SUMMARY
The common belief that endonucleolytic cleavage is the initial, rate-determining step of mRNA decay in Escherichia coli fails to explain the influence of 5’ termini on the half-lives of primary transcripts. We have re-examined the initial events of RNA degradation in that organism by devising an assay to probe the 5’ phosphorylation state of RNA and by employing a self-cleaving hammerhead ribozyme to investigate the degradative consequences of an unphosphorylated 5’ end. These studies have identified a previously unrecognized prior step in decay that triggers subsequent internal cleavage by the endonuclease RNase E and thereby governs RNA longevity: the rate-determining conversion of a triphosphorylated to a monophosphorylated 5’ terminus. Our findings redefine the role of RNase E in RNA degradation and explain how unpaired 5’-terminal nucleotides can facilitate access to internal cleavage sites within primary transcripts. Moreover, these results reveal a striking parallel between the mechanisms of mRNA decay in prokaryotic and eukaryotic organisms.
Keywords: mRNA degradation, RNA stability, triphosphate, monophosphate, RNase E, RNA I, rpsT, RNA ligation, hammerhead, ribozyme
INTRODUCTION
Messenger RNA degradation is an important regulatory mechanism that directly affects gene expression by limiting the number of times that a message can be translated into protein. Its impact can be quite pronounced, with mRNA lifetimes differing by as much as two orders of magnitude within a single cell. In Escherichia coli, for example, where the average message decays with a half-life of about 5 minutes, individual mRNA half-lives can be as short as several seconds or as long as an hour (Gerdes et al., 1990; Baumeister et al., 1991; Bernstein et al., 2002).
E. coli appears to lack a 5’ exoribonuclease, and its mRNAs typically end in a protective stem-loop structure that impedes 3’ exoribonuclease attack. As a result, mRNA decay in that organism has been thought generally to begin with endonucleolytic cleavage at one or more internal sites (Apirion, 1973; Deana and Belasco, 2005; Deutscher, 2006). The endonuclease principally responsible for such cleavage events is RNase E, whose inactivation stabilizes most mRNAs in E. coli (Ono and Kuwano, 1979; Mudd et al., 1990; Babitzke and Kushner, 1991; Melefors and von Gabain, 1991; Taraseviciene et al., 1991). RNase E cuts RNA within single-stranded segments that are AU-rich (McDowall et al., 1994). The resulting RNA fragments are then swiftly degraded to mononucleotides by a combination of endonucleolytic and 3’ exonucleolytic digestion. The rapidity with which the 3’ cleavage products decay is due in part to the marked preference of RNase E for RNA substrates bearing a single phosphate group at the 5’ terminus (Mackie, 1998; Spickler et al., 2001), a property that results from the ability of monophosphorylated 5’ ends to bind this endonuclease and increase its catalytic activity (Jiang and Belasco, 2004; Callaghan et al., 2005).
Although endonuclease cleavage sites can be found at diverse locations throughout RNA transcripts, differences in bacterial mRNA longevity are commonly determined by features of the 5’ untranslated region (UTR). Thus, the lifetimes of labile messages can be markedly increased simply by replacing the 5’ UTR with that of a long-lived mRNA, such as the E. coli ompA transcript or the Bacillus subtilis aprE transcript (Belasco et al., 1986; Bechhofer and Dubnau, 1987; Sandler and Weisblum, 1988; Hansen et al., 1994; Hue et al., 1995; Hambraeus et al., 2000). The stabilizing influence of the ompA and aprE leader regions results from two key features: a 5’-terminal stem-loop structure and a high-affinity ribosome-binding site (Emory et al., 1992; Arnold et al., 1998; Hambraeus et al., 2000; Hambraeus et al., 2002). The protective effect of the ompA 5’ stem-loop derives not from its sequence or shape but rather from its location at the RNA 5’ end; indeed, the addition of unpaired nucleotides upstream of this RNA hairpin structure is as destabilizing as deletion of the stem-loop (Emory et al., 1992; Arnold et al., 1998). The presence of a stem-loop at the 5’ terminus is not only necessary for mRNA stabilization by the ompA or aprE 5’ UTR but also sufficient to prolong the life spans of various short-lived RNAs that normally lack such a structure (Bouvet and Belasco, 1992; Emory et al., 1992; Baker and Mackie, 2003; Sharp and Bechhofer, 2005). These findings have led to the conclusion that there is a major pathway for bacterial mRNA decay in which endonuclease access to internal cleavage sites is governed by the structure of the RNA 5’ terminus. According to this model, such access is facilitated by the presence of several unpaired nucleotides at the 5’ end (Bouvet and Belasco, 1992; Emory et al., 1992). Consistent with the 5’-access model is the observation that circularization of E. coli rpsT mRNA makes that message more resistant to endonuclease cleavage, presumably by depriving it of a 5’ end (Mackie, 2000).
Despite persuasive evidence for the existence of a 5’-end-dependent pathway for endonucleolytic degradation of primary transcripts in E. coli, its mechanism has never been explained. Although the propensity of RNase E to cut 5’ monophosphorylated RNAs much faster than their triphosphorylated counterparts is thought to account for the comparative instability of 3’-terminal cleavage products (Mackie, 1998; Spickler et al., 2001), the triphosphorylated 5’ ends of primary transcripts appear to be incapable of interacting productively with RNase E, as evidenced by the inability of substrates bearing anything other than a 5’ monophosphate (e. g., a 5’ triphosphate, cap, or hydroxyl) to enhance the catalytic activity of that enzyme (Mackie, 1998; Jiang et al., 2000; Tock et al., 2000). Consequently, 5’-terminal base pairing would seem to be irrelevant to RNase E cleavage of primary transcripts.
Here we describe an investigation of the mechanism by which 5’ termini govern access of RNase E to internal cleavage sites within primary transcripts. Our re-examination of the initial events in E. coli RNA degradation provides evidence contrary to the established view that this important cellular process begins with endonucleolytic cleavage. By revealing a previously unrecognized 5’-terminal event that triggers RNase E cleavage and controls the rate of RNA decay, these findings offer an explanation for the 5’-end-dependent degradation of primary transcripts.
RESULTS
The ability of several unpaired 5’-terminal nucleotides to facilitate RNase E cleavage of primary transcripts, irrespective of the sequence of those nucleotides or their distance from the cleavage site, is well illustrated by the degradation of RNA I, an untranslated RNA that controls the copy number of ColE1-related plasmids (Figure 1A). This 108-nt primary transcript has proven to be a very useful model system for studying RNase E-mediated RNA degradation in E. coli. Its decay is thought to begin with cleavage by RNase E five nucleotides from the 5’ end to generate an intermediate (RNA I-5) that is then rapidly degraded (Lin-Chao and Cohen, 1991). Previously, we have shown that the degradation of RNA I is 5’-end-dependent, as it can be stabilized in E. coli by adding a stem-loop upstream of the single-stranded segment at its 5’ end (RNA I+hp) (Bouvet and Belasco, 1992). The protective effect of such a stem-loop is abolished when it is preceded by a single-stranded RNA segment (RNA I.613) (Figure 1A), provided that this segment is not itself preceded by a 5’-terminal stem-loop (RNA I.613+hp) (Figure 1B). These changes in the rate of RNA I decay are not accompanied by any detectable alteration in the preferred site of RNase E cleavage.
Figure 1. 5’-end-dependent decay of RNA I and 5’ extension variants thereof.

