Abstract
We used molecular xenomonitoring (MX, detection of filarial DNA in mosquitoes) to evaluate the impact of mass drug administration (MDA) in sentinel locations in Egypt with high (11.5%) and low (4.1%) baseline microfilaria prevalence rates. Blood-fed Culex pipiens were pooled by household and tested for Wuchereria bancrofti DNA by PCR. There was no significant relationship between the infection status of household residents and parasite DNA status of mosquitoes from the same houses. After 5 MDA rounds, parasite DNA rates in mosquitoes in high- and low-prevalence areas were reduced by 93.8% and 100% to 0.19% (95% CI: 0.076–0.382%) and 0% (95% CI: 0–0.045%), respectively. These changes were consistent with decreases in microfilaria prevalence rates in these sites; they provide insight regarding the minimal mosquito DNA rates necessary for sustained transmission of filariasis in Egypt. We conclude that MX is a powerful tool for monitoring the impact of MDA on filariasis endemicity and transmission.
INTRODUCTION
Lymphatic filariasis (LF) is a major tropical disease with an estimated 120 million people infected in 83 countries and some 1,300 million at risk of acquiring the infection.1 The World Health Assembly resolved to eliminate lymphatic filariasis as a public health problem in 1997.2 The Global Program for Elimination of Lymphatic Filariasis (GPELF) is based on a strategy of mass drug administration (MDA) of 4–6 annual rounds of antifilarial medications with the goal of reducing the reservoir of blood microfilariae (MF) below the level required for transmission by mosquitoes.3
Filariasis is focally endemic in Egypt. The mosquito vector responsible for transmission of filariasis in Egypt is Culex pipiens. This mosquito is widely distributed and extremely abundant throughout the country. It is mainly anthropophagic, endophagic, and endophilic.4 Prior to the initiation of MDA in 2000, ~137,000 people in 181 villages and towns were infected with Wuchereria bancrofti, and 2.7 million were at risk of acquiring the infection.5,6 Egypt was one of the first countries that initiated a National Program for Elimination of Lymphatic Filariasis (NPELF) based on WHO guidelines. The program comprised 5 annual rounds of MDA with diethylcarbamazine (DEC, 6 mg/kg) and albendazole (400 mg) in all known endemic areas in the country. MDA was distributed in September of each year from 2000 through 2004 to all eligible people in endemic areas (excluding pregnant women and children < 2 years of age). Reported coverage rates for the program exceeded 90% in all years. Approximately 2.7 million people were targeted in the 5th round of MDA (100% of the at-risk population in 2004).6
Molecular xenomonitoring (MX) employs PCR to detect filarial DNA in wild-caught mosquitoes. Prior studies have suggested the potential of this method for assessing the success of filariasis elimination.5,7 Our early studies showed that MX could be used to estimate the relative prevalence of W. bancrofti infection in villages with high and low filariasis prevalence rates.8 These studies used the SspI PCR assay to test pools of indoor-resting Cx. pipiens. Although dissection can be used to detect filarial parasites in mosquitoes, MX becomes particularly valuable after implementation of MDA programs, when mosquito infection rates are reduced to levels that cannot be accurately assessed by dissection. MX has the potential to be a sensitive method for detecting persistent filarial parasites in communities. It should also provide an indirect indication of the potential for ongoing filariasis transmission.9,10
The purpose of this study was to evaluate MX as a tool for assessing the impact of a national filariasis elimination program in well-characterized sentinel sites. Our results show that MX can be a practical and efficient tool for assessing the impact of MDA in communities; it may also be useful for determining endpoints for LF elimination programs.
