Abstract
Rats of the PVG.RT1u strain develop autoimmune diabetes when thymectomized at 6 wk of age and are rendered relatively lymphopenic by a cumulative dose of 1,000 rads 137Cs γ-irradiation given in four split doses. Previous studies have shown that the disease is prevented by the intravenous injection of 5 × 106 CD4+ CD45RC− TCRαβ+ RT6+ peripheral T cells from normal syngeneic donors. These cells have a memory phenotype and are presumably primed to some extrathymic antigen. However, we now report that the CD4+ CD8− population of mature thymocytes is a very potent source of cells, with the capacity to prevent diabetes in our lymphopenic animals. As few as 6 × 105 of these cells protect ∼50% of recipients and the level of protection increases with cell dose. It appears that one characteristic of the intrathymic selection of the T cell repertoire is the generation of cells that regulate the autoimmune potential of peripheral T cells that have been neither clonally deleted intrathymically nor rendered irreversibly anergic in the periphery.
Discussions of the mechanisms whereby T cell responses are made to foreign antigens but not to self usually classify autoantigens into two groups, those that are intrathymic and those that are extrathymic. There is compelling evidence that for those self-antigens expressed within the thymus, the corresponding T cells are clonally deleted (1) at a stage in their development at which encounter with specific antigen induces cell death. While this mode of tolerance induction is conceptually attractive as far as intrathymic self-antigens are concerned, it does not address the question as to how the immune system achieves tolerance to tissue-specific peripheral self-antigens. The processes that mediate tolerance to these are regarded as occurring solely extrathymically and depend on a distinction being made between the way that self-antigens and foreign antigens are presented to peripheral T cells. Whereas foreign antigens are presented by appropriate antigen-presenting cells that induce specific T cell activation and, consequently, adaptive immunity, self-antigens are believed to be presented by cells that lack the relevant costimulatory molecules (2, 3) with the result that the potentially autoreactive T cells are either rendered anergic (2) or simply fail to respond at all, a process known as clonal indifference (4).
A common feature of all of these mechanisms is that they are all passive in the sense that they depend either on the intrathymic autolysis of autoreactive T cells at a crucial developmental stage or on processes that extrathymically induce intrinsic nonresponsiveness of cells that are not deleted. However there is a considerable amount of experimental data that casts doubt on the completeness of the forgoing explanation of self-tolerance. The data support the view that self-tolerance to at least some autoantigens also requires the presence of T cells, which play an active role in suppressing the autoreactive potential of cells that have neither been clonally deleted nor become anergic (5–11, reviewed in 12). These experiments, while all compatible with the view that the prevention of autoimmune responses requires the active participation of T cells that play some protective or regulatory role, raise a number of questions with regard to the way such a population of regulatory T cells (hereafter abbreviated to Treg) are generated. In an earlier series of experiments, we showed that a very high incidence of autoimmune diabetes developed in rats that were thymectomized as young adults and then made lymphopenic by sublethal γ-irradiation (9). Most of the data reported in that paper concerned the characterization of the subset of CD4+ peripheral T cells that, on transfer from normal syngeneic donors, prevented the development of the disease. However, in a small study involving a few animals it was also found that the transfer of CD4+CD8− thymocytes was similarly protective. We now report the results of more extensive experiments, in which a comparison has been made between the number of CD4+CD8− thymocytes and of peripheral CD4+ T cells required to achieve protection from diabetes. The data show that the CD4+CD8− thymocyte subset is particularly potent in this comparison. Consequently, the results are not compatible with the concept that the protective subset of peripheral cells is generated by an extrathymic antigen-driven clonal expansion of Treg from intrathymic precursors present at low frequency. Rather, the selection of this regulatory subset is an intrathymic event. Possible mechanisms are discussed.
Materials and Methods
Animals and Induction of Diabetes.
6- to 12-wk-old PVG.RT1c or PVG.RT1u strain rats of either sex were used, and these were bred in the specific pathogen-free unit of the Medical Research Council Cellular Immunology Unit (Oxford, UK). To induce diabetes, PVG.RT1u rats were thymectomized at 6 wk of age and, starting 2 wk later, were given four equal doses of 250 rads 137Cs γ-irradiation at 2-wk intervals. Over the ensuing 10–12 wk, virtually all the males (exclusively used in this study) and 70% of the females developed autoimmune diabetes (9). Rats were periodically bled and blood glucose levels assayed using a glucose hexokinase (HK) reagent (Sigma Diagnostics, Poole, UK). Rats were classed diabetic if weight loss was associated with blood glucose levels in excess of 400 mg/dL.
mAbs.
