Abstract
Transforming growth factor β1 (TGF-β1) regulates leukocytes and epithelial cells. To determine whether the pleiotropic effects of TGF-β1, a cytokine that is produced by both keratinocytes and Langerhans cells (LC), extend to epidermal leukocytes, we characterized LC (the epidermal contingent of the dendritic cell [DC] lineage) and dendritic epidermal T cells (DETC) in TGF-β1 null (TGF-β1 −/−) mice. I-A+ LC were not detected in epidermal cell suspensions or epidermal sheets prepared from TGF-β1 −/− mice, and epidermal cell suspensions were devoid of allostimulatory activity. In contrast, TCR-γδ+ DETC were normal in number and appearance in TGF-β1 −/− mice and, importantly, DETC represented the only leukocytes in the epidermis. Immunolocalization studies revealed CD11c+ DC in lymph nodes from TGF-β1 −/− mice, although gp40+ DC were absent. Treatment of TGF-β1 −/− mice with rapamycin abrogated the characteristic inflammatory wasting syndrome and prolonged survival indefinitely, but did not result in population of the epidermis with LC. Thus, the LC abnormality in TGF-β1 −/− mice is not a consequence of inflammation in skin or other organs, and LC development is not simply delayed in these animals. We conclude that endogenous TGF-β1 is essential for normal murine LC development or epidermal localization.
Transforming growth factor-β (TGF-β) is comprised of three isoforms (β1, β2, and β3) that are frequently coexpressed, that bind to the same receptor complex, and that initiate similar biochemical changes in target cells in vitro (1, 2). Thus, it is surprising that the phenotype of the homozygous null mutant of the murine gene encoding one of these proteins, TGF-β1, is catastrophic. Although 50% of TGF-β1 −/− and 25% of TGF-β1+/− embryos experience prenatal demise, at birth TGF-β1 −/− mice are normally formed and indistinguishable from +/+ and +/− littermates until postnatal d 10 (3–5). Thereafter, TGF-β1 −/− mice exhibit a progressive wasting syndrome that results from inflammation involving several organs that invariably eventuates in death by postnatal d 30 (4, 5). The inflammation in TGF-β1 −/− mice is lymphocyte and macrophage predominant, involves lungs, heart, liver, and other organs (4, 5), and is associated with production of various autoantibodies (6, 7). Abnormalities in cytokine production (3), MHC antigen expression (8), and nitric oxide metabolism (9) have also been identified in TGF-β1 −/− mice, but the relationship of these findings to the genesis of the phenotype is uncertain.
The epidermal microenvironment is TGF-β1 rich; Langerhans cells (LC) as well as keratinocytes produce this pleiotropic cytokine (10–12). Previous studies of TGF-β1 −/− mice demonstrated that TGF-β1 plays an important role in epidermal homeostasis. Keratinocytes of TGFβ1 −/− mice are hyperproliferative in vivo as well as in vitro, and are predisposed to undergo malignant transformation (10, 13). To determine whether abnormalities in epidermal homeostasis extend to resident leukocytes in skin, we characterized epidermal LC and dendritic epidermal T cells (DETC) in TGF-β1 −/− mice. Our results implicate TGF-β1 as a critical endogenous regulator of murine epidermal LC.
Materials and Methods
Mice.
TGF-β1 −/− and littermate control (+/+) mice were derived from matings of mice that were heterozygous for a null mutation of the TGF-β1 gene (generated [4] and supplied by A. Kulkarni and S. Karlsson [National Institute of Neurological Disorders and Stroke, Bethesda, MD]). Since their derivation, heterozygous males have been backcrossed to C57BL/6 females four times and the colony has been maintained by interbreeding. Thus, although mice in this colony express only H-2b MHC antigens, each animal has a mixture of 129Sv and C57BL/6 background genes and is unique. TGF-β1 −/− and littermate control mice used in these studies were reared in a pathogen-free facility and were used at 8–20 d of age. All animals were housed and used in experiments in accordance with institutional guidelines.
Preparation of Epidermal Cell Suspensions and Epidermal Sheets.