(A) RNA I and its derivative RNA I.613. The principal site of RNase E cleavage is marked with an arrow. RNA I+hp and RNA I.613+hp are identical to RNA I and RNA I.613 except for the addition of a 5’-terminal stem-loop (GAUCGCCCACCGGCAGCUGCCGGUGGGCGAUC or AGAGCGGCUUCGGCCGCUCU, respectively)
(B) Decay of RNA I, RNA I+hp, RNA I.613, and RNA I.613+hp. At time intervals after inhibiting transcription, total RNA was isolated from E. coli containing plasmid pRNAI, pRNAI+hp, pRNAI.613, or pRNAI.613+hp and analyzed by Northern blotting. tRNACys served as an internal standard. The measured half-lives were 1.8 ± 0.1 min for RNA I, 3.5 ± 0.1 min for RNA I+hp, 0.9 ± 0.1 min for RNA I.613, and 2.8 ± 0.2 min for RNA I.613+hp.
The mechanism by which 5’-terminal base pairing can slow the degradation of primary RNA transcripts such as RNA I has never been determined. Although the preference of RNase E for monophosphorylated RNAs helps to explain the lability of the monophosphorylated 3’-terminal decay intermediates generated by endonucleolytic cleavage, that enzyme appears to be unable to interact productively with the triphosphorylated 5’ ends of primary transcripts, whether or not they are single-stranded. This conundrum raised the possibility that an important step in RNA degradation had been overlooked. Although it has long been assumed that RNase E acts directly to cleave primary transcripts in E. coli, we reasoned that the destabilizing effect of an unpaired 5’ end could be understood if cleavage of primary transcripts by this endonuclease is preceded by removal of the gamma and beta phosphates from the 5’ terminus. (We will refer to this hypothetical event as “pyrophosphate removal” without meaning to imply whether it occurs in a single step or by sequential loss of individual phosphate groups.) A prior step of this kind could explain the destabilizing effect of single-stranded 5’ ends, as monophosphorylated 5’ termini can activate RNase E only if they are unpaired (Mackie, 1998).
Assay for examining the 5’ phosphorylation state of RNA
To learn whether uncut transcripts that are monophosphorylated exist transiently in cells as decay intermediates, we devised an assay – Phosphorylation Assay By Ligation of Oligonucleotides (PABLO) – that makes it possible to estimate the percentage of a particular RNA that is monophosphorylated at the 5’ end. This method is based on the known ability of T4 DNA ligase to join a DNA oligonucleotide (oligo X) to a monophosphorylated RNA, but not one having another 5’ phosphorylation state, when their ends are juxtaposed via annealing to a bridging oligonucleotide (oligo Y) (Figure 2A) (Kleppe et al., 1970; Fareed et al., 1971; Nath and Hurwitz, 1974; Moore and Sharp, 1992; Moore and Query, 2000). The ligation yield for any RNA of interest can then be determined by using gel electrophoresis and blotting to resolve the ligation product from the unligated RNA.
Figure 2. PABLO analysis of RNA synthesized in vitro.

(A) Method for analyzing the 5’ phosphorylation state of RNA (PABLO). T4 DNA ligase selectively joins oligo X to 5’-monophosphorylated, but not 5’-triphosphorylated, RNA annealed to the bridging oligo Y, thereby producing an extended DNA-RNA chimera detectable by Northern blotting.
(B) Specificity of PABLO. Internally radiolabeled RNA I.613 bearing a 5’ monophosphate or a 5’ triphosphate was prepared by in vitro transcription in the presence or absence of excess AMP. These RNAs were then subjected to PABLO with RNA I.613-specific oligonucleotides, and the effect of changing or omitting various reaction components was determined. The two different oligonucleotides X that were used, X22 (lanes 1, 2, 3, 5, and 7) and X32 (lane 4), were 22 or 32 nucleotides long, respectively.
(C) Confirmation of the 5’ phosphorylation state of in vitro transcripts. The 5’ phosphorylation state of the RNA I.613 transcripts examined in (B) was corroborated by digestion with a monophosphate-dependent 5’ exonuclease.
(D) Effect of suboptimal end juxtaposition on PABLO ligation yields. The monophosphorylated RNA I.613 sample examined in (B) was subjected to PABLO with a set of bridging Y oligonucleotides that either perfectly juxtaposed the 3’ end of X32 with the 5’ end of the RNA (gap size = 0), left them separated by 1-3 nucleotides (gap size = +1, +2, or +3), or caused them to overlap by one nucleotide (gap size = -1).
The specificity of the assay was demonstrated by performing PABLO analysis on in vitro transcribed RNA I.613 bearing a monophosphate or triphosphate at the 5’ end. As expected, monophosphorylated RNA I.613 was ligated efficiently in the presence of properly matched DNA oligonucleotides (Figure 2B). The reaction yield of ~50% was typical of what we have seen for other fully monophosphorylated RNAs analyzed in this manner and was not due to incomplete monophosphorylation, as evidenced by the full susceptibility of the same RNA sample to degradation by a 5’-monophosphate-dependent exonuclease (Figure 2C). By contrast, triphosphorylated RNA I.613 was completely unreactive (Figure 2B, C). Ligation of monophosphorylated RNA I.613 was abolished when, upon annealing to oligo Y, its 5’ end and the 3’ end of oligo X were separated by as few as two nucleotides or overlapped by just a single nucleotide (Figure 2D). Interestingly, some ligation did occur when the ends of the annealed substrates were separated by one nucleotide, though much less than when those ends were precisely juxtaposed.
Phosphorylation state of RNA I and RNA I.613 in E. coli
To determine whether monophosphorylated full-length transcripts are present as potential degradation intermediates in E. coli, we examined the 5’ phosphorylation state of RNA I and RNA I.613, each of which has a unique unstructured 5’ end (Figure 1A). A substantial ligation yield (9-16%) was observed for both RNA I and RNA I.613 when these RNAs were extracted with total cellular RNA from a log-phase E. coli culture and subjected to PABLO analysis with transcript-specific oligonucleotides (Figure 3). As a control, we also examined the 5’ phosphorylation state of the endonucleolytic cleavage product RNA I-5, which should be entirely monophosphorylated. RNA I-5 ligated with a high efficiency (47%) similar to that for fully monophosphorylated RNA I.613 transcribed in vitro. By comparing the ligation yields for RNA I and RNA I.613 to those for fully monophosphorylated RNAs, we estimate that about 18-32% of RNA I and RNA I.613 is monophosphorylated at steady-state. The reproducibility of the PABLO ligation yields obtained with multiple RNA preparations, irrespective of the method of RNA extraction (Figure S1), indicated that the monophosphorylated RNAs have a natural origin.
Figure 3. 5’ phosphorylation state of RNA I and RNA I.613 in E. coli.