The mouse monoclonal antibodies (mAbs) used in these studies were as follows: W3/13 (anti-rat Leukosialin) (13); W3/ 25 (anti-rat CD4) (13); OX22 (anti-rat exon C of CD45) (14, 15); OX32 (anti-rat exon C of CD45, noncompetitive with OX22) (14, 16); OX12 (anti-rat Igκ chain) (17); OX-6 (anti-rat MHC class II) (18); OX-8 (anti-rat CD8) (19); OX-21 (anti-human C3b inactivator) (20). Rabbit anti–mouse Ig (RAM–Ig) and FITC-conjugated Fab fragments of RAM Ig (RAM–Fab–FITC) were prepared by S. Simmonds in this laboratory and PE-conjugated rabbit anti–mouse Ig (RAM–PE) was from Serotec (Kidlington, UK). OX12 IgG was iodinated (125I) as already described (21).
Isolation of T Cells and Thymocyte Subpopulations.
Rat thoracic duct lymphocytes (TDLs) were obtained by cannulation of the duct (22). Cells were collected at 4°C overnight into flasks containing PBS and 20 U/ml heparin. Thymocytes were prepared by the pressing of thymus fragments through a stainless steel mesh, filtering off the debris through a cotton wool or lens tissue filter, and washing the single cell suspension so obtained in ice-cold cellhandling medium.
Rat lymphocyte populations were negatively selected from TDLs or thymocytes using a rosetting technique as described elsewhere (23). CD4+CD8− thymocytes, together with small numbers of CD4−CD8− cells were isolated by depletion of CD8+ cells using the mAb OX8. B cells were obtained from TDLs by depleting T cells using W3/13 mAb. CD4+ T cells were isolated by depletion of B cells and CD8+ T cells using the mAbs OX12, OX8, and OX6. To obtain CD4+CD45RC− T cells TDLs were depleted using the mAbs OX22, OX32, and OX8. The purity of all isolated cells was analyzed on a FACScan® (Becton Dickinson, Palo Alto, CA) by labeling pre- and postdepletion samples with RAM–PE. The percentage of contaminated cells was between 0.5 and 5%.
In Vivo B Cell Help Assay.
Primed B and T cells were purified from the TDLs of PVG.RT1c rats immunized intraperitoneally with 1 ml of 10% SRBC 6–10 wk previously. Irradiated (800 rads, 24 h previously) syngeneic recipient rats were reconstituted with purified B cells (3 × 107) and 1ml of 10% SRBC. Different groups of rats also received one of three cells doses (20, 10, or 5 × 106 cells) of either CD4+CD8− thymocytes or primed peripheral CD4+ T cells. Control rats received no additional cells. Sera from these animals were taken after 7 d, heat-inactivated, and assayed for anti-SRBC antibody using a radioimmunoassay as already described (24). In brief, 50 μl of 109 SRBC/ml were plated out into the wells of a microtiter plate. SRBC were incubated with diluted sera in triplicate for 2 h at 4°C, followed by four washes with PBS containing 0.05% BSA, and a further 2 h incubation with 125I–OX12 IgG (100 ng/ml) also followed by four washes. The SRBC were harvested and counted in a Rackgamma counter (1261 MULTIGAMMA, Pharmacia LKB, Uppsala, Sweden) using 15 s counts/tube.
Results
Previous studies in our laboratory have demonstrated that PVG.RT1u rats, a normal strain with no tendency to develop autoimmunity, spontaneously develop fatal insulin-dependent diabetes by 8–10 wk after the final dose of irradiation (9) when thymectomized at 6 wk of age and then subjected to four doses of 250 rads 137Cs γ-irradiation at 2-wk intervals. The disease can be completely prevented by the intravenous injection of a particular subset of peripheral CD4+ T cells from syngeneic normal donors. The phenotype of the disease-preventing T cells has been determined: the cells are CD4+ CD45RC− TCR αβ+ RT6+ (9) and a dose of 5 × 106 cells protects 100% of recipients. The most direct interpretation of these findings is that normal PVG.RT1u rats have T cells that have a diabetogenic potential, but that this potential is only revealed by a radiation-induced lymphopenia that abrogates the protective action of the CD4+ CD45RC− TCR αβ+ RT6+ regulatory T cells.
