Abstract
Ceramides are intramembrane diffusible mediators involved in transducing signals originated from a variety of cell surface receptors. Different adaptive and differentiative cellular responses, including apoptotic cell death, use ceramide-mediated pathways as an essential part of the program. Here, we show that human dendritic cells respond to CD40 ligand, as well as to tumor necrosis factor-α and IL-1β, with intracellular ceramide accumulation, as they are induced to differentiate. Dendritic cells down-modulate their capacity to take up soluble antigens in response to exogenously added or endogenously produced ceramides. This is followed by an impairment in presenting soluble antigens to specific T cell clones, while cell viability and the capacity to stimulate allogeneic responses or to present immunogenic peptides is fully preserved. Thus, ceramide-mediated pathways initiated by different cytokines can actively modulate professional antigen-presenting cell function and antigen-specific immune responses.
Dendritic cells (DCs) represent key players in the immune response (1). They capture and process antigens in nonlymphoid tissues, then move into T cell–dependent areas of secondary lymphoid organs to prime naive T cells and initiate the immune response (2). Along this process, DCs lose antigen-capturing ability as they differentiate into mature, fully stimulatory antigen-presenting cells (APCs) (3). The recent establishment of an in vitro system that allows human DCs to be mantained in culture preserving their immature phenotype, i.e., efficient antigen uptake and processing, has revealed a unique tool to gain insights into the basic mechanisms governing the differentiation of DCs (4). Evidence has been provided, in fact, that tumor necrosis-factor-α (TNF-α) (via the p55 TNF-R1), IL-1β, CD40 ligand (CD40L), and bacterial lipopolysaccaride (LPS) can promote DC differentiation in vitro, resulting in irreversible structural and functional changes associated with a mature DC phenotype, including downregulation of antigen uptake and processing capacity (5). However, little is known about the intracellular signals that are responsible for mediating these changes in DCs after cytokines or bacterial products exposure.
Sphingomyelin breakdown by sphingomyelinases, with resulting ceramide release, is a major signaling event that follows both TNF-R1 and IL-1β receptor engagement by their respective ligands (6, 7). Ceramide diffuses within membranes activating downstream effectors, including different protein kinases and phosphatases, eventually leading to a variety of adaptive and differentiative cellular responses (8–10). Intriguingly, LPS, also a potent inducer of DC differentiation (5), mimics many of the cellular responses initiated by TNF-α and IL-1β, without inducing sphingomyelin hydrolysis but likely becouse of its structural analogy with ceramide itself (11, 12). Therefore, we investigated whether some of the differentiative changes induced in DCs by inflamatory cytokines and LPS could be mediated by ceramides.
Materials and Methods
In Vitro Culture of Human DCs.
PBMCs obtained by standard Ficoll–Paque method (Organon Teknika, Durham, NC) were separated on multistep Percoll gradients (Pharmacia Fine Chemicals, Uppsala, Sweden) and the light density fraction from the 42.5–50% interface was recovered and depleted of CD19+ and CD2+ cells using magnetic beads coated with specific antibodies (Dynal, Oslo, Norway). The remaining cells were cultured in RPMI-1640 supplemented with 2 mM l-glutamine, 1% nonessential aminoacids, 1% pyruvate, 50 μg/ml kanamicin, 5 × 10−5 M 2-ME (GIBCO BRL, Gaithersburg, MD) + 10% FCS (Hyclone Laboratories, Inc., Logan, UT) in the presence of 50 ng/ml GM–CSF and 1000 U/ml IL-4 (provided by Dr. A. Lanzavecchia, Basel Institute for Immunology, Switzerland). Cultured DCs were routinely >90% CD1+, HLA-DR+, CD14−, and were used after 5–7 d of culture.
Ceramide Mass Measurement Assay.
After stimulation, lipids were extracted and then incubated with Escherichia coli diacylglycerol kinase. Ceramide phosphate was then isolated by TLC using CHCl3/CH3OH/CH3COOH (65:15:5) as solvent (13, 14). Authentic ceramide-1-phosphate was identified by autoradiography at Rf 0.25. Quantitative results for ceramide production are expressed as pmol ceramide-1-phosphate/106 cells.
Endocytosis and Antigen Presentation Assays.