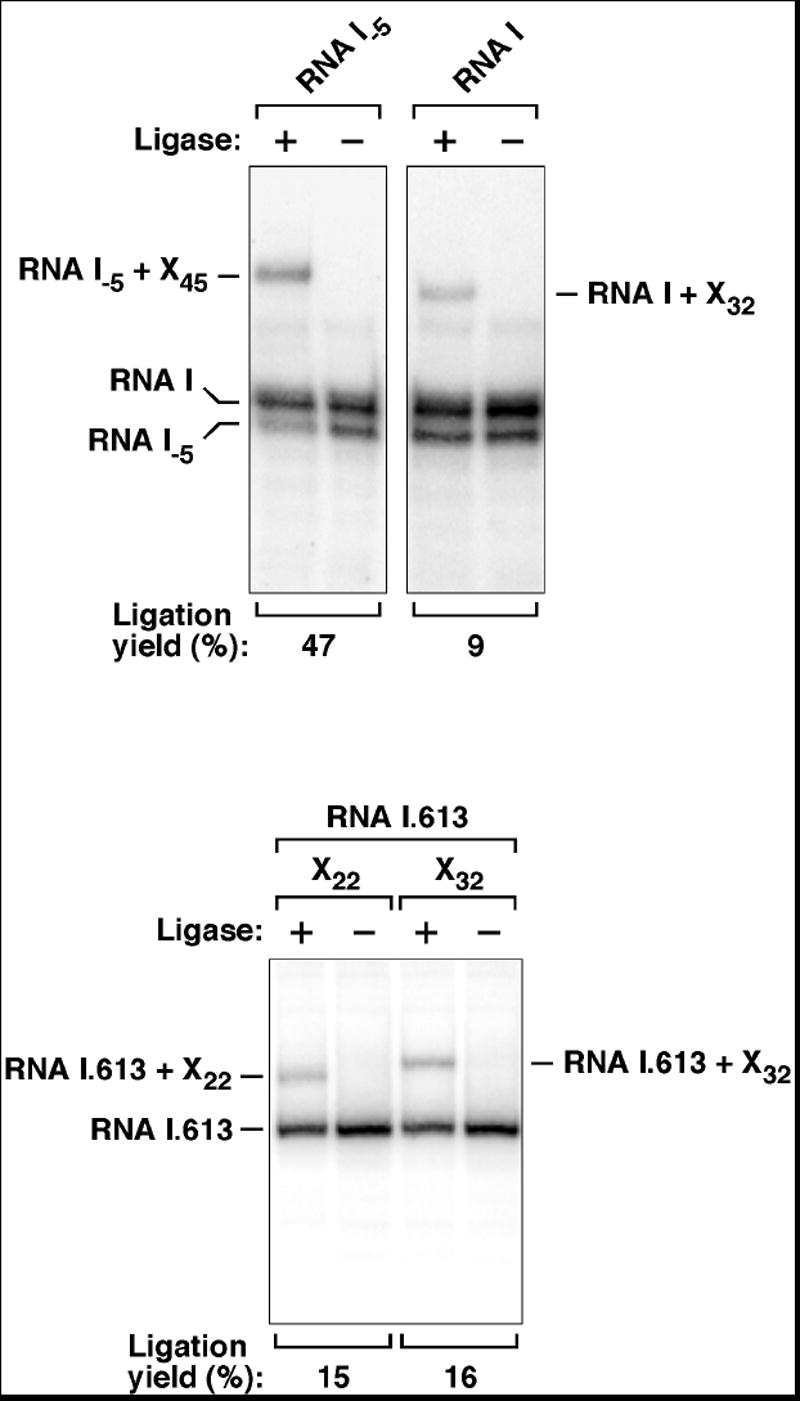
Total RNA extracted from E. coli containing plasmid pRNAI or pRNAI.613 was subjected to PABLO analysis of RNA I or RNA I.613. As a positive control, the 5’ phosphorylation state of the fully monophosphorylated degradation intermediate RNA I-5 was also examined. Oligos X of various lengths were used (XN, where N corresponds to the length in nucleotides). In multiple experiments, the ligation yields were 9 ± 1% and 14 ± 2% for RNA I and RNA I.613, respectively. The radiolabeled probe used to detect RNA I.613 did not detect its 3’ cleavage product (RNA I-5) due to the complementarity of the probe to the 5’-terminal segment of the transcript.
Preferential degradation via a monophosphorylated intermediate
The concentration of monophosphorylated RNA I and RNA I.613 in E. coli may be limited by the rapid degradation of these RNA species soon after they form, as RNAs bearing a 5’ monophosphate are the preferred substrates of RNase E. To address this point, we impaired RNase E cleavage of RNA I.613 in E. coli by mutating the primary cleavage site (RNA I.613-8C; see Figure S2). As expected, PABLO analysis revealed an increase in the relative concentration of the monophosphorylated form of the RNA (Figure 4A). Thermally inactivating a temperature-sensitive variant of RNase E had a similar effect (data not shown).
Figure 4. Origin and fate of monophosphorylated RNA I.613 in E. coli.

(A) Accumulation of monophosphorylated RNA I.613 when RNase E cleavage is impeded. PABLO was performed to compare the 5’ phosphorylation state of RNA I.613 and RNA I.613-8C, a variant in which the primary RNase E cleavage site of RNA I.613 had been mutated to impede cleavage (ACAGUAUUU → ACCCCCCCC; see Figure 1A). In multiple experiments in E. coli, the ligation yield consistently doubled when RNase E cleavage was inhibited.
(B) Phosphorylation state of the 38-nt 5’-terminal decay intermediate produced in E. coli by RNase E cleavage of RNA I.613 (left) or in vitro by RNase E cleavage of fully monophosphorylated, internally radiolabeled RNA I.613 (right). In multiple experiments in E. coli, the PABLO ligation yields were 14 ± 2% and 55 ± 7% for full-length RNA I.613 and its 5’ cleavage product, respectively.
(C) Invariant ratio of monophosphorylated to triphosphorylated RNA I.613 following transcription inhibition. (Left) PABLO analysis of RNA I.613 isolated from E. coli at time intervals after inhibiting transcription. (Right) Concentration of monophosphorylated RNA I.613 (ligation product) and triphosphorylated RNA I.613 (unligated) as a function of time after transcription inhibition.
If the predominant degradation pathway for an RNA in E. coli involves pyrophosphate removal from the primary transcript prior to RNase E cleavage, then the 5’-terminal fragments that result from such cleavage should mostly be monophosphorylated. To test this prediction, we examined the phosphorylation state of the 5’ fragment generated in E. coli by cleavage of RNA I.613 by RNase E. This 38-nt decay intermediate (Figure 1A) was detectable by Northern blotting as a band whose presence was abolished by RNase E inactivation or by mutation of the RNase E cleavage site and whose electrophoretic mobility was reduced when its internal stem-loop was extended (Figure S2). The PABLO ligation yield for this 5’ cleavage product was 43-53% (Figure 4B), significantly higher than that for its full-length precursor (15-16%) and similar to the ligation yield observed for both fully monophosphorylated RNA I.613 and its 5’ cleavage product synthesized in vitro (55-56%; Figure 4B). That this decay intermediate was almost completely monophosphorylated in vivo implies that the preferred degradation pathway for RNA I.613 in E. coli involves RNase E cleavage of full-length transcripts that bear only one phosphate at the 5’ end.
Conversion of triphosphorylated RNA to monophosphorylated RNA
In principle, the monophosphorylated full-length RNAs that we have observed could originate in any of three ways: as products of 5’ pyrophosphate removal, as primary transcription products, or as RNA fragments produced by endonucleolytic cleavage of longer precursors. The third possibility was ruled out by the identical 5’ sequence of the monophosphorylated and triphosphorylated forms and the complete absence of both when the proximal promoter was mutated (data not shown). The other two possibilities led to distinct predictions as to the effect of transcription inhibition on the relative abundance of monophosphorylated and triphosphorylated RNA. If the monophosphorylated full-length RNAs arise solely as primary transcription products resulting from initiation of RNA synthesis with a nucleoside monophosphate, then the preference of RNase E for monophosphorylated substrates should cause the ratio of monophosphorylated to triphosphorylated RNA to decline once further RNA synthesis is blocked. Conversely, if the monophosphorylated RNAs arise by pyrophosphate removal from triphosphorylated precursors, then the continuous resupply of the monophosphorylated intermediates should prevent that ratio from decreasing after transcription ceases.
