 38% | 77% | 1% |
Results are presented as lytic units at 30% per 107 cells. The data are from one of two similar experiments performed.
Ly-49D+ NK Cells Lyse Con A Blasts of Varied H-2 Haplotypes.
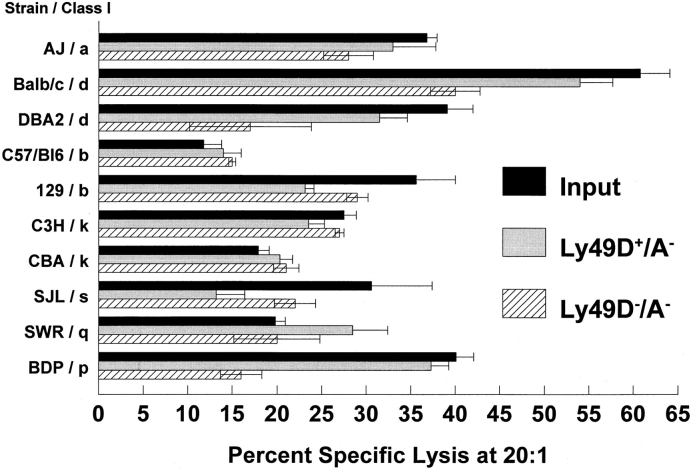
In further attempts to establish any possible class I restriction of the lytic capacity of Ly-49D+ NK cells, Con A lymphoblasts from seven different H-2 haplotypes were examined. Ly-49D+ cells were isolated as described above and expanded in IL-2 for 6–10 d. Splenic Con A lymphoblasts were generated for 48 h and labeled with either 51Cr or 111In, and were used as targets in a 4-h cytotoxicity assay. The data presented in Fig. 4 demonstrates that targets expressing H-2a, H-2b, H-2d, H-2k, H-2s, H-2q, and H-2p are all lysed by Ly-49D+ NK cells. In general, the patterns of lysis observed indicated that Ly-49D+ NK cells are comparable to both the Ly-49D− and input cells against the majority of the blast targets. These results support the lytic data using tumor targets that Ly-49D+ NK cells do not appear to be inhibited by any of the class I–expressing target cells tested to date. One conclusion from this data is that the glycoprotein encoded by the Ly-49 gene may not interact with class I MHC. If recognition does occur between Ly-49D–encoded proteins and class I, signals that inhibit NK lysis are not generated.
Figure 4.
Ly-49D+/A− and Ly-49D−/A− NK cells lyse Con A blasts of multiple H-2 haplotypes. Ly49D+/A− NK cells were sorted from Ly-49D−/A− NK cells after partial depletion of the Ly-49C+/A+ and G2+ cells, as described in Materials and Methods. Cells were cultured for 6 d in high dose IL- 2. Con A lymphoblasts were prepared from a variety of H-2 haplotypes representing H-2a, H-2d, H- 2k, H-2s, H-2a, and H-2q. Blasts were labeled with 51Cr and used as targets in a 4-h cytotoxicity assay. E/T ratios of 20:1, 7:1, 2:1, and 0.7:1 were assayed, and the percent of specific lysis at 20:1 is shown. Spontaneous release of targets varied from 19 to 35%. Results also are presented for the input population that was partially depleted of Ly-49A, C, and G2+ cells. The resulting Ly-49 phenotypes of these populations are the same as in the legend to Table 1. These results represent one of two such experiments performed.
Ly-49D+ NK Cells Mediate Reverse Antibody-dependent Cellular Cytotoxicity (RADCC) in the Presence of mAB 12A8.