2 × 105 DCs were resuspended in 200 μl RPMI buffered with 25 mM Hepes + 10% FCS. C2-ceramide and C2-dihydroceramide (Sigma Immunochemicals, St. Louis, MO) were reconstituted in EtOH at 15 mM and stored at −20°C until use. Diacylglycerol was purchased from Amersham (Buckingham, England). Lucifer yellow (LY), FITC–dextran (DX) (Molecular Probes, Inc., Eugene, OR), or HRP (Sigma Immunochemicals) were reconstituted in PBS, stored at 4°C, and spun in a microfuge before use to eliminate aggregates. To quantify LY and FITC–DX, cells were washed four times with cold PBS containing 1% FCS and 0.01% NaN3 and analyzed on a FACScan® (Becton Dickinson, Mountain View, CA), using propidium iodide to exclude dead cells. For horseradish peroxidase (HRP) quantification, cells were washed four times as above then four times with PBS alone with one tube change, lysed with 0.05% Triton X-100 in 10 mM Tris buffer pH 7.4 for 30 min, and the enzyme activity of the lysate was measured using O-phenilendiamine and H2O2 as substrates with reference to a standard curve. Tetanus toxoid (TT)-specific T cell clones KS140 and KB24 and TT peptide P2 (residues 830–843) were provided by Dr. A. Lanzavecchia. TT antigen was purchased from Connaught (Ontario, Canada).
For antigen presentation assays, 4 × 104 T cells were cultured with 104 irradiated DCs in 200 μl RPMI with 10% FCS in flatbottomed microplates. [3H]thymidine incorporation was measured at day 2. For MLR, 1.5 × 105 responding cells from allogeneic adult PBMCs were cultured with different numbers of irradiated DCs. [3H]thymidine incorporation was measured at day 5. Parietaria judaica pollen (Allergon, Angelholm, Sweden) was extracted with bicarbonate buffer 0.125 M pH 8. PjE-specific clone P6.2 was isolated from an atopic patient (15).
DNA Labeling and Flow Cytometry Analysis.
2 × 105 DCs were treated in 200 μl RPMI 10% FCS with 80 μM C2-ceramide for 10 min on ice, then for 1 h at 37°C. After incubation, cells were washed and left in culture for 48 h. Cells were then recovered and processed for propidium iodide staining and FACS® analysis as previously described (13).
Other Reagents.
Supernatant from J558L cells stably expressing a chimeric mCD40L–mCD8α construct, provided by Dr. P. Lane, (Basel Institute for Immunology) was used as a source of CD40L. Recombinant human TNF-α with R32W and S86T substitutions (TNF-R1 specific) and TNF-α with D143N and A145R substitutions (TNF-R2 specific) were provided by Drs. W. Lesslauer and H. Loetscher (Hoffman La Roche, Ltd., Basel, Switzerland). IL-1β was provided by Dr. L. Melli (IRIS, Siena, Italy).
Results and Discussion
Cytokines that Induce Maturation, Signal Ceramide Accumulation in DCs.
IL-1β and TNF-α have been shown to induce transient ceramide accumulation in tumor cell lines (16, 17). It was not known whether CD40, like other TNF receptor family members such as TNF-R1 p55, Fas/APO-1, or NGF-R p75 (13, 16, 18), also signaled through ceramide generation. Therefore, we investigated whether cross-linking of CD40 was able to induce ceramide accumulation in cultured immature DCs. Fig. 1 shows that CD40L, as well as IL-1β and TNF-α, engaging TNF-R1 p55 but not TNF-R2 p75, were all potent inducers of ceramide generation in cultured DCs. Because CD40L, IL-1β, and TNF-α trigger in vitro maturation of DCs (5), as does LPS, which is structurally analogous to ceramide itself (11), these results raised the possibility that a common ceramide-mediated pathway, mimicked by LPS, could be responsible for some of the functional changes observed during in vitro maturation of DCs.
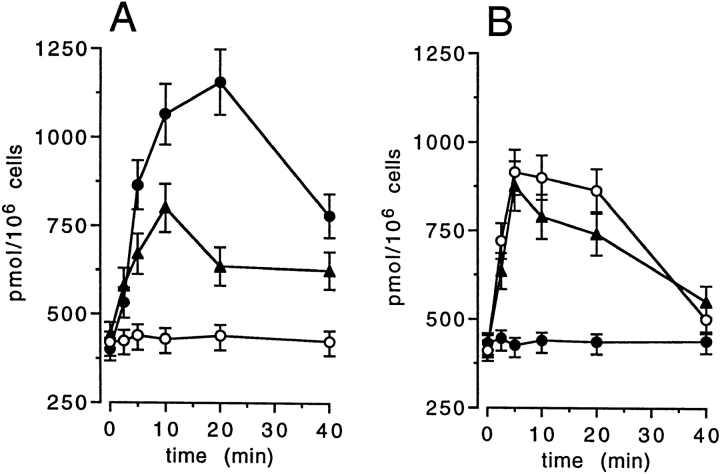
Figure 1.