To distinguish between these two possibilities, we performed PABLO analysis on RNA I.613 samples isolated at time intervals after inhibiting transcription with rifampicin. As RNA decay proceeded following the cessation of RNA synthesis, no significant change was observed in the ratio of triphosphorylated to monophosphorylated full-length transcripts (Figure 4C), consistent with a precursor-product relationship. The invariance of this ratio conformed precisely to the kinetics expected for a two-step reaction in which the first step (pyrophosphate removal) is rate-determining and in which the process begins with a steady-state mixture of the starting material and the labile intermediate (Pilling and Seakins, 1995). We conclude that monophosphorylated RNA I.613 is produced in E. coli by pyrophosphate removal from primary transcripts, thereby allowing the relative abundance of the monophosphorylated RNA to be sustained after transcription is inhibited. The conclusion that little if any monophosphorylated RNA I.613 is created via transcription initiation with a nucleoside monophosphate is consistent with the vast excess (37:1) of ATP over AMP in E. coli cells (Glembotski et al., 1981).
5’ pyrophosphate removal from messenger RNA
To ascertain whether translated messages are likewise subject to degradation by a mechanism involving initial pyrophosphate removal, we examined rpsT mRNA, which encodes ribosomal protein S20. The E. coli rpsT gene is transcribed from two promoters (P1 and P2) to generate a pair of transcripts (Figure 5A) that each begin with several unpaired nucleotides and are rapidly degraded by RNase E (Mackie and Parsons, 1983; Mackie, 1991; Mackie, 1992; Ow et al., 2003). Previous studies have shown that a P2-like rpsT transcript can be stabilized by the addition of a 5’-terminal stem-loop or by circularization to eliminate the 5’ end altogether (Mackie, 2000; Baker and Mackie, 2003).
Figure 5. Phosphorylation state of the rpsT P1 transcript and its HO-rpsT counterpart in E. coli.

(A) 5’ phosphorylation state of rpsT mRNA. (Left) The E. coli rpsT transcriptional unit. Jagged lines ending in arrowheads, primary transcripts. Gray rectangle, protein-coding region. (Right) PABLO analysis of the chromosomal rpsT P1 transcript in E. coli. In multiple experiments, the ligation yield was 25 ± 2% for the rpsT P1 transcript.
(B) Generation of HO-rpsT mRNA by self-cleavage of the hammerhead-containing rpsT.HH transcript. Only the 5’-terminal segment of the transcript is shown.
(C) 5’ phosphorylation state of HO-rpsT mRNA. (Left) The rpsT.HH transcriptional unit. (Right) PABLO analysis of HO-rpsT mRNA in E. coli. The plasmid-encoded rpsT P1 transcript and its self-cleaved HO-rpsT P1 counterpart were detected with a radiolabeled probe complementary to a sequence tag inserted into the 3’ UTR.
We performed PABLO to examine the role of pyrophosphate removal in the degradation of the E. coli rpsT P1 transcript. As expected for a degradation mechanism that proceeds via a monophosphorylated intermediate, a substantial fraction of wild-type P1 transcripts were found to be monophosphorylated at their unique 5’ end (approximately half, as estimated from the ligation yield of 23%) (Figure 5A).
Longevity of RNA bearing a 5’ hydroxyl
If conversion of a triphosphorylated to a monophosphorylated 5’ end controls the rate of rpsT mRNA decay in E. coli, then preventing the formation of such a monophosphorylated intermediate should impede RNase E cleavage and prolong the lifetime of the transcript. To render the rpsT message incapable of attaining a monophosphorylated state, we replaced its triphosphate with a 5’ hydroxyl group by taking advantage of the chemistry of hammerhead ribozymes, which undergo self-cleavage at a defined site to produce a 3’ fragment that begins with a 5’ hydroxyl (Long and Uhlenbeck, 1993). In E. coli, a plasmid-encoded RNA comprising a hammerhead ribozyme fused to the 5’ end of the rpsT P1 transcript (rpsT.HH) underwent self-cleavage to generate an RNA species (HO-rpsT) identical to the natural, triphosphorylated rpsT P1 transcript but for the presence of a 5’ hydroxyl (Figure 5B, C). No monophosphorylation of HO-rpsT mRNA was evident by PABLO analysis, consistent with the absence of polynucleotide kinase activity in E. coli. Autocatalytic cleavage of rpsT.HH was so rapid in E. coli that the hammerhead-containing precursor transcript was virtually undetectable (Figure 6A). HO-rpsT mRNA decayed with a half-life (5.3 ± 0.1 min) that was six times longer than that of the triphosphorylated rpsT P1 transcript (0.9 ± 0.1 min) (Figure 6A). The greatly increased lifetime of the HO-rpsT message compared to its triphosphorylated counterpart attests to the importance of pyrophosphate removal in determining the rate of RNase E-mediated RNA degradation in E. coli, as RNase E alone cannot distinguish between cognate substrates bearing a 5’ hydroxyl or 5’ triphosphate (Figure 6B) and therefore cannot account for the enhanced longevity of HO-rpsT mRNA.
Figure 6. Stabilization of rpsT mRNA in E. coli by a 5’ hydroxyl.

(A) Decay of HO-rpsT mRNA in E. coli. At time intervals after inhibiting transcription, total RNA was isolated from E. coli containing plasmid pRPST1 (top left) or pRPST.HH (bottom left) and analyzed by Northern blotting with a radiolabeled probe complementary to a 3’ UTR sequence tag. Degradation rates were calculated for rpsT P1 mRNA bearing a 5’-terminal hydroxyl (HO-rpsT; half-life = 5.3 ± 0.1 min) or a 5’-terminal triphosphate (TriP-rpsT; half-life = 0.9 ± 0.1 min) by plotting band intensity as a function of time (right). In each case, the triphosphorylated P2 transcript (internal standard) decayed with a half-life of 1.2 ± 0.3 min.
(B) Equivalent RNase E cleavage rates in vitro for rpsT RNAs bearing a 5’ triphosphate or a 5’ hydroxyl. Equal amounts of radiolabeled, in vitro synthesized rpsT RNA bearing a 5’ monophosphate, 5’ triphosphate, or 5’ hydroxyl were treated with purified N-RNase E under identical conditions, and samples quenched at time intervals were analyzed by gel electrophoresis and autoradiography.
DISCUSSION
The mechanism by which 5’-terminal structure influences the lifetimes of primary RNA transcripts in E. coli has long remained a mystery not accounted for by the established view that RNA decay begins with endonucleolytic cleavage at internal sites. Our findings now provide an explanation by revealing a key degradative event that precedes RNase E cleavage: the rate-determining conversion of a triphosphorylated to a monophosphorylated 5’ end (Figure 7). Due to the preference of RNase E for substrates bearing a 5’ monophosphate, this modification can trigger rapid decay of the entire transcript by setting off a cascade of endonucleolytic and 3’ exonucleolytic events (Mackie, 1998). By contrast, sequestering the monophosphorylated 5’ end of the RNA in secondary structure would be expected to impede subsequent endonucleolytic cleavage by making this end inaccessible to RNase E, whose catalytic activation by a 5’ monophosphate requires an unpaired 5’ terminus (Mackie, 1998). A corollary of the discovery of this key step in degradation is that E. coli must contain an as yet unidentified enzyme that initiates RNA decay by removing the gamma and beta phosphates from the 5’ end of primary transcripts, either sequentially or as pyrophosphate. (Purified RNase E lacks such activity [data not shown].)
Figure 7. Mechanism of the 5’-end-dependent pathway for RNA degradation in E. coli.

An RNA pyrophosphohydrolase (hatchet) removes the gamma and beta phosphates at the 5’-terminus of a triphosphorylated primary transcript, either simultaneously (as shown) or sequentially. The resulting monophosphorylated decay intermediate is then rapidly cleaved by RNase E (scissors linked to a 5’-sensor domain), an endonuclease with a marked preference for RNA substrates bearing a single phosphate group at the 5’ end.
