Abstract
Polymeric micelles were constructed from poly(L-lactic acid) (PLA; Mn 3K)-b-poly(ethylene glycol) (PEG; Mn 2K)-b-poly(L-histidine) (polyHis; Mn 5K) as a tumor pH-specific anticancer drug carrier. Micelles (particle diameter: ~ 80 nm; critical micelle concentration (CMC): 2 µg/ml) formed by dialysis of the polymer solution in dimethylsulfoxide (DMSO) against pH 8.0 aqueous solution, are assumed to have a flower-like assembly of PLA and polyHis blocks in the core and PEG block as the shell. The pH-sensitivity of the micelles originates from the deformation of the micellar core due to the ionization of polyHis at a slightly acidic pH. However, the co-presence of pH-insensitive lipophilic PLA block in the core prevented disintegration of the micelles and caused swelling/aggregation. A fluorescence probe study showed that the polarity of pyrene retained in the micelles increased as pH was decreased from 7.4 to 6.6, indicating a change to a more hydrophilic environment in the micelles. Considering that the size increased up to 580 nm at pH 6.6 from 80 nm at pH 7.4 and that the transmittance of micellar solution increased with decreasing pH, the micelles were not dissociated but rather swollen/aggregated. Interestingly, the subsequent decline of pyrene polarity below pH 6.6 suggested re-self-assembly of the block copolymers, most likely forming a PLA block core while polyHis block relocation to the surface. Consequently, these pH-dependent physical changes of the PLA-b-PEG-b-polyHis micelles provide a mechanism for triggered drug release from the micelles triggered by the small change in pH (pH 7.2-6.5).
Keywords: Poly(L-histidine)-b-PEG-b-poly(L-lactic acid) triblock copolymer, Flower-like micelle, Tumor pH, Triggered drug release
1. Introduction
Recently pharmaceutical applications of polymeric micelles [1–3] designed to enable spatial site-specific drug delivery [4] have burst onto a scene with challenging innovations. In tumor treatment the micelles system has proven effective in regression of tumor size while minimizing damage to the healthy tissues [4] and is being actively investigated in conjunction with hyperthermia [5], ultrasound [6], specific enzyme-induced cleavable bonds [7–9], and specific ligands or antibodies [10–13] for active targeting purposes.
More recently, pH-sensitive polymeric carriers have been used in targeted antitumor drug delivery [14–25], based on intrinsic differences between various solid tumors and the surrounding normal tissues in terms of their relative acidity [26]. The extracellular pH (pHe) in most tumors is more acidic (pH 6.5-7.2) than in normal tissues [27–29]. In all measurements (involving either invasive or noninvasive methods) of the pHe of human and animal solid tumors, more than 80% of all measured values consistently fall below a pH of 7.2 [27–29]. The development of pH-sensitive drug-carriers therefore maybe a unique strategy and confer advantages over using external stimuli, such as hyperthermia [5] and ultrasound [6] which require exact location of tumors for triggered release [30].
One study for tumor pHe targeting concerns pH-labile chemical bonds. Hydrazone and acetal bonds between the drug and the micelles can be cleaved by acidic pH [14–16], however these bonds are more labile at endo/lysosomal pH (pH 5.0-5.5) than at tumor pHe to accelerate drug release from the micelles [14–16]. On the other hand, smart micelles showing a pH-induced interior structural change [17–18] or physical destabilization [19–25] have recently been investigated and showed a high sensitivity to tumor pHe. The physical change in the drug-carrier [17–25] seems more tunable to recognize small differences in pH like tumor pHe, compared to the systems that rely on chemical bonds [14–16]. Furthermore, translating the non-specific nature of cationic HIV Tat peptide to acidic solid tumor-specific by pH-dependent electrostatic shielding/deshielding with polymeric micelles is an attractive concept [31]. At normal blood pH, the poly(sulfonamide) is negatively charged and shields Tat peptide on micellar surface by electrostatic interactions. In the tumor pHe, disrupting the ionic interactions is triggered by lower pH in the tumor milieu due to deionized poly(sulfonamide), leading to the detachment of poly(sulfonamide) from the micelles and exposure of the hidden Tat peptide in tumoral environments. This system targeted realistic tumor pHe.
Poly(L-histidine) (polyHis) [19–24] is a promising biomaterial for the construction of such pH-sensitive polymeric carriers. The imidazole ring (pKb ~ 6.5) of polyHis has lone pairs of electrons on the unsaturated nitrogen that endow it with pH-dependent amphoteric properties [19–20]. The ionization of polyHis (below pKb) switches the nature of this material from lipophilic to lipophobic [19–20]. In addition, the polyHis presents a strong endosomolytic property by its proton sponge effect and/or its interaction with anionic phospholipids comprising the endosomal compartments [20–21, 23, 32]. These properties of polyHis contributed to the development of a smart micelle system responsive to tumor pHe or more acidic endosomal pH tunable by a mixed micelle approach with poly(L-lactic acid) (PLA)-b-PEG [19–21, 23–24].
In this study, we prepared a flower-like pH-sensitive polymer micelle from biodegradable PLA-b-PEG-b-polyHis to replace the mixed micelles approach [20] where phase separation between two block copolymers was observed by polyHis ionization. As shown in Fig. 1, the flower-like micelles are expected to be formed from PLA-b-PEG-b-polyHis and be responsive to tumor pHe. Switching the drug release kinetics from slow during circulation to fast at tumor sites by an acidic pH-induced micelles-swelling mechanism (due to ionization of the polyHis) may enhance the effectiveness of the chemotherapy. For the proof of this concept, we preferentially examined the pH-sensitive properties of the flower-like micelles with a fluorescence probe technique, particle size measurement, and a transmittance study. In addition, doxorubicin (DOX: a model anticancer drug in this study)-loaded flower-like micelles were evaluated by monitoring pH-dependent drug release rate and cytotoxicity against the wild breast tumor cell line (MCF-7).