Ceramide production in DCs following CD40L, IL-1β (A), and TNF-α exposure (B). DCs were isolated and cultured as described (4), then left untreated (A, open circles) or stimulated for the indicated times with proper amounts of soluble CD40L (31) (A, closed circles), 104 U/ml IL-1β (A, closed triangles), 200 ng/ml TNF-α (B, closed triangles), 200 ng/ ml TNF-α with R32W and S86T substitutions (TNF-R1 specific) (B, open circles), and 200 ng/ml TNF-α with D143N and A145R substitutions (TNF-R2 specific) (B, closed circles) (32). Lipids were extracted and incubated with E. coli diacylglycerol kinase for ceramide quantitation (13). Mean data ± one SD were obtained from three experiments from three donors.
Ceramides Down-modulate Macromolecule Uptake by DCs.
To test this hypothesis directly, we investigated whether exposure to exogenous cell-permeant C2-ceramide could down-modulate DC antigen uptake ability. DCs capture antigen either via macropinocytosis, a cytoskeleton-dependent type of fluid phase endocytosis initiated by membrane ruffling and formation of large vesicles, or receptor-mediated endocytosis through Fcγ and mannose receptors (5). As shown in Fig. 2, C2-ceramide could inhibit the uptake of three different classical endocytosis markers and their time-dependent accumulation into DCs. Both macropinocytosis, as assessed by LY and FITC-DX, and receptormediated endocytosis, as assessed by limiting amounts of HRP, were significantly affected. Comparable results were also obtained using C6-ceramide, a longer acyl chain ceramide analogue (data not shown). By contrast, C2-dihydroceramide, a structural analogue of C2-ceramide that lacks a double bond at the 4–5 position in the sphingoid base, was ineffective. Similarly, exposure to other diffusible signaltransducing lipid mediators such as diacylglycerol, did not affect macromolecule uptake ability of DCs (Fig. 2, A, C, E).
Figure 2.
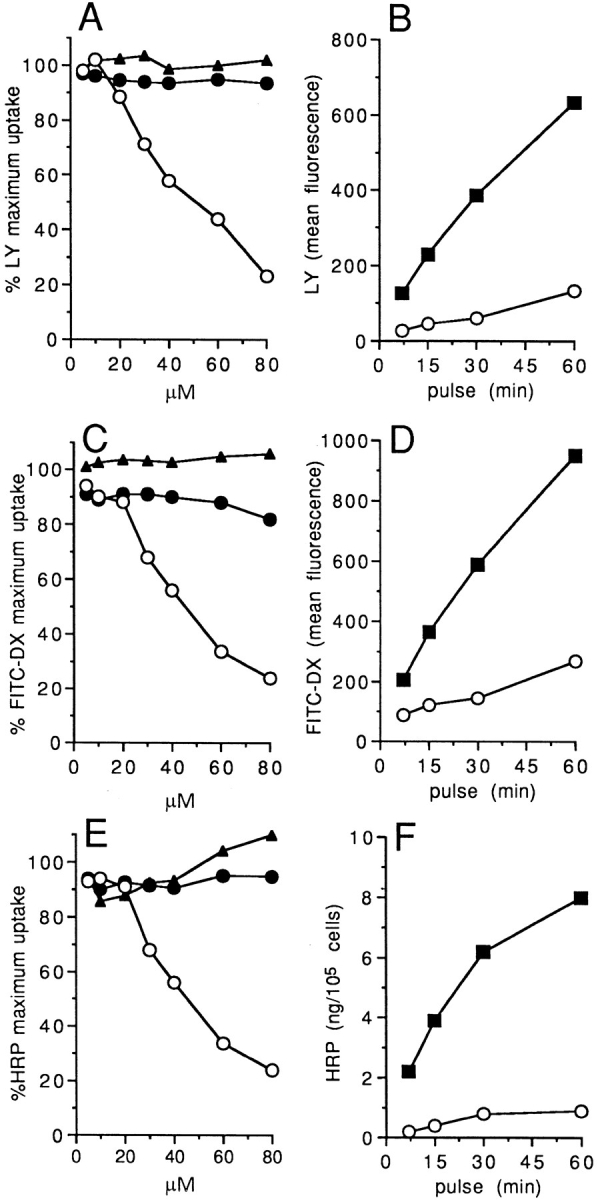
