Abstract
A key rate-limiting step in the adaptive immune response at peripheral challenge sites is the transmission of antigen signals to T cells in regional lymph nodes. Recent evidence suggests that specialized dendritic cells (DC) fulfill this surveillance function in the resting state, but their relatively slow turnover in most peripheral tissues brings into question their effectiveness in signaling the arrival of highly pathogenic sources of antigen which require immediate mobilization of the full range of host defenses for maintenance of homeostasis. However, the present report demonstrates that recruitment of a wave of DC into the respiratory tract mucosa is a universal feature of the acute cellular response to local challenge with bacterial, viral, and soluble protein antigens. Consistent with this finding, we also demonstrate that freshly isolated respiratory mucosal DC respond in vitro to a variety of CC chemokines as well as complementary cleavage products and N-formyl-methionyl-leucine-phenylalanine. This suggests that rapid amplification of specific antigen surveillance at peripheral challenge sites is an integral feature of the innate immune response at mucosal surfaces, and serves as an “early warning system” to alert the adaptive immune system to incoming pathogens.
The host response to invading pathogens is classically viewed as a two-tiered system, comprising a series of innate (inflammatory) and acquired (adaptive) immune mechanisms which operate over distinct time scales (1). Thus, the first line of defense is provided by the rapid recruitment of phagocytic granulocytes (usually neutrophils) into sites of tissue injury in response to locally produced chemotactic factors. This is followed up to 48 h later by a second wave of mononuclear cells containing large numbers of macrophages which are effective in both the uptake of persisting antigen and its subsequent presentation to the T cell system. In this classical scheme, the adaptive immune system serves as an optional backup to innate host defenses, and is called upon only in situations where significant amounts of antigen persist at the challenge site beyond the time frame of the acute inflammatory response.
While this scheme appears inherently economical, it could equally be viewed as flawed in at least one respect; viz, the delayed recruitment of adaptive immune mechanisms into the host response provides a temporal window for the establishment and spread of incoming pathogens. This danger would appear to be greatest in the case of pathogens not previously encountered by the host, i.e., for which neither antibody nor T-effector memory cells are available. Under such circumstances, host survival may ultimately be determined by the rapidity with which naive T cells are primed against antigens displayed by the pathogen at the challenge site, a process which occurs initially in local draining lymph nodes.
Recent evidence suggest that dendritic cells (DC) function as first-line sentinels in immune surveillance of peripheral tissues (2), including mucosal surfaces such as those in the lung and airways (3, 4). These DC migrate into peripheral tissues from a circulating monocytelike precursor pool, and differentiate locally to a stage in which they are specialized for acquisition and processing of antigen, but remain unable to effectively present the antigen locally to T cells (2). This latter function, in particular a unique capacity for potent activation of naive T cells, is acquired after their migration to regional lymph nodes (2).
Thus, individual DC present a “snapshot”of the antigens encountered during their transient sojourn through their respective peripheral tissue sites, presumably including those antigens derived from incoming pathogens.
The effectiveness of such a surveillance system in the context of infectious disease is presumably a direct function of the DC traffic (i.e., cell number/unit time) between peripheral tissue sites and their respective regional lymph nodes. In relatively quiescent tissues such as skin and muscle, mean DC transit times are estimated to normally be in the order of weeks (5–7). However, DC turnover is considerably more rapid at the main mucosal surfaces in direct contact with the outside environment (viz, the gastrointestinal and respiratory tracts) where resident populations are renewed every 3–4 d (7, 8).
Materials and Methods
Experimental Animals.
Specified pathogen-free adult PVG rats were supplied by the Animal Resource Centre (Murdoch, Western Australia). All animal experimentation was carried out with the prior approval of the Institute for Child Health Animal Ethics and Experimentation Committee which complies with conditions set down by the Australian National Health and Medical Research Council.
Bacterial Models.