Epidermal cell suspensions were prepared from trunk skin by limited trypsinization (0.25% trypsin [USB, Cleveland, OH] in HBSS without calcium or magnesium at 4°C for 18 h) and trituration in HBSS, 0.05% DNase, 30% FCS. Epidermal sheets were prepared from ear skin by incubation in 0.5 M ammonium thiocyanate (37°C for 20 min), fixed in acetone (−20°C for 30 min), and rehydrated in PBS (14).
Flow Cytometry and Immunofluorescence Microscopy.
Hybridomas secreting mAb Y3-P (anti-I-Ab), MK-D6 (anti-I-Ad), and N418 (anti-CD11c) were obtained from American Type Culture Collection (Rockville, MD). G8.8 (anti-gp40) was produced and characterized as described (15). mAbs Y3-P, MK-D6, and N418 were purified from hybridoma supernatants by protein A affinity chromatography (Pierce Chemical Co., Rockford, IL); G8.8 was purified using protein G (Pierce Chemical). These mAb were modified with FITC (Sigma) or NHS–LC–biotin (Bio; Pierce Chemical) as described (16). The following mAbs were purchased from PharMingen (San Diego, CA): FITC–anti-TCR-αβ (H57-597), FITC–anti-TCR-γδ (GL3), FITC–Thy-1.2 (30-H12), Bio–anti-CD45 (30F11.1), and relevant isotype controls. Phycoerythrin– streptavidin (PE–SA) was purchased from TAGO, Inc. (Burlingame, CA).
For flow cytometry, cells were suspended in cold PBS containing 5% FCS and 0.01% NaN3 and preincubated with saturating concentrations of 2.4G2 (anti-FcRγII [17]) followed by FITC–mAb, Bio–mAb, and PE–SA. Surface antigen expression was analyzed using a FACScan® flow cytometer equipped with Research Software (Becton Dickinson, Mountain View, CA). Propidium iodide permeable cells were excluded by live gating. Epidermal sheets were stained for LC and DETC with FITC–anti-I-A or FITC–anti-TCR mAb diluted in PBS/FCS/NaN3, washed, and analyzed by epifluorescence microscopy.
Primary Allogeneic Reactions.
Epidermal cells derived from trunk skin of TGF-β1 −/− and control mice were cocultured in flatbottomed 96-well plates with 2 × 105 accessory cell–depleted T cells prepared from the skin-associated lymph nodes of female BALB/c mice (18) for 120 h at 37°C. [3H]TdR (1 μCi/well) was added for the final 12 h of the culture period. Cell-associated radioactivity was determined by direct β counting.
Immunohistochemistry.
Cells reactive with various lineageselective mAbs were localized in lymph nodes using a three-step immunohistochemical procedure (19). In brief, acetone-fixed frozen sections of tissues were washed with PBS and incubated with hybridoma supernatants. After washing, mAbs were detected using digoxigenin-modified anti-rat (or anti-hamster) IgG Ab (Pierce Chemical), peroxidase-conjugated sheep anti-digoxigenin Fab, and 3,3′-diaminobenzidine. Digoxigenin-3-O-methylcarbonyl-ε-aminocaproic acid, N-hydroxy-succinimidyl ester, and anti-digoxigenin Ab were purchased from Boehringer Mannheim (Indianapolis, IN) and used as suggested by the manufacturer.
Rapamycin Treatment.
Rapamycin (Wyeth-Ayerst; Princeton, NJ) was dissolved in 0.2% high viscosity carboxymethyl cellulose (Sigma)/0.25% polysorbate 80 in dH20 and administered to all progeny resulting from the mating of several breeding pairs of TGF-β1+/− mice by i.p. injection (4 mg/kg) on postnatal day 10 and 3×/wk thereafter.
Results
Epidermal Leukocytes in TGF-β1 −/− Mice.