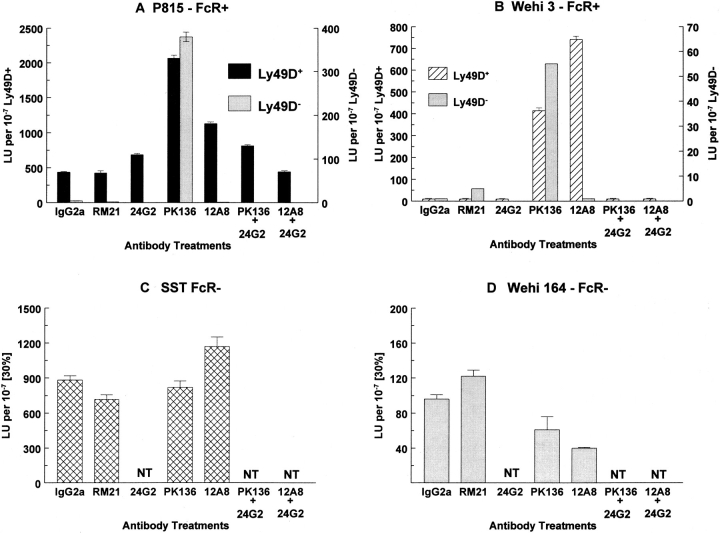
Since Ly-49D+ NK cells failed to exhibit any pattern of class I restriction, we decided to examine mAb 12A8 for its ability to activate the lytic potential of Ly-49D+ NK cells. A panel of different tumor target cells, including both FcγR+ and FcγR− targets, were examined for susceptibility to lysis by Ly-49D+ and Ly-49D− NK cells in the presence of various antibodies. Fig. 5 presents the data obtained from a representative redirected lysis (RADCC) assay. The addition of mAb 12A8 to Ly-49D+, but not Ly-49D−, NK cells, results in augmented lysis of FcγR+ target cells. Both P815 (Fig. 5 A) and WEHI-3 (Fig. 5 B) targets (FcγR+) are lysed much more efficiently in the presence of mAb 12A8 when compared to either media alone, a negative control mAb (Rγ2A), or mAbs reactive with NK cells (e.g., RM21 and 2.4G2). This RADCC effect has been reported for mAb PK136, which detects the NK-1.1 molecule (17), and can be blocked by the addition of mAb 2.4G2, which is specific for FcγRII/III (18). As a further control in these experiments, it can be seen that mAb PK136 is capable of augmenting the lysis of both the Ly49D+ and D− subsets (>95% NK-1.1+) against the FcγR+ targets P815 and WEHI-3. The Ly-49D− subset, however, is not augmented in the presence of mAb 12A8 to lyse these same targets. These results also reveal that the augmented lysis observed in the presence of mAb 12A8 can be abrogated upon addition of mAb 2.4G2 (identical to that seen using PK136 + 2.4G2). Similar results have been obtained upon pretreatment and washing of Ly-49D+ NK cells with mAb 12A8 before performing cytotoxicity assays (data not shown). FcγR− target cells such as SST (Fig. 5 C) and WEHI 164 (Fig. 5 D) do not demonstrate any significant augmentation of lysis by Ly-49D+ NK cells in the presence of mAb 12A8. The data shown in Fig. 5 (C) shows that mAb 12A8 weakly augments killing by Ly-49D+ NK cells against the SST target. This finding has not been reproducible in other experiments using this target and was not considered significant. Based on the above experiments, we conclude that mAb 12A8 engagement of the Ly-49D molecule augments the lytic function of NK cells. These results establish for the first time that a member of the Ly-49 gene family may upregulate NK lytic function upon binding the appropriate ligand.
Figure 5.
mAb 12A8 mediates RADCC of FcγR+ target cells by Ly-49D+, but not Ly-49D−, NK cells. Splenic NK cells were depleted of cells expressing Ly-49A, C, and G2. Ly-49D+ and Ly-49D− cells were sorted (using biotinylated 12A8 followed by streptavidin PE) into populations that were >98% 12A8+ (Ly-49D+) or 12A8− (Ly-49D−). These subsets were cultured in high dose IL-2 for 9 d, washed, and tested against the indicated targets in a 4-h 51Cr-release assay at E/T ratios starting at 10:1. mAbs were added at a final concentration of 2 μg/well. Data are presented as lytic units per 107 cells at 30% lysis. This experiment is one of at least three representative assays (NT, not tested). Both the Ly-49D+ and Ly-49D− populations were <5% Ly49A, C, and G2+ at the time of testing.