Fig. 1.

Schematic diagram depicting the central concept of flower-like pH-sensitive micelles based on PLA-b-PEG-b-polyHis triblock copolymer. During circulation in blood micelles preserve a flower-like shape but at the tumor site micelles undergo interior swelling and triggers antitumor drug release.
2. Materials and Methods
2.1. Materials
Succinic anhydride, 4-dimethylaminopyridine (DMAP), pyridine, triethylamine (TEA), dimethylformamide (DMF), diethyl ether, N-hydroxysuccinimide (NHS), N, N-dicyclohexylcarbodiimide (DCC), dichloromethane (DCM), dimethylsulfoxide (DMSO), N-(2-aminoethyl)maleimide (AEM) trifluoroacetate salt, anhydrous liquid ammonia, sodium, cystamine, tris (2-carboxyethyl)-phosphine hydrochloride (TCEP), tetrazolium salt MTT, L-glutamine, n-propyl galate, glycerol, and DOX•HCl were purchased from Sigma-Aldrich (St.Louis, MO, USA). Penicillin–streptomycin, Tris–HCl (pH 8.4), fetal bovine serum (FBS), 0.25% (w/v) trypsin–0.03% (w/v) EDTA solution, and RPMI1640 medium were purchased from Gibco (Uxbridge, UK). Poly(imbenzyl-L-histidine) (average repeating unit number: 36) was synthesized as described in detail in our previous reports [19, 22]. PLA (Mn 3K)-b-PEG (Mn 2K)-COOH (mono-carboxylated PLA-b-PEG) was prepared by a conventional method [19, 23, 33].
2.2. Synthesis of PLA-b-PEG-b-polyHis
In order to synthesize an ABC type triblock copolymer of PLA-b-PEG-b-polyHis, synthetic routes were tried as shown in Scheme 1. The terminal amine group of poly(imbenzyl-L-histidine) (20 mmol) was modified with succinic anhydride (24 mmol), DMAP (24 mmol), and TEA (24 mmol) in DMF (30 ml)/pyridine (10ml) at room temperature for 1 day. After the reaction, the carboxylated poly(imbenzyl-L-histidine) (yield: 98±3 wt. %) was obtained after re-precipitation from excess diethyl ether. The conversion of the terminal amino group of poly(imbenzyl-L-histidine) into a carboxyl group was confirmed by the transfer of ¹H NMR peak at δ 3.97 (-CH-NH2 at the terminal site of poly(imbenzyl-L-histidine)) to δ 4.92 (-CH-NH-CO- at the conjugation site of poly(imbenzyl-L-histidine) and succinic anhydride) (data not shown). In order to substitute carboxyl group in poly(imbenzyl-L-histidine) with AEM, the carboxylated poly(imbenzyl-L-histidine) (20 mmol) was activated using NHS (24 mmol) and DCC (24 mmol) in DMF (30 ml). After carrying out the reaction for 1 day at room temperature, dicyclohexylurea (DCU) was removed by filtration and then AEM (40 mmol) in TEA (40 mmol) was added to the filtrate for coupling reaction between poly(imbenzyl-L-histidine) and AEM (reaction time: one day). The solution was dialyzed to remove unconjugated AEM. Poly(imbenzyl-L-histidine)-AEM (yield: 94±5 wt. %) was obtained after freeze-drying. The conjugation of AEM to poly(imbenzyl-L-histidine) was confirmed by ¹H NMR peak transfer of δ 4.12 (-CH2-COOH, terminal group of carboxylated poly(imbenzyl-L-histidine)) to δ 2.04 (-CH2-CO-NH-, conjugation site of poly(imbenzyl-L-histidine)-AEM) (data not shown). The percentage conversion of poly(imbenzyl-L-histidine) to poly(imbenzyl-L-histidine)-AEM was 95±3 wt. %, as calculated by the comparison of two ¹H NMR peaks (δ 4.12 and δ 2.04). Subsequently, poly(imbenzyl-L-histidine)-AEM (2 g) was treated with finely cut metallic sodium (0.4 g) under anhydrous liquid ammonia (40 ml) to remove benzyl groups in poly(imbenzyl-L-histidine) (reaction time: 30 min) [19]. After evaporating all liquid ammonia, 1 N HCl (15 ml) was added to dissolve the residue. This solution was filtered to remove insoluble materials and extracted three times with diethyl ether (40 ml). PolyHis-AEM (yield: 87±6 wt. %) was collected after freeze-drying for 2 days. The ¹H-NMR peaks confirmed the removal of benzyl group and the presence of same AEM content on polymer (data not shown).
Scheme 1.