To begin to characterize epidermal leukocytes in TGF-β1 −/− mice, cells were prepared from the trunk skin of −/− and +/+ littermates and examined for the simultaneous expression of CD45 and I-A antigens, and CD45 and TCR-γδ (or Thy1.2) via multicolor flow cytometry. Although TGF-β1 +/+ mice contained a normal contingent of LC (CD45+ I-Ab+ cells), LC could not be identified in TGF-β1 −/− epidermis (see Fig. 1 for results with 17-d-old mice). However, the frequency of DETC (CD45+ TCR-γδ+ cells) among TGF-β1 −/− epidermal cells was normal. In addition, essentially all of the leukocytes (CD45+ cells) present in TGF-β1 −/− epidermis were DETC. This latter result excludes the existence of a significant population of immature LC (CD45+ I-Ab− TCR-γδ− cells) in TGF-β1 −/− epidermis. The CD45− I-A+ cells detected in TGF-β1 −/− epidermis (see Fig. 1 a) represent keratinocytes that inappropriately express class II MHC antigens; this finding is consistent with the previous report of disordered MHC Ag expression in TGF-β1 −/− mice (8). The LC deficiency in TGF-β1 −/− mice was confirmed in six additional experiments with mice ranging in age from 7–18 d (Table 1). Younger animals were not studied because normal numbers of epidermal LC would not be expected before postnatal day 7. Older animals were not studied because the health status of TGF-β1 −/− mice deteriorates rapidly after 18 d.
Figure 1.
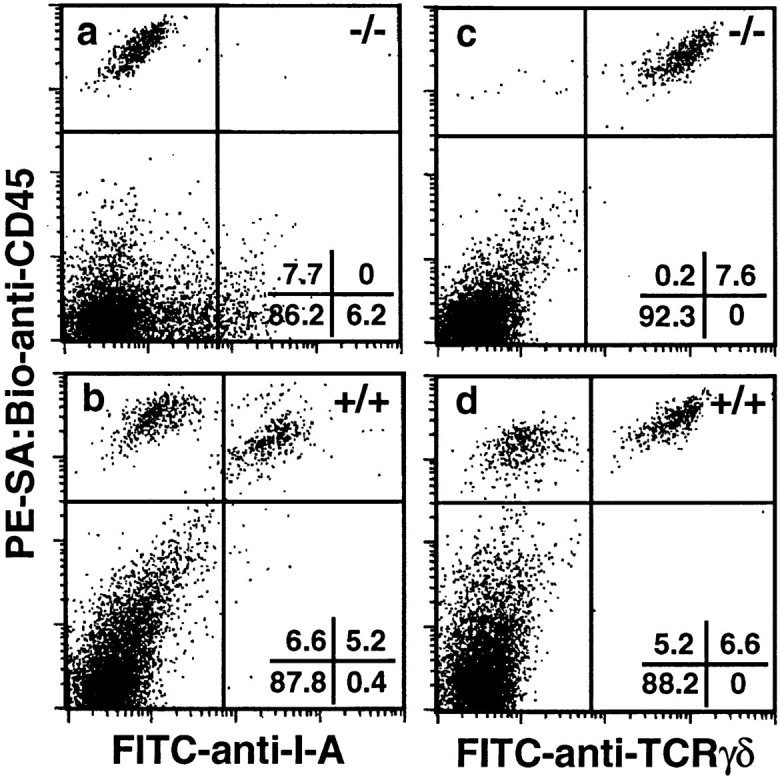
TGF-β1 −/− mice lack epidermal LC. Epidermal cell suspensions were prepared from the trunk skin of 17-d TGF-β1 −/− and littermate control mice, stained for CD45 and I-A antigens (or CD45 and TCR-γδ), and analyzed using multicolor flow cytometry. Nonviable cells were excluded during data acquisition. Markers were adjusted such that cells stained with isotype control reagents were contained within the left lower quadrant of each panel.
Table 1.