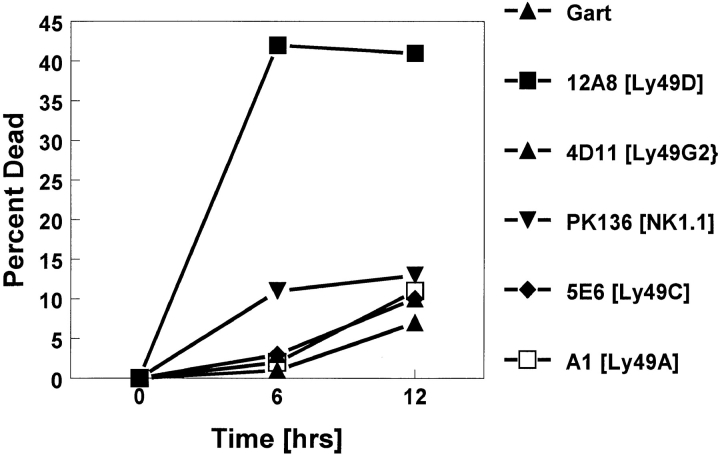
mAb 12A8 Induces Apoptosis of Ly-49D+ NK Cells.
The ability of mAb 12A8 to mediate RADCC in Ly-49D+ NK cells presents functional data for involvement of the Ly-49D molecule in activating the lytic mechanism of NK cells. We also have obtained evidence that the Ly-49D molecule may be involved in triggering apoptotic events in NK cells that lead to cell death. The results shown in Fig. 6 demonstrate that mAb 12A8 induces apoptosis in ∼40% of bulk NK cells by 6 h. Although some variability was observed in different experiments, none of the other Ly49–specific antibodies induced any consistent or potent apoptosis. Since mAb 12A8 stains ∼60% of bulk NK cells, of which up to 20% may represent cross-reacting Ly-49A+ cells, it appears that most Ly-49D+ NK cells are susceptible to mAb 12A8–induced apoptosis. Of interest, mAb PK136 that recognizes the NK-1.1 antigen (mouse NKR-P1) was not very effective at inducing apoptosis. Only 10–12% of NK cells demonstrated PI uptake after the addition of PK136. These results support the hypothesis that Ly-49D represents an activation molecule on NK cells. Although Ly-49A, C, and G2 have been demonstrated to be class I inhibitory receptors on NK cells, cross-linking of their specific receptors does not induce apoptotic associated events in NK cells. Therefore, intracellular signaling events mediated by Ly-49D appear to be different from those mediated by Ly-49A, C, and G2. Apoptotic-associated events also have been observed using the CellFit program (Becton Dickinson) to measure the DNA index of murine NK cells after appropriate stimulation and cross-linking of the Ly-49 mAb. Using this methodology, we have observed increases in abnormal DNA content in NK cells treated with mAb 12A8, but not with 5E6 or 4D11 (data not shown).
Figure 6.
mAb 12A8 induces apoptosis in Ly-49D+ NK cells. This graph presents the data obtained from a representative experiment in which percent cell death (PI uptake) is calculated after 6 and 12 h of antibody treatment. Cell death is calculated over that observed with media alone (33, 35 & 38% respectively). Maximum cell death was observed after 6 h of treatment with mAb 12A8 and cross-linking. This is a representative experiment of eight similar experiments performed.