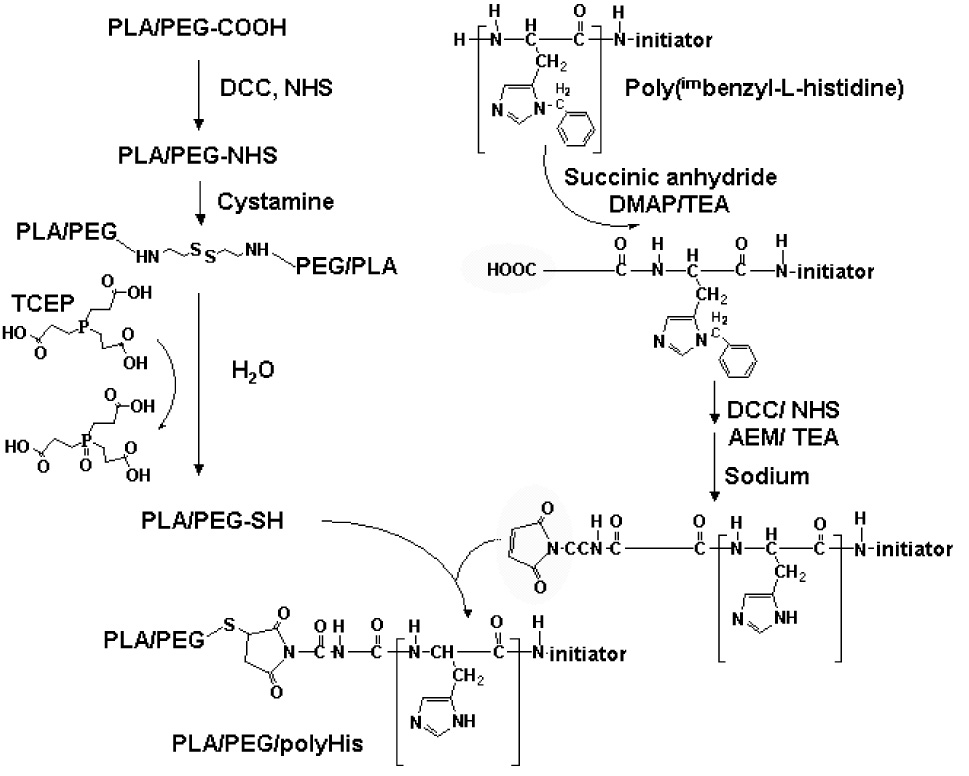
Overall scheme for the synthesis of PLA-b-PEG-b-polyHis
On the other hand, PLA-b-PEG-COOH (20 mmol) activated with NHS (24 mmol) and DCC (24 mmol) in DCM (30 ml) for 1 day was filtered to remove DCU and then diethyl ether (240 ml) was added to precipitate PLA-b-PEG-NHS. Dried PLA-b-PEG-COOH was reacted with cystamine (40 mmol) in DMSO (15 ml), in the presence of TEA (40 mmol). After 1 day, TCEP (80 mmol) dissolved in deionized water (30 ml) was mixed with the solution for the reduction of PLA-b-PEG-cystamine-PEG-b-PLA or PLA-b-PEG-cystamine (reaction time: 4 hours). The solution was then dialyzed to remove unconjugated cystamine and TCEP. PLA-b-PEG-SH (yield: 70±8 wt. %) was lyophilized. ¹H-NMR showed peak transfer of δ 4.23 (-CH2-COOH, terminal group of PLA-b-PEG-COOH) to δ 2.83 (-CH2-CO-NH-, conjugation site of PLA-b-PEG-SH) (data not shown). The percentage conversion of PLA-b-PEG-COOH to PLA-b-PEG-SH was 82±5 wt. %, as calculated by the comparison of two ¹H NMR peaks (δ 4.23 and δ 2.83).
Finally, the coupling of polyHis-AEM (10 mmol) and PLA-b-PEG-SH (10 mmol) was carried out in DMSO (10 ml)/deionized water (10 ml) overnight. Resulting solution was dialyzed in a dialysis membrane tube (Spectra/Pore; MWCO 8K) to remove unconjugated polymers. After freeze-drying, PLA (Mn 3,012)-b-PEG (Mn 2,000)-b-polyHis (Mn 5,132) triblock copolymer (yield: 85±3 wt.%) was obtained. The complete coupling of PLA-b-PEG and polyHis was confirmed by the transfer of ¹H NMR peak at δ6.95 (-CH=CH- at maleimide of polyHis-AEM) to δ 3.24 (-CH-CH2-) (data not shown).
The molecular weight distribution of PLA-b-PEG-b-polyHis was determined with MALDI TOF mass spectra acquired with a Micromass TOFSPEC mass spectrometer in the reflection mode.
2.3. Acid-base titration
The PLA-b-PEG-b-polyHis and NaCl (control) were dissolved in 35 ml of deionized water (30 mmol/l) and the solution was adjusted to pH 12 with 1 N NaOH. The diluted solution was titrated by the stepwise addition of 0.1 N HCl to obtain the titration profile.
2.4. Preparation of polymeric micelle
The PLA-b-PEG-b-polyHis (20 mg) dissolved in DMSO (20 ml) was transferred to a preswollen dialysis membrane tube (Spectra/Por; MWCO 15K) and dialyzed against HCl (or NaOH)–Na2B4O7 buffer solution (pH 7.4, 20 mM) for 24 hours. The outer phase was replaced three times with fresh Na2B4O7 buffer solution. The solution was then mixed with phosphate buffer saline (PBS) solution (pH 7.4). The solution (ionic strength: 0.15) was subsequently lyophilized after filtering through a 0.8 µm syringe filter. The yield (wt.%) of micelles was 89±6 wt.%, as calculated by weighing the freeze–dried micelles powder.
2.5. Fluorescence spectroscopy
For the measurement of steady-state fluorescence spectra, pyrene solution (6.0×10−2 M) in acetone was added to deionized water to attain a pyrene concentration of 12×10−7 M. The solution was then distilled under vacuum at 60 °C for 1 hour to remove acetone from the solution. The acetone-free pyrene solution was mixed with the polymeric micelles (1×10−4~1×10−1g/l), yielding final pyrene concentration of 6.0 × 10−7 M. The change of the intensity ratio (I337/I334) of the pyrene with polymer concentration was plotted from excitation spectra from 300 to 360 nm and at emission wavelength 390 nm [17–18, 33]. The CMC at pH 7.4 was determined from crossover point at low polymer concentration from this plot [33].
In order to quantify the polarity around the pyrene molecules retained in the polymeric micelles (0.1 g/l) with pH, the intensity ratio (I337/I334, higher ratios means a less polar environment) of the pyrene at different pHs (pH 7.4-5.5) was measured.