C2-ceramide inhibits both fluid phase and mannose receptor mediated endocytosis by DCs. (A, C, E) 2 × 105 DCs were incubated with different concentrations of C2-ceramide (open circles), C2-dihydroceramide (closed circles), or diacylglycerol (closed triangles) for 10 min on ice, then transferred at 37°C and LY (1 mg/ml) (A), FITC–DX (1 mg/ml) (C), or HRP (0.1 μg/ml) (E) were added for 30 min. Results are expressed as percent of maximum uptake. The background (cells pulsed at 0°C) was less than 1% of the uptake at 37°C in all the experiments. (B, D, F) 2 × 105 DCs were pretreated with 80 μM C2-ceramide (open circles) or medium (closed squares) for 10 min on ice, then transferred at 37°C and LY (B), FITC–DX (D) or HRP (F) accumulation was measured at different times. Results are expressed as mean fluorescence intensity (B, D) or as amount of cell-associated HRP (F). Comparable results were obtained using five different DC preparations. The vehicle did not affect endocytosis (data not shown).
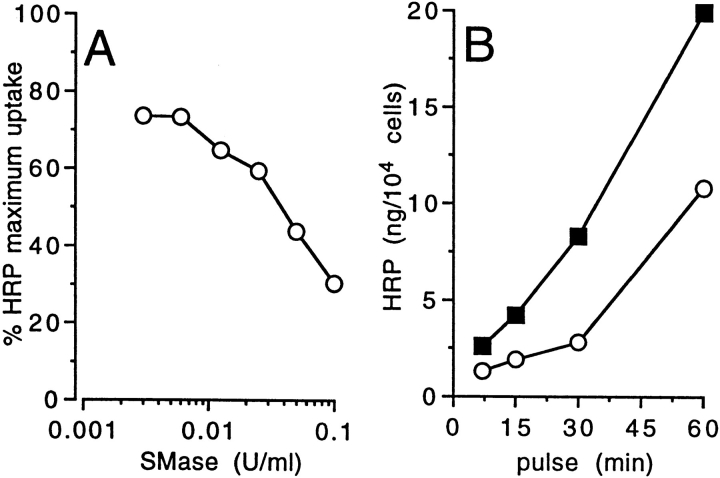
We then tested whether the endogenous production of ceramide would result in a similar inhibition of the endocytic ability of DCs. Exposure of cultured DCs to exogenous sphingomyelinase, which results in intracellular ceramide accumulation (data not shown), also induced a dose-dependent inhibition of HRP uptake (Fig. 3 A) and substantially retarded its time-dependent accumulation (Fig. 3 B). Taken together, these results indicated that ceramide could specifically mediate inhibition of macromolecules uptake by DCs.
Figure 3.
Endogenously produced ceramide inhibits endocytosis by DCs. 2 × 105 DCs were incubated with Streptomyces sp. SMase (open circles) at different concentrations (A) or at 0.1 U/ml (B) for 1 h at 37°C, then HRP was added at the final concentration of 1 mg/ml for 30 min (A) or for different times (B). The results are expressed as percentage of maximum HRP uptake (A) or as the amount of cell associated HRP and compared with untreated cells (closed squares) (B).
Ceramides Down-modulate Soluble Antigen Presentation by DCs.