Leukocytes in Epidermal Cell Suspensions from TGF-β1 Null and Control Mice
| Experiment | Age | Genotype | LC (%)* | DETC (%)* | Leukocytes (%)* | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8d | −/− | 0.2 | ND | 2.5 | |||||
| +/+ | 5.5 | ND | 6.5 | |||||||
| 2 | 10d | −/− | 0.2 | 2.7 (3.2) | 3.6 | |||||
| +/+ | 5.4 | 3.8 (4.2) | 8.5 | |||||||
| 3 | 18d | −/− | 0 | 4.9 | 4.8 | |||||
| +/− | 3.8 | 3.5 | 6.3 | |||||||
| +/+ | 4.5 | 4.4 | 10.5 | |||||||
| 4 | 17d | −/− | 0.1 | ND | 6.7 | |||||
| +/− | 6.1 | ND | 13.0 | |||||||
| +/+ | 6.5 | ND | 11.9 | |||||||
| 5 | 14d | −/− | 0.1 | ND | 6.7 | |||||
| +/− | 5.4 | ND | 10.6 | |||||||
| +/+ | 8.9 | ND | 13.7 | |||||||
| 6 | 17d | −/− | 0 | 7.6 | 7.6 | |||||
| +/+ | 5.2 | 6.6 | 12.4 | |||||||
| 7 | 14d | −/− | 0 | ND | 6.9 | |||||
| +/+ | 3.0 | ND | 6.5 | |||||||
| Summary** | ||||||||||
| −/− | 0.1 ± 0.1 (n = 7) | 5.1 ± 2.0 (n = 3) | 5.5 ± 1.8 (n = 7) | |||||||
| +/− | 5.1 ± 1.0 (n = 3) | 3.5 (n = 1) | 10.0 ± 2.8 (n = 3) | |||||||
| +/+ | 5.6 ± 1.7 (n = 7) | 4.9 ± 1.2 (n = 3) | 10.0 ± 2.7 (n = 7) |
Epidermal cell suspensions were prepared from TGF-β1 −/− mice and littermate controls and stained for CD45 and I-A antigens (LC), CD45 and TCRγδ (DETC), and CD45 and isotype control (Leukocytes), and analyzed using multicolor flow cytometry. In some experiments, DETC were also identified as CD45+Thy-1.2+ cells (% in parentheses). Nonviable cells were excluded by live gating. Data are presented as percentages of events accumulated and represent individual determinations (*) or the mean ± SEM (**) of individual determinations.
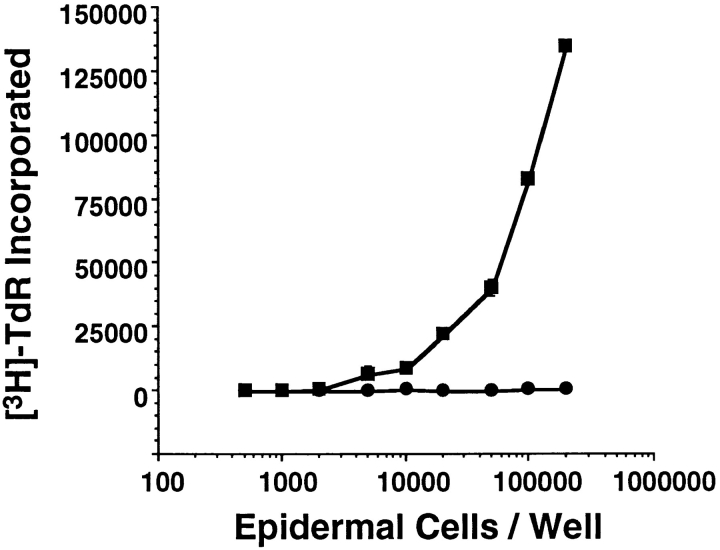
LC were also quantitated in TGF-β1 −/− and wildtype epidermis in situ. Immunofluorescence microscopy of epidermal sheets prepared from the ear skin of TGF-β1 −/− mice confirmed that LC were absent; DETC were normal in number and morphology (data not shown). In addition, epidermal cell suspensions from TGF-β1 −/− mice failed to initiate a proliferative response in naive BALB/c T cells (Fig. 2). Thus, LC (or LC precursors) could not be detected in the epidermis of TGF-β1 −/− mice, or in epidermal cell suspensions prepared from these animals, using assays of LC surface phenotype or function.
Figure 2.
TGF-β1 −/− epidermis is deficient in allostimulatory activity. Epidermal cells from the trunk skin of TGF-β1 −/− and +/+ mice were incubated with BALB/c lymph node T cells (2 × 105/well) in flatbottomed microtiter plates for 120 h. [3H]TdR was added (1 μCi/well) for the last 12 h of the incubation. Cell-associated radioactivity was measured by direct β counting. Closed circles, epidermal cells from TGF-β1 −/− mice; closed squares, epidermal cells from +/+ controls.
Lymphoid Dendritic Cells in TGF-β1 −/− Mice.