2.6. Photon correlation spectroscopy (PCS) by Zetasizer
PCS was conducted using a Zetasizer 3000 (Malatvern Instruments, USA) with a He-Ne Laser beam at a wavelength of 633 nm, and a fixed scattering angle of 90°. The polymeric micelles (0.1 g/l, ionic strength: 0.15) were exposed to different pH values (pH 7.4-5.5) at 37 °C for 24 hours before measurement of the particle size and particle size distribution.
2.7. Transmittance of micellar solution
The transmittance was measured using a Varian CARY 1E UV-Vis spectrophotometer. Before the test, all micellar solutions (0.1 g/l, ionic strength: 0.15) with different pHs (pH 7.4-5.5) were stabilized at 37 °C for 24 hours. Relative transmittance of micellar solution was measured in the selected pH change with respect to a transmittance at pH 7.4.
2.8. DOX loading to micelles
DOX•HCl (1 mol) was stirred with TEA (2 mol) in DMSO overnight, in order to remove HCl salt and make DOX lipophilic [20–24]. DOX (10 mg) was blended with PLA-b-PEG-b-polyHis (50 mg) in DMSO (20 ml). The mixture was transferred to a preswollen dialysis membrane (Spectra/Por molecular weight cut-off 15,000) and dialyzed against HCl (or NaOH)–Na2B4O7 buffer solution (pH 7.4, 20 mM) for 24 hours. The dialysis medium was changed several times and the contents inside the dialysis tube were subsequently lyophilized. The amount of entrapped DOX was determined by measuring the UV absorbance at 481 nm of the drug-loaded polymeric micelles dissolved in DMSO. The DOX loading efficiency was 81±5 wt. %, as calculated by dividing the loading DOX content by the feeding DOX content.
2.9. pH-dependent drug release from micelle
For drug release test, DOX-loaded micelles were dispersed in PBS (1 ml, ionic strength: 0.15) with different pHs (pH 7.4, 7.0, 6.8, 6.4, and 6.0), and then transferred in a dialysis membrane tube (Spectra/Por MWCO 10K). This membrane tube was immersed in a vial containing 10 ml of phosphate buffered saline (PBS) solution adjusted to different pHs. The release of DOX from micelles was tested with mechanical shaking (100 rev. /min) at 37 °C. The outer phase of the dialysis membrane was withdrawn and replaced with fresh buffer solution at predetermined time intervals in order to maintain sink condition. Free DOX inside and outside the dialysis membrane tube were analyzed to ensure the amount of DOX released from micelles. The measurement of DOX concentration by a UV/Vis spectrophotometer.
2.10. pH-dependent cell cytotoxicity
Human breast adenocarcinoma (MCF-7) cells were obtained from ATCC. These cells were maintained in RPMI-1640/PBS (pH 7.4-6.0) medium with 2 mM L-glutamine, 5% penicillin–streptomycin, 20% fetal bovine serum in a humidified incubator at 37 °C and 5% CO2 atmosphere. Before test, the cells (1 ×104 cells/ml) were harvested by 0.25% (w/v) trypsin–0.03% (w/v) EDTA solution and seeded in a 96-well plate for 24 hours.
For cytotoxicity tests, free DOX (10 µg/ml) or equivalent DOX-loaded micelles in RPMI-1640/PBS medium were added to the medium-removed 96-well palate. The pH of culture medium was readjusted with 0.1 N HCl or 0.1 N NaOH. During 24 hours incubation, no considerable pH shift in the culture medium was observed. After the incubation, chemosensitivity was measured using tetrazolium salt MTT assay [20–24]. The absorbance of each well was read on a microplate reader using a test wavelength of 570 nm and a reference wavelength 630 nm. The cell viability expressed in this study is relative to those at each pH in the absence of DOX and micelles and the direct pH effect on the cell viability was not monitored with the MCF-7 cell line. The change in pH from 7.4 to 6.0 may influence cell surface charges and cellular physiology and viability, noting that the low pH is a favorable environment for tumor cells but has detrimental effect on normal cells [26–29].
2.11. Confocal microscopy
The tumoral DOX accumulation by the PLA-b-PEG-b-polyHis micelles with pH change was observed with the cells grown on a Lab-Tek II chamber slide (Nalge Nunc, Napevillem, IL, USA). The DOX concentration used was 1 µg/ml in the micellar solution with pH 7.4 and 7.0. After 5 hours incubation, the cells treated with the micelles were washed three times with PBS (pH 7.4) and fixed with 1% formaldehyde in PBS for 10 min at room temperature. A coverslip was mounted on a microscope slide with a drop of anti-fade mounting media (5% N-propyl galate, 47.5% glycerol and 47.5% Tris–HCl, pH 8.4) to reduce fluorescence photo bleaching. The DOX accumulation in MCF-7 cells was examined by a confocal microscope (Leica TCS NT, Leica, Germany).
3. Results and discussion
3.1. Characterization of PLA-b-PEG-b-polyHis
The PLA (Mn 3K)-b-PEG (Mn 2K)-b-polyHis (Mn 5K) was prepared by coupling thiol group at the end of PEG block of PLA-b-PEG to the maleimide of polyHis block, after functionalization (Scheme 1) of PLA-b-PEG and polyHis synthesized as described in our previous reports [19, 22–23, 33]. The molecular weight distribution was 1.37 as determined from MALDI TOF mass spectra (data not shown). The apparent pKb of this polymer was found to be around 7.0 (Fig. 2). In addition, this polymer exhibited a pH-buffering region of pH 7-5 (this is a inherent property of polyHis) [19].
Fig. 2.

The pH-profile of PLA-b-PEG-b-polyHis tribock copolymer (●) and NaCl (■) by acid-base titration. The average value from triplicate titrations was plotted.
