Abstract
Previous studies have suggested that granulated metrial gland (GMG) cells are bone marrow– derived lymphoid cells, which differentiate in situ in the mouse pregnant uterus into natural killer (NK)–like cells. Similar to NK cells, GMG cells express an abundant level of cytolytic mediators such as perforin. The factor(s) regulating the differentiation of GMG cells remain(s) to be identified, although cytokines previously implicated in the stimulation/activation of NK cells (e.g., IL-2, IL-6, IL-7, and IL-12) can be considered as potential candidates. Recently, IL-15, a novel cytokine, which displays biological activities similar to IL-2, has also been shown to be capable of activating NK cells. Using reverse transcription–polymerase chain reaction (RT-PCR) analysis, we have demonstrated in the present study that IL-15 and its cognate receptor, but not the other cytokines, are expressed in the mouse pregnant uterus, with a time course concomitant with those of cytolytic mediators in differentiated GMG cells. Moreover, IL-15, though not IL-2, is capable of inducing the expression of perforin and granzymes in pregnant uterine tissues explanted in vitro. Data obtained from in situ hybridization study have suggested that the macrophages present in the pregnant uterus may be responsible for the production of IL-15. These results suggest that IL-15 is involved in regulating the differentiation of GMG cells during mouse pregnancy.
The metrial gland is a uterine tissue that develops adjacent to the placenta in pregnant rodents (1, 2). This specialized tissue consists primarily of fibroblasts and a conspicuous population of granulated cells called granulated metrial gland (GMG) cells (1, 2). Cells similar to GMG cells, termed endometrial granulocytes, have also been found in humans and other primates (3). Previous studies conducted by us and others have indicated that GMG cells and endometrial granulocytes belong to the NK cell lineage, bearing both surface markers (e.g., asialo GM-1 and LGL-1) and cytolytic mediators (e.g., perforin and granzymes) expressed by NK cells (4, 5). Although a number of hypotheses regarding the biological function of perforin-expressing GMG cells in the materno–fetal junction have been proposed (6), this issue remains elusive. Moreover, although GMG cells appear to differentiate and proliferate in situ in the uterus during pregnancy, the factors initiating and/or regulating their development have not been identified. However, our previous study using pseudopregnant mice suggested that maternal, rather than fetal, factors associated with pregnancy are responsible for inducing the differentiation of GMG cells (7). Considering that GMG cells are NK-like cells expressing high levels of cytolytic mediators, it is reasonable to speculate that agents capable of stimulating NK cells and/or inducing cytolytic mediators may be involved in controlling the development of GMG cells.
Several cytokines have been shown to be able to induce the differentiation and activation of NK cells and cytotoxic T lymphocytes, and to induce and upregulate the expression of perforin and granzymes in these cells. These include IL-2, IL-6, IL-7, IL-12 (8–10), and a recently identified cytokine designated IL-15 (11, 12). IL-15 belongs to the four-helix bundle cytokine family and exhibits some biological activities previously identified for IL-2 (13). Moreover, the receptor complexes for IL-15 and IL-2 share two common subunits, the β chain and the γc chain (13). In spite of its similarities to IL-2, IL-15 is unique in its cell and tissue distribution and probably possesses distinctive functions. While IL-2 is produced predominantly by activated T cells, IL-15 appears to be produced by a variety of cell types and tissues, but not by T cells (13). The mRNA coding for IL-15 has been detected in cells such as monocytes–macrophages, fibroblasts, and epithelial cell lines, as well as tissues and organs including spleen, skeletal muscle, and placenta (13). The broad distribution of IL-15 suggests that it may substitute for IL-2 in executing certain biological functions in tissues where IL-2 is absent or present in low concentrations. In view of the abundant expression of IL-15 mRNA in placenta, it can be postulated that IL-15 may be involved in regulating the differentiation of GMG cells during pregnancy.
The present study is designed to investigate the possible function of IL-15 in the mouse pregnant uterus. The results show that the expression of IL-15 correlates closely with the differentiation of GMG cells, and that IL-15 can enhance the expression of perforin in pregnant uterine tissues maintained in short-term in vitro culture.
Materials and Methods
Animals and Tissues.