(a) Moraxella catarrhalis was grown in Mueller Hinton broth, washed extensively with saline, and suspended at ∼109 CFU/ml. The suspension was heated at 60°C for 1 h and passed through a 26 gauge needle several times to break up any bacterial clumps. Rats were exposed by aerosol to the suspension for 1 h using a Tri-R inhalation exposure apparatus (Tri-R Instruments, New York). (b) Bordetella pertussis (Welcome strain 28; provided by Dr. P. Novotny, Kent, England) was grown on Charcoal agar, washed in sterile PBS, and ∼109 organisms in 50 μl were deposited directly onto the tracheal surface of adult rats by intratracheal intubation.
Viral Model.
Sendai virus was provided by Dr. Jane Allan (University of Western Australia, Perth, Western Australia) and grown for 3 d in the allantoic cavity of eggs. Allantoic fluid was stored at −70°C until used. Adult rats were inoculated intranasally with 103 HAU in 50 μl of virus containing allantoic fluid. Control animals were similarly inoculated with virus-free allantoic fluid. Infection of airway epithelium was confirmed by staining with mAb WS16 against the nucleoprotein antigen of Sendai virus (provided by Dr. A. Portner, St. Jude Children's Research Hospital, Memphis, TN). There was no influx of DC or T cells into the airway epithelium of control animals.
Antigen Sensitization.
For antigen sensitization experiments, animals were intraperitoneally primed with 100 μg of ovalbumin (OVA; Sigma Chemical Co., St. Louis, MO) in 0.5 ml PBS containing 10 mg aluminium hydroxide (Wyeth Amphojel). 14 d later, the animals were challenged with a 30 min aerosol of 1% (wt/vol) OVA in PBS.
Immunohistochemical Analysis.
Tracheas were removed and immediately fixed in cold ethanol for 30 min. The tissue was then rehydrated in PBS, embedded in 100% OCT, and frozen in liquid nitrogen–cooled iso-pentane. Tangential sections 8–10 μm were cut on a cryostat and immunostained as detailed in reference 4. Eosinophils were identified by cytochemical staining (4). Sections were counterstained with hematoxylin, dehydrated, and mounted. Primary antibodies used were Ox6 (rat MHC class II; 9), Ox12 (rat kappa light chains on B cells; 9), R73 (rat TcRαβ; 10), ED2 (rat macrophage; 11), and RP3 (rat neutrophils; 12). DC identification criteria were pleiomorphic morphology, together with positive staining with Ox6 and negative staining for Ox12, R73, and ED2, as detailed previously (4, 13).
DC Chemotaxis.
The assay system was based upon that described in 14, except that 6.5 mm Costar Transwells (Costar Corp., Cambridge, MA) with polycarbonate membranes (3-μm pore size) were used. Briefly, DC were enriched to 75–82% purity from collagenase digests of respiratory tract tissue by flow cytometry gating for Ox6+, Ox12−, and ED2−-cells, as previously described (13). 600 μl medium (RPMI plus 2.5% fetal calf serum) containing putative chemoattractant was placed in the lower chamber, and 105 enriched DC in 100 μl medium were placed in the insert; after incubation for 1 h at 37°C, the top surface of the inserts was washed free of cells and the insert was fixed in cold ethanol for 10 min. The polycarbonate membranes were excised, and the contralateral surface immunostained with MoAb Ox6; migrating cells were observed under a ×25 objective and enumerated as mean number cells/high power field.
The chemoattractants used were 10% Zymosan–activated normal rat serum (the active agents being complement cleavage products, in particular C5a), N-formyl-methionyl-leucine-phenylalanine (f MLP; 10−8 M; Sigma Chemical Co.), rat MCP-1, GRO/KC, GROβ (all at 100 ng/ml), rat RANTES (200 ng/ ml), and murine eotaxin (100 ng/ml) from Peprotech (London, UK), human MCP-4 (100 ng/ml; Glaxo Wellcome, Geneva, Switzerland), and human IL-8 (100 ng/ml; Genzyme Corp., Boston, MA). The human IL-8 used here was shown to chemoattract rat neutrophils in this assay, in preliminary experiments.
Results and Discussion
The present study sought to ascertain whether a similar DC response occurred after challenge of the airways with live infectious organisms (both viral and bacterial pathogens) to which animals had not been previously exposed, and an inert protein antigen (OVA) to which the animals were presensitized by parenteral immunization.