3.2. Micelle formation
The micelle formation by the self-assembly of PLA-b-PEG-b-polyHis was monitored by fluorometry in the presence of pyrene as a fluorescent probe. Pyrene is highly hydrophobic and preferentially migrates into the lipophilic micellar core in an aqueous solution [33]. When pyrene is located in a non-polar environment (like a lipophilic core in micelle), it shows strong fluorescence. However, in a polar environment it displays a weak fluorescent intensity and a shift of excitation peak. In addition, the change in the intensity ratio (I337/I334) of the pyrene extracted from the excitation graph indicates whether the polymer forms micelles or exists as a unimer [17–18, 33]. Fig. 3 shows that the critical micelle concentration (CMC) of PLA-b-PEG-b-polyHis was approximately 2 µg/ml, as estimated from the crossover point of the intensity ratio (I337/I334). This ABC type polymer structure (PLA-b-PEG-b-polyHis) employing a flower-like assembly in an aqueous solution, yielded lower CMC at pH 7.4 (> pKb of polyHis) than those of polyHis-b-PEG (10 µg/ml) [19] and PLA-b-PEG (4.5 µg/ml) [33], which is due to increased lipophilicity by the presence of two lipophilic blocks (PLA and polyHis). This low CMC value may lead to maintenance of good stability of the micelles in blood stream.
Fig. 3.

The determination of the CMC from the fluorescence intensity ratio I337/I334 from excitation spectra vs. log concentration of the PLA-b-PEG-b-polyHis micelles (pH 7.4).
Fig. 4 shows that the PLA-b-PEG-b-polyHis micelles had an average 80 nm size with a unimodal size distribution, based on the intensity-average diameter (Fig. 4). In addition, the transmittance of the micelles at pH 7.4 was 92.5±0.4% of a blank solution (data not shown), which would be due to scattered light by micelles.
Fig. 4.
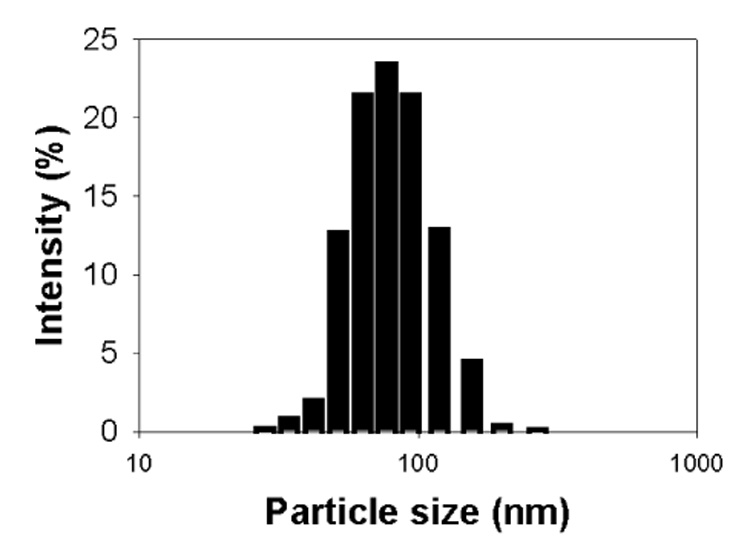
The particle size distribution of the PLA-b-PEG-b-polyHis micelles (pH 7.4)
3.3. pH-dependent structural change of micelle
The micropolarity change of the micellar core by pH was monitored by employing a pyrene probe (Fig. 5(a)). The decrease in the pyrene intensity ratio (I337/I334) at a same polymer concentration of 0.1 g/l indicates an increase in polarity of micellar core. The PLA-b-PEG-b-polyHis micelles showed a gradual increase in polarity as the pH decreased from 7.4 to 6.6, suggesting pH-dependent micellar core structural change such as swelling.
Fig. 5.

The pH-sensitivity of the PLA-b-PEG-b-polyHis micelles from (a) a plot of the intensity ratio I337/I334 (n=3), (b) the relative transmittance change (n=3), and (c) the particle size change (n=3), according to the pH of micellar solution. The micellar solution (ionic strength: 0.15) was kept to 0.1 g/l.
Figs. 5(b) and (c) show that the relative transmittance of the micellar solution decreased and the size of the micelles increased with decreasing pH. In particular, the change of the particle size from 80 nm at pH 7.4 to 580 nm at pH 6.6 exhibits approximately 380-fold volume transition, suggesting pH-dependent physical changes in the micelles. Similar results have been observed with BAB (hydrophobic B blocks and hydrophilic A block) type amphiphilic copolymers which form flower-like micelles and often lead to micellar bridging (resulting in micelle aggregation) depending on environmental conditions [34–38]. For example, the formation of three-dimensional networks by bridging between micelles has been induced by high polymer concentration, salts and pH [34–38]. It is expected that as the pH of solution decreases, the PLA-b-PEG-b-polyHis micelles swell (Fig. 5(a)) as a result of the ionization of polyHis and some lipophilic end chains disengaged from the micelles causes the bridging between micelles. Unlike polyHis-b-PEG micelles and mixed micelles of polyHis-b-PEG/PLA-b-PEG [19–21], the PLA-b-PEG-b-polyHis micelles resisted disintegration upon ionization of polyHis, but became swollen and further aggregated.
It is interesting to note that below pH 6.6, the PLA-b-PEG-b-polyHis micelles exhibited a gentle rise of the pyrene intensity ratio (I337/I334): p<0.05 (at pH 6.0 or 5.5) compared to at pH 6.6, analyzed by student t-test. This suggests the transfer of pyrene from a polar to a non-polar environment and possible formation of secondary particles composed of the lipophilic PLA block domains and hydrophilic PEG-b-polyHis domains. Considering the size of particles below pH 6.6 was around 900 nm in diameter (Fig. 5(c)), this particle must be in a form of super-aggregate during re-organization of PLA-b-PEG-b-polyHis at low pH from initially stable micelles at high pH.