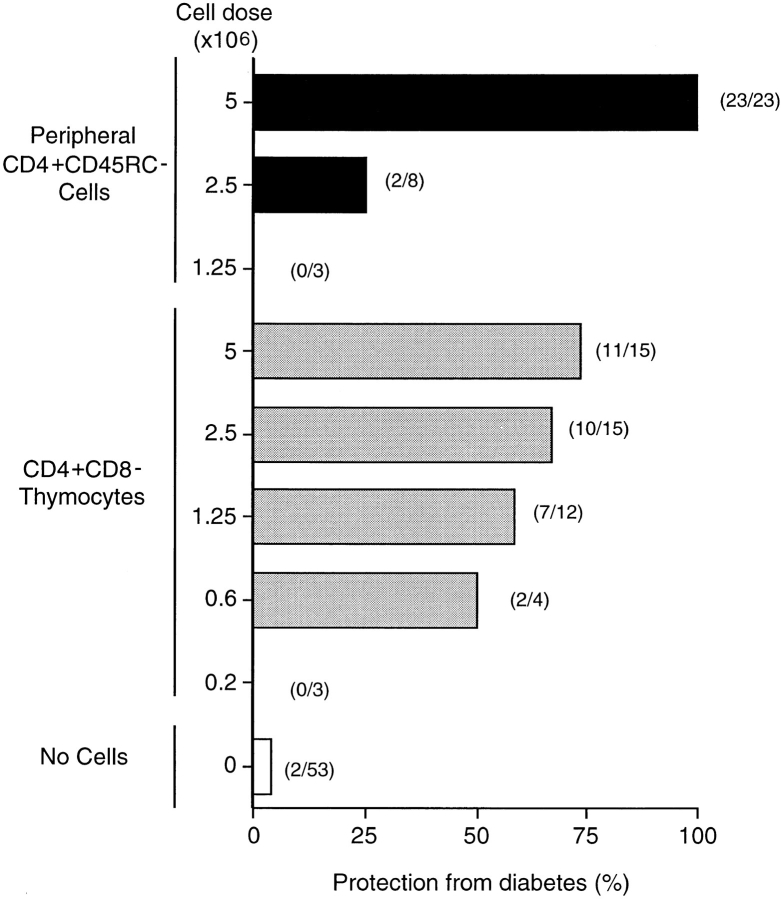
Given that the peripheral CD4+ T cell subset that prevents diabetes has a primed T cell phenotype, and that insulin-dependent diabetes is a tissue-specific autoimmune disease, it was anticipated that the priming of the protective T cells took place in the periphery, possibly in the lymph nodes draining the pancreas. Such a hypothesis would predict that CD4+CD8− thymocytes, being a naive population, would be far less potent at controlling the diabetes than the clonally expanded peripheral T cells would be. However, this prediction was not confirmed by experiment. Assays of the ability of twofold decreasing doses of CD4+CD8− thymocytes to protect prediabetic recipients from disease produced a very flat dose/response curve. While 5 × 106 of these cells protected 11 of 15 recipients, significant protection was still observed at a dose eight times lower than this (Fig. 1). In contrast, when 5 × 106 CD4+CD45RC− peripheral cells were transferred into prediabetic recipients, all animals (23/23) were protected from disease, but at one-half this cell dose, protection was almost completely lost, with six of eight recipients becoming diabetic. A further twofold reduction in the dose of donor cells failed to protect any recipients (Fig. 1).
Figure 1.
CD4+CD8− thymocytes are more potent than peripheral CD4+CD45RC− T cells in protecting from diabetes. T cells subsets were negatively selected from TDLs or thymus from normal PVG.RT1u rats by rosette depletion as described in Material and Methods. Three different doses of CD4+CD45RC− peripheral T cells and five doses of CD4+ CD8− thymocytes were injected i.v. into recipient groups of prediabetic rats shortly after their last irradiation. Results are expressed as percentage of animals that are protected from development of diabetes and are a compilation of 10 individual experiments involving, with controls, a total of 136 animals. Numbers in parenthesis represent the aggregate number of animals in each group. The purity of isolated cells was >95%.