LC represent the epidermal contingent of the dendritic cell (DC) lineage (20). To determine whether lymphoid DC were present in TGF-β1 −/− mice, we examined skin-draining lymph nodes for several leukocyte subpopulations. Axillary lymph nodes were sectioned and stained with several lineage-selective mAbs (see Fig. 3). CD11c+ (N418-reactive) DC were readily identified in T cell–dependent regions of lymph nodes from TGF-β1 −/− as well as control mice. In contrast, cells reactive with anti-gp40 (G8.8), another DC-selective mAb (21), were absent from TGF-β1-deficient lymph nodes. F4/80+ macrophages were similarly represented and distributed in both TGF-β1 −/− and TGF-β1 +/+ tissue. These results suggest that although lymphoid DC are present in TGF-β1 null mice, all subpopulations may not be normally represented. Alternatively, TGF-β1 may be required for expression of certain lymphoid DC differentiation antigens.
Figure 3.
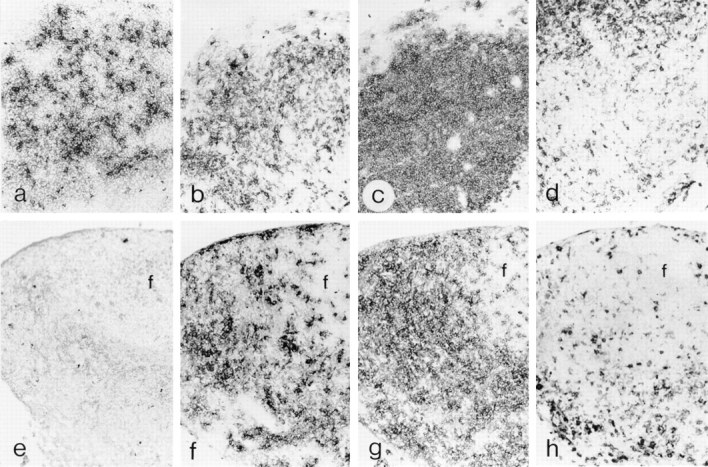
Lymphoid dendritic cells are present in TGF-β1 −/− mice. Frozen sections of axillary lymph nodes from a 14-d TGF-β1 −/− mouse and a +/+ littermate control were stained with several lineage-selective markers using a three-step immunohistochemical technique. (a–d) sections from littermate control. (e–h) sections from TGF-β1 −/− mouse. (a and e) anti-gp40 (G8.8). (b and f ) anti-CD11c (N418). (c and g) anti-CD3ε (145-2C11). (d and h) anti-macrophage (F4/80).
Dissociation of the Langerhans Cell Deficiency from Other Aspects of the TGF-β1 −/− Phenotype.
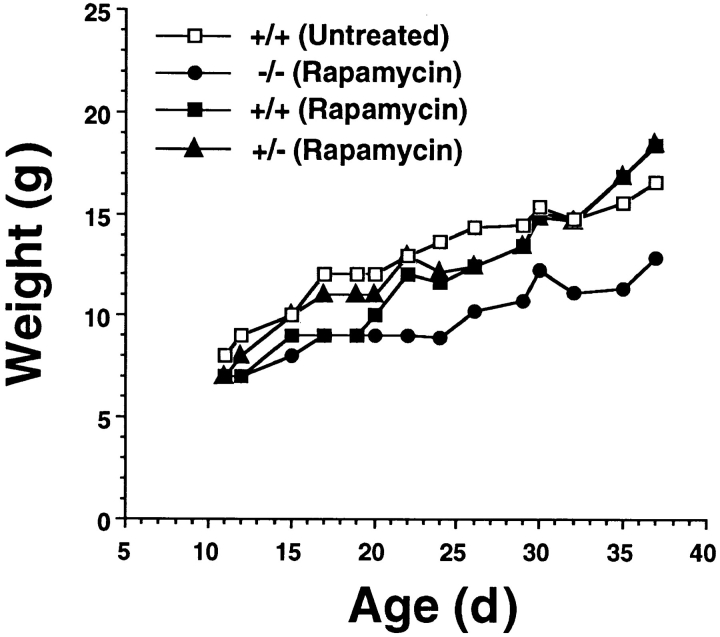
The TGF-β1 −/− phenotype is characterized by marked inflammation of several major organs, wasting, and death at an early age (3, 4). The skin is not prominently involved in this inflammatory syndrome. Histopathologic examination of 17 d TGF-β1 −/− skin revealed only a sparse interstitial inflammatory infiltrate that was difficult to distinguish from the minimal infiltrate present in controls (data not shown). Nonetheless, to exclude the possibility that aberrant cytokine secretion associated with inflammation (in skin or elsewhere) influenced the ability of LC (or LC precursors) to develop or localize in TGF-β1 −/− epidermis, we assessed the LC content of the skin of TGF-β1 −/− mice that were treated with the immunosuppressive rapamycin (22). Rapamycin (4 mg/kg) was administered i.p. to littermates born to TGF-β1 heterozygotes beginning at 10 d of age and 3×/wk thereafter. This regimen obviated the wasting that is invariably present in untreated TGF-β1 −/− mice older than 14 d (Fig. 4), and almost completely inhibited the inflammation in lungs, heart, and liver that is characteristic of TGF-β1 −/− mice (data not shown).