Considering that the micelles formed aggregates whose size exceeds tumor vascular pore size (average 400–600 nm) [39], PLA-b-PEG-b-polyHis micelles may be useful for extending micelle resident time in tumor sites after extravasation. It was reported that large cluster formation between extravasated nanoparticles increased the retention time of nanoparticles in tumor [40–41]. The proof of this hypothesis will require further in vivo investigation.
3.4. pH-dependent DOX release and cytotoxicity
The swelling of micelles in acidic pH may be closely linked to the release rate of incorporated DOX. Fig. 6(a) shows that during micelles swelling the release of DOX from the micelles was accelerated. This may be due to that DOX carrying capacity of micellar core decreases as a result of the ionization polyHis in the micellear core. The difference of DOX release with pH was remarkable. For 5 hours, 20 wt. % of DOX was released from the micelles at pH 7.4, whereas 40 wt. % at pH 7.0. Furthermore, for 24 hours the micelles released 32 wt. % of DOX at pH 7.4, and 55 wt. % at pH 7.0, 60 wt. % at pH 6.8, 67 wt. % at pH 6.4 and 72 wt. % at pH 6.0. In particular, these micelles responded very little a pH difference between pH 7.4 and 7.0, which is comparable with that the mixed micelles of polyHis-b-PEG/PLA-b-PEG recognized a pH difference between pH 7.4 and 6.8 [19–21].
Fig. 6.
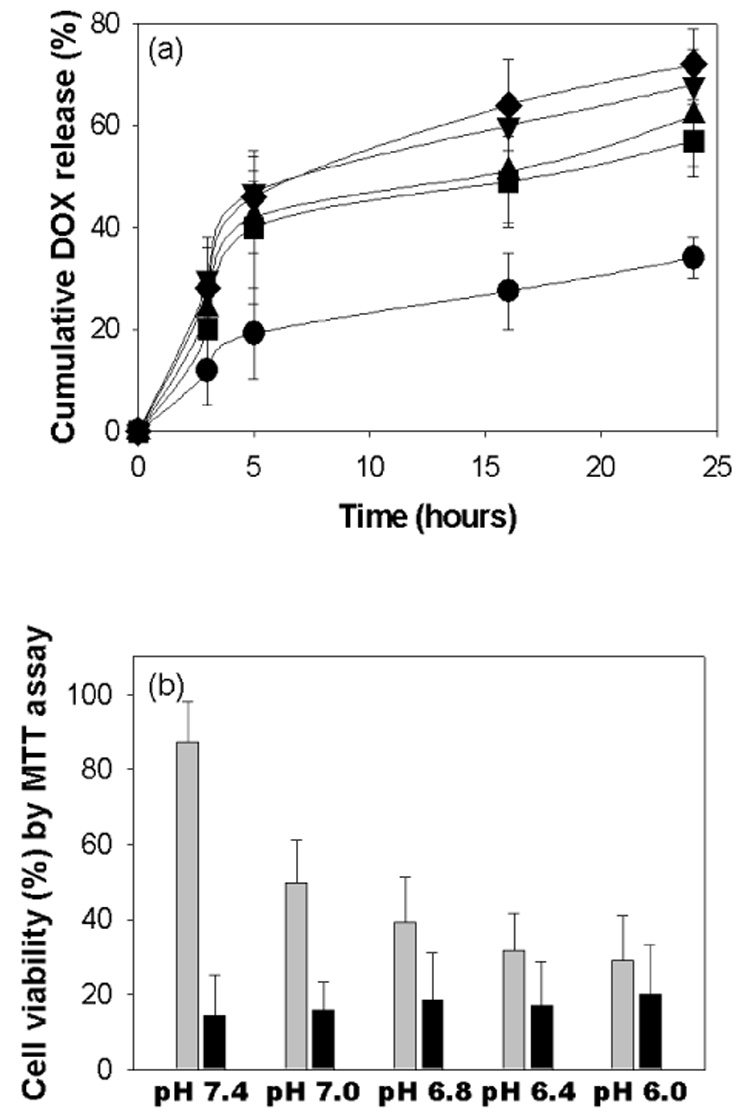
(a) The pH-dependent cumulative DOX release from the PLA-b-PEG-b-polyHis micelle: pH 7.4 (●), pH 7.0 (■), pH 6.8 (▲), pH 6.4 (▼), pH 6.0 (◆) (n=3), and (b) the pH-dependent cytotoxicity of DOX loaded flower-like micelles (■) or free DOX (■) against MCF-7 cells (n=3).
An in vitro cell cytotoxicity test performed with the DOX-loaded PLA-b-PEG-b-polyHis micelles (Fig. 6(b)) demonstrated that the micelles actively killed MCF-7 cells at lower pHs because of enhanced amount of DOX released. The viability of MCF-7 cells treated with the DOX-loaded PLA-b-PEG-b-polyHis micelles was 87% at pH 7.4, 40% at pH 6.8, 30% at pH 6.4 and 26% at pH 6.0. No cytotoxicity was observed up to 100 µg/ml of the blank micelles of PLA-b-PEG-b-polyHis for MCF-7 cells after 24 hours incubation, regardless of culture medium pH (data not shown).
Confocal images of MCF-7 cells treated with the DOX-loaded PLA-b-PEG-b-polyHis micelles are present in Fig. 7. The DOX accumulation in MCF-7 cells differed with pH. At pH 7.0, a high intracellular DOX concentration was visualized with red intensity of DOX. This is due to the accelerated DOX release from the micelles at this pH, while at pH 7.4 the tumor cells exhibited limited internalization of DOX.