Multiple lines of evidence indicate that this 5’-end-dependent pathway is the principal mechanism by which RNA I.613 and rpsT mRNA are degraded in E. coli. For example, nearly all of the 5’ fragment produced by RNase E cleavage of RNA I.613 in E. coli is monophosphorylated whereas much less than half of uncut RNA I.613 is monophosphorylated, suggesting that degradation of RNA I.613 via pyrophosphate removal and subsequent RNase E cleavage is significantly faster than direct cleavage of the triphosphorylated transcript by Rnase E. This conclusion is consistent with our finding that RNA I.613 can be stabilized by adding a 5’-terminal stem-loop. Pyrophosphate removal appears to be even more pivotal for triggering the decay of the rpsT P1 transcript, as indicated by its marked degree of stabilization by a 5’ hydroxyl.
Despite the importance of the 5’-end-dependent decay pathway, RNase E is also able to cut RNAs whose 5’ terminus is sequestered, albeit at a reduced rate, as evidenced by the slow degradation of RNA I+hp and RNA I.613+hp to produce the labile decay intermediate RNA I-5. Moreover, the prolonged but finite longevity of RNA I and rpsT mRNA in cells lacking RNase E activity (Lin-Chao and Cohen, 1991; Bouvet and Belasco, 1992; Ow et al., 2003) implies that these RNAs can also be slowly degraded via one or more secondary pathways involving other ribonucleases, such as RNase G (a low-abundance RNase E paralog), RNase III (an endonuclease specific for double-stranded RNA), RNase Z (a tRNA-processing endonuclease), or polynucleotide phosphorylase (a 3’ exonuclease implicated in degrading 3’ cleavage products of RNA I and rpsT mRNA) (Mackie, 1989; Xu and Cohen, 1995; Binnie et al., 1999; Jiang et al., 2000; Tock et al., 2000; Ow et al., 2003; Perwez and Kushner, 2006). Interestingly, the conversion of a triphosphorylated to a monophosphorylated 5’ end would be expected to expedite degradation via two of these alternative pathways, either directly by catalytic activation (RNase G) or as an indirect result of improved 3’-terminal polyadenylation (polynucleotide phosphorylase) (Feng and Cohen, 2000; Jiang et al., 2000; Tock et al., 2000).
Key to the utility of PABLO analysis for examining the 5’ phosphorylation state of cellular transcripts is the quantitative nature of this assay, which makes it possible not only to detect monophosphorylated RNAs but also to estimate their relative abundance. This attribute is a consequence of the high ligation yields that can be obtained by using T4 DNA ligase to join ends selectively juxtaposed via base pairing to a bridging oligonucleotide and of the direct comparison of band intensities made possible by Northern blot analysis (rather than PCR, for example). Furthermore, the specificity of PABLO ligation and the use of Northern hybridization to detect ligation products makes it straightforward to distinguish the phosphorylation state of an uncut transcript from that of a processing product truncated at the 5’ or 3’ end.
A number of E. coli transcripts have been reported to decay via a 5’-end-dependent mechanism (Emory et al., 1992; Bouvet and Belasco, 1992; Bricker and Belasco, 1999; Mackie, 2000; Baker and Mackie, 2003), suggesting that 5’ pyrophosphate removal initiates the degradation of many translated and untranslated RNAs. A similar mechanism may also contribute to 5’-end-dependent RNA decay in bacterial species that lack RNase E, such as B. subtilis, where pyrophosphate removal could instead facilitate RNA degradation by RNase J1, which preferentially degrades monophosphorylated RNAs via both endonucleolytic and 5’-exonucleolytic mechanisms (Hambraeus et al., 2002; Sharp and Bechhofer, 2005; Even et al., 2005; Mathy et al., 2007). (No homolog of that ribonuclease exists in E. coli.) Interestingly, mRNA decay triggered by 5’ pyrophosphate removal is analogous to the mechanism of nonsense-mediated mRNA decay in yeast, which begins with 5’ cap removal to generate a monophosphorylated intermediate that is rapidly degraded via 5' exonuclease digestion (Muhlrad and Parker, 1994). Cap removal followed by 5’-to-3’ digestion can likewise result from the deadenylation of eukaryotic messages (Muhlrad et al., 1994). If viewed as the prokaryotic equivalent of a decapping reaction, the ability of E. coli to trigger the decay of primary transcripts by removing pyrophosphate from the 5’ end reveals an intriguing similarity between prokaryotic and eukaryotic mechanisms of mRNA degradation, which were previously thought to be distinct.
EXPERIMENTAL PROCEDURES
Strains and plasmids
Measurements of RNA lifetime and phosphorylation state were performed in Escherichia coli K-12 strain N3433 (lacZ43 thi-1 relA1 spoT1) and derivatives thereof bearing either a temperature-sensitive rne (RNase E) allele (ams-1) (Ono and Kuwano, 1979; Arnold et al., 1998) or a deletion of the poly(A) polymerase gene (pcnB∷kan) (Masters et al., 1993). RNA from phoA- cells was extracted from E. coli strain DHB4 (Boyd et al., 1987).
Plasmid pRNAI was constructed by replacing the promoter of the pBR322 RNA I gene with a β-lactamase (bla) promoter and then inserting this chimeric gene between EcoRI and BamHI sites of pCM128 (Tucker et al., 1984), a pSC101 derivative whose copy number is RNA I-independent. Plasmid pRNAI+hp is identical to pRNAI but for the insertion of a self-complementary sequence (5’ GATCGCCCACCGGCAGCTGCCGGTGGGCGATC 3’) at the beginning of the segment encoding RNA I. Plasmids pRNAI.613 and pRNAI.613+hp are derivatives of pRNAI that bear a 33-bp (5’ AGACCCCCACCCCGATCCCGCTCGAGCGGGATC 3’) or 53-bp (5’ AGAGCGGCTTCGGCCGCTCTAGACCCCCACCCCGATCCCGCTCGAGCGGGATC 3’) insert at the beginning of the segment encoding RNA I and an improved RNA I.613 promoter with modifications in both the -10 region (GACAAT → TATAAT) and the -35 region (TTCAAA → TTGACA). Plasmids pRNAI.613-8C and pRNAI.613mut-10 are variants of pRNAI.613 with mutations in the segments corresponding to either the RNase E cleavage site (ACAGTATTT → ACCCCCCCC) or the -10 promoter region (TATAAT → CCCCCC) of RNA I.613, respectively. Plasmid pRNAI.713 is a derivative of pRNAI.613 bearing a self-complementary extension of the segment corresponding to the first stem-loop of RNA I.613 (GATCCCGCTCGAGCGGGATC → GATCCCGCTCGAGCAGCTGCTCGAGCGGGATC) and an unimproved bla promoter. Plasmid pET-RNAI.613 is a derivative of pET-28a (Novagen) that, when linearized with HpaI, allows RNA I.613 to be transcribed from a T7 ϕ2.5 promoter that efficiently produces A-initiated transcripts (Coleman et al., 2004).
Plasmid pRPST1 is a derivative of pPM30 (Meacock and Cohen, 1980) that contains a complete copy of the E. coli rpsT gene, including both promoters and the transcription terminator, as well as an 18-bp sequence tag (5’ AGACCCCCACCCCGATCT 3’) inserted into the rpsT 3’ untranslated region between the HindIII site and the transcription termination signal. Plasmid pRPST.HH was derived from pRPST1 by inserting a DNA fragment containing a hammerhead sequence (5’ ATTATAATAAGATCCCGCTCGAGCGGGATCAGTGATCTGAAGAGCCGAAAGGCGAA ACACGCGTAAGCGTGTC 3’) at the 5’ end of the rpsT P1 transcription unit and by replacing the rpsT P1 promoter with a bla promoter. Plasmid pET-RPST1 is similar to pET-RNAI.613 except that the T7 ϕ2.5 promoter is fused to the E. coli rpsT P1 transcription unit, whose second nucleotide (thymine) has been changed to guanine to allow transcription by T7 RNA polymerase.