In principle, groups of normal adult rats were subjected to local airways challenge with aerosol or a liquid bolus delivered directly onto the airway surface, containing either heat-killed or live bacteria (M. catarrhalis or B. pertussis, respectively), live parainfluenza type1 virus (Sendai), or OVA; for the latter stimulus, comparisons were made between OVA-preprimed and naive animals. Animals were killed in groups at strategic (generally daily) intervals, and tracheal tissue samples cryopreserved for subsequent immunohistochemical analysis of cell populations within the airway epithelium, using methods detailed previously (4).
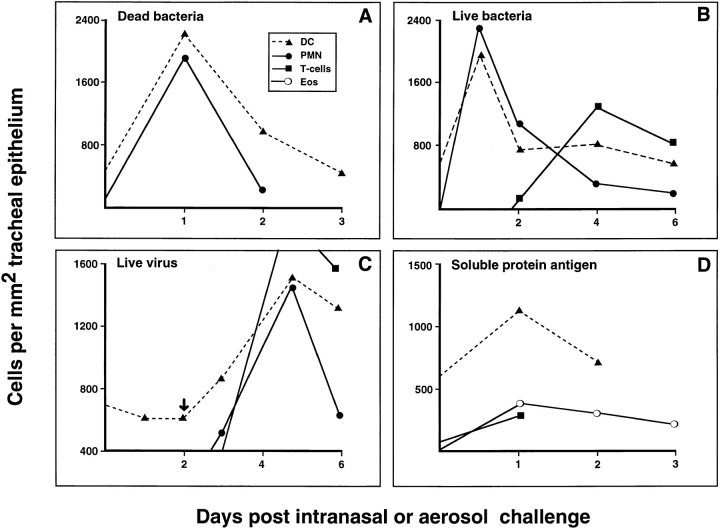
The time frame of interest in the present studies was the duration of the host acute cellular inflammatory responses triggered by the various stimuli, and the latter was defined in each model by a series of initial trials. Representative data are shown in Fig. 1. The acute cellular response to microbial agents depicted in A–C of Fig. 1 demonstrate the transient influx of PMN, which is the hallmark of the acute host response to this class of stimuli. These cells are enumerated in tangential sections through the tracheal epithelium after immunostaining with the PMN-specific mAb RP3 (12). The PMN response is most rapid following challenge with proinflammatory bacterial cell wall extract (Fig. 1 A). In the case of intranasally delivered live virus, we were able to identify the time of onset of the PMN response (starting on day 3) as occurring within 24 h of the first detectable expression of viral protein within infected airway epithelial cells (Fig. 1 C, arrow). It was of interest to note the intensity of the T cell chemotactic responses, which were initiated in both systems which used live pathogens (see particularly the virus model in Fig. 1 C), in contrast to the inflammatory response to bacterial cell wall products which did not include a significant T cell component.
Figure 1.
Quantitative cellular responses within tracheal epithelium during inflammation. Data shown are from representative experiments (⩾5 for each model); each point is a mean value derived from four to six animals. (A) Inhalation of aerosolized heat-killed M. catarrhalis produced an acute inflammatory reaction in which Ox6+ DC and RP3+ neutrophils were the only inflammatory cell type detected in the epithelium. Peak numbers were detected at 1 d after aerosol. (B) Live B. pertussis organisms inoculated intratracheally resulted in an influx of Ox6+ DC and RP3+ neutrophils with maximum cell numbers occurring at day 1. The T cell influx began at day 2 and peaked at day 4. (C ) Intranasal Sendai virus initiated a cellular response which commenced with an Ox6+ DC influx shortly after the earliest detection of intraepithelial Sendai virus nucleoprotein immunostaining at day 2 (C, arrow) and shortly before changes could be detected in other cell types. Maximum DC numbers were found at day 5, and coincided with maximal neutrophil and T cell numbers. (D) After intraperitoneal priming with ovalbumin and Al(OH)6, animals were challenged 14 d later with an aerosol of ovalbumin and cellular changes within the epithelium measured. In this instance Ox6+ DC, eosinophil, and T cell numbers peaked at day 1 after challenge.