Fig. 7.
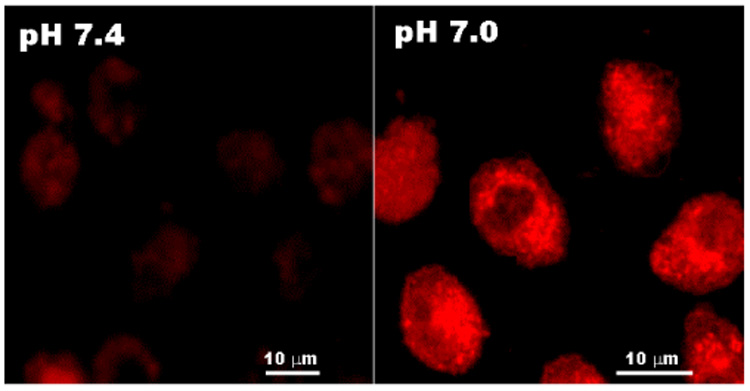
Confocal images of MCF-7 cells treated with the DOX-loaded PLA-b-PEG-b-polyHis micelles at different pH condition (pH 7.4 and 7.0).
These results confirmed that the PLA-b-PEG-b-polyHis micelles distinguish tumor extracellular pH by an accelerated drug release rate.
4. Conclusion
PLA-b-PEG-b-polyHis was constituted to a novel pH-sensitive flower-like polymeric micelle. The micelles (~ 80 nm in diameter) showed triggered release of DOX on decreasing pH from 7.4 to 6.0. From an in vitro cell viability study, it was found that DOX-loaded micelles were advantageous for tumor cells killing because the triggering pH for DOX release was around tumor pHe (pH 7.2-6.6) and there was minimal cytotoxicity at pH 7.4. This approach may provide maximal therapeutic efficacy in the tumor site resulting from increased drug accumulation, while having the lowest probability of drug accumulation in normal tissues for reduced side effects. However, this hypothesis requires further investigation for proof.
Acknowledgments
The authors would like to thank Ajay Taluja (University of Utah) for carefully editing this manuscript. This work was supported by NIH CA101850.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J. Control. Release. 2005;101:59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Nakanishi T, Fukushima S, Okamoto K, Suzuki M, Matsumura Y, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Development of the polymer micelle carrier system for doxorubicin. J. Control. Release. 2001;74:295–302. doi: 10.1016/s0168-3659(01)00341-8. [DOI] [PubMed] [Google Scholar]
- 3.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. Targeted polymeric micelles for delivery of poorly soluble drugs. Cell Mol. Life Sci. 2004;61:2549–2559. doi: 10.1007/s00018-004-4153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponce AM, Vujaskovic Z, Yuan F, Needham D, Dewhirst MW. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperthermia. 2006;22:205–213. doi: 10.1080/02656730600582956. [DOI] [PubMed] [Google Scholar]
- 6.Pitt WG, Husseini GA, Staples BJ. Ultrasonic drug delivery-a general review. Expert Opin. Drug Deliv. 2004;1:37–56. doi: 10.1517/17425247.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berret JF, Schonbeck N, Gazeau F, EI Kharrat D, Sandre O, Vacher A, Airiau M. Controlled clustering of superparamagnetic nanoparticles using block copolymers: design of new contrast agents for magnetic resonance imaging. J. Am. Chem. Soc. 2006;128:1755–1761. doi: 10.1021/ja0562999. [DOI] [PubMed] [Google Scholar]
- 8.Veronese FM, Schiavon O, Pasut G, Mendichi R, Andersson L, Tsirk A, Ford J, Wu G, Kneller S, Davies J, Duncan R. PEG-doxorubicin conjugates: influence of polymer structure on drug release, in vitro cytotoxicity, biodistribution, and antitumor activity. Bioconjug. Chem. 2005;16:775–784. doi: 10.1021/bc040241m. [DOI] [PubMed] [Google Scholar]
- 9.Khandare JJ, Jayant S, Singh A, Chandna P, Wang Y, Vorsa N, Minko T. Dendrimer versus linear conjugate: Influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjug. Chem. 2006;17:1464–1472. doi: 10.1021/bc060240p. [DOI] [PubMed] [Google Scholar]
- 10.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberq B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc. Natl. Acad. Sci. USA. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park EK, Kim SY, Lee SB, Lee YM. Folate-conjugated methoxy poly(ethylene glycol)/poly(epsilon-caprolactone) amphiphilic block copolymeric micelles for tumor-targeted drug delivery. J. Control. Release. 2005;109:158–168. doi: 10.1016/j.jconrel.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Liu SQ, Wiradharma N, Gao SJ, Tong YW, Yang YY. Bio-functional micelles self-assembled from a folate-conjugated block copolymer for targeted intracellular delivery of anticancer drugs. Biomaterials. 2007;28:1423–1433. doi: 10.1016/j.biomaterials.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Vinogradov S, Batrakova E, Li S, Kabanov A. Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells. Bioconjug. Chem. 1999;10:851–860. doi: 10.1021/bc990037c. [DOI] [PubMed] [Google Scholar]
- 14.Gillies ER, Fréchet JM. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconjug. Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]
- 15.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “Smart” drug delivery systems: double-targeting pH-responsive pharmaceutical nanocarriers. Bioconjug. Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bae Y, Nishiyama N, Fukushima S, Koyama H, Yasuhiro M, Kataoka K. Preparation and biological characterization of polymeric micelle drug carriers with intracellular pH-triggered drug release property: tumor permeability controlled subcellular drug distribution, and enhanced in vivo antitumor efficacy. Bioconjug. Chem. 2005;16:122–130. doi: 10.1021/bc0498166. [DOI] [PubMed] [Google Scholar]