Figure 4.
Rapamycin abrogates the fatal wasting syndrome that results from TGF-β1 deficiency. Progeny of TGF-β1 +/− × +/− matings were treated with rapamycin (4 mg/kg i.p.) on postnatal day 10 and 3×/ wk thereafter. Serial weights were determined before each treatment and representative data are depicted.
Rapamycin-treated TGF-β1 −/− mice not only survived indefinitely, but gained weight for the duration of the experiments (39 and 42 d). Although the most dramatic components of the TGF-β1 −/− phenotype were eliminated by rapamycin treatment, epidermal sheets from ears of rapamycin-treated TGF-β1 −/− mice were devoid of LC (data not shown), as were epidermal cell suspensions prepared from trunk skin of these mice (Table 2). Rapamycin treatment had no effect on LC in TGF-β1 +/+ or +/− mice and, as in the case of untreated mice, DETC were the only leukocytes present in appreciable numbers in TGF-β1 −/− epidermis. Thus, the LC deficiency in TGF-β1 −/− mice does not result from the inflammatory syndrome that causes the demise of these animals, and cannot be attributed simply to delayed development.
Table 2.
Epidermal Leukocytes in Rapamycin-treated TGF-β1 Null and Control Mice
| Group | Genotype (Age) | Treatment | LC (%) | DETC (%) | Leukocytes (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | −/− (39 d) | Rapamycin | 0 | 1.2 | 0.9 | |||||
| −/− (42 d) | Rapamycin | 0 | 0.8 | 0.7 | ||||||
| −/− (42 d) | Rapamycin | 0 | 0.6 | 0.4 | ||||||
| 2 | +/+ (39 d) | Rapamycin | 1.5 | 1.4 | 2.8 | |||||
| +/+ (42 d) | Rapamycin | 2.5 | 2.1 | 4.4 | ||||||
| 3 | +/+ (42 d) | Rapamycin | 0.8 | 0.9 | 1.7 | |||||
| +/+ (39 d) | None | 1.6 | 1.7 | 3.1 | ||||||
| +/− (42 d) | None | 2.6 | 3.4 | 4.8 |
Mice were treated with rapamycin (4 mg/kg i.p.) on postnatal day 10 and 3×/wk thereafter. Data represent percentages of viable cells expressing the indicated antigens in suspensions of cells derived from trunk epidermis of individual mice. Subpopulations of epidermal leukocytes were identified as described in the legend for Table 1.
Discussion
TGF-β1 −/− mice represent the first animal model in which an absolute deficiency of LC (or other nonlymphoid DC) has been identified. Decreased numbers of LC were previously documented in the op/op mouse (23), a null mutant of the M-CSF (CSF-1) gene, but the LC deficiency in the TGF-β1 −/− mouse is much more profound. Although the LC deficiency is the most obvious alteration in the DC lineage in TGF-β1 −/− mice, lymphoid DC may also be abnormal. DC expressing gp40 (15, 21) were absent from skin-draining lymph nodes of TGF-β1 −/− mice, whereas CD11c+ DC were readily detected. Perhaps gp40-bearing DC in lymph nodes represent a distinct subpopulation of DC that in this site are derived exclusively from epidermal LC. Alternatively, TGF-β1 may modulate cell surface expression of certain DC antigens. The deficiency of epidermal LC in TGF-β1 −/− mice reported here also constitutes one of the most striking abnormalities in hematopoetic cells that has been documented in these animals. Previous studies focused primarily on abnormalities in the representation of mononuclear cell subpopulations in lymphoid tissues, and the immune dysregulation that underlies the inflammatory wasting syndrome that is associated with this genotype (6, 7, 24). The relationship of the LC/DC abnormalities in TGF-β1 −/− mice to other features of the phenotype is unknown, although abnormalities in DC may contribute to the decreased T cell mitogenic responses that are seen in TGF-β1 −/− mononuclear cells (24).