These responses contrast with that of animals challenged with an inert protein antigen to which they are presensitised (Fig. 1 D); the acute phase of this latter response was characterized by the influx of eosinophils as opposed to PMN, and a relatively small number of T cells. No cellular response was seen in unprimed animals. Macrophage influx was not a significant feature of the initial cellular response in any of these models.
However, the feature common to all these challenge models is the transient waves of DC which are recruited into the epithelium coincident with the early phase of respective cellular inflammatory responses. The most intense response is that triggered by high-level local stimulation with pro-inflammatory bacterial cell wall extract, in which intraepithelial airway DC density transiently increases to levels up to threefold those of resting tissue (Fig. 1 A), before their migration on to regional lymph nodes (13). The more subtle stimuli, such as infection with live virus and bacteria and even inhalation of inert nominal antigen, also clearly evoke qualitatively similar responses, involving the transient build up of intraepithelial DC numbers in challenged airway tissue to levels that are two- to threefold those of resting tissues.
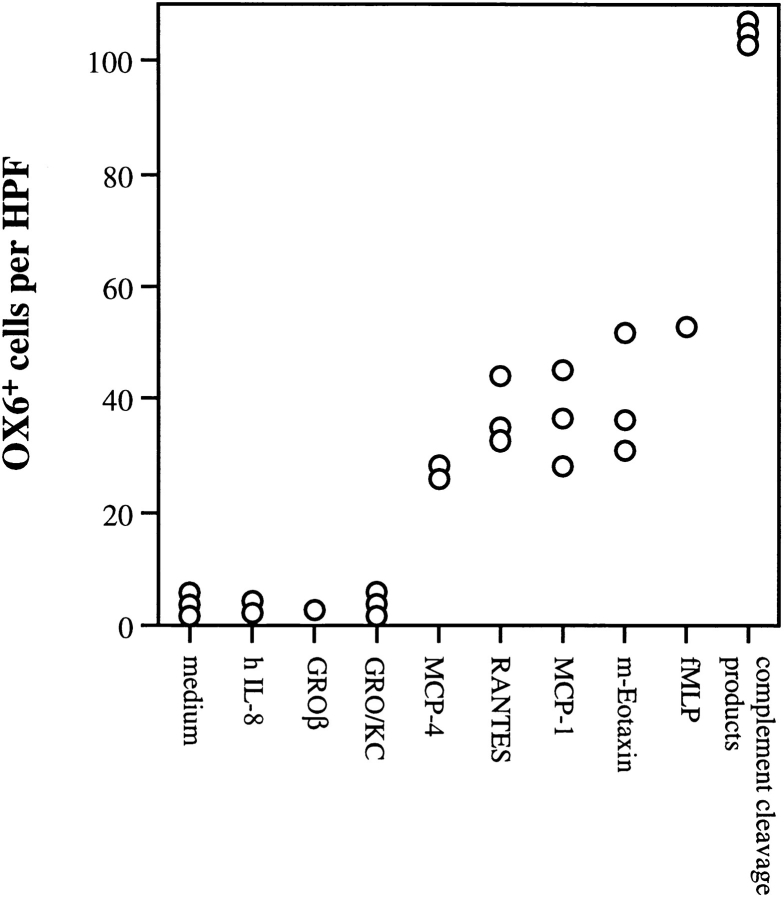
We have additionally screened a broad panel of chemotactic agents for capacity to attract respiratory mucosal DC in vitro (Fig. 2). Consistent with findings in Fig. 1 indicating rapid in vivo recruitment of DC in response to bacterial stimuli, complement cleavage products, and f MLP exhibit potent chemoattractant activity in vitro. Additionally, MCP-1, -4, RANTES, and eotaxin, all of which are members of the CC chemokine family, exhibited significant chemoattractant activity, whereas the CXC chemokines IL-8, GRO/KC, and GRO/β, were inactive. These findings are comparable to those recently reported using putative DC cultured from human blood in the presence of GM-CSF and IL-4, which also responded to a broad spectrum of chemotactic stimuli (14); however, the blood-derived DC were unresponsive to MCP-1, which may reflect subtle species differences in receptor expression, or possibly variations related to the degree of maturation of respective DC populations at the time of assay.
Figure 2.