RNA extraction from E. coli
Total cellular RNA was harvested from E. coli growing exponentially in MOPS glucose at 37°C, except for the experiments in which rne+ and rnets strains were grown at 30°C and then shifted for 10 min to 44°C (Arnold et al., 1998). RNA was isolated by the hot-phenol extraction procedure as previously described (Emory and Belasco, 1990), except that the sodium acetate/sucrose buffer used to resuspend bacterial pellets also contained EDTA (50 mM). For one experiment shown in Figure S1, RNA was instead isolated by the RNeasy procedure (Qiagen).
RNA synthesis by in vitro transcription
Internally radiolabeled triphosphorylated RNA I.613 was synthesized by in vitro transcription. The reaction mixture (40 μl) contained Tris Cl (40 mM, pH 7.9), MgCl2 (6 mM), NaCl (10 mM), dithiothreitol (10 mM), spermidine (2 mM), GTP (1 mM), CTP (1 mM), UTP (1 mM), ATP (1 mM), [α-32P]CTP (50 μCi), RNasin (20 units; Promega), plasmid pET-RNAI.613 linearized by HpaI cleavage (0.5 μg), and T7 RNA polymerase (40 units; New England Biolabs). After incubation for 4 h at 37°C, the RNA was purified by electrophoresis on a 6% polyacrylamide / 8 M urea gel, eluted from a crushed gel fragment by agitation for 18 h at 4°C in a solution (1 ml) containing NaCl (150 mM) and RNasin (20 units/ml), filtered, and ethanol precipitated. To produce monophosphorylated RNA I.613, in vitro transcription was performed in the presence of AMP (15 mM) and a reduced concentration of ATP (0.25 mM). Cleavage of this RNA by purified N-RNase E was performed as previously described (Jiang et al., 2000).
Internally radiolabeled rpsT RNAs bearing a 5’ triphosphate or 5’ monophosphate were prepared in a similar manner, except that the template was plasmid pET-RPST1 linearized by HindIII cleavage. An otherwise identical rpsT RNA bearing a 5’ hydroxyl was produced by treating the monophosphorylated transcript with calf intestine alkaline phosphatase (New England Biolabs).
The 5’ phosphorylation state of the in vitro transcripts was confirmed by treatment with a 5’ exonuclease specific for monophosphorylated RNA. The reaction mixture (20 μl) contained Tris Cl (50 mM, pH 8.0), MgCl2 (2 mM), NaCl (100 mM), internally radiolabeled RNA I.613 (0.7 pmol), and Terminator exonuclease (0.1 unit; Epicentre). After 2 h at 30°C, the reaction products were analyzed by electrophoresis on a 6% polyacrylamide / 8 M urea gel.
Measurement of RNA lifetimes
Total cellular RNA was extracted from E. coli at time intervals after inhibiting transcription with rifampicin (0.2 mg/ml), and equal amounts (10 μg) were subjected to gel electrophoresis on polyacrylamide (6-11%) containing 8 M urea. The RNA was transferred to a Hybond-XL membrane (Amersham) by electroblotting and UV crosslinking and probed with a 5’-radiolabeled oligodeoxynucleotide complementary to an internal segment of RNA I, RNA I.613, and derivatives thereof (P1: 5’ TCAAGAGCTACCAACTC 3’), a sequence tag inserted into the 3’ UTR of rpsT (P4: 5’ CAAAGATCGGGGTGGGGGTCTAAG 3’), or a cysT tRNA loading control (P5: 5’GGAGTCGAACCGGACTAGACGG). Radioactive bands were visualized with a Molecular Dynamics Storm 820 PhosphorImager, and the band intensities were quantified by using ImageQuant software (Molecular Dynamics). RNA half-lives were calculated by linear regression analysis of data, which were obtained from at least 2-3 independent experiments.
Relative rates of RNA cleavage by purified N-RNase E were measured in vitro as previously described (Jiang et al., 2000).
PABLO analysis
Total RNA from E. coli (20 μg) was combined with oligo X (0.4 μM) and oligo Y (0.08 μM) in a final volume of 50.5 μl, heated to 75°C for 5 min, slowly cooled to 30°C, and then chilled on ice for at least 1 min. A buffer (29.5 μl) containing Tris Cl (108 mM, pH 7.8), MgCl2 (27 mM), dithiothreitol (27 mM), ATP (1.9 mM), RNasin (4 units/μl; Promega), and T4 DNA ligase (0.4 Weiss units/μl; Fermentas) was added, and the ligation reaction was allowed to proceed for 4 h at 37°C. The reaction was stopped by adding EDTA (1 μl, 0.5 M), and the products were phenol extracted, ethanol precipitated, and analyzed by Northern blotting as described for the RNA half-life measurements. The RNAs and their ligation products were detected by probing with a 5’-radiolabeled oligonucleotide: P1 for RNA I and RNA I-5 (5’ TCAAGAGCTACCAACTC 3’, complementary to an internal segment of both RNAs), P2 for RNA I.613 (5’ GATCGGGGTGGGGGTCT 3’, complementary to the 5’-terminal segment), P3 for chromosomal rpsT (5’ GTCCAACTCCCAAATGTGTTC 3’, complementary to a segment shared by both transcripts), or P4 for plasmid-encoded rpsT (5’ CAAAGATCGGGGTGGGGGTCTAAG 3’, complementary to a sequence tag inserted into the 3’ UTR). Ligation yields were calculated from the ratio of ligated RNA to ligated plus unligated RNA. Mean values and standard deviations were calculated from yields measured in at least three independent experiments. PABLO analysis of internally radiolabeled RNA I.613 transcribed in vitro was performed in a similar manner, except that the monophosphorylated and triphosphorylated transcripts (0.7 pmol) were first combined with total RNA from E. coli cells that lacked plasmid pRNAI.613 and autoradiography was performed directly on the polyacrylamide gel without blotting.
The Y oligos used to examine the phosphorylation state of E. coli transcripts were designed to juxtapose the 3’ end of oligo X and the 5’ end of the RNA under investigation. These RNA 5’ ends were identified by primer extension or by sequencing cDNAs obtained via RT-PCR amplification of total cellular RNA that had or had not been treated with tobacco acid pyrophosphatase prior to ligation to an oligoribonucleotide with T4 RNA ligase. Contrary to a previous report (Mackie and Parsons, 1983), the 5’-terminal sequence of the rpsT P1 transcript was found to be 5’ AUCACUACGUAA ….