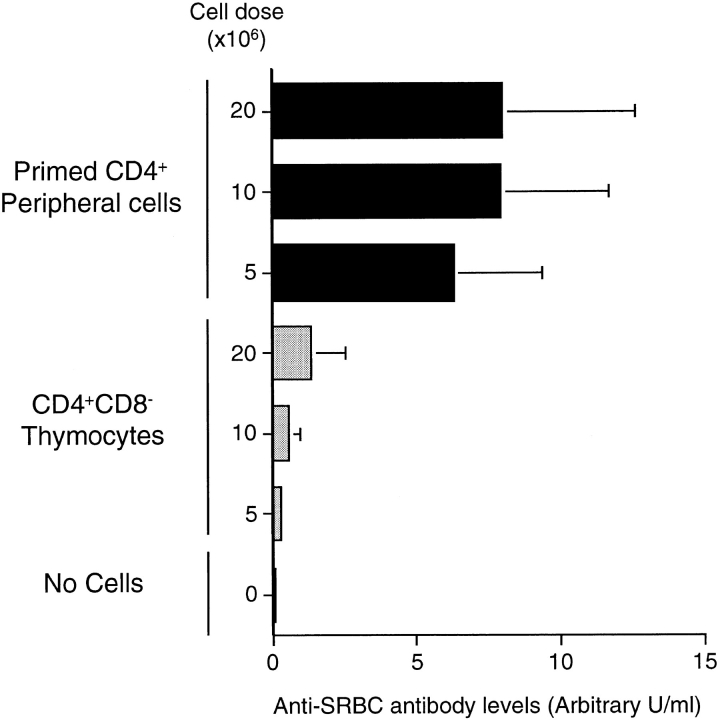
To exclude the possibility that thymocytes were generally more potent at mediating T cell functions when transferred into lymphopenic recipients, we compared, in an adoptive transfer system, the ability of CD4+CD8− thymocytes and SRBC-primed CD4+ peripheral T cells to provide primed B cells with help to produce specific antibody. PVG.RT1c rats were injected with 1 ml of 10% SRBC and cannulated between 6 and 8 wk after immunization. Primed B cells and CD4+ T cells were purified from the TDLs of these animals and 3 × 107 B cells were injected into irradiated (800 rads) syngeneic recipients with further antigen (1 ml of 10% SRBC) and different doses of CD4+ primed T cells or CD4+CD8− thymocytes from normal PVG.RT1c donors. Sera were taken from these animals 1 wk later and assayed for antibodies specific for SRBC using a radioimmunoassay. The data in Fig. 2 show that in contrast with their potency in the prevention of diabetes, the CD4+CD8− thymocytes were less active than CD4+ primed peripheral T cells in providing B cells with help for antibody production. Repeating this experiment using unprimed rather than primed peripheral CD4+ T cells showed that these too were more potent at providing B cell help (data not shown).
Figure 2.
Naive CD4+CD8− thymocytes are less potent in providing in vivo B cell help than primed peripheral CD4+ T cells. Primed CD4+ T cells and primed B cells were negatively selected from TDLs from PVG.RT1c rats primed with 108 SRBC 4–9 wk before cannulation. Naive CD4+CD8− thymocytes were negatively selected from thymus of normal PVG.RT1c rats by rosette depletion as described in Materials and Methods. Syngeneic recipients were given 800 rads and injected i.v. 1 d later with 108 SRBC, 3 × 107 SRBC-primed B cells and either three different doses of SRBC-primed CD4+ peripheral T cells or three different doses of naive CD4+CD8− thymocytes. The controls were given 108 SRBC and 3 × 107 SRBC-primed B cells. The recipients were bled 7 d later and the anti-SRBC antibody titers determined by radioimmunoassay. The results are expressed in arbitrary units made by comparison with a pool of sera from rats immunized with SRBC.
Discussion
Taken together, the foregoing observations are not compatible with the concept that the peripheral T cells that prevent diabetes are generated by an antigen-driven expansion of naive T cells derived from thymocyte precursors that exist at a low frequency. While in principle it is possible that the thymocyte transfer experiments are artifactual, in that there is no direct evidence that the regulatory thymocytes we assay normally leave the thymus, we have shown that the cells express the l-selectin homing receptor (25), and it is difficult to conceive of an intrathymic role for such expression. Instead, it seems more likely that they are the precursors of the peripheral T cells that prevent diabetes on adoptive transfer.