METHODS
Study sites
Mosquito collections were carried out in 2 sentinel study sites at approximately the same time as blood surveys that were also conducted by our group. The study sites included a high-prevalence area in Giza governorate (Giz) and a low-prevalence area in Qalubiya governorate (Qal) that had MF prevalence rates of 11.5% and 3.1% and community MF loads of 0.534 and 0.114, respectively, just prior to the first round of MDA in 2000.11 The Giz study area (45 km south of Cairo) included the 2 contiguous villages of Kafr Bahary (KB) with 1,057 houses and Kafr Qebly (KQ) with 1,014 houses. These villages had no significant antifilarial treatment prior to initiation of MDA. The Qal study area included two adjacent villages (Kafr Tahoria, KT, with 208 houses and Tahoria, TH, with 852 houses) in the southeastern Nile delta ~35 km NE of Cairo. The Qal villages were more typical of localities included in the Egyptian NPELF than the Giz villages because they had low infection rates and a history of treatment of filariasis prior to initiation of the national program in 2000. We have reported results of prior studies of filariasis in the Qal villages.12,13 KT was mass-treated with DEC (6 mg/kg) in 1998; ~20% of TH residents were screened in the same year, and infected subjects were selectively treated with DEC. Population estimates for KB, KQ, and TH are between 5,000 and 6,000; ~1,500 people live in KT. Study sites were mapped, and houses were sequentially numbered. Households were randomly selected for assessment of filarial infections using EpiInfo software, version 6.14 Approximately 10–20% of the houses in these villages were studied each year.
Blood collection and screening for W. bancrofti infection
Population surveys were conducted 2–4 months prior to the first round of MDA and repeated each year just before the next round of MDA for 5 consecutive years as previously described.11 Briefly, subjects were tested for circulating filarial antigen (CFA) with a rapid-format card test; persons with positive antigen tests were tested for microfilaremia by membrane filtration of 1 mL of night blood. Persons with negative antigen tests were considered to be amicrofilaremic.15
Mosquito collections
Mosquitoes were collected in 150–200 houses per year in each of the 2 study areas, with new houses sampled each year. Collections were carried out from May to August for 6 successive years (from 2000 to 2005), 1–4 months pre-MDA, and 8–11 months after each of 5 annual rounds of MDA. Individual houses were visited on a weekly basis for 1 month (4–5 visits per house). Sampling was conducted in each village approximately the same time each year. Trained field technicians aspirated resting mosquitoes on walls and beneath furniture in bedrooms from 10 PM to 1 AM (about 10 minutes/house). Female Cx. pipiens were counted and classified according to their abdominal appearance as blood-fed, gravid, or empty (neither blood-fed nor gravid) and transferred into labeled 1.5-mL Eppendorf tubes that were stored at −70°C for later detection of W. bancrofti DNA. Only blood-fed and gravid mosquitoes were retained for PCR analysis; empty mosquitoes were discarded. Mosquitoes from houses with > 25 fed or gravid females were divided into equal pools for PCR testing.
Detection of W. bancrofti DNA in Cx. Pipiens
We used a standard PCR protocol for detecting W. bancrofti DNA in mosquitoes.9 This method uses NV-1 and NV-2 primers to amplify the SspI DNA repeat sequence.16 The test detects 1 pg of genomic W. bancrofti DNA, which is < 1% of the DNA content of a single microfilaria. The 188-bp amplified product was detected by agarose gel electrophoresis. Each PCR run included 2 types of negative control (DNA from unfed Cx. pipiens and a no-template control). Three types of positive controls were also tested in each PCR run: a dissected mosquito that contained 1 or more filarial worms, DNA from non-blood-fed mosquitoes that was spiked with purified W. bancrofti DNA, and purified SspI-DNA PCR product.
Estimation of filarial DNA rates in resting mosquitoes
Parasite DNA rates in mosquitoes (maximum likelihood with 95% CI) were estimated by PoolScreen 2.0 software as previously described.17 We also analyzed PCR results in terms of the percent of positive pools and percent of houses with positive mosquitoes. The distribution of filarial infections within each study site was assessed by scoring houses as being in the “core” or “periphery” of the village. Peripheral houses face vacant lots or agricultural land on at least one side, whereas core houses are surrounded by other houses.
Statistical analysis
Data were analyzed with SPSS 11.0 software (SPSS, Inc., Chicago, IL). Proportions were compared by χ2 or Fisher exact tests. The Mann–Whitney U test was used to compare group means for nonparametric data.
Ethical clearance
This study was reviewed and approved by institutional review boards at Ain Shams University and at Washington University School of Medicine. The Egyptian Ministry of Health and Population also approved the study.