The Y oligos used for PABLO analysis of E. coli RNA were YRNA I.613 (5’ ATCGGGGTGGGGGTCTCAAGTTATCATTCATATTGTTC 3’), YRNA I (5’ GCTTCAGCAGAGCGCAGATACCAAATACTGTCAAGTTATCATTCATATTGTTC 3’), YRNA I-5 (5’ GCTTCAGCAGAGCGCAGATACCAAATGAACAATATGAATGATAACTTG 3’), and YrpsT-P1 (5’ ACTCGTTACGTAGTGATCAAGTTATCATTCATATTGTTC 3’). The Y oligos used for PABLO analysis of in vitro transcripts were YRNA I.613, YGAP-1 (5’ ATCGGGGTGGGGGTC_CAAGTTATCATTCATATTGTTC 3’), YGAP+1 (5’ ATCGGGGTGGGGGTCTACAAGTTATCATTCATATTGTTC), YGAP+2 (5’ ATCGGGGTGGGGGTCTAACAAGTTATCATTCATATTGTTC 3’), and YGAP+3 (5’ ATCGGGGTGGGGGTCTAAACAAGTTATCATTCATATTGTTC 3’). The X oligos used for PABLO analysis were X22 (5’ GAACAATATGAATGATAACTTG 3’), X32 (5’ AAAAAAAAAAGAACAATATGAATGATAACTTG 3’), X45 (5’ AAAAAAAAAAAAAAAAAAAAAAACAAGTTATCATTCATATTGTTC 3’), and X90 (5’ CCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCCC CCCCCCCCCCGAACAATATGAATGATAACTTG 3’).
Supplementary Material
Acknowledgments
We thank Huiqing Zeng for pilot studies that identified experimental conditions for PABLO analysis and Jhilya Mayas for comments on the manuscript. This research was supported by a grant to J. G. B. from the National Institutes of Health (GM35769).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apirion D. Degradation of RNA in Escherichia coli. A hypothesis. Mol Gen Genet. 1973;122:313–322. doi: 10.1007/BF00269431. [DOI] [PubMed] [Google Scholar]
- Arnold TE, Yu J, Belasco JG. mRNA stabilization by the ompA 5’ untranslated region: two protective elements hinder distinct pathways for mRNA degradation. RNA. 1998;4:319–330. [PMC free article] [PubMed] [Google Scholar]
- Babitzke P, Kushner SR. The Ams (altered mRNA stability) protein and ribonuclease E are encoded by the same structural gene of Escherichia coli. Proc Natl Acad Sci USA. 1991;88:1–5. doi: 10.1073/pnas.88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KE, Mackie GA. Ectopic RNase E sites promote bypass of 5’-end-dependent mRNA decay in Escherichia coli. Mol Microbiol. 2003;47:75–88. doi: 10.1046/j.1365-2958.2003.03292.x. [DOI] [PubMed] [Google Scholar]
- Baumeister R, Flache P, Melefors Ö, von Gabain A, Hillen W. Lack of a 5’ non-coding region in Tn1721 encoded tetR mRNA is associated with a low efficiency of translation and a short half-life in Escherichia coli. Nucleic Acids Res. 1991;19:4595–4600. doi: 10.1093/nar/19.17.4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechhofer DH, Dubnau D. Induced mRNA stability in Bacillus subtilis. Proc Natl Acad Sci USA. 1987;84:498–502. doi: 10.1073/pnas.84.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco JG, Nilsson G, von Gabain A, Cohen SN. The stability of E. coli gene transcripts is dependent on determinants localized to specific mRNA segments. Cell. 1986;46:245–251. doi: 10.1016/0092-8674(86)90741-5. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci USA. 2002;99:9697–9702. doi: 10.1073/pnas.112318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnie U, Wong K, McAteer S, Masters M. Absence of RNase III alters the pathway by which RNAI, the antisense inhibitor of ColE1 replication, decays. Microbiology. 1999;145:3089–3100. doi: 10.1099/00221287-145-11-3089. [DOI] [PubMed] [Google Scholar]
- Bouvet P, Belasco JG. Control of RNase E-mediated RNA degradation by 5’-terminal base pairing in E. coli. Nature. 1992;360:488–491. doi: 10.1038/360488a0. [DOI] [PubMed] [Google Scholar]
- Boyd D, Manoil C, Beckwith J. Determinants of membrane protein topology. Proc Natl Acad Sci USA. 1987;84:8525–8529. doi: 10.1073/pnas.84.23.8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker AL, Belasco JG. Importance of a 5’ stem-loop for longevity of papA mRNA in Escherichia coli. J Bacteriol. 1999;181:3587–3590. doi: 10.1128/jb.181.11.3587-3590.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan AJ, Marcaida MJ, Stead JA, McDowall KJ, Scott WG, Luisi BF. Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature. 2005;437:1187–1191. doi: 10.1038/nature04084. [DOI] [PubMed] [Google Scholar]
- Coleman TM, Wang G, Huang F. Superior 5’ homogeneity of RNA from ATP-initiated transcription under the T7 ϕ2.5 promoter. Nucleic Acids Res. 2004;32:e14. doi: 10.1093/nar/gnh007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana A, Belasco JG. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 2005;19:2526–2533. doi: 10.1101/gad.1348805. [DOI] [PubMed] [Google Scholar]
- Deutscher MP. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34:659–666. doi: 10.1093/nar/gkj472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory SA, Belasco JG. The ompA 5’ untranslated RNA segment functions in Escherichia coli as a growth-rate-regulated mRNA stabilizer whose activity is unrelated to translational efficiency. J Bacteriol. 1990;172:4472–4481. doi: 10.1128/jb.172.8.4472-4481.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emory SA, Bouvet P, Belasco JG. A 5’-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Genes Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- Even S, Pellegrini O, Zig L, Labas V, Vinh J, Brechemmier-Baey D, Putzer H. Ribonucleases J1 and J2: two novel endoribonucleases in B. subtilis with functional homology to E. coli RNase E. Nucleic Acids Res. 2005;33:2141–2152. doi: 10.1093/nar/gki505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed GC, Wilt EM, Richardson CC. Enzymatic breakage and joining of deoxyribonucleic acid. J Biol Chem. 1971;246:925–932. [PubMed] [Google Scholar]
- Feng Y, Cohen SN. Unpaired terminal nucleotides and 5’ monophosphorylation govern 3’ polyadenylation by Escherichia coli poly(A) polymerase I. Proc Natl Acad Sci USA. 2000;97:6415–6420. doi: 10.1073/pnas.120173797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes K, Thisted T, Martinussen J. Mechanism of post-segregational killing by the hok/sok system of plasmid R1: sok antisense RNA regulates formation of a hok mRNA species correlated with killing of plasmid-free cells. Mol Microbiol. 1990;4:1807–1818. doi: 10.1111/j.1365-2958.1990.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Glembotski CC, Chapman AG, Atkinson DE. Adenylate energy charge in Escherichia coli CR341T28 and properties of heat-sensitive adenylate kinase. J Bacteriol. 1981;145:1374–1385. doi: 10.1128/jb.145.3.1374-1385.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambraeus G, Karhumaa K, Rutberg B. A 5’ stem-loop and ribosome binding but not translation are important for the stability of Bacillus subtilis aprE leader mRNA. Microbiology. 2002;148:1795–1803. doi: 10.1099/00221287-148-6-1795. [DOI] [PubMed] [Google Scholar]