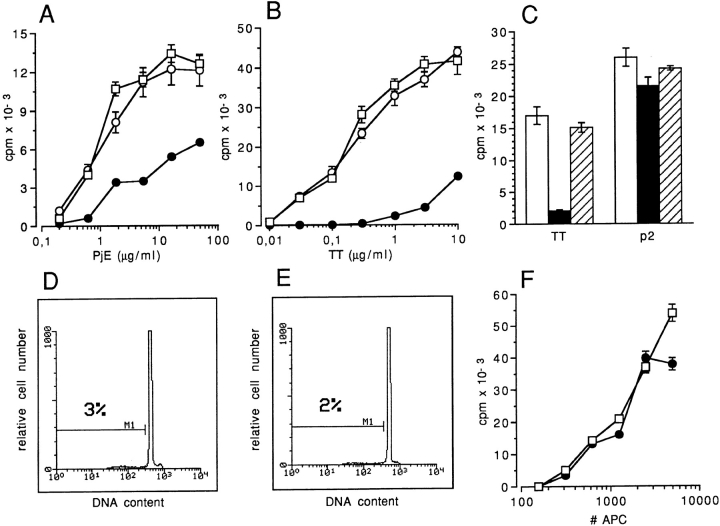
Cultured DCs are extremely efficient at presenting soluble antigen to specific T cells (2). In vitro maturation of DCs promoted by short term exposure to TNF-α results in a severalfold decrease of the antigen presentation capacity, associated with an increase in T cell stimulatory ability (4). Therefore, we tested whether ceramide could be sufficient for effectively modulating antigen presentation to T cells by using two different soluble antigens, TT and a soluble extract of P. judaica pollen (PjE). Cultured DCs were exposed to C2-ceramide and then pulsed with TT or PjE, before being used to challenge antigen-specific T cell clones (15, 19). Fig. 4 shows that C2-ceramide induced a ∼50fold reduction in the ability of DCs to present PjE, and ∼100-fold reduction in the ability to present TT to their respective T cell clones (Fig. 4, A and B). By contrast, DCs treated with C2-ceramide were at least as efficient as untreated DCs in presenting nonprocessed antigen, i.e., in presenting an immunogenic TT peptide to the same TTspecific T cell clone (Fig 4 C). Ceramide analogue C2-dihydroceramide was ineffective in blocking the response to soluble antigens (Figs. 4, A, B, and C).
Figure 4.
C2-ceramide inhibits presentation of native antigen but does not inhibit presentation of peptide and alloantigens. (A) Proliferative response of CD4+ T cell clone P6.2 to different concentrations of PjE. DCs were left untreated (open squares), pretreated with 80 μM C2-ceramide (closed circles), or with C2-dihydroceramide (open circles). (B) Proliferative response of CD4+ T cell clone KB24 to different concentrations of soluble TT. DCs were left untreated (open squares), pretreated with 80 μM C2-ceramide (closed circles), or with C2dihydroceramide (open circles). (C) Proliferative response of CD4+ T cell clone KS140 to 1 μg/ml of soluble TT and 10 ng/ml TT peptide P2 (residues 830–843) (19). DCs were left untreated (open bars), pretreated with 80 μM C2-ceramide (closed bars), or with C2-dihydroceramide (hatched bars). In all experiments, DCs were pulsed for 1 h with antigen, washed, irradiated, and mixed at 1:4 ratio with T cells. [3H]thymidine incorporation (1 μCi/ well, sp act 5 Ci/mMol) was measured on day 2 and results are expressed as mean of triplicate cultures ± one SD. (D and E) FACS® histograms of propidium iodide–stained nuclei from untreated DCs (D) or DCs treated with 80 μM C2-ceramide and then cultured for 48 h (E). (F) Adult PBMCs were cultured with different number of allogeneic DCs treated with 80 μM C2-ceramide (closed circles) or left untreated (open squares). [3H]thymidine incorporation were measured after 5 d and results are expressed as mean of triplicate cultures ± one SD.
C2-ceramide is known to induce apoptotic cell death when administered to hemopoietic tumor cell lines or to in vivoactivated primary lymphoid cells within 6–12 h (20–22). Therefore, we checked whether the observed changes in antigen-processing capacity were due to loss of cell viability. C2-ceramide–treated DCs cultured for as long as 48 h excluded Trypan blue, displayed normal morphology, and did not show any DNA fragmentation by propidium iodide staining and FACS® analysis (Fig. 4, D and E). Moreover, C2-ceramide treatment did not affect the ability of DCs to stimulate allogeneic T cells (Fig. 4 F).
Finally, we investigated whether the endogenous production of ceramide would affect the ability of DCs to present soluble antigen to T cells. Cultured DCs were treated with exogenous sphingomyelinase before being pulsed with TT, or with a TT peptide, and used to challenge a TT-specific T cell clone. Fig. 5 shows that endogenous ceramide production almost completely prevented presentation of soluble TT antigen, but had no inhibitory effect on TT peptide presentation by DCs, to the same TT-specific T cell clone.