The nature of the requirement for TGF-β1 as a regulator of LC has not been determined. TGF-β1 may be required for proliferation or differentiation of LC precursors. This suggestion is compatible with the recent report that TGF-β1 promotes the in vitro expansion of DC in serumfree cultures of human CD34+ hematopoetic progenitors (25). Alternatively, TGF-β1 may influence the ability of LC precursors (or LC) to localize in epidermis or facilitate LC survival. Candidate mechanisms for TGF-β1 action include modulation of cytokine production (3), induction of growth factors or growth factor receptors (26), and regulation of adhesion molecule expression or function (27). Regulation could occur at transcriptional or posttranscriptional levels. Interestingly, there are a number of similarities between TGF-β1 null mice and mice that are deficient in the transcription factor relB (28, 29). Inflammatory syndromes and DC abnormalites are present in both models. RelB −/− mice have LC, but lack lymphoid DC. In contrast, TGF-β1 −/− mice have lymphoid DC, but lack LC. These results, in light of the recent determination that TGF-β1 influences the activity of NF-κB/Rel proteins in murine B cells by regulating levels of the inhibitor IκBα (30), suggest that TGF-β1 may regulate DC via a similar pathway. Resolution of these questions should result in elucidation of mechanisms by which murine LC/DC are regulated in vivo.
Acknowledgments
The authors thank Vivian McFarland for technical assistance, Harry Schaefer for help preparing the figures, Dr. Suren Sehgal (Wyeth-Ayerst) for supplying rapamycin, and Drs. George L. Barnes, Thilo Jakob, Stephen I. Katz, and Kim B. Yancey for reviewing the manuscript.
This work was supported in part by grants from the National Institutes of Health (AI 24137 and AG 04350 to A.G. Farr).
References
- 1.Sporn MB, Roberts AB. Transforming growth factor-β: recent progress and new challenges. J Cell Biol. 1992;119:1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kingsley DM. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes & Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 3.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature (Lond) 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni AB, Huh C-G, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickson MC, Martin JS, Cousins FM, Kulkarni AB, Karlsson S, Akhurst RJ. Defective haematopoiesis and vasculogenesis in transforming growth factor-β1 knock out mice. Development. 1995;121:1845–1854. doi: 10.1242/dev.121.6.1845. [DOI] [PubMed] [Google Scholar]
- 6.Dang H, Geiser AG, Letterio JJ, Nakabayshi T, Kong L, Fernandes G, Talal N. SLE-like autoantibodies and Sjogrens syndrome–like lymphoproliferation in TGFβ knockout mice. J Immunol. 1995;155:3205–3212. [PubMed] [Google Scholar]
- 7.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffmann R, Payne S, Longnecker G, Moses E, Karlsson S. Autoimmune manifestations in the transforming growth factor-β1 knockout mouse. Blood. 1996;87:1439–1445. [PubMed] [Google Scholar]
- 8.Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor β1 (TGF-β1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-β1 null mouse. Proc Natl Acad Sci USA. 1993;90:9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vodovotz Y, Geiser AG, Chesler L, Letterio JJ, Campbell A, Lucia MS, Sporn MB, Roberts AB. Spontaneously increased production of nitric oxide and aberrant expression of the inducible nitric oxide synthase in vivo in the transforming growth factor β1 null mouse. J Exp Med. 1996;183:2337–2342. doi: 10.1084/jem.183.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glick AB, Kulkarni AB, Tennebaum T, Hennings H, Flanders KC, O'Reilly M, Sporn MB, Karlsson S, Yuspa SH. Loss of expression of transforming growth factor β in skin and skin tumors is associted with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci USA. 1993;90:6076–6080. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gruschwitz MS, Hornstein OP. Expression of transforming growth factor type beta on human epidermal dendritic cells. J Invest Dermatol. 1992;99:114–116. doi: 10.1111/1523-1747.ep12611890. [DOI] [PubMed] [Google Scholar]