- 17.Na K, Lee ES, Bae YH. Adriamycin loaded pullulan acetate /sulfonamide conjugate nanoparticles responding to tumor pH: pH-dependent cell interaction, internalization and cytotoxicity in vitro. J. Control. Release. 2003;87:3–13. doi: 10.1016/s0168-3659(02)00345-0. [DOI] [PubMed] [Google Scholar]
- 18.Na K, Lee KH, Bae YH. pH-sensitivity and pH-dependent interior structural change of self-assembled hydrogel nanoparticles of pullulan acetate/oligo-sulfonamide conjugate. J. Control. Release. 2004;97:513–525. doi: 10.1016/j.jconrel.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Lee ES, Shin HJ, Na K, Bae YH. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release. 2003;90:363–374. doi: 10.1016/s0168-3659(03)00205-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 21.Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- 22.Kim GM, Bae YH, Jo WH. pH-induced micelle formation of poly(histidine-cophenylalanine)-block-poly(ethylene glycol) in aqueous media. Macromol. Biosci. 2005;5:1118–1124. doi: 10.1002/mabi.200500121. [DOI] [PubMed] [Google Scholar]
- 23.Gao ZG, Lee DH, Kim DI, Bae YH. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J. Drug Targeting. 2005;13:391–397. doi: 10.1080/10611860500376741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ES, Na K, Bae YH. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release. 2005;103:405–418. doi: 10.1016/j.jconrel.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Licciardi M, Giammona G, Du J, Armes SP, Tang Y, Lewis AL. New folate-functionalized biocompatible block copolymer micelles as potential anti-cancer drug delivery systems. Polymer. 2006;47:2946–2955. [Google Scholar]
- 26.Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumors. Int. J. Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- 27.Leeper DB, Engin K, Thistlethwaite AJ, Hitchon HD, Dover JD, Li DJ, Tupchong L. Human tumor extracellular pH as a function of blood glucose concentration. Int. J. Radiat. Oncol. Biol. Phys. 1994;28:935–943. doi: 10.1016/0360-3016(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 28.Ojugo ASE, Mcsheehy PMJ, Mcintyre DJO, Mccoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extraceullar pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous 19F and 31P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdán S, Galons JP, Gillies RJ. In vivo imaging of extracellular pH using ¹H MRSI. Magn. Reson. Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Oh KT, Yin H, Lee ES, Bae YH. Polymeric nanovehicles for anticancer drugs with triggering release mechanisms. J. Mater. Chem. (submitted) [Google Scholar]
- 31.Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control. Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benns JM, Choi JS, Mahato RI, Park JS, Kim SW. pH-sensitive cationic polymer gene delivery vehicle: N-Ac-poly(L-histidine)polyHis–graft–poly(L-lysine) comb shaped polymer. Bioconjug. Chem. 2000;11:637–645. doi: 10.1021/bc0000177. [DOI] [PubMed] [Google Scholar]
- 33.Han SK, Na K, Bae YH. Sulfonamide based pH-sensitive polymeric micelles: physicochemical characteristics and pH-dependent aggregation. Colloids Surf. A: Physicochem. Eng. Aspects. 2003;214:49–59. [Google Scholar]
- 34.Preuschen J, Menchen S, Winnik MA, Heuer A, Spiess HW. The aggregation behavior of a symmetric, fluorinated, telechelic polymer system studied by 19F NMR relaxation. Macromolecules. 1999;32:2690–2695. [Google Scholar]
- 35.Berret JF, Calvet D, Collet A, Viguier M. Fluorocarbon associative polymers. Curr. Opin. Colloid Interf. Sci. 2003;8:296–306. [Google Scholar]
- 36.Grassl B, Billon L, Borisov O, Francois J. Poly(ethylene oxide)- and poly(acrylamide)-based water-soluble associative polymers: synthesis, characterization, properties in solution. Polym. Int. 2006;55:1169–1176. [Google Scholar]
- 37.Gourier C, Beaudoin E, Duval M, Sarazin D, Maitre S, Francois J. A light scattering study of the association of hydrophobically modified poly(ethylene oxide) in water. J. Colloid Interface Sci. 2000;230:41–52. doi: 10.1006/jcis.2000.7033. [DOI] [PubMed] [Google Scholar]
- 38.Bossard F, aubry T, Gotzamanisb G, Tsitsilianis C. pH-Tunable rheological properties of a telechelic cationic polyelectrolyte reversible hydrogel. Soft Matter. 2006;2:510–516. doi: 10.1039/b601435f. [DOI] [PubMed] [Google Scholar]
- 39.Campbell RB. Tumor physiology and delivery of nanopharmaceuticals. Anti-cancer agents in medical chemistry. 2006;6:503–512. doi: 10.2174/187152006778699077. [DOI] [PubMed] [Google Scholar]
- 40.Park KH, Song HC, Na K, Bom HS, Lee KH, Kim S, Kang D, Lee DH. Ionic strength-sensitive pullulan acetate nanoparticles (PAN) for intratumoral administration of radioisotope; ionic strength-dependent aggregation behavior and 99mTechnetium retention property. Colloids Surfaces B: Biointerfaces. doi: 10.1016/j.colsurfb.2007.04.010. (in press) [DOI] [PubMed] [Google Scholar]
- 41.Song HC, Na K, Park KH, Shin CH, Bom HS, Kang D, Kim S, Lee ES, Lee DH. Intratumoral administration of rhenium-188 labeled pullulan acetate nanoparticles (PAN) in mice bearing CT-26 cancer cells for suppression of tumor growth. J. Microbiol Biotechn. 2006;16:1491–1498. [Google Scholar]