In vitro responses of respiratory tract DC to different chemotactic agents. Freshly prepared respiratory tract DC were assessed for responsiveness to a range of chemotactic agents in a 1 h chemotaxis assay, as detailed in Materials and Methods. Each data point represents results from an individual experiment, each of which included complementary cleavage products plus the medium control.
These findings indicate that current perceptions of the scope of innate host defense responses need to be broadened to include the rapid activation of an in-built “early warning system” at sites of inflammation to facilitate the most rapid possible flow of information on the presence of previously unencountered antigens to the adaptive immune system. This mechanism fulfills an essential prediction of the model proposed recently (1, 15) for the evolution of the immune system, viz, that DC may be evolution's answer to the problem posed by organisms that evade primitive (innate) first-line defense systems.
Footnotes
This work was supported by Glaxo Wellcome, the Raine Foundation of Western Australia, and the National Health and Medical Research Council of Australia.
References
- 1.Janeway CA. The immune response evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 3.Holt PG, Schon-Hegrad MA, Oliver J. MHC class II antigen-bearing dendritic cells in pulmonary tissues of the rat: regulation of antigen presentation activity by endogenous macrophage populations. J Exp Med. 1988;167:262–274. doi: 10.1084/jem.167.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schon-Hegrad MA, Oliver J, McMenamin PG, Holt PG. Studies on the density, distribution, and surface phenotype of intraepithelial class II MHC antigen (Ia)-bearing dendritic cells (DC) in the conducting airways. J Exp Med. 1991;173:1345–1356. doi: 10.1084/jem.173.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart DNJ, Fabre JW. Demonstration and characterization of Ia-positive dendritic cells in the interstitial connective tissues of rat heart and other tissues, but not brain . J Exp Med. 1981;154:347–361. doi: 10.1084/jem.154.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H-D, Ma C, Yuan J-T, Wang Y-K, Silvers WK. Occurrence of donor Langerhans cells in mouse and rat chimeras and their replacement in skin grafts. J Invest Dermatol. 1986;86:630–633. doi: 10.1111/1523-1747.ep12275627. [DOI] [PubMed] [Google Scholar]
- 7.Fossum S. Dendritic leukocytes: features of their in vivophysiology. Res Immunol. 1989;140:883–891. doi: 10.1016/0923-2494(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 8.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol. 1994;153:256–261. [PubMed] [Google Scholar]
- 9.Mason DW, Arthur RP, Dallman MJ, Green JR, Spickett GP, Thomas ML. Functions of rat T-lymphocyte subsets isolated by means of monoclonal antibodies. Immunol Rev. 1983;74:57–82. doi: 10.1111/j.1600-065x.1983.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 10.Hünig T, Wallny HJ, Hartley JK, Lawetzky A, Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989;169:73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damoiseaux JG, Dopp EA, Neefjes JJ, Beelen RH, Dijkstra CD. Heterogeneity of macrophages in the rat evidenced by variability in determinants: two new anti–rat macrophage antibodies against a heterodimer of 160 and 95 kd (CD11/CD18) J Leukocyte Biol. 1989;46:556–564. doi: 10.1002/jlb.46.6.556. [DOI] [PubMed] [Google Scholar]
- 12.Sekiya S, Gotoh S, Yamashita T, Watanabe T, Saitoh S, Sendo F. Selective depletion of rat neutrophils by in vivo administration of a monoclonal antibody. J Leukocyte Biol. 1989;46:96–102. doi: 10.1002/jlb.46.2.96. [DOI] [PubMed] [Google Scholar]
- 13.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sozzani S, Sallusto F, Luini W, Zhou D, Piemonti L, Allavena P, Van Damme J, Valitutti S, Lanzavecchia A, Mantovani A. Migration of dendritic cells in response to formyl peptides, C5a, and a distinct set of chemokines. J Immunol. 1995;155:3292–3295. [PubMed] [Google Scholar]
- 15.Ibrahim MAA, Chain BM, Katz DR. The injured cell: the role of the dendritic cell system as a sentinel receptor pathway. Immunol Today. 1995;16:181–186. doi: 10.1016/0167-5699(95)80118-9. [DOI] [PubMed] [Google Scholar]