Discussion
Eight distinct members of the Ly-49 gene family have been identified (Ly-49A–H), although specific mAbs that recognize Ly-49A (A1/YE1-32 and 48), Ly-49C (5E6), and Ly-49G2 (4D11) are the only ones available. The availability of these mAbs has helped to further define the functional role of NK cells that express these receptors. The importance of generating mAbs against the remaining, undefined members of the Ly-49 gene family is paramount to understanding the functional role that these molecules play in NK cell biology. Investigation of the proposed class I inhibitory properties mediated by members of the Ly-49 gene family may be facilitated by acquiring such reagents. The prototype molecule, Ly-49A, when expressed on NK cells, has been shown to bind the class I molecules H2-Dd and Dk. This recognition of class I ligand by the Ly-49A receptor has been shown to inhibit the lytic properties of Ly-49A+ NK cells. A similar recognition process has been proposed for Ly-49G2+ NK cells. Inhibition of lysis by Ly49G2+ NK cells, however, has only been observed against selected target cells that appear to express high levels of H2-Dd and/or Ld (8, 18). Experiments to determine if Ly49G2 specifically binds to H2-Dd and/or Ld are now in progress. Brennan et al. (9) have observed binding of Ly49C+ cells to multiple H-2 haplotypes, including H-2d, H-2k, H-2s, and H-2b. Furthermore, Ly-49C+ NK cells have been demonstrated to be the subset of NK cells that is responsible for rejecting H-2d, but not H-2b, bone marrow allografts (11). These experiments imply that Ly-49C+ NK cells also may be capable of upregulating NK cell function upon appropriate receptor/ligand interaction. A recent report by Stoneman et al. (19) demonstrates that glycosylation of the Ly-49C core protein varies in different mouse strains. They have implied that the extent to which the Ly49C molecule is glycosylated may explain the functional discrepancies observed by Ly-49C+ NK cells from different mouse strains. Of further interest is the recent data from Yu et al. that describes the role of Ly-49A+ and C+ NK cells in mediating hybrid resistance (10). These authors conclude that class I inhibition of Ly-49A+ and C+ NK cells support the “missing self” and not the “hemopoietic histocompatibility antigen” hypothesis for hybrid resistance. The data from this group also suggested that allelic differences in Ly49C+ NK cells may control which class I molecules inhibit their lytic function.
Our laboratory has generated an mAb designated 12A8 that recognizes the Ly-49D molecule expressed on the surface of B6 NK cells. Functional analysis of Ly-49D+ NK cells demonstrate that they do not appear to be restricted in their lytic capacity against either tumor targets or Con A blasts of different H-2 haplotypes. More significantly, we have demonstrated that the protein receptor encoded by Ly-49D is capable of enhancing the lytic potential of NK cells. In the presence of FcγR+ target cells, mAb 12A8 triggers Ly49D+ NK cells to lyse these targets in a RADCC fashion similar to that which occurs with the mAb PK136 against the NK-1.1 (NKR-P1) molecule (17). Furthermore, crosslinking of mAb 12A8 on the surface of NK cells using a secondary anti-rat antibody induces IL-2–activated NK cells to undergo apoptotic events resulting in programmed cell death. Studies with both T cells (20, 21) and human NK cells (22) have revealed that IL-2–activated cells can undergo apoptosis when stimulated through activating surface receptors such as CD3 and CD16, respectively. The significance of this finding is that mAb specific for the Ly-49A, C, and G2 proteins did not induce positive signaling events leading to apoptosis. Therefore, our data provide strong evidence that Ly-49D is not an inhibitory receptor on NK cells as are Ly-49A, C, and G2. Ly-49D appears capable of enhancing lytic function, and possibly signals NK cells through alternate pathways.
A question arises as to whether or not the observed RADCC results obtained with mAb 12A8 on Ly-49D+ NK cells could be an intrinsic function of the mAb itself, rather than that of the Ly-49D receptor. It would be tempting to determine if Ly-49A+ NK cells could be induced to mediate RADCC by mAb 12A8 because of its cross-reactivity. The results of such an experiment could not be interpreted, however, because the percentage of Ly49A+ NK cells that coexpress Ly-49D is unknown. Until an antibody is produced that specifically reacts with Ly49D, we must at least entertain the possibility that mAb 12A8 could have some intrinsic capacity to activate Ly-49 molecules.
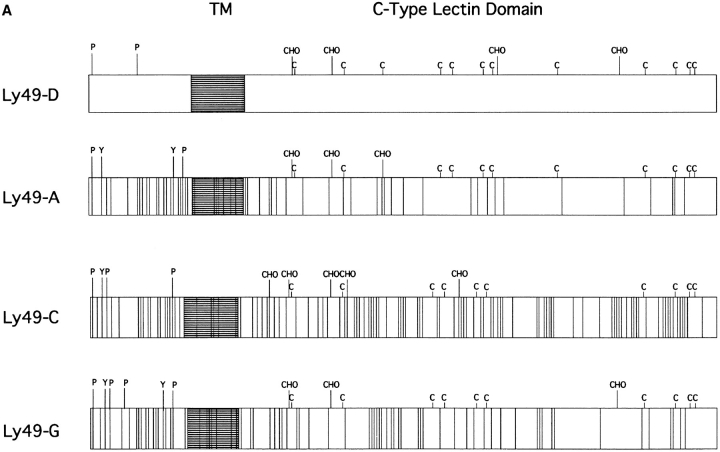
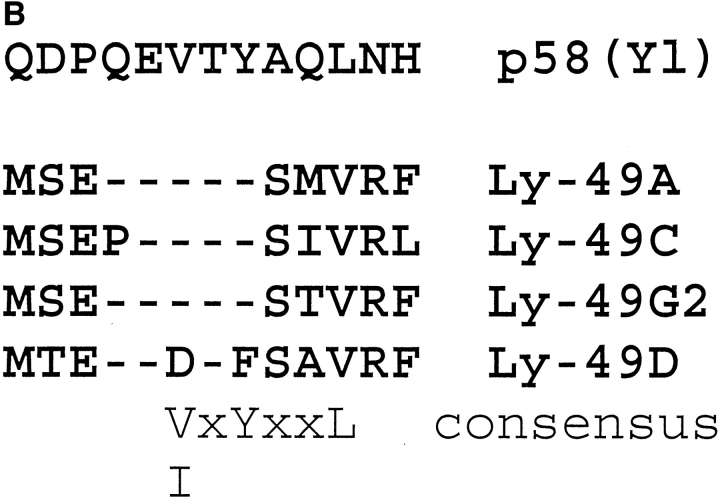
Because of the unique activating properties of Ly-49D+ NK cells compared to the inhibitory properties observed in Ly-49A, C, and G2+ NK cells, we compared the amino acid homology of these proteins. Fig. 7 A is a schematic comparison of Ly-49D vs. Ly-49 A, C, and G2, based on the proposed amino acid sequence of these molecules (3). There is obviously a high degree of homology between Ly49D and A that is highest in the extracellular domain (86%) and least homologous in the cytoplasmic domain (51%). The structural homology between Ly-49A and D in their extracellular domains probably accounts for the cross-reactivity that is observed with mAb 12A8, since it can bind to both Ly-49D and Ly-49A. Furthermore, we can also speculate that the lack of homology in the cytoplasmic region of these molecules accounts for the different signaling properties of these proteins. The cytoplasmic domains of Ly-49A, C, and G2 all share at least two potential serine/ threonine phosphorylation sites (P). These three molecules also share similar sites for tyrosine phosphorylation (Y) in their intracellular domains. A significant divergence in phosphorylation motifs is seen in the cytoplasmic domain of Ly-49D. Ly-49D contains only one potential serine/ threonine phosphorylation motif, and none of the tyrosine phosphorylation motifs found in either Ly-49A, C, or G2. Fig. 7 B presents a 13–amino acid consensus sequence comparing the Ly-49A, C, G2 and D molecules with a similar cytoplasmic region of the human p58 inhibitory receptors. Long et al. (23) have recently described a V/IxYxxL consensus sequence in p58 proteins that may represent an immunoreceptor tyrosine-based inhibitory motif (ITIM). This V/IxYxxL motif is present in the Ly-49 inhibitor receptors A, C, and G2. However, the Ly-49D receptor does not contain this ITIM motif, which further supports our functional data that Ly-49D is an activation receptor on murine NK cells.
Figure 7.
(A) Amino acid homology of Ly-49D vs. Ly-49A, C, and G2. This figure is a schematic representation of the proposed amino acid sequences of Ly-49D, A, C, and G2, as described by Smith et al. (3) The highly conserved extracellular cysteine residues are represented (C) along with the proposed glycosylation sites (CHO). Potential serine/threonine phosphorylation sites are labeled in the cytoplasmic region (P). Possible tyrosine phosphorylation sites (Y) are also designated in the cytoplasmic domain. Open spaces in these schematics represent areas of homology between these four proteins, whereas the perpendicular lines shown in Ly-49A, C, and G2 represent individual amino acid differences compared to Ly-49D. (B) Homologous regions from the intracellular domains of the four Ly-49 molecules compared to the human p58 inhibitory receptor. Dashes represent amino acids identical to the p58 sequence. A consensus sequence (V/IxYxxL) for a possible immunoreceptor tyrosine inhibitory motif found in p58 is also present in Ly-49A, C, and G2, but not in Ly-49D.
In summary, the present study describes the first member of the Ly-49 family of NK receptors that appears to positively regulate NK function, rather than to inhibit it. The detailed structure/function relationship of the ITIMs of Ly-49 family members will greatly aid in the understanding of the mechanism of action of these novel NK cell receptors.
Acknowledgments
We would like to thank Jeanette Higgins and Louise Finch from the Clinical Services Program, SAIC, for their expert technical assistance in cell sorting, and Susan Charbonneau and Joyce Vincent for typing and editing this manuscript.
Footnotes
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 86-23, 1985).
1 Abbreviations used in this paper: ab+c′, antibody plus complement; FCA, flow cytometry analysis; ITIM, immunoreceptor tyrosine-based inhibitory motif; IP, immunoprecipitation; NWNAD, nylon wool–nonadherent (cells); PI, propidium iodide; RADCC, reverse antibody-dependent cellular cytotoxicity.
References
- 1.Ljunggren HG, Karre K. In search of the “missing self ”: MHC molecules and NK cell recognition. Immunol Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 2.Wong S, Freeman D, Kellcher C, Mager D, Takei F. Ly-49 multigene family. New members of a superfamily of type II-membrane proteins with lectin-like domains. J Immunol. 1991;147:1417–1423. [PubMed] [Google Scholar]
- 3.Smith HRC, Karlhofer FM, Yokoyama WM. Ly-49 multigene family expressed by IL-2 activated NK cells. J Immunol. 1994;153:1068–1079. [PubMed] [Google Scholar]
- 4.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+IL-2 activated natural killer cells. Nature (Lond) 1992;358:21–22. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 5.Kane KP. Ly-49 mediates EL4 lymphoma adhesion to isolated class I major histocompatibility complex molecules. J Exp Med. 1994;179:1011–1015. doi: 10.1084/jem.179.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama WM, Kehn PJ, Cohen DI, Shevach EM. Chromosomal localization of the Ly-49 (A1, YE1/48) multigene family. Genetic association with the NK1.1 antigen. J Immunol. 1990;145:2353–2358. [PubMed] [Google Scholar]
- 7.Daniel BF, Nakamura MC, Rosen SD, Yokoyama WM, Seaman WE. Ly-49A, a receptor for H-2Dd, has a functional carbohydrate recognition domain. Immunity. 1994;1:789–792. doi: 10.1016/s1074-7613(94)80020-0. [DOI] [PubMed] [Google Scholar]