The antigen specificity of the regulatory cells is unknown. In particular, it is not known whether they respond to environmental antigens or to self, or even possibly to both (26–28). However, in other experimental systems allogeneic, or even xenogeneic, thymic epithelium transplantation can give rise to the generation of regulatory T cells that mediate dominant tolerance to antigens expressed on the transplanted thymic epithelia (5, 6, 29). These data indicate that the role played by the thymus in preventing peripheral immunity to intrathymic self-antigens is not readily accounted for solely in terms of the generation of a T cell repertoire that is clonally deleted for self and reactive to non-self. Instead, the results would seem to require that thymic epithelium facilitates the generation of T cells that can actively control potentially autoreactive T cells. The mRNA for several putatively tissue-specific self-antigens is also expressed intrathymically (30), and this expression has been found to occur in thymic epithelial cells (31, 32). It has been shown that such mRNA can lead to gene expression in that a number of transgenes placed under promoters that are nominally tissue specific have been shown to be expressed intrathymically (32, 33). These observations may bear on the question of the nature of the autoantigen in the lymphopenia-induced diabetes that we study. It has been shown that rats injected at birth with B chain peptides of rat insulin are resistant to the subsequent induction of diabetes (31), and similar findings have been reported in the NOD mouse (34, 35). These results, together with those indicating the expression of the insulin gene in the thymus (32, 36), suggest that insulin itself may be a target autoantigen in insulin-dependent diabetes in rodents and, given the results obtained with thymic epithelium transplantation referred to above, that at least some of the regulatory T cells that protect lymphopenic rats from diabetes may be specific for insulin.
Although CD4+CD8− thymocytes could protect a significant proportion of recipients from diabetes, even at a dose of 6 × 105 cells, an inoculum almost ten times larger failed to prevent disease in 100% of animals (Fig. 1). Similar partial protection has also been observed by unfractionated peripheral CD4+ T cells (9), and the failure to protect all recipients has been ascribed to an antagonistic effect of the CD45RC+ subset in the inoculum on the protective action of the CD45RC− subset (9). This interpretation is supported by earlier observations that the CD45RC+ subset is autoreactive (37). The data reported herein, on the gradual increase in the level of protection against diabetes obtained with increasing doses of CD4+CD8− thymocytes, has been analyzed theoretically using the assumption that a recipient of such cells will be protected from diabetes if, by chance, the inoculum contains more protective thymocytes than diabetogenic thymocytes and, conversely, that disease will develop if the opposite balance occurs. The ratio of the sizes of these two antagonistic populations will be subject to statistical variation and this fact forms the essential element of the theoretical analysis. Details of this analysis are presented in the Appendix but the findings are summarized in Table 1. It will be seen that the theoretical prediction, developed retrospectively, fits the data very well; in fact, rather better than one might expect on the basis of a theory that depends on statistically evaluated chance. The conclusion that one can draw is that the analysis supports the hypothesis but, by the very nature of such an approach, it cannot be claimed to have proved it. Indeed, while the data clearly support the conclusion that the thymus is a potent source of CD4+CD8− thymocytes with the capacity to prevent diabetes on transfer into prediabetic recipients, they do not exclude the possibility that the induction of the regulatory phenotype is a postthymic event. The mathematical model is equally applicable to such a hypothesis but the physiological implications are quite different. As has been reported, the peripheral CD4+ cell that prevents diabetes on adoptive transfer has a primed phenotype, which suggests that it has encountered its specific antigen after leaving the thymus. This observation allows the possibility that the inductive effect that gives rise to Treg occurs at a postthymic stage of T cell differentiation and is compatible with data that show, in other experimental systems, selftolerance requires the presence of the relevant autoantigen in the periphery (38) as well as in the thymus (29, 39). Further experiments are planned, specifically to test the prediction of the theory that at very high thymocyte doses, protection from diabetes should be observed in almost all recipients.
Table 1.
Comparison of Experimental and Theoretical Results on Control of Diabetes by CD4+CD8− Thymocytes
| Thymocyte dose | Frequency of protected rats | |||
|---|---|---|---|---|
| Observed | Calculated | |||
| × 106 | ||||
| 5.0 | 11/15 | 11/15 (76%) | ||
| 2.5 | 10/15 | 10/15 (69%) | ||
| 1.25 | 7/12 | 8/12 (64%) | ||
| 0.625 | 2/4 | 2/4 (59%) | ||
| 25.0 | ND | 19/20 (94%) | ||
The expected number of prediabetic rats protected from development of diabetes by different doses of CD4+CD8− thymocytes was calculated using the statistical analysis detailed in the Appendix. The level of protection observed experimentally (Observed) is compared with predicted values (Calculated). Percentages in parentheses indicate the calculated frequencies of protection from diabetes. As indicated in the Appendix, to calculate the percentage of rats protected from diabetes by thymocyte transfer requires that values be ascribed to the frequency of diabetogenic cells and regulatory ones. For example, if the frequency of diabetogenic cells is assumed to be 1:32,000, then the frequency of regulatory cells is just 8% greater than this. It is evident that the theoretical predictions fit the experimental data only if the excess of regulatory cells over diabetogenic ones is small.
Irrespective of the nature of the underlying mechanism that gives rise to the gradual increase in the level of protection from diabetes mediated by increasing doses of CD4+ CD8− thymocytes, this observation provides a straightforward explanation for the induction of autoimmunity in mice thymectomized within the first few days of life (40–42). Our findings suggest that until the number of thymocytes exported into the periphery reaches some critical number, the regulatory mechanism that prevents autoimmunity is not intact. Because the neonatal mouse is immunologically immature, this critical number will not be attained until some time in the postnatal period, and thymectomy during this interval would be expected to cause a breakdown in self-tolerance.
Acknowledgments
We thank Steve Simmonds and Mike Puklavec for technical assistance and Phil Stumbles, Francisco Ramirez, and Vicky Heath for discussion and assistance.
Appendix
A theoretical analysis of the data in Fig. 1 has been developed based on the hypothesis that the inocula of CD4+CD8− thymocytes used to prevent diabetes contains two minority populations, one that can cause the disease and one that can prevent it. The further assumption is made that an inoculum prevents diabetes if it contains more protective cells than diabetogenic ones but that protection fails if the ratio is reversed. Given that in any inoculum the numbers of protective and diabetogenic cells will be subject to independent statistical variation, it is possible to calculate the probable proportion of recipients that will be protected from diabetes at any cell dose. This proportion depends on two numbers: the frequency of diabetogenic cells and the ratio of this frequency to that of the regulatory cells. These two variables are not independent, in that if a value for the first is assumed then there is only one value of the second that will give an agreement between theory and experiment.
The essential feature of the analysis is illustrated by noting that as the thymocyte dose increases, the standard deviation in the number of diabetogenic cells, expressed as a fraction of the total of these cells, decreases. Consequently, if the frequency of regulatory (protective) thymocytes is larger than that of the diabetogenic ones, the proportion of recipients that are protected from diabetes will become larger as the number of thymocytes injected is increased.
Formally, the proportion of animals that fail to be protected is given by the double integral:

where P is the proportion of rats that receive more diabetogenic cells than regulatory ones; μ is the mean number of diabetogenic CD4+CD8− thymocytes in a given sample of these cells, i.e., μ = nq, where n = total number of CD4+CD8− thymocytes in the sample and q is the frequency of diabetogenic cells among CD4+CD8− thymocytes; (1 + α) is the ratio of regulatory cells to diabetogenic cells among CD4+ CD8− thymocytes.
Footnotes
A. Saoudi is supported by a postdoctoral fellowship from the Wellcome Trust and from the Fondation pour la Recherche Medicale of France. B. Seddon is supported by a Wellcome Trust Prize Studentship.
A. Saoudi and B. Seddon contributed equally to this work.
References
- 1.Kappler JW, Roehm M, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 2.Mueller DL, Jenkins MK, Schwartz RH. Clonal expansion versus functional clonal inactivation: a costimulatory signalling pathway determines the outcome of T cell antigen receptor occupancy. Annu Rev Immunol. 1989;7:445–480. doi: 10.1146/annurev.iy.07.040189.002305. [DOI] [PubMed] [Google Scholar]
- 3.Nickoloff BJ, Turka LA. Immunological functions of non-professional antigen-presenting cells: new insights from studies of T-cell interactions with keratinocytes. Immunol Today. 1994;15:464–469. doi: 10.1016/0167-5699(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 4.Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, Malissen B, Zinkernagel RM, Hengartner H. Ablation of "tolerance" and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho A, Salaun J, Corbel C, Bandeira A, Le Douarin N. The role of thymic epithelium in the establishment of transplantation tolerance. Immunol Rev. 1993;133:225–240. doi: 10.1111/j.1600-065x.1993.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 6.Zamoyska R, Waldmann H, Matzinger P. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur J Immunol. 1989;19:111–117. doi: 10.1002/eji.1830190118. [DOI] [PubMed] [Google Scholar]
- 7.Fukuma K, Sakaguchi S, Kuribayashi K, Chen WL, Morishita R, Sekita K, Uchino H, Masuda T. Immunologic and clinical studies on murine experimental autoimmune gastritis induced by neonatal thymectomy. Gastroenterology. 1988;94:274–283. doi: 10.1016/0016-5085(88)90413-1. [DOI] [PubMed] [Google Scholar]
- 8.Stumbles PA, Penhale WJ. IDDM in rats induced by thymectomy and irradiation. Diabetes. 1993;42:571–578. doi: 10.2337/diab.42.4.571. [DOI] [PubMed] [Google Scholar]
- 9.Fowell D, Mason DW. Evidence that the T cell repertoire of normal rats contains cells with the potential to cause diabetes. Characterization of the CD4+T cell subset that inhibits this autoimmune potential. J Exp Med. 1993;177:627–636. doi: 10.1084/jem.177.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cairns L, Rosen FS, Borel Y. Mice naturally tolerant to C5 have T cells that suppress the response to this antigen. Eur J Immunol. 1986;16:1277–1282. doi: 10.1002/eji.1830161015. [DOI] [PubMed] [Google Scholar]
- 11.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 12.Saoudi A, Seddon B, Heath V, Fowell V, Mason D. The physiological role of regulatory T cells in the prevention of autoimmunity: the function of the thymus in the generation of the regulatory T cell subset. Immunol Rev. 1996;149:195–216. doi: 10.1111/j.1600-065x.1996.tb00905.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams AF, Galfré G, Milstein C. Analysis of cell surface by xenogeneic myeloma-hybrid antibodies: differentiation antigens of rat lymphocytes. Cell. 1977;12:663–673. doi: 10.1016/0092-8674(77)90266-5. [DOI] [PubMed] [Google Scholar]
- 14.McCall MN, Shotton DM, Barclay AN. Expression of soluble isoforms of rat CD45. Analysis by electron microscopy and use in epitope mapping of anti-CD45R monoclonal antibodies. Immunology. 1992;76:310–317. [PMC free article] [PubMed] [Google Scholar]
- 15.Spickett GP, Brandon MR, Mason DW, Williams AF, Woollett GR. MRC OX-22, a monoclonal antibody that labels a new subset of T lymphocytes and reacts with the high molecular weight form of the leukocyte-common antigen. J Exp Med. 1983;158:795–810. doi: 10.1084/jem.158.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woollett GR, Barclay AN, Puklavec M, Williams AF. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985;15:168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- 17.Hunt SV, Fowler MH. A repopulation assay for B and T lymphocyte stem cells employing radiation chimaeras. Cell Tissue Kinetics. 1981;14:445–464. doi: 10.1111/j.1365-2184.1981.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 18.McMaster WR, Williams AF. Identification of Ia glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol. 1979;9:426–433. doi: 10.1002/eji.1830090603. [DOI] [PubMed] [Google Scholar]
- 19.Brideau RJ, Carter PB, McMaster WR, Webb M. Two subsets of rat T lymphocytes defined with monoclonal antibodies. Eur J Immunol. 1980;10:609–615. doi: 10.1002/eji.1830100807. [DOI] [PubMed] [Google Scholar]
- 20.Hsiung LM, Barclay AN, Brandon MR, Sim E, Porter RR. Purification of human C3b inactivator by monoclonal-antibody affinity chromatography. Biochem J. 1982;203:293–298. doi: 10.1042/bj2030293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensenius JC, Williams AF. The binding of anti-immunoglobulin antibodies to rat thymocytes and thoracic duct lymphocytes. Eur J Immunol. 1974;4:91–97. doi: 10.1002/eji.1830040207. [DOI] [PubMed] [Google Scholar]
- 22.Gowans JL, Knight EJ. The role of re-circulation of lymphocytes in the rat. Proc R Soc Lond B Biol Soc. 1964;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- 23.Mason, D.W., W.J. Penhale, and J.D. Sedgwick. 1987. Preparation of lymphocyte subpopulations. In Lymphocytes. 1 ed. G.G.B. Klaus, editor. IRL Press, Oxford. 35–54.