- 12.Granucci F, Girolomoni G, Lutz MB, Foti M, Marconi G, Gnocchi P, Nolli L, Ricciardi-Castagnoli P. Modulation of cytokine expression in mouse dendritic cell clones. Eur J Immunol. 1994;24:2522–2526. doi: 10.1002/eji.1830241039. [DOI] [PubMed] [Google Scholar]
- 13.Glick AB, Lee MM, Darwiche N, Kulkarni AB, Karlsson S, Yuspa SH. Targeted deletion of the TGF-β1 gene causes rapid progression to squamous cell carcinoma. Genes & Dev. 1994;8:2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- 14.Caughman SW, Sharrow SO, Shimada S, Stephany D, Mizouchi T, Rosenberg A, Katz SI, Singer A. Ia+ murine epidermal Langerhans cells are deficient in surface expression of the class I major histocompatibility complex. Proc Natl Acad Sci USA. 1986;83:7438–7442. doi: 10.1073/pnas.83.19.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farr A, Nelson A, Truex J, Hosier S. Epithelial heterogeneity in the murine thymus: A cell surface glycoprotein expressed by subcapsular and medullary epithelium. J Histochem Cytochem. 1991;39:645–653. doi: 10.1177/39.5.2016514. [DOI] [PubMed] [Google Scholar]
- 16.Holmes K, Fowlkes BJ. Preparation of cells and reagents for flow cytometry. Curr Prot Immunol. 1991;1:5.3.2–5.3.4. doi: 10.1002/0471142735.im0503s44. [DOI] [PubMed] [Google Scholar]
- 17.Unkeless J. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang A, Udey MC. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation: ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J Immunol. 1991;146:3347–3355. [PubMed] [Google Scholar]
- 19.Lee M-G, Sharrow SO, Farr AG, Singer A, Udey MC. Expression of the homophilic adhesion molecule E-cadherin by immature thymocytes and thymic epithelial cells. J Immunol. 1994;152:5653–5659. [PubMed] [Google Scholar]
- 20.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 21.Borkowski TA, Nelson AJ, Farr AG, Udey MC. Expression of gp40, the murine homolog of human epithelial adhesion molecule (Ep-CAM), by murine dendritic cells. Eur J Immunol. 1996;26:110–114. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- 22.Kunz J, Hall MN. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem Sci. 1993;18:334–338. doi: 10.1016/0968-0004(93)90069-y. [DOI] [PubMed] [Google Scholar]
- 23.Witmer-Pack MD, Hughes DA, Schuler G, Lawson L, McWilliam A, Inaba K, Steinman RM, Gordon S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci. 1993;104:1021–1029. doi: 10.1242/jcs.104.4.1021. [DOI] [PubMed] [Google Scholar]
- 24.Christ M, McCartney-Francis NL, Kulkarni AB, Ward JM, Mizel DE, Mackall CL, Gress RE, Hines KL, Tian H, Karlsson S, Wahl SM. Immune dysregulation in TGF-β1-deficient mice. J Immunol. 1994;153:1936–1946. [PubMed] [Google Scholar]
- 25.Strobl H, Riedl E, Schneinecker C, Bello-Fernandez C, Pickl WF, Rappersberger K, Majdic O, Knapp W. TGFβ1 promotes the development of dendritic cells from CD34+progenitors. J Immunol. 1996;157:1499–1507. [PubMed] [Google Scholar]
- 26.Keller JR, Jacobsen SEW, Sill KT, Ellingsworth LR, Ruscetti FW. Stimulation of granulopoiesis by transforming growth factor β: synergy with granulocyte/ macrophage–colony-stimulating factor. Proc Natl Acad Sci USA. 1991;88:7190–7194. doi: 10.1073/pnas.88.16.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LWMM. Control of lymphocyte recirculation in man II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissueselective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 28.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck R-P, Lira S A, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/ Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 29.Burkly L, Hession C, Ogata L, Relly C, Marconl LA, Olson D, Tizard R, Cate R, Lo D. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature (Lond) 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 30.Arsura M, Wu M, Sonenshein GE. TGF-β1 inhibits NF-κB/Rel activity inducing apoptosis of B cell: transcriptional activation of IκBα. Immunity. 1996;5:31–40. doi: 10.1016/s1074-7613(00)80307-6. [DOI] [PubMed] [Google Scholar]