- Hambraeus G, Persson M, Rutberg B. The aprE leader is a determinant of extreme mRNA stability in Bacillus subtilis. Microbiology. 2000;146:3051–3059. doi: 10.1099/00221287-146-12-3051. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Chen L-H, Fejzo MLS, Belasco JG. The ompA 5’ untranslated region impedes a major pathway for mRNA degradation in Escherichia coli. Mol Microbiol. 1994;12:707–716. doi: 10.1111/j.1365-2958.1994.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Hue KK, Cohen SD, Bechhofer DH. A polypurine sequence that acts as a 5’ mRNA stabilizer in Bacillus subtilis. J Bacteriol. 1995;177:3465–3471. doi: 10.1128/jb.177.12.3465-3471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Belasco JG. Catalytic activation of multimeric RNase E and RNase G by 5’-monophosphorylated RNA. Proc Natl Acad Sci USA. 2004;101:9211–9216. doi: 10.1073/pnas.0401382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Diwa A, Belasco JG. Regions of RNase E important for 5’-end-dependent RNA cleavage and autoregulated synthesis. J Bacteriol. 2000;182:2468–2475. doi: 10.1128/jb.182.9.2468-2475.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe K, Van de Sande JH, Khorana HG. Polynucleotide ligase-catalyzed joining of deoxyribo-oligonucleotides on ribopolynucleotide templates and of ribo-oligonucleotides on deoxyribopolynucleotide templates. Proc Natl Acad Sci USA. 1970;67:68–73. doi: 10.1073/pnas.67.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Chao S, Cohen SN. The rate of processing and degradation of antisense RNA I regulates the replication of ColE1-type plasmids in vivo. Cell. 1991;65:1233–1242. doi: 10.1016/0092-8674(91)90018-t. [DOI] [PubMed] [Google Scholar]
- Long DM, Uhlenbeck OC. Self-cleaving catalytic RNA. FASEB J. 1993;7:25–30. doi: 10.1096/fasebj.7.1.8422971. [DOI] [PubMed] [Google Scholar]
- Mackie G. Specific endonucleolytic cleavage of the mRNA for ribosomal protein S20 of Escherichia coli requires the project of the ams gene in vivo and in vitro. J Bacteriol. 1991;173:2488–2497. doi: 10.1128/jb.173.8.2488-2497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GA. Stabilization of the 3’ one-third of Escherichia coli ribosomal protein S20 mRNA in mutants lacking polynucleotide phosphorylase. J Bacteriol. 1989;171:4112–4120. doi: 10.1128/jb.171.8.4112-4120.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie GA. Secondary structure of the mRNA for ribosomal protein S20. Implications for cleavage by ribonuclease E. J Biol Chem. 1992;267:1054–1061. [PubMed] [Google Scholar]
- Mackie GA. Ribonuclease E is a 5’-end-dependent endonuclease. Nature. 1998;395:720–723. doi: 10.1038/27246. [DOI] [PubMed] [Google Scholar]
- Mackie GA. Stabilization of circular rpsT mRNA demonstrates the 5’-end dependence of RNase E action in vivo. J Biol Chem. 2000;275:25069–25072. doi: 10.1074/jbc.C000363200. [DOI] [PubMed] [Google Scholar]
- Mackie GA, Parsons GD. Tandem promoters in the gene for ribosomal protein S20. J Biol Chem. 1983;258:7840–7846. [PubMed] [Google Scholar]
- Masters M, Colloms MD, Oliver IR, He L, Macnaughton EJ, Charters Y. The pcnB gene of Escherichia coli, which is required for ColE1 copy number maintenance, is dispensable. J Bacteriol. 1993;175:4405–4413. doi: 10.1128/jb.175.14.4405-4413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathy N, Bénard L, Pellegrini O, Daou R, Wen T, Condon C. 5’-to-3’ exoribonuclease activity in bacteria: role of RNase J1 in rRNA maturation and 5’ stability of mRNA. Cell. 2007;129:681–692. doi: 10.1016/j.cell.2007.02.051. [DOI] [PubMed] [Google Scholar]
- McDowall KJ, Lin-Chao S, Cohen SN. A+U content rather than a particular nucleotide order determines the specificity of RNase E cleavage. J Biol Chem. 1994;269:10790–10796. [PubMed] [Google Scholar]
- Meacock PA, Cohen SN. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980;20:529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- Melefors Ö, von Gabain A. Genetic studies of cleavage-initiated mRNA decay and processing of ribosomal 9S RNA show that the Escherichia coli ams and rne loci are the same. Mol Microbiol. 1991;5:857–864. doi: 10.1111/j.1365-2958.1991.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Query CC. Joining of RNAs by splinted ligation. Methods Enzymol. 2000;317:109–123. doi: 10.1016/s0076-6879(00)17009-0. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Sharp PA. Site-specific modification of pre-mRNA: the 2’-hydroxyl groups at the splice sites. Science. 1992;256:992–997. doi: 10.1126/science.1589782. [DOI] [PubMed] [Google Scholar]
- Mudd EA, Krisch HM, Higgins CF. RNase E, an endoribonuclease, has a general role in the chemical decay of E. coli mRNA: evidence that rne and ams are the same genetic locus. Mol Microbiol. 1990;4:2127–2135. doi: 10.1111/j.1365-2958.1990.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Decker CJ, Parker R. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5’→3’ digestion of the transcript. Genes Dev. 1994;8:855–866. doi: 10.1101/gad.8.7.855. [DOI] [PubMed] [Google Scholar]
- Muhlrad D, Parker R. Premature translational termination triggers mRNA decapping. Nature. 1994;370:578–581. doi: 10.1038/370578a0. [DOI] [PubMed] [Google Scholar]
- Nath K, Hurwitz J. Covalent attachment of polyribonucleotides to polydeoxyribonucleotides catalyzed by deoxyribonucleic acid ligase. J Biol Chem. 1974;249:3680–3688. [PubMed] [Google Scholar]
- Ono M, Kuwano M. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of mRNA. J Mol Biol. 1979;129:343–357. doi: 10.1016/0022-2836(79)90500-x. [DOI] [PubMed] [Google Scholar]
- Ow MC, Perwez T, Kushner SR. RNase G of Escherichia coli exhibits only limited functional overlap with its essential homologue, RNase E. Mol Microbiol. 2003;49:607–622. doi: 10.1046/j.1365-2958.2003.03587.x. [DOI] [PubMed] [Google Scholar]
- Perwez T, Kushner SR. RNase Z in Escherichia coli plays a significant role in mRNA decay. Mol Microbiol. 2006;60:723–737. doi: 10.1111/j.1365-2958.2006.05124.x. [DOI] [PubMed] [Google Scholar]
- Pilling MJ, Seakins PW. Reaction Kinetics. New York: Oxford University Press; 1995. [Google Scholar]
- Sandler P, Weisblum B. Erythromycin-induced stabilization of ermA messenger RNA in Staphylococcus aureus and Bacillus subtilis. J Mol Biol. 1988;203:905–915. doi: 10.1016/0022-2836(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Sharp JS, Bechhofer DH. Effect of 5’-proximal elements on decay of a model mRNA in Bacillus subtilis. Mol Microbiol. 2005;57:484–495. doi: 10.1111/j.1365-2958.2005.04683.x. [DOI] [PubMed] [Google Scholar]
- Spickler C, Stronge V, Mackie GA. Preferential cleavage of degradative intermediates of rpsT mRNA by the Escherichia coli RNA degradosome. J Bacteriol. 2001;183:1106–1109. doi: 10.1128/JB.183.3.1106-1109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraseviciene L, Miczak A, Apirion D. The gene specifying RNase E (rne) and a gene affecting mRNA stability (ams) are the same gene. Mol Microbiol. 1991;5:851–855. doi: 10.1111/j.1365-2958.1991.tb00758.x. [DOI] [PubMed] [Google Scholar]
- Tock MR, Walsh AP, Carroll G, McDowall KJ. The CafA protein required for the 5’-maturation of 16 S rRNA is a 5’-end-dependent ribonuclease that has context-dependent broad sequence specificity. J Biol Chem. 2000;275:8726–8732. doi: 10.1074/jbc.275.12.8726. [DOI] [PubMed] [Google Scholar]
- Tucker WT, Miller CA, Cohen SN. Structural and functional analysis of the par region of the pSC101 plasmid. Cell. 1984;38:191–201. doi: 10.1016/0092-8674(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Xu F, Cohen SN. RNA degradation in Escherichia coli regulated by 3’ adenylation and 5’ phosphorylation. Nature. 1995;374:180–183. doi: 10.1038/374180a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