RESULTS
General description of study areas
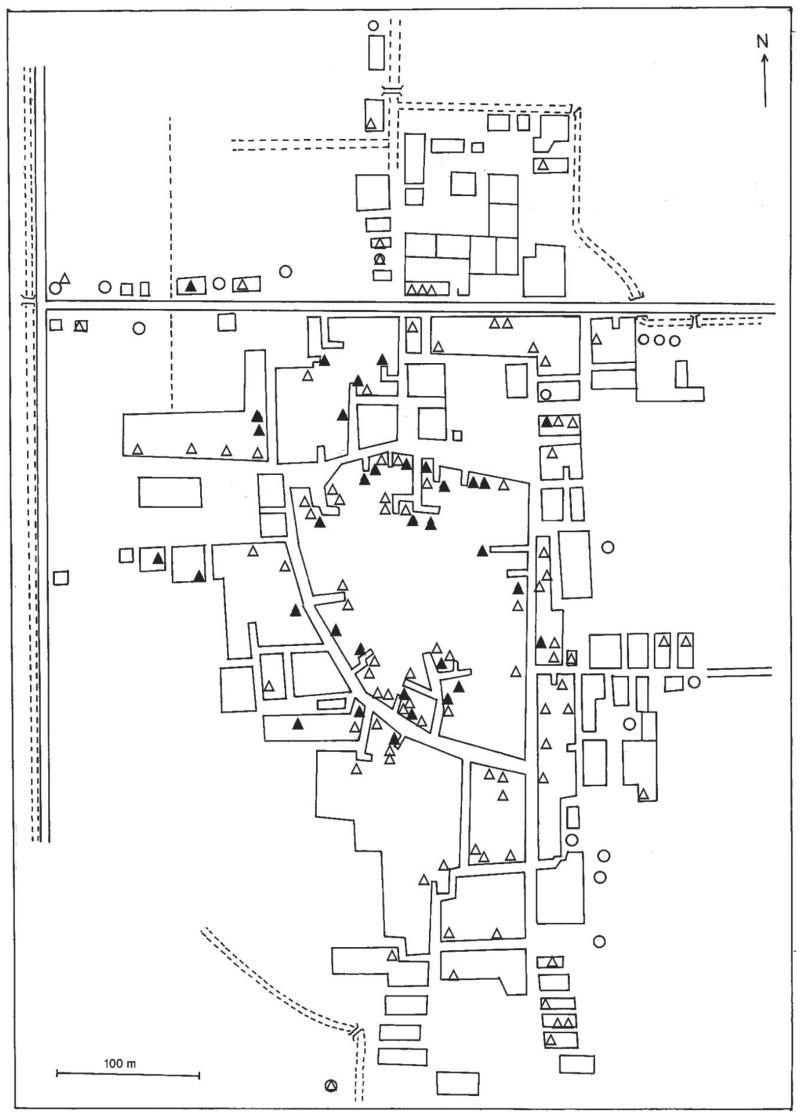
There were 1,060 houses in the Qal study area. Figure 1 shows that the 130 houses surveyed before the first round of MDA in TH were distributed throughout the village. The major mosquito breeding sites identified in TH were 2 cesspools in the northern and NE edges of the village. The major mosquito breeding sites in KT were 2 abandoned wells near the edge of the village in the northeast sector. The Giz study area consisted of 2,071 houses. The main mosquito breeding site in Giz is a polluted drainage canal that runs along the east side of the study area.
Figure 1.
Map of Tahoria village in the Qal study area, showing households where mosquitoes were collected for PCR testing prior to the first round of mass drug administration (MDA): ▲, houses with mosquitoes positive for filarial DNA by PCR; △, houses with mosquitoes negative for filarial DNA by PCR; housing blocks are shown as squares; dashed lines represent irrigation and drainage canals.
Mosquito collection and the influence of the number of mosquitoes per pool on detection of W. bancrofti
Cx. pipiens comprised > 99% of indoor-resting mosquitoes collected in both study areas. Other species included Ochlerotatus caspius, Anopheles tenebrosus, and Anopheles pharoensis. In the months just prior to the first round of MDA, 6,571 female Cx. pipiens were collected from 223 houses in Giz in 946 house-nights (7.0 ± 5.6 [SD] mosquitoes per house-night). Of these, ~50.0% were blood-fed or gravid (14.7 ± 8.7 per house collected in 4 or 5 nights). In Qal, 2,671 Cx. pipiens females were captured from 179 houses in 728 house-nights (3.7 ± 3.0 per house-night), and 44.9% of the females were blood-fed or gravid (6.6 ± 4.4 per house collected in 4 or 5 nights). Thus mosquito densities were higher in Giz than in Qal (P < 0.001). The number of mosquitoes collected per house-night tended to increase slightly over the course of the study (data not shown). Therefore, decreased infection rates in humans and mosquitoes after MDA did not result from changes in mosquito densities in the study areas.