Pregnant C57BL/6 mice were used. The morning a vaginal plug was found was designated as day 0.5 of gestation. On specified days of gestation, animals were killed and the entire uterus (for gestation days 3–6; with the embryos) or the utero–placental tissues at implantation sites (for gestation days 8–18; without the fetus; hereafter referred to as the utero– placental units) were aseptically removed. For extraction of RNA or proteins, the removed tissues were immediately frozen and stored at −70°C until use. For tissue explant culture, the fetusdepleted utero–placental units were minced into 2–4-mm pieces, and cultured in α-modified minimum essential medium (αMEM) supplemented with 5% fetal bovine serum (FBS; Hyclone Laboratory, Logan, UT) and antibiotics. In some cultures, cytokines (IL-15 or IL-2) were added to a final concentration of 300 ng/ml. After a 24-h incubation, the cells and tissues were harvested, washed with PBS, and frozen for subsequent extraction of RNA or proteins.
Cytokines.
Recombinant human IL-15 and IL-2, were provided by Immunex Co. (Seattle, WA) and Cetus Co. (Emeryville, CA), respectively. These two cytokines have similar molecular masses (14–15 kD) and have been shown to be capable of functioning across species on murine cells (13).
Antibodies and cDNA Clone.
A polyclonal rabbit anti–mouse perforin antiserum was prepared and characterized in our previous study (7). A neutralizing anti-human IL-15 antiserum and a cDNA clone encoding murine IL-15 (13) were obtained from Immunex.
Western Blot Analysis.
Frozen uterine tissues were thawed in PBS containing 0.5% NP-40, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mM EDTA. After homogenization, the homogenates were clarified by centrifugation and the protein concentrations were determined using the BCA protein assay kit (Pierce, Rockfold, IL). Aliquots of the homogenates, containing the same amounts of protein, prepared from the tissues of different gestational age were subjected to Western blot analysis using an antiserum specific for mouse perforin, according to the established protocol.
RT-PCR.
Total RNA was prepared from the uterus or the utero–placenta unit using the RNAzol B solution (Tel-Test, Inc., Friendswood, TX), according to the instructions of the manufacturer. Complementary DNA (cDNA) was synthesized from 1 μg of the isolated RNA using Moloney murine leukemia virus reverse transcriptase (Perkin-Elmer, Norwalk, CT). PCR using the paired 5′ and 3′ primers for specific cytokine or cytolytic mediator was performed using the following protocol: 1 cycle of 3 min at 94°C, 1 min at 55°C, and 2 min at 72°C; and 35 cycles of 1 min at 94°C, 1 min at 55°C, and 1.5 min at 72°C. A negative control reaction in which no RNA template was added was included in each experiment. In each PCR reaction, a trace amount of [32P]dCTP (2 × 105 cpm/mixture) was included to label the PCR product. The PCR products were electrophoresed on 1% agarose gels containing ethidium bromide and visualized by UV illumination. After photodocumentation, the agarose gels were fixed in 10% TCA, washed, and dried under vacuum. The dried gels were exposed to autoradiographic films or subjected to image analysis using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
In Situ Hybridization.
The expression of IL-15 in the utero– placental unit was examined by in situ hybridization using a riboprobe specific for murine IL-15. In brief, cryostat sections (6 μm) of the utero–placental units taken from a day-9 pregnant mouse were fixed with 4% paraformaldhyde, rinsed, dehydrated, denatured, and hybridized with either the dioxigenin-labeled antisense or sense IL-15 riboprobe at 42°C overnight. After washing, slides were incubated with an alkaline phosphatase–conjugated antidioxigenin antibody for 1 h at room temperature. After washing, the slides were incubated with a substrate solution containing 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt to visualize the cells that hybridized with the riboprobe. The preparation of dioxigenin-labeled antisense and sense riboprobes from the murine IL-15 cDNA and the visualization of the hybridized cells were performed using the Genius Dig RNAlabeling kit (Boehringer Mannheim, Indianapolis, IN).
Results and Discussion
Time Course of the Expression of the mRNAs Encoding Cytokines and Cytolytic Mediators in the Pregnant Uterus.