Figure 5.

Endogenous ceramide inhibits presentation of soluble TT antigen but not of TT peptides by DCs. Proliferative response of CD4+ T cell clone KS140 to 1 μg/ml of soluble TT and 10 ng/ml TT peptide P2. DCs were pretreated for 2 h with 0.1 U/ml Streptomyces sp. SMase (closed bars), or left untreated (open bars), pulsed for 1 h with antigen or peptide, then mixed at 1:4 ratio with T cells. [3H]thymidine incorporation was measured on day 2. Results are expressed as mean of triplicate cultures ± one SD.
In this paper, we provide evidence that ceramides inhibit the antigen-capturing ability of cultured DCs, thereby suggesting a common molecular basis for CD40L, TNF-α, and IL-1β, or bacterial products such as LPS, to downmodulate antigen presentation by professional APC (5). In fact, we show that CD40L, as well as TNF-α or IL-1β, were all strong inducers of ceramide accumulation in DCs. Ceramides may specifically control antigen capturing and processing by DCs, as other cytokine-mediated differentiation events, i.e., upregulation of LFA1, B7-1, ICAM-1, and MHC molecules, were not consistently affected by ceramide exposure (data not shown). Accordingly, the enhanced immunostimulatory ability of mature DCs could not be promoted by exogenous ceramides, suggesting that additional intracellular mediators participate in the maturative process. Importantly, specific immunoefficiency of DCs can be inhibited without affecting cell viability or the ability to present nonprocessed antigen.
A possible explanation for these findings may reside in the capacity of endogenously released ceramides to interfere with vesicular trafficking. In fact, ceramides have been shown to directly inhibit endocytosis (23) and glycoprotein transport through the Golgi complex in CHO cells (24). Perturbing anterograde transport through the Golgi may prevent newly synthesized MHC class II molecules to reach endosomal compartments to be loaded with peptides derived from hydrolyzed antigen. Interestingly, the fungal antibiotic brefeldin A (BFA), a classic inhibitor of both endogenous and exogenous antigen processing and presentation (25, 26), which causes disassembly of the Golgi apparatus (27) and its fusion with the ER and with early endosomes (28, 29), also triggers sphingomyelin hydrolysis resulting in ceramide production (30). Therefore, it is likely that the capacity of BFA to modulate antigen presentation is mediated by endogenously released ceramide.
Ceramides are emerging as intramembrane messengers involved in a variety of cellular adaptive and differentiative responses. Here, we provide evidence for a novel important function of ceramides in highly specialized cells such as DCs, which is modulation of soluble antigen presentation. Moreover, our data suggest that the capacity of ceramides to perturb intracellular membrane trafficking may be exploited by extracellular ligands able to trigger sphingomyelin hydrolysis, or by bacterial products that mimic ceramide, such as LPS, in order to regulate professional APC function and antigen-specific immune responses.
Acknowledgments
We thank Drs. A. Lanzavecchia, P. Lane, W. Lesslauer, and H. Loetscher for reagents.
Footnotes
This work has been supported by Istituto Superiore di Sanitá (Progetto Tubercolosi), Associazione Nazionale Ricerca sul Cancro, CNR (Progetto Citochine), MURST, and European Community (Projects Human Capital and Mobility and Biomed 2). R. De Maria is an AIRC fellowship holder.