- 24.Bunce JV, Mason DW. The tolerization of rat thymocytes to xenogeneic erythrocytes: kinetics of induction and recovery. Eur J Immunol. 1981;11:889–896. doi: 10.1002/eji.1830111108. [DOI] [PubMed] [Google Scholar]
- 25.Seddon, B., A. Saoudi, M. Nicholson, and D. Mason. 1996. CD4+CD8- thymocytes that express L-Selectin protect rats from diabetes upon adoptive transfer. Eur. J. Immunol. In press. [DOI] [PubMed]
- 26.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science (Wash DC) 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 27.Heath WR, Miller JF. Expression of two alpha chains on the surface of T cells in T cell receptor transgenic mice. J Exp Med. 1993;178:1807–1811. doi: 10.1084/jem.178.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason D. Allelic exclusion of α chains in TCRs. Int Immunol. 1994;6:881–885. doi: 10.1093/intimm/6.6.881. [DOI] [PubMed] [Google Scholar]
- 29.Le Douarin N, Corbel C, Bandeira A, Thomas-Vaslin V, Modigliani Y, Coutinho A, Salaün J. Evidence for a thymus-dependent form of tolerance that is not based on elimination or anergy of reactive T cells. Immunol Rev. 1996;149:35–54. doi: 10.1111/j.1600-065x.1996.tb00898.x. [DOI] [PubMed] [Google Scholar]
- 30.Jolicoeur C, Hanahan D, Smith KM. T–cell tolerance toward a transgenic beta-cell antigen and transcription of endogenous pancreatic genes in thymus. Proc Natl Acad Sci USA. 1994;91:6707–6711. doi: 10.1073/pnas.91.14.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowell, D., F. Powrie, A. Saoudi, B. Seddon, V. Heath, and D. Mason. 1995. The role of subsets of CD4+ T cells in autoimmunity. In T Cell Subsets in Infectious and Autoimmune Diseases. D. Chadwick and C. Cardew, editors. John Wiley & Sons, Ltd., Chichester, UK. 173–182. [DOI] [PubMed]
- 32.Antonia SJ, Geiger T, Miller J, Flavell RA. Mechanisms of immune tolerance induction through the thymic expression of a peripheral tissue-specific protein. Int Immunol. 1995;7:715–725. doi: 10.1093/intimm/7.5.715. [DOI] [PubMed] [Google Scholar]
- 33.Heath WR, Allison J, Hoffmann MW, Schonrich G, Hammerling G, Arnold B, Miller JFAP. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature (Lond) 1992;359:547–549. doi: 10.1038/359547a0. [DOI] [PubMed] [Google Scholar]
- 34.Muir A, Peck A, Clare-Salzler M, Song YH, Cornelius J, Luchetta R, Krischer J, Maclaren N. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferongamma transcription. J Clin Invest. 1995;95:628–634. doi: 10.1172/JCI117707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parish NM, Hutchings PR, O'Reilly L, QuarteyPapafio R, Healey D, Ozegbe P, Cooke A. Tolerance induction as a therapeutic strategy for the control of autoimmune endocrine disease in mouse models. Immunol Rev. 1995;144:269–300. doi: 10.1111/j.1600-065x.1995.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 36.Morahan G, Allison J, Miller JFAP. Tolerance of class I histocompatibility antigens expressed extrathymically. Nature (Lond) 1989;339:622–624. doi: 10.1038/339622a0. [DOI] [PubMed] [Google Scholar]
- 37.Powrie F, Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22lowsubset. J Exp Med. 1990;172:1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eishi Y, McCullagh P. Acquisition of immunological self-recognition by the fetal rat. Immunology. 1988;64:319–323. [PMC free article] [PubMed] [Google Scholar]
- 39.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin N. Tolerance induced by thymic epithelium grafts in birds. Science (Wash DC) 1987;237:1032–1035. doi: 10.1126/science.3616623. [DOI] [PubMed] [Google Scholar]
- 40.Kojima A, Tanaka-Kojima Y, Sakakura T, Nishizuka Y. Spontaneous development of autoimmune thyroiditis in neonatally thymectomized mice. Lab Invest. 1976;34:550–557. [PubMed] [Google Scholar]
- 41.Taguchi O, Nishizuka Y, Sakakura T, Kojima A. Autoimmune oophoritis in thymectomized mice: detection of circulating antibodies against oocytes. Clin Exp Immunol. 1980;40:540–553. [PMC free article] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–1586. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]