GMG cells are bone marrow–derived NK-like cells that differentiate in situ within the pregnant uterus and reside predominantly in the metrial gland and decidua basalis (14, 15). In mouse, the differentiation of these cells begins at the time when implantation of the embryo takes place (day 4.5 of gestation), peaks around days 12–14 of gestation, and subsides thereafter (15). The differentiation of GMG cells is accompanied by the increased expression of cytolytic mediators, e.g., perforin, in these cells (15, 16). The factor(s) or mechanism(s) controlling this pregnancy-associated biological process remain(s) elusive. During pregnancy, the uterine endometrium undergoes remarkable cellular changes referred to as decidualization (17). Cell populations in the decidualized uterine tissue are heterogeneous, including gland epithelial cells, fibroblasts, macrophages, lymphoid cells, and other stromal cells (17). Many of these cells are capable of secreting cytokines which in term may influence the development or functional activities of other cell populations in a paracrine manner (18–22). Because the appearance of GMG cells in the pregnant uterus coincides with decidualization of the uterine tissue (15), it is conceivable that cytokines or other humoral factors (e.g., gestational hormones [23]) present locally in the decidual tissue are involved in initiating or regulating the differentiation of GMG cells. In view of the close relationship between GMG cells and NK cells in terms of their morphological, phenotypical, and biochemical features, these two cell types may be subjected to similar biological stimuli. We therefore sought to examine whether cytokines previously implicated in the activation of NK cells, including IL-2, IL-6, IL-7, IL-12, and IL-15, are expressed in the mouse pregnant uterus and, if so, whether these cytokines are involved in regulating GMG cell differentiation.
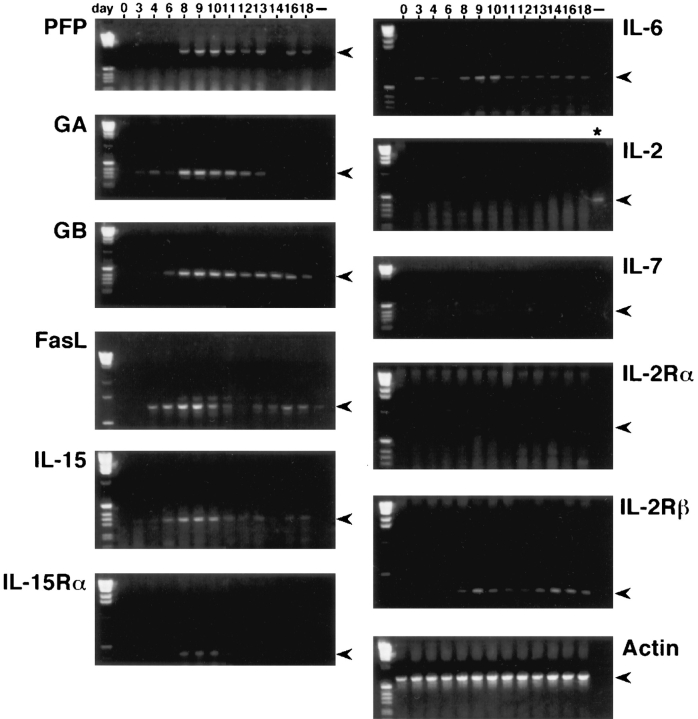
The expression of mRNAs encoding IL-2, IL-6, IL-7, IL-12, and IL-15 in the pregnant uterus was investigated by RT-PCR analysis, using RNA extracted from the entire uterus (before pregnancy or on gestation day 3, 4, and 6) or the utero–placental units (gestation day 8 onward). The products of RT-PCR were examined by visualization on electrophoresed gels (Fig. 1). The results obtained demonstrated that, while the mRNAs coding for IL-2 and IL-7 were essentially undetectable throughout the gestational period investigated, the mRNAs for IL-6 and IL-12 appeared to be constantly present in the pregnant uterus (data for IL-12 not shown). Although the message levels for IL-6 and IL-12 fluctuated to some extent during gestation, no distinct peaks for their expression were observed. In contrast, the expression of IL-15 seemed to be restricted to the late–early to mid gestational period, ranging from gestation day 6 to day 11 (Fig. 1). The two essential components of the IL-15 receptor complex, the α chain (IL-15Rα) and the β chain (IL-15/IL-2Rβ), were also expressed in the pregnant uterus, with the former expressed concomitantly with IL-15. In contrast, the α chain of the IL-2 receptor complex (IL-2Rα), which is required for the high affinity binding of IL-2, was absent in the pregnant uterus (Fig. 1). These latter findings further suggested that IL-15, but not IL-2, may be responsible for executing immunoregulatory function(s) in the pregnant uterus.
Figure 1.
Expression of mRNAs encoding lymphocyte cytolytic mediators, cytokines, and cytokine receptors in the pregnant uterus, investigated by RT-PCR analysis (see Materials and Methods).
The expression of mRNAs encoding cytolytic mediators in the pregnant uterus was similarly examined using RT–PCR analysis. Interestingly, the expression of mRNAs coding for perforin, granzyme A (GA), granzyme B (GB), and Fas ligand (FasL) appeared to commence on day 6 and reached the maximum on day 9 of gestation (Fig. 1). The time frames of cytolytic mediator mRNA expression in the pregnant uterus correlate well with those of IL-15 and IL-15Rα, suggesting a role for IL-15 as an inducer of the expression of these cytolytic mediators in this unique local environment. Since IL-6 and IL-12, two other cytokines capable of inducing cytolytic mediator expression (9, 10), are also present in the pregnant uterus (Fig. 1; data shown only for IL-6), it is possible that they may act in conjunction with IL-15 to achieve a full-scale immunoregulation.
Effects of IL-15 on the Expression of Perforin in the Utero– Placental Explants.
The above-described concomitant expression of IL-15 and different cytolytic mediators by GMG cells provides supporting yet circumstantial evidence that IL-15 may be responsible for the differentiation of these cells during pregnancy. To obtain additional evidence attesting to this hypothesis, we sought to investigate directly the biological effects of IL-15 on GMG cells. Previous studies, however, have demonstrated that GMG cells are very fragile and can be easily damaged during various mechanical or enzymatic manipulations essential for their isolation from the pregnant uterus (24). To circumvent this technical problem which may thwart the availability of intact GMG cells for further studies in vitro, we opted to utilize explant culture prepared from pregnant uteri. Based on the previous studies indicating that GMG cells represent the dominant, if not the exclusive, cell population producing cytolytic mediators in the pregnant uterus (15, 16), the results obtained using the pregnant uterus explants can be considered as being representative of those obtained using cultured GMG cells.
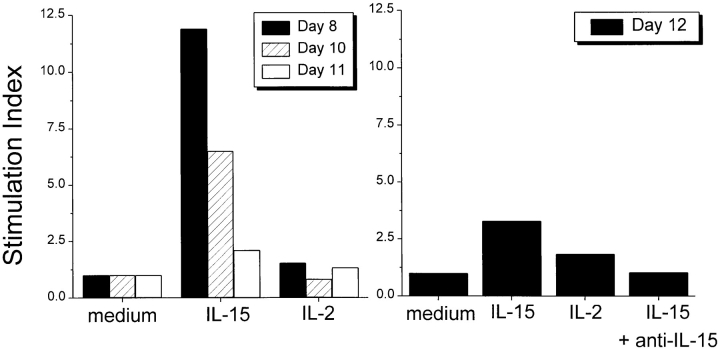
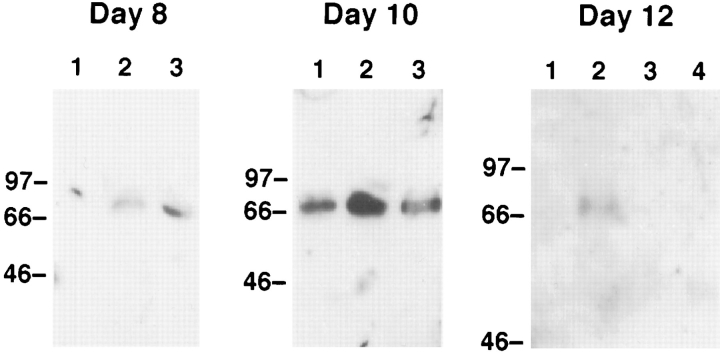
Minced pieces (2–4 mm) of the uterus (prior to gestation day 6) or the utero–placental unit (gestation day 8 and afterwards) were maintained at 37°C in medium with or without the indicated cytokine. IL-15 or IL-2 was used at 300 ng/ml, a concentration previously demonstrated to be effective in stimulating cytotoxic lymphocytes including NK cells (12). After a 24-h culture, the tissues and cells migrating out from the explants were harvested and their RNAs and proteins were extracted. To monitor the differentiation of GMG cells, the induction/enhancement of perforin expression in the cultured explants was examined. Using RT–PCR coupled with a semiquantitative Phosphorimager analysis, it was determined that the explants cultured in the presence of IL-15 (300 ng/ml) expressed two- to tenfold more perforin mRNA than those cultured in the absence of cytokine (Fig. 2). In contrast, IL-2 (300 ng/ml) exerted no detectable effect on the explants similarly prepared. This in vitro induction effect by IL-15 was observed only in the explants prepared from pregnant mice at gestation ages between day 8 and day 12. No significant induction or increase of perforin expression could be detected in the explants of pregnant uterus removed before gestation day 6 or after gestation day 13 (data not shown). These results suggested that cells present in the explants, probably GMG cells, are susceptible to the stimulation by IL-15 within a limited time period during pregnancy. This is consistent with the above-described finding that mRNA coding for IL-15Rα, which is imperative for cellular responses specific to IL-15, was expressed in the pregnant uterus in vivo between gestation day 8 through day 12 (see Fig. 1). The IL-15-induced expression of perforin could also be detected by Western blot analysis of the proteins extracted from the explants (Fig. 3). Moreover, the induction of perforin by IL-15 was completely abolished by the addition of a neutralizing anti-IL-15 antibody (Figs. 2 and 3; data shown only for the day 12 explant). Induction of GA and GB by IL-15, but not IL-2, in the explants removed from mice between gestation day 8 and day 12 was also observed. However, the extent of the induction of these two mediators was always smaller (approximately twofold; data not shown) than those of perforin. Interestingly, in these experiments, it was found that the biological effects of IL-15 were more evident at the earlier stage during the gestation period (10-fold increase of perforin mRNA in day 8 explants versus twofold increase in day 12 explants). These differential effects were probably due to the less advanced differentiation and the lower endogenous perforin level of day 8 GMG cells, which rendered these cells more responsive to IL-15 than the highly differentiated day 12 GMG cells.
Figure 2.
Effects of IL-15 and IL-2 on the expression of perforin mRNA in pregnant uterus. The explants of pregnant uterus prepared on the indicated days of gestation were cultured for 24 h in medium alone, or in medium supplemented with either IL-15 or IL-2. The RNAs prepared from the cultured explants were subjected to RT-PCR and PhosphorImager analysis. The level of the expression of perforin mRNA in the explants obtained on the indicated day of gestation was determined based on the ratio of the PCR product specific for perforin to that for β-actin. The perforin/β-actin ratios obtained in the RNAs prepared from the explants cultured in the presence of cytokines were then normalized against those obtained from the explants cultured in medium alone.
Figure 3.
Effects of IL-15 and IL-2 and the production of perforin protein in pregnant uterus. Proteins extracted from the cultured explants of pregnant uterus were subjected to Western blot analysis using an anti-perforin antiserum. Lanes 1, with medium; lane 2, with IL-15; lane 3, with IL-2; lane 4, with IL-15 and anti-IL-15.
These in vitro studies, together with the investigation on the correlative expression of mRNAs encoding IL-15, IL-15Rα, and cytolytic mediators in vivo (see Fig. 1), suggest rather strongly the role of IL-15 as the principal inducer/ regulator of differentiation of GMG cells in the pregnant uterus. Although it has been reported that IL-2, at high concentrations, could stimulate the cytolytic activity of GMG cells in vitro (25), our study indicates that IL-2 is probably not involved in regulating the differentiation of GMG cells in vivo.
Localization of IL-15-producing Cells in the Pregnant Uterus.
Another question pertinent to the biological function of IL-15 during pregnancy is the following: which cell population present in the pregnant uterus is responsible for the production of this cytokine? Since both macrophages and fibroblasts, two cell types known to produce IL-15, have been shown to be present in large numbers in the pregnant uterine tissue, they may be responsible for producing IL-15 in the local environment. Other cells, such as uterine epithelial cells and trophoblasts, as well as GMG cells themselves, have also been reported to be capable of secreting cytokines (18–20). The possibility that these cells may also contribute to the production of IL-15 in the pregnant uterus therefore can not be excluded. To address this issue, we examined the sections of the utero–placental units by in situ hybridization using riboprobes specific for IL-15. The results showed that some cells residing in the decidua basalis could hybridize to the antisense probe for IL-15 (Fig. 4). According to their anatomical localization, these IL-15expressing cells appeared to be macrophages, but not GMG cells. Moreover, cells expressing IL-15 messages were not detected in the labrynthine placenta, excluding the possibility that trophoblasts may be an important source of IL-15 (data not shown). Studies aimed at elucidating more precisely the identity of the IL-15-expressing cells (e.g., immunohistocytochemical staining using antibodies specific for macrophage surface markers) are underway.
Figure 4.
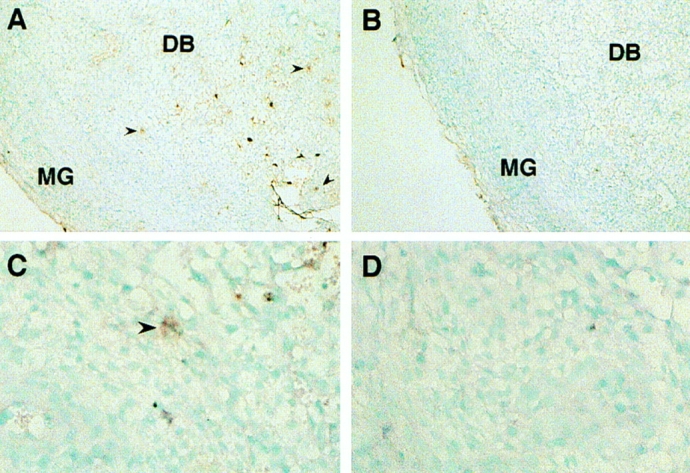
Expression of IL-15 in the pregnant uterus analyzed by in situ hybridization. Sections of an implantation site in a pregnant uterus of day 9 of gestation were hybridized with dioxygenin-labeled antisense (A and C) or sense (B and D) riboprobe specific for murine IL-15. Some cells residing in the decidua basalis (DB) (arrows) appeared to hybridize specifically with the antisense probe, indicating the expression of IL-15 mRNA in these cells.
Acknowledgments
We thank Drs. R. Steinman, A. Granelli-Piperno, and P. Haslett for critical reading of this manuscript.
Footnotes
This work is supported in part by grants from the National Institutes of Health (CA-47307) and the Cancer Research Institute. C.-C. Liu is supported by a Career Scientist Award from the Irma T. Hirschl Trust and an Established Investigatorship from the American Heart Association (National Center).
References
- 1.Stewart IJ, Peel S. The structure and differentiation of granulated metrial gland cells of the pregnant mouse uterus. Cell Tissue Res. 1977;184:517–527. doi: 10.1007/BF00220975. [DOI] [PubMed] [Google Scholar]
- 2.Liu C-C, Parr EL, Young JD-E. Granulated lymphoid cells of the pregnant uterus: morphological and functional features. Int Rev Cytol. 1994;153:105–136. doi: 10.1016/s0074-7696(08)62189-0. [DOI] [PubMed] [Google Scholar]
- 3.Bulmer JN, Sunderland CA. Bone-marrow origin of endometrial granulocytes in the early human placenta bed. J Reprod Immunol. 1983;5:383–387. doi: 10.1016/0165-0378(83)90247-4. [DOI] [PubMed] [Google Scholar]
- 4.Mukhtar DDY, Stewart IJ, Croy BA. Leucocyte membrane antigens on mouse granulated metrial gland cells. J Reprod Immunol. 1989;15:269–279. doi: 10.1016/0165-0378(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 5.Parr EL, Parr MB, Young JD-E. Localization of a pore-forming protein (perforin) in granulated metrial gland cells. Biol Reprod. 1987;37:1327–1336. doi: 10.1095/biolreprod37.5.1327. [DOI] [PubMed] [Google Scholar]
- 6.Croy BA. Granulated metrial gland cells: hypotheses concerning possible functions during murine gestation. J Reprod Immunol. 1994;27:85–94. doi: 10.1016/0165-0378(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 7.Zheng LM, Joag SV, Parr MB, Parr EL, Young JD-E. Perforin-expressing granulated metrial gland cells in murine deciduomata. J Exp Med. 1991;174:1221–1226. doi: 10.1084/jem.174.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C-C, Rafii S, Granelli-Piperno A, Trapani JA, Young JD-E. Perforin and serine esterase gene expression in stimulated human T cells: kinetics, mitogen requirements, and effect of cyclosporin A. J Exp Med. 1989;170:2105–2118. doi: 10.1084/jem.170.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth MJ, Ortaldo JR, Bere W, Yagita H, Okumura K, Young HA. Il-2 and IL-6 synergize to augment the pore-forming protein gene expression and cytotoxic potential of human peripheral blood T cells. J Immunol. 1990;145:1159–1166. [PubMed] [Google Scholar]
- 10.Salcedo TW, Azzoni L, Wolf SF, Perussia B. Modulation of perforin and granzyme messenger RNA expression in human natural killer cells. J Immunol. 1993;151:2511–2520. [PubMed] [Google Scholar]
- 11.Gamero AM, Ussery D, Reintgen DS, Puleo CA, Djei JY. Interleukin 15 induction of lymphokine-activated killer cell function against autologous tumor cells in melanoma patient lymphocytes by a CD18-dependent, perforinrelated mechanism. Cancer Res. 1995;55:4988–4994. [PubMed] [Google Scholar]
- 12.Ye, W., J.D.-E Young, and C.-C. Liu. 1996. Interleukin-15 induces the expression of mRNAs of cytolytic mediators and augments cytotoxic activities in primary murine lymphocytes. Cell. Immunol. In press. [DOI] [PubMed]
- 13.Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, et al. Cloning of a T cell growth factor that interacts with the beta chain of the interleukin-2 receptor. Science (Wash DC) 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 14.Peel S, Stewart I, Bulmer D. Experimental evidence for the bone marrow origin of granulated metrial gland cells of the mouse uterus. Cell Tissue Res. 1983;223:647–656. doi: 10.1007/BF00212232. [DOI] [PubMed] [Google Scholar]
- 15.Zheng LM, Liu C-C, Ojcius DM, Young JD-E. Expression of lymphocyte perforin in the mouse uterus during pregnancy. J Cell Sci. 1991;99:317–323. doi: 10.1242/jcs.99.2.317. [DOI] [PubMed] [Google Scholar]
- 16.Parr EL, Young LHY, Parr MB, Young JD-E. Granulated metrial gland cells of pregnant mouse uterus are NK-like cells that contain perforin and serine esterases. J Immunol. 1990;145:2365–2372. [PubMed] [Google Scholar]
- 17.Tarchand U. Decidualisation: origin and role of associated cells. Biol Cell. 1986;57:9–16. doi: 10.1111/j.1768-322x.1986.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 18.Hunt JS. Cytokine networks in the utero placental unit: macrophages as pivotal regulatory cells. J Reprod Immunol. 1989;16:1–17. doi: 10.1016/0165-0378(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 19.Guilbert L, Robertson SA, Wegmann TG. The trophoblast as an integral component of a macrophage– cytokine network. Immunol Cell Biol. 1993;71:49–57. doi: 10.1038/icb.1993.5. [DOI] [PubMed] [Google Scholar]
- 20.Croy BA, Guilbert LJ, Browne MA, Gough NM, Stinchcomb DT, Reed N, Wegmann TG. Characterization of cytokine production by the metrial gland and granulated metrial gland cells. J Reprod Immunol. 1991;19:149–166. doi: 10.1016/0165-0378(91)90014-h. [DOI] [PubMed] [Google Scholar]
- 21.De M, Sanford TR, Wood GW. Expression of interleukin 1, interleukin 6 and tumor necrosis factor alpha in mouse uterus during the peri-implantation period of pregnancy. J Reprod Fertil. 1993;97:83–89. doi: 10.1530/jrf.0.0970083. [DOI] [PubMed] [Google Scholar]
- 22.Parr EL, Chen H-L, Parr MB, Hunt JS. Synthesis and granular localization of tumor necrosis factor-alpha in activated NK cells in the pregnant mouse uterus. J Reprod Immunol. 1995;28:31–40. doi: 10.1016/0165-0378(94)00905-m. [DOI] [PubMed] [Google Scholar]
- 23.Stewart I. Differentiation of granulated metrial gland cells in ovariectomized mice given ovarian hormones. J Endocrinol. 1987;112:23–26. doi: 10.1677/joe.0.1120023. [DOI] [PubMed] [Google Scholar]
- 24.Parr EL, Szary A, Parr MB. Measurement of natural killer activity and target cell binding by mouse metrial gland cells isolated by enzymic or mechanical methods. J Reprod Fert. 1990;88:283–294. doi: 10.1530/jrf.0.0880283. [DOI] [PubMed] [Google Scholar]
- 25.Croy BA, Reed N, Malashenko BA, Kim K, Kwon BS. Demonstration of YAC target cell lysis by murine granulated metrial gland cells. Cell Immunol. 1991;133:116–126. doi: 10.1016/0008-8749(91)90184-d. [DOI] [PubMed] [Google Scholar]