References
- 1.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Austyn JM. Antigen uptake and presentation by dendritic leukocytes. Semin Immunol. 1992;4:227–236. [PubMed] [Google Scholar]
- 3.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolesnick R, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 7.Heller RA, Krönke M. Tumor necrosis factor– mediated signaling pathways. J Cell Biol. 1994;126:5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannun YA. The sphingomyelin cycle and second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 9.Kolesnick R, Fuks Z. Ceramide: a signal for apoptosis or mitogenesis? . J Exp Med. 1995;181:1949–1952. doi: 10.1084/jem.181.6.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Testi, R. 1996. Sphingomyelin breakdown and cell fate. Trends Biochem. Sci. In press. [DOI] [PubMed]
- 11.Joseph CK, Wright SD, Bornmann WG, Randolph JT, Kumar ER, Bittman R, Liu J, Kolesnick RN. Bacterial lipopolysaccaride has structural similarity to ceramide and stimulates ceramide-activated protein kinase in myeloid cells. J Biol Chem. 1994;269:17606–17610. [PubMed] [Google Scholar]
- 12.Wright S, Kolesnick RN. Does endotoxin stimulate cells by mimicking ceramide? . Immunol Today. 1995;16:297–302. doi: 10.1016/0167-5699(95)80185-5. [DOI] [PubMed] [Google Scholar]
- 13.Cifone MG, De Maria R, Roncaioli P, Rippo MR, Azuma M, Lanier LL, Santoni A, Testi R. Apoptotic signaling through CD95 (Fas/APO-1) activates an acidic sphingomyelinase. J Exp Med. 1994;180:1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cifone MG, Roncaioli P, De Maria R, Camarda G, Santoni A, Ruberti G, Testi R. Multiple signaling originate at the Fas/Apo-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO (Eur Mol Biol Organ) J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallusto F, Corinti S, Pini C, Biocca MM, Bruno G, Di Felice G. Parietaria judaica-specific T cell clones from atopic patients: heterogeneity in restriction, Vβ usage and cytokine profile. J Allergy Clin Immunol. 1996;97:627–637. doi: 10.1016/s0091-6749(96)70308-1. [DOI] [PubMed] [Google Scholar]
- 16.Kim M-Y, Linardic C, Obeid L, Hannun Y. Identification of sphingomyelin turnover as an effector mechanism for the action of tumor necrosis factor α and γ-interferon. Specific role in cell differentiation. J Biol Chem. 1991;266:484–489. [PubMed] [Google Scholar]
- 17.Mathias S, Younes A, Kan C-C, Orlow I, Joseph C, Kolesnick RN. Activation of the sphingomyelin signaling pathway in intact EL4 cells and in a cell-free system by IL-1β. Science (Wash DC) 1993;259:519–522. doi: 10.1126/science.8424175. [DOI] [PubMed] [Google Scholar]
- 18.Dobrowsky RT, Werner MH, Castellino AM, Chao MV, Hannun YA. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science (Wash DC) 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 19.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature (Lond) 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 20.Obeid LM, Linardic CM, Karolak LA, Hannun YA. Programmed cell death induced by ceramide. Science (Wash DC) 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 21.Jarvis WD, Kolesnick RN, Fornari FA, Traylor RS, Gerwitz DA, Grant S. Induction of apoptotic damage and cell death by activation of the sphingomyelin pathway. Proc Natl Acad Sci USA. 1994;91:73–77. doi: 10.1073/pnas.91.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Maria R, Boirivant M, Cifone MG, Roncaioli P, Hahne M, Tschopp J, Pallone F, Santoni A, Testi R. Functional expression of Fas and Fas ligand on human gut lamina propria lymphocytes. A potential role for the acidic sphingomyelinase pathway in normal immunoregulation. J Clin Invest. 1996;97:316–322. doi: 10.1172/JCI118418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen C-S, Rosenwald AG, Pagano RE. Ceramide as a modulator of endocytosis. J Biol Chem. 1995;270:13291–13297. doi: 10.1074/jbc.270.22.13291. [DOI] [PubMed] [Google Scholar]
- 24.Rosenwald AG, Pagano RE. Intracellular transport of ceramide and its metabolites at the Golgi complex: insights from short-chain analogs. Adv Lipid Res. 1993;26:101–118. [PubMed] [Google Scholar]
- 25.Yewdell JW, Bennink JR. Brefeldin A specifically inhibits presentation of protein antigens to cytotoxic T lymphocytes. Science (Wash DC) 1989;244:1072–1075. doi: 10.1126/science.2471266. [DOI] [PubMed] [Google Scholar]
- 26.Adorini L, Ullrich SJ, Appella E, Fuchs S. Inhibition by brefeldin A of presentation of exogenous protein antigens to MHC class II–restricted T cells. Nature (Lond) 1990;346:63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- 27.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 28.Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 30.Linardic CM, Jayadev S, Hannun YA. Brefeldin A promotes hydrolysis of sphingomyelin. J Biol Chem. 1992;267:14909–14911. [PubMed] [Google Scholar]
- 31.Lane P, Brocker T, Hubele S, Padovan E, Lanzavecchia A, McConnell F. Soluble CD40 ligand can replace the normal T cell–derived CD40 ligand signal to B cells in T cell–dependent activation. J Exp Med. 1993;177:1209–1213. doi: 10.1084/jem.177.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor α (TNF-α)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]