Figure 2 shows the relationship between the number of mosquitoes tested per house and the percent of houses with mosquitoes that contained W. bancrofti DNA for each year of the study. The pre-MDA data show that the percent of positive pools increased with pool size in both study areas. The proportion of positive pools decreased after MDA for both large and small mosquito pools. These differences were significant for pool sizes of 10 or more mosquitoes in both areas (P ≤ 0.01). We consider pools with < 10 mosquitoes to be suboptimal for MX assessment of filarial DNA in mosquitoes by household.
Figure 2.
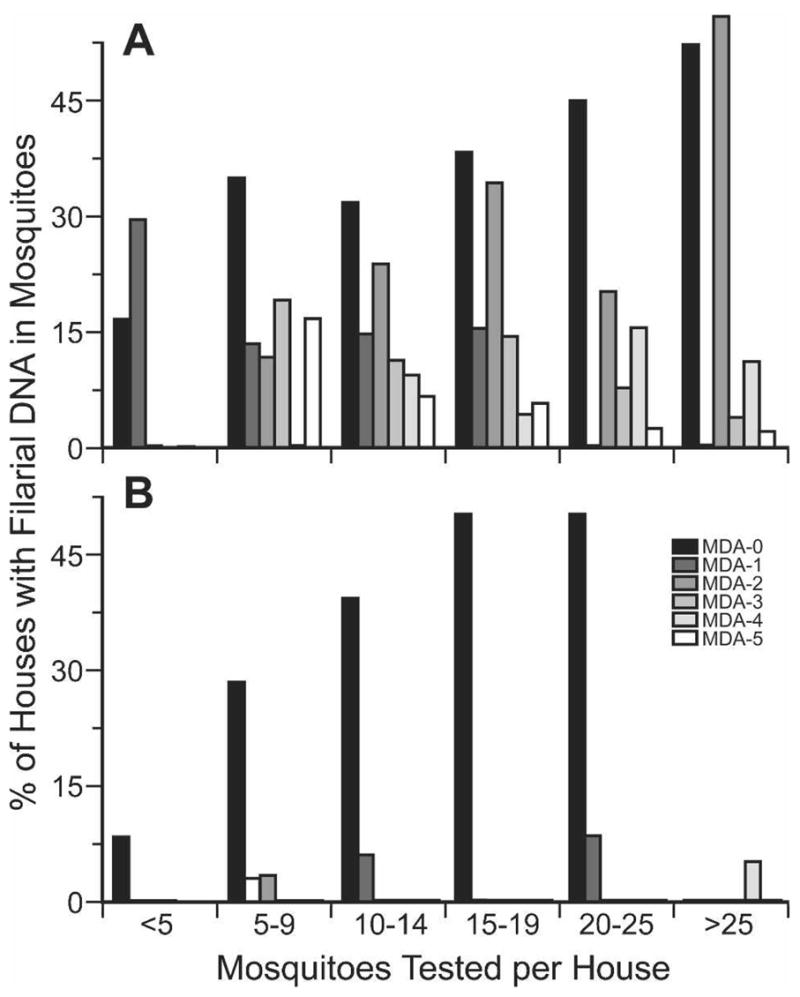
Effect of mosquito sample size on the percentage of houses with Wuchereria bancrofti DNA in Culex pipiens in the Giz (A) and Qal (B) study areas. Histograms show results obtained before the first round of mass drug administration (MDA) and after each round of MDA.
Figure 3 shows frequency distributions for the number of blood-fed or gravid mosquitoes collected in houses in the 2 study areas, pre-MDA. Despite 4–5 visits per house, < 10 females were recovered in many of the houses in both study areas (74.3% of 179 houses studied pre-MDA in Qal, and 35.0% of 223 houses in Giz). This is a significant limitation for using the percent of positive houses to follow parasite DNA rates in mosquitoes in endemic areas. In contrast, PoolScreen 2.0 takes pool size into account for estