Abstract
Two cell permeable peptide fluoromethyl ketone inhibitors of Interleukin-1β converting enzyme (ICE) family proteases were tested as inhibitors of apoptotic cell death of T lymphocytes at various stages of differentiation. The CPP-32–like protease activity in apoptotic cell lysates was blocked by both the ICE inhibitor Cbz-Val-Ala-Asp(OMe)-fluoromethyl ketone (ZVADFMK) as well as its truncated analog Boc-Asp(OMe)-fluoromethyl ketone (BD-FMK), which failed to block ICE. In vitro apoptotic death in murine thymocytes triggered by the independent agents dexamethasone, etoposide, radiation, anti-Fas, and anti-CD3 was blocked equally well by BD-FMK and ZVAD-FMK, but not by the control reagent Cbz-Phe-Ala-fluoromethyl ketone. In activated T cell blasts, while anti-CD3/ Fas-induced death was almost completely inhibited by both ZVAD-FMK and BD-FMK, death induced by dexamethasone, etoposide, or irradiation was more sensitive to inhibition by BD-FMK. In the murine T cell line CTLL-2, apoptotic death induced by IL-2 withdrawal, etoposide, or dexamethasone was inhibited by BD-FMK, while ZVAD-FMK was without effect. These data indicate that ICEfamily proteases comprise a common functional step in distinct T cell apoptotic death pathways, but suggest that different family members are likely to be critical in various differentiated T cell types, even when triggered by the same stimulus.
While programmed cell death has become recognized as an important component of normal development and immune function, the biochemical pathways leading to such cell death remain poorly defined. However, the recent demonstration that the nematode death gene ced-3 encodes a cysteine protease related to the mammalian interleukin-1β converting enzyme (ICE) has led to the identification of a family of cysteine proteases related by sequence homology (1). This ICE-family of proteases has an unusual substrate cleavage specificity for aspartic acid residues at the P1 position. Studies of sequence homology and fine specificity of substrate cleavage suggest there are two to three subfamilies (2, 3): The ICE-like subfamily prefers substrates with hydrophobic amino acids at P4 (such as Tyr-ValAla-Asp [YVAD]), the CPP-32–like subfamily has less sequence homology to ICE and prefers substrates with acidic amino acids at P4 (such as Asp-Glu-Val-Asp [DEVD]), and a potential ICH-1–like subfamily remains poorly characterized. In the case of death induced by Fas cross-linking, there is evidence for a proteolytic cascade involving sequential activation of ICE-like enzymes and CPP-32–like enzymes (4, 5).
Convincing evidence for a functional role of ICE family proteases in programmed cell death has come from several strategies designed to selectively inactivate these proteases, particularly the expression of the virally encoded protein inhibitors CrmA and Baculovirus p35 (reviewed in reference 1). Peptide-based inhibitors of ICE family proteases have also been shown to block apoptotic death in vivo and in vitro, but their membrane permeability is sometimes a problem, and their specificity has not always been adequately established.
We report here the ability of two newly developed cell permeant peptide-fluoromethyl ketone inhibitors of ICE family proteases to specifically block in vitro apoptotic death processes in T lymphocytes triggered by different input pathways. These results indicate that this protease family comprises a common downstream step in apoptotic T cell death pathways. The ICE inhibitor Cbz-Val-Ala-Asp(OMe)- fluoromethyl ketone (ZVAD-FMK) specifically blocks most examples of T lymphocyte apoptotic death. However, several examples of T cell death which are resistant to ZVADFMK were blocked by the homologous inhibitor BDFMK, which blocks CPP-32–like proteases but not ICE. These results suggest that for a single apoptotic stimulus, different members of the ICE family are functionally important in different types of T cells, and show the use of peptide-FMK reagents as probes of the role of ICE family proteases in in vitro cell death systems.
Materials and Methods
Reagents.
The protease inhibitors Cbz-Val-Ala-Asp-(OMe)- fluoromethyl ketone (ZVAD-FMK), Boc-Asp(OMe)-fluoromethyl ketone (BD-FMK), Cbz-Asp(OMe)-Glu(OMe)-Val-Asp (OMe)-fluoromethyl ketone (ZDEVD-FMK), Cbz-Phe-Ala-fluoromethyl ketone (ZFA-FMK), Cbz-Ala-Ala-Asp-chloromethyl ketone (ZAAD-CMK) and the CPP-32 substrate Cbz-AspGlu-Val-Asp-7-amino-4-trifluoromethyl coumarin (ZDEVD-AFC) were purchased from Enzyme Systems Products (Dublin, CA), dissolved as stock solutions of 50 mM in DMSO, and stored at −70°C. Fixed Staphylococcal aureus (Sansorbin) was obtained from Calbiochem Corp. (La Jolla, CA). Polyclonal anti–human IL-1β was purchased from R&D Systems Inc. (Minneapolis, MN), mouse anti–human Fas (CH-11) from Upstate Technologies Inc. (Waltham, MA), and hamster anti–mouse Fas (Jo2) from PharMingen (San Diego, CA). Dexamethasone, etoposide (VP16), and Hoechst 33342 were obtained from Sigma Chemical Co. (St. Louis, MO). FITC-Annexin V was purchased from Brand Applications B. V. (Maastricht, Netherlands).
Granzyme B Activity.
Granzyme B activity was measured in detergent extracts of cloned murine CTL, provided by Dr. Martha Alexander-Miller (National Cancer Institute). Extracts were prepared by treating 1 × 107 CTL with 1 ml of 1% Triton X-100 in assay buffer at 0°C for 10 min, followed by centrifugation at 11,000 g for 10 min. This extract was treated with the haloketone reagents at the indicated concentrations for 2 h at room temperature, followed by the activity assay, which was carried out with 1 × 105 cell equivalents of extract/well in flat-bottom microtiter plates using Boc-Ala-Ala-Asp-S-benzyl (BAAD-S-Bzl) (Enzyme Systems Products) at a final concentration of 125 μM in 0.5 M NaCl, 0.1 M Hepes, 1 mM EDTA, and 0.5 mM 5,5′-dithiobis(2-nitrobenzoic acid) DTNB, pH 7.5. Activity was calculated from initial slopes of plots of A414 versus time, relative to controls with no inhibitor.
CPP-32-like DEVDase Activity.
Jurkat cells (5 × 106/ml) were incubated with 1 μg/ml CH-11 anti-Fas IgM on ice for 15 min, and then at 37°C for 45 min. After centrifugation, the cell pellets were lysed in ICE buffer (1 × 106 cells in 200 μl 50 mM Hepes buffer, pH 7.5, 10% sucrose, and 0.1% Triton X-100) for 20 min on ice. After centrifugation at 10,000 g for 10 min at 4°C, 200 μl of supernatant was transferred to a tube containing 2 μl 1 M dithiothreitol. Peptide-FMKs were then added at the indicated concentrations and incubated on ice for 15 min followed by ZDEVD-AFC to a final concentration of 50 μM. After incubation at room temperature for 1 h, each tube was diluted with 2 ml PBS, and fluorescence measured (excitation, 400 nm, emission, 505 nm). With the amount of cell extracts used, DEVD-ase activity was linear.
IL-1β Processing.
IL-1 secretion from blood monocytes was measured as previously described (6). Briefly, blood was preincubated with peptide-FMKs for one h, followed by addition of 50 μc/ml [35S]methionine and fixed S. aureus (Sansorbin; Calbiochem; 300 μg/ml). After 5 h of incubation at 37°C, supernatants were immunoprecipitated with anti–IL-1β, and analyzed on 14% nonreducing SDS gels.
Induction of T Cell Death.
T cells were obtained from C57Bl/ 10 mice ( Jackson Laboratory, Bar Harbor, ME). T cell blasts were generated by activating splenic T cells with PMA (2 ng/ml) and ionomycin (1 μg/ml) for 48 h, followed by at least 24 h of culture in complete medium (RPMI-1640 supplemented with 10% FCS, 100 IU penicillin, and 10 μg/ml streptomycin) with 10 U/ml of human recombinant IL-2 (Cetus Corp., Emeryville, CA). CTLL-2 cells (American Type Culture Collection, Rockville, MD) were carried in complete medium supplemented with 2 U/ml IL-2. The protease inhibitors were added at the initiation of 200 μl cultures in all experiments described.
Thymocyte death was induced by culture (106/ml complete medium) with the following stimuli: 0.01 μM dexamethasone, 2 μg/ml etoposide, immobilized anti-CD3, and immobilized anti-Fas, or they were exposed to 5Gy of γ-irradiation before culture. Immobilized antibodies were precoated on wells (5 μg/ml of 2C11 anti-CD3 or 10 μg/ml of anti-Fas) followed by washing. T cell blasts (0.5 × 106/ml of IL-2 containing complete medium) were cultured with 0.1 μM dexamethasone, 2 μg/ml etoposide, and immobilized anti-CD3, or exposed to 5Gy irradiation before culture to induce apoptotic death by 18 h. CTLL-2 cells (2.5 × 105/ml) were cultured in IL-2 containing medium with 5 μg/ml etoposide or 50 μM dexamethasone for 18 h to induce death. For IL-2 withdrawal, cells were washed three times in complete medium and cultured at 0.25 × 106 cells/ml in the absence or presence of IL-2.
Assays of Apoptotic Cell Death.
To measure apoptotic nuclear morphology, cells were harvested and stained with 4 μg/ml Hoechst 33342 for 10 min at 37°C before fluorescence microscopy. Nuclei were scored as apoptotic if they displayed chromatin condensation and/or nuclear fragmentation. For propidium iodide staining, samples were counterstained with 5 μg/ml of propidium iodide. A minimum of 200 cells were scored for each data point. In some experiments membrane damage was quantitated by the trypan blue uptake of dead cells.
For analyzing the sub-G0 DNA content, cells were harvested by centrifugation and the cell pellets resuspended in lysis buffer (0.1% sodium citrate and 0.1% Triton X-100) containing 50 μg/ml of propidium iodide. Samples were stored at 4°C for 18 h before FACScan® analysis using CELL Quest software.
To detect phosphotidylserine exposure on cell membranes, cells were stained with FITC-Annexin V (25 ng/ml) for 10 min at room temperature in the dark. Annexin V high cells were measured on a flowcytometer by gating on FSC/SSC settings for control untreated cells.
Results
ZVAD-FMK and BD-FMK Specifically Block ICE family Proteases, but not Granzyme B.
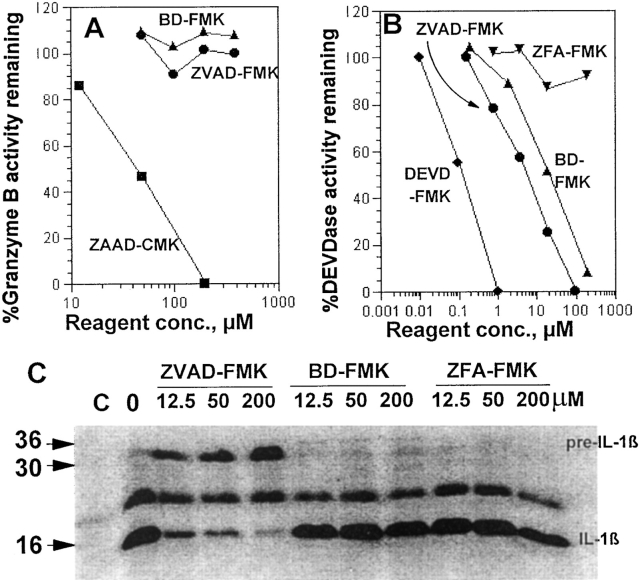
Although appropriate peptide-chloromethyl ketones irreversibly inactivate both serine and cysteine proteases, the less reactive fluoromethyl ketones are reported to be selective for cysteine proteases (7). To confirm the selectivity of ZVAD-FMK and BD-FMK for cysteine proteases, we tested their ability to inactivate granzyme B, a serine protease recognizing asp-containing peptides generally similar to those recognized by ICE family members. Fig. 1 A shows that these FMK reagents fail to detectably inactivate granzyme B in CTL extracts at concentrations up to 400 μM. In contrast, ZAAD-CMK blocks this enzyme, showing that peptide-asp-FMK reagents are selective for cysteine over serine proteases as expected.
Figure 1.
Reactivity of peptide-FMK reagents. (A) BD-FMK and ZVAD-FMK do not inactivate Granzyme B. Detergent extracts of cloned murine CTL were used as a source of enzyme with the thiobenzyl ester BAAD-S-Bzl as substrate. The indicated final concentrations of inhibitors were preincubated with enzyme for 2 h at 37°C before addition of substrate. ZAAD-CMK was tested as a positive control. (B) P1-Asp FMKs inactivate apoptotic DEVDase from Jurkat. A detergent extract of antiFas–treated Jurkat cells was used as a source of active CPP-32 or similar ICE family protease with a fluorometric assay of ZDEVD-AFC hydrolysis. The indicated concentrations of peptide-FMK reagents were preinbucated with the extract at 0°C for 15 min before addition of substrate to begin the assay. (C ) Peptide-FMK inhibition of stimulated human monocyte IL-1β processing. An SDS gel of secreted and immunopreciptated 35S-labeled IL-1β is shown. The control lane C received no S. aureus. The nonspecific band at 22 kD was not seen in all experiments.
Anti-Fas treated Jurkat T cells rapidly acquire a CPP32-like ability to cleave DEVD peptide substrates (8), and we have measured this activity in extracts of such cells using the fluorogenic substrate AcDEVD-AFC. Fig. 1 B shows the effect of peptide-FMKs on this activity. As expected, the homologous ZDEVD-FMK is a potent inhibitor, with ZVAD-FMK and BD-FMK also active with about 40 and 150 times less potency, respectively. In contrast, the control ZFA-FMK reagent lacking aspartate at P1 failed to show significant inhibition.
Fig. 1 C shows that ZVAD-FMK inhibits the conversion of pre–IL-1β (31 kD) to active IL-1β (17 kD) in monocytes stimulated by S. aureus. The shorter reagent BDFMK is seen to be at least 100 times less potent in inhibiting this enzyme as is the control reagent ZFA-FMK, lacking aspartate at the P1 position crucial for recognition by ICE famliy proteases.
Inhibition of Thymocyte Apoptotic Death By ZVAD-FMK and BD-FMK.
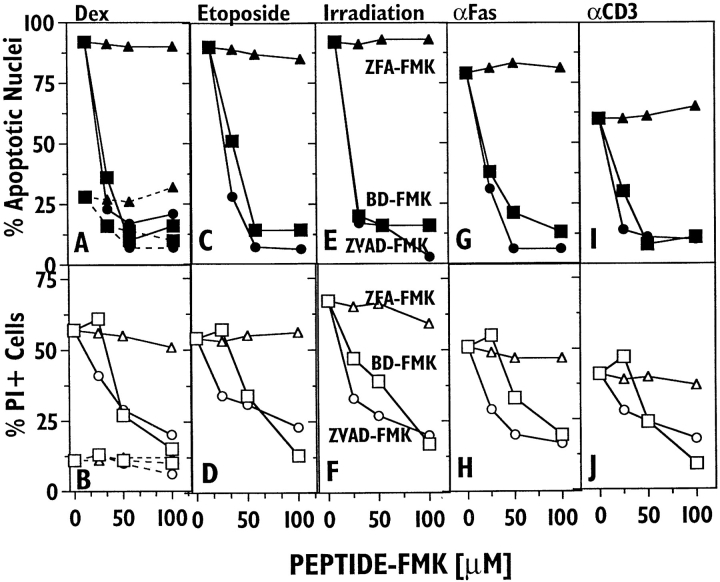
Thymocytes provide a well studied model system for programmed cell death, which can be triggered in vitro by four independent input pathways: corticosteroid, DNA damage by the DNA topoisomerase II inhibitor etoposide or radiation, anti-Fas, and anti-CD3. Fig. 2 shows the ability of ZVAD-FMK and BD-FMK to inhibit such death. A shows that the spontaneous development of apoptotic nuclear morphology at 18 h is ∼70% blocked by 100 μM ZVAD-FMK or BD-FMK, but not by an equal concentration of ZFA-FMK. At this time, there is little spontaneous death as judged by propidium iodide (PI) staining (B). Dexamethasone potently induces apoptotic nuclear morphology, an effect blocked to control levels by 50 μM ZVAD-FMK or BD-FMK, but unaffected by ZFA-FMK. Cell death as assessed by PI (B) shows a similar specific inhibition by both ZVAD-FMK or BD-FMK, but somewhat more reagent is required to achieve levels of inhibition comparable to apoptotic nuclei. C–F show the similar ability of peptide-FMKs to block thymocyte death by etoposide and γ-irradiation. Previous studies have shown both these death systems to be dependent on p53 (9). Thymocyte death induced by immobilized anti-Fas antibody (Fig. 2, G–H ) is also effectively and specifically blocked by ZVAD-FMK and BD-FMK, confirming the ability of other ICE inhibitors to block anti-Fas triggered cell death (10, 11). Finally, immobilized anti-CD3 antibody induces somewhat less death in these cells than other stimuli (12), but both death readouts again showed specific inhibition by both ZVAD- or BD-FMK(I and J ). Thus, thymocyte death triggered by four independent input pathways is specifically inhibited by peptide-FMK inhibitors of ICE family proteases, with similar titration curves.
Figure 2.
ZVAD-FMK and BD-FMK block murine thymocyte death induced by different agents in vitro. The effects of ZVAD-FMK (circles), BD-FMK (squares), and ZFA-FMK (triangles) on thymocyte death after 18 h of culture in the presence of the following: A and B, spontaneous death (dotted lines) and dexamethasone (solid lines); C and D, etoposide; E and F, prior γ-irradiation; G and H, immobilized anti-Fas; I and J, immobilized anti-CD3. Cell death was assessed by apoptotic nuclear morphology (top row) and staining with propidium iodide (bottom row).
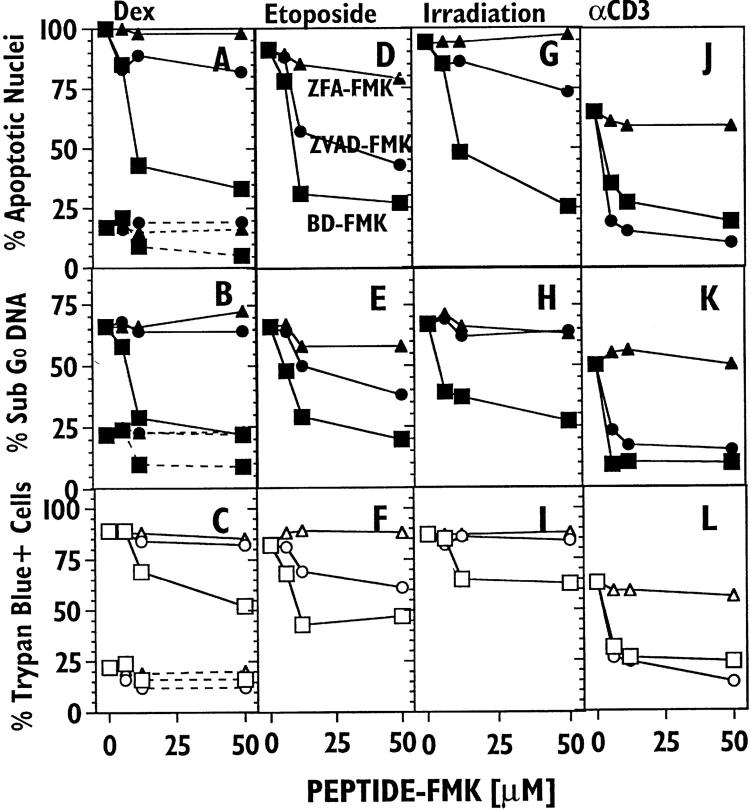
Diminished Blocking of T Lymphoblast Apoptotic Death by ZVAD-FMK, but not ZFA-FMK.
Upon activation, peripheral T cells acquire sensitivity to death by TCR crosslinking. Fig. 3 shows an experiment examining the ability of peptide-FMK reagents to block T lymphoblast death induced by dexamethasome (A–C ), etoposide (D–F ), γ-irradiation (G–I ), and anti-CD3 ( J–L). The two independent measurements of apoptotic nuclear damage gave very similar results (top two rows). As was the case for thymocytes, the control ZFA-FMK reagent was without significant effect. However, for these blasts, the inhibition patterns of ZVADFMK and BD-FMK are significantly different from those found in thymocytes in several respects: (a) ZVAD-FMK shows clearly different inhibition patterns with different apoptotic stimuli, (b) BD-FMK is a generally better blocker of apoptotic nuclear damage and cell death measured by membrane integrity than is ZVAD-FMK, and (c) with dexamethasone and irradiation, BD-FMK prevented apoptotic nuclear damage in some cells which nevertheless died as assessed by trypan blue. Although the latter comprise a minority of the cells which died, there is no evidence that their death occurred via a pathway dependent upon ICE family proteases.
Figure 3.
Inhibition of the apoptotic death of murine T cell blasts by peptide-FMKs. Splenic T cell blasts were cultured for 18 h in the presence of: dexamethasone (A–C); etoposide (D–F); after γ-irradiation(G–I ); and immobilized anti-CD3 ( J–L) in the indicated concentration of ZVADFMK (circles), BD-FMK (squares) and ZFA-FMK (triangles). Cell death was assessed by apoptotic nuclear morphology (top row), quantitation of sub-G0 DNA by flow cytometry staining (middle row) and with trypan blue (bottom row).
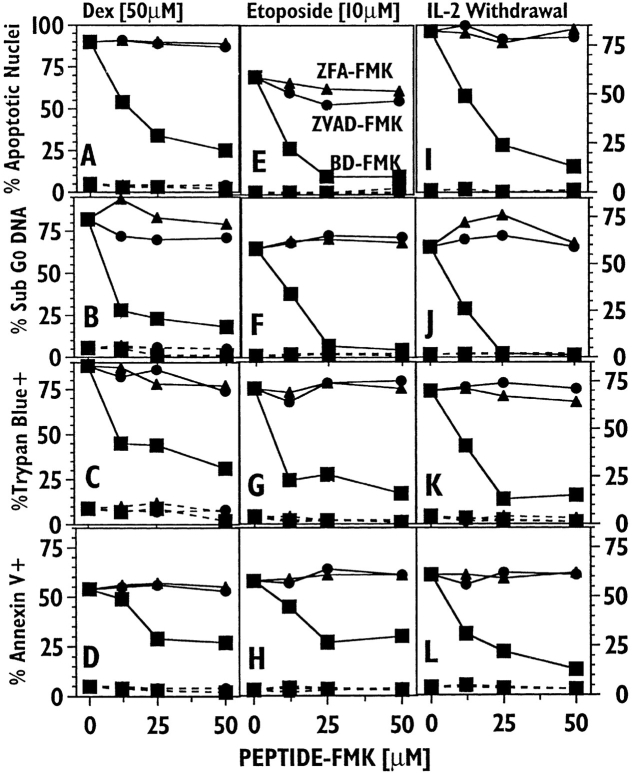
BD-FMK, but not ZVAD-FMK, Blocks Apoptotic Death in the IL-2–dependent CTTL-2 Cell Line.
Fig. 4 shows experiments examining the ability of ZVAD-FMK and BDFMK to inhibit apoptotic death in the IL-2–dependent murine T cell line CTTL-2. In these experiments we also measured the binding of FITC-Annexin V to the membrane, another nonnuclear apoptotic damage reflecting external phosphotidyl serine exposure (13). A previous report showed that ICE inhibitors fail to block death mediated by IL-2 withdrawal in this cell line (14). Fig. 4 confirms these results with the ICE inhibitor ZVAD-FMK, which, like ZFA-FMK, fails to block any of the death readouts induced by dexamethasone (A–D), etoposide (E–H ), or IL-2 withdrawal (I–L) in these cells. Unlike ZVAD-FMK, BDFMK inhibited CTLL-2 death with patterns quite similar to those seen with thymocytes and lymphoblasts. Apoptotic nuclear damage was completely inhibited by this reagent while nonnuclear death criteria were in some cases less completely blocked, reflecting the behavior seen with blasts.
Figure 4.
BD-FMK, but not ZVAD-FMK, blocks apoptotic death in CTLL-2 cells induced by several agents. Murine CTLL-2 cells were cultured with ZVAD-FMK (circles), BD-FMK (squares), or ZFA-FMK (triangles), and in the absence (dashed lines) or presence (solid lines) of the following death stimuli: A–D, dexamethasone (50 μM); E–H, etoposide (10 μM) and I–L: IL-2 withdrawal for 18 h. Cell death was measured by apoptotic nuclear morphology (A, E, I ), FACS®-analyzed sub-G0 DNA (B, F, J ), trypan blue staining (C, G, K ); and FACS®-analyzed FITC-Annexin V staining (D, H, L).
Discussion
While blocking in vivo and in vitro programmed cell death by expressing viral inhibitors of ICE family proteases has given convincing evidence of their general functional importance (1), such inhibitors are difficult to use experimentally to implicate these proteases in many important death systems. To probe T lymphocyte apoptotic death in vitro, we have studied two different peptide-FMK reagents designed to inactivate ICE family proteases. While further work is clearly needed to define the family members reactive with these reagents, the functional effects reported here allow insight into the changes in functional death pathways which accompany T cell differentiation. A combination of these experiments with future biochemical studies on ICE family proteases should lead to a more exact definition of molecular death pathways in T cells.
Both ZVAD-FMK and BD-FMK contain aspartate in the P1 position and are designed to irreversibly inactivate ICE family cysteine proteases in intact cells by alkylating the active site cysteine (7). Unlike other available peptide-based inhibitors of ICE family proteases, these FMK reagents can penetrate membranes readily because the aspartate carboxylate side chain is esterified and is expected to undergo cytoplasmic hydrolysis to the free aspartate derivative. Inactivation of a cellular process by a P1 asp-containing peptide–FMK reagent, but not a control reagent such as ZFA-FMK, shows that a member of the ICE family is functionally required, based on the unusual asp-peptide cleavage specificity of the ICE family and the selectivity of FMK reagents for cysteine proteases.
Because ICE-like proteases may become activated and in turn process CPP-32-like proteases (4, 5), it has been suggested that ZVAD-FMK inhibits death by selectively blocking the former (15). In support of this, 10 μM ZVADFMK was reported not to inhibit the ability of such CPP32-like proteases to cleave poly-ADP-ribose polymerase (15). However, the data of Fig. 1 suggest that ZVAD-FMK could block death by directly inactivating CPP-32–like members of the ICE family, and indicate that further studies with purified enzymes are required. Furthermore, Fig. 2 shows that the ICE inhibitor ZVAD-FMK and the nonICE inhibitor BD-FMK block thymocyte death about equally well, suggesting that ZVAD-FMK's ability to inhibit the ICE-like protease subfamily may not be the mechanism by which it blocks death. Cysteine protease inhibitors containing only a P1 asp have been previously shown to block some cell deaths (16, 17), but their reactivity with ICE family proteases remains to be established. In contrast to BD-FMK, a more reactive chloromethyl ketone analog blocks ICE (18). While more experiments are needed to assess the activity of BD-FMK with other ICE-family proteases, the results in Fig. 1 indicate that the ability of this reagent to block cell death could be due to its ability to directly inactivate some CPP-32-like ICE-family proteases.
The similarity of the inhibition titration curves of thymocyte death induced by the four independent input pathways tested in Fig. 2 is striking. In no case was significant inhibition seen with the control ZFA-FMK, indicating that asparatate at the P1 position is important. For apoptotic morphology, half maximal inhibition occurred at 15–40 μM ZVAD-FMK or BD-FMK, while comparable inhibition of death assessed by PI required about twice as much reagent. The similarity of the inhibition curves suggests that the same ICE family protease is the target of these reagents in inhibiting these four separate input pathways of thymocyte death.
Peptide FMKs inhibit the apoptotic death of activated peripheral T cells with a significantly different pattern than was found in thymocytes. ZVAD-FMK blocks Fas-induced death effectively, but has a variable effect on other death pathways in blasts. These experiments show several novel aspects of the role of ICE family proteases in T lymphocyte apoptotic death. For a single input pathway such as corticosteroid, T cell maturation is accompanied by a markedly diminished sensitivity to the ICE-inhibitor ZVAD-FMK, while sensitivity to BD-FMK is largely maintained. This is most readily explained by a shift in the particular ICE family protease members functional in the death pathway as the cells mature. However, in T cell blasts, the CD3-triggered Fas death pathway maintains its sensitivity to ZVAD-FMK, arguing that ICE-like target proteases are still expressed and accessible to the reagent. Thus the linkages between the different death signaling input pathways and ICE family proteases undergo interesting, and hitherto unappreciated, changes with differentiation of T cells.
The experiments with CTLL-2 in Fig. 4 show a more dramatic example of the different behavior of BD-FMK and ZVAD-FMK, with the latter completely ineffective in blocking any manifestation of death triggered by any death stimulus tested. These data are compatible with the findings of Vasilakos et al. (14) using other ICE inhibitors on the death of CTLL-2 cells by IL-2 withdrawal. However, our findings indicate that this is a property of CTLL-2 cells more than growth factor withdrawal, and our data with BDFMK indicate that other ICE-family proteases are part of all these death pathways. The behavior of CTLL-2 cells appears to be unusual among T cell death systems we have examined, and such ZVAD-FMK resistant apoptotic death processes may be uncommon generally, as this reagent and related ICE-specific inhibitors have been found to block a number of nonlymphoid in vitro and in vivo death systems (19–23).
The findings presented here make two related but distinct points: (a) they argue strongly that ICE family proteases comprise a common step(s) in the apoptotic death of most T lymphoid cells triggered by distinct input pathways, and (b) they show that death pathways in T cells at different stages of differentiation seem to use different ICE family members when death is triggered by the same input pathway. Many important issues remain to be elucidated regarding the functional ICE family proteases including the elements controlling their activation, interactions amongst family members, and their functional substrates leading to cell death. Our results show that peptide-FMK inhibitors of ICE family proteases permit simple functional experiments which can begin to shed light on some of these issues.
Acknowledgments
We would like to thank Drs. Charles Zacharchuk and David Segal for their helpful comments on the manuscript.
References
- 1.Henkart PA. ICE family proteases: mediators of all apoptotic cell death? . Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 2.Duan H, Chinnaiyan AM, Hudson PL, Wing JP, He WW, Dixit VM. ICE-LAP3, a novel mammalian homologue of the Caenorhabditis eleganscell death protein Ced-3 is activated during Fas- and tumor necrosis factor– induced apoptosis. J Biol Chem. 1996;271:1621–1625. doi: 10.1074/jbc.271.3.1621. [DOI] [PubMed] [Google Scholar]
- 3.Xue D, Shaham S, Horvitz HR. The Caenorhabditis eleganscell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 4.Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 5.Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature (Lond) 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 6.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, Miller DK, Molineaux SM, Weidner JR, Aunins J, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature (Lond) 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 7.Shaw E. Cysteinyl proteinases and their selective inactivation. Adv Enzymol Relat Areas Mol Biol. 1990;63:271–346. doi: 10.1002/9780470123096.ch5. [DOI] [PubMed] [Google Scholar]
- 8.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW. CPP32/apopain is a key interleukin-1–beta converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 9.Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature (Lond) 1993;363:849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- 10.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature (Lond) 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 11.Los M, Van de Craen M, Penning LC, Schenk H, Westendorp M, Baeuerle PA, Dröge W, Krammer PH, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1–mediated apoptosis. Nature (Lond) 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 12.Tadakuma T, Kizaki H, Odaka C, Kubota R, Ishimura Y, Yagita H, Okumura K. CD4+CD8+thymocytes are susceptible to DNA fragmentation induced by phorbol ester, calcium ionophore and anti-CD3 antibody. Eur J Immunol. 1990;20:779–784. doi: 10.1002/eji.1830200411. [DOI] [PubMed] [Google Scholar]
- 13.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 14.Vasilakos JP, Ghayur T, Carroll RT, Giegel DA, Saunders JM, Quintal L, Keane KM, Shivers BD. IL-1–beta converting enzyme (ICE) is not required for apoptosis induced by lymphokine deprivation in an IL-2–dependent T cell line. J Immunol. 1995;155:3433–3442. [PubMed] [Google Scholar]
- 15.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO (Eur Mol Biol Organ) J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mashima T, Naito M, Kataoka S, Kawai H, Tsuruo T. Aspartate-based inhibitor of interleukin-1–beta converting enzyme prevents antitumor agent–induced apoptosis in human myeloid leukemia U937 cells. Biochem Biophys Res Comm. 1995;209:907–915. doi: 10.1006/bbrc.1995.1584. [DOI] [PubMed] [Google Scholar]
- 17.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science (Wash DC) 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estrov Z, Black RA, Sleath PR, Harris D, Van Q, LaPushin R, Estey EH, Talpaz M. Effect of interleukin-1–beta converting enzyme inhibitor on acute myelogenous leukemia progenitor proliferation. Blood. 1995;86:4594–4602. [PubMed] [Google Scholar]
- 19.Zhu HJ, Fearnhead HO, Cohen GM. An ICE-like protease is a common mediator of apoptosis induced by diverse stimuli in human monocytic THP.1 cells. FEBS Lett. 1995;374:303–308. doi: 10.1016/0014-5793(95)01116-v. [DOI] [PubMed] [Google Scholar]
- 20.Cain K, Inayat-Hussain SH, Couet C, Cohen GM. A cleavage-site–directed inhibitor of interleukin 1βconverting enzyme-like proteases inhibits apoptosis in primary cultures of rat hepatocytes. Biochem J. 1996;314:27–32. doi: 10.1042/bj3140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science (Wash DC) 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- 22.Milligan CE, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz LC, Tomaselli KJ, Oppenheim RW, Schwartz LM. Peptide inhibitors of the ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson MD, Weil M, Raff MC. Role of Ced3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]