- 8.Mason LH, Ortaldo JR, Young HA, Kumar V, Bennett M, Anderson SK. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2) J Exp Med. 1995;182:293–303. doi: 10.1084/jem.182.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan J, Mager D, Jefferies W, Takei F. Expression of different members of the Ly-49 gene family define distinct natural killer cell subsets and cell adhesion properties. J Exp Med. 1994;180:2287–2295. doi: 10.1084/jem.180.6.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu YLL, George T, Dorfman JR, Roland J, Kumar V, Bennett M. The role of Ly-49A and 5E6 (Ly49C) molecules in hybrid resistance mediated by murine natural killer cells against normal T cell blasts. Immunity. 1996;4:67–76. doi: 10.1016/s1074-7613(00)80299-x. [DOI] [PubMed] [Google Scholar]
- 11.Sentman CL, Hackett J, Jr, Kumar V, Bennett M. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1bbone marrow grafts. J Exp Med. 1989;170:191–202. doi: 10.1084/jem.170.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy WJ, Raziuddin A, Mason L, Kumar V, Bennett M, Longo D L. NK cell subsets in the regulation of murine hematopoiesis. I. 5E6+ NK cells promote hematopoietic growth in H-2dstrain mice. J Immunol. 1995;155:2911–2917. [PubMed] [Google Scholar]
- 13.Mason LH, Giardina SL, Hecht T, Ortaldo J, Mathieson BJ. LGL-1: a non-polymorphic antigen expressed on a major population of mouse natural killer cells. J Immunol. 1988;140:4403–4412. [PubMed] [Google Scholar]
- 14.Chadwick BS, Miller RG. Hybrid resistance in vitro. Possible role of both class I MHC and self peptides in determining the level of target cell sensitivity. J Immunol. 1992;148:2307–2313. [PubMed] [Google Scholar]
- 15.Aruffo, A. 1992. Transient expression of protein in Cos cells. In Current Protocols in Molecular Biology. Vol. 2. John Wiley & Sons, New York. 16.12.1.
- 15a.Ortaldo, J.R., R.T. Winkler-Pickett, S. Nagata, and C.F. Ware. 1996. Fas involvement in human NK cell apoptosis: lack of a requirement for CD16 mediated events. J. Leukoc. Biol. In press. [DOI] [PubMed]
- 16.Yokoyama WM, Jacobs LB, Kanagawa O, Shevach EM, Cohen DI. A murine T lymphocyte antigen belongs to a supergene family of type II integral membrane proteins. J Immunol. 1989;143:1379–1386. [PubMed] [Google Scholar]
- 17.Karlhofer FM, Yokoyama WM. Stimulation of murine natural killer (NK) cells by a monoclonal antibody specific for the NK-1.1 antigen. IL-2 activated NK cells possess additional specific stimulation pathways. J Immunol. 1991;146:3662–3673. [PubMed] [Google Scholar]
- 18.Mason LH, Mathieson BJ, Ortaldo JR. Natural killer (NK) cell subsets in the mouse: NK-1.1+/LGL-1+ cells restricted to lysing NK targets, whereas NK-1.1+/LGL-1−cells generate lymphokine-activated killer cells. J Immunol. 1990;145:751–759. [PubMed] [Google Scholar]
- 19.Stoneman ER, Bennett M, An J, Chestnut KA, Wakeland EK, Sheerer JB, Siciliano MJ, Kumar V, Mathew PA. Cloning and characterization of 5E6 (Ly49C), a receptor molecule expressed on a subset of murine natural killer cells. J Exp Med. 1995;182:305–313. doi: 10.1084/jem.182.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenardo MJ. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature (Lond) 1991;353:858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- 21.Critchfield JH, Racke MK, Zuniga-Pflucker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ. T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science (Wash DC) 1994;263:1139–1143. doi: 10.1126/science.7509084. [DOI] [PubMed] [Google Scholar]
- 22.Ortaldo JR, Mason AT, O'Shea JJ. Receptorinduced death in human natural killer cells: involvement of CD16. J Exp Med. 1995;181:339–344. doi: 10.1084/jem.181.1.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burshtyn DN, Scharenberg AM, Wagtmann N, Rajagopalan S, Berrada K, Yi T, Kinet JP, Long EO. Recruitment of tyrosine phosphatase HCP by the killer cell inhibitor receptor. Immunity. 1996;4:77–85. doi: 10.1016/s1074-7613(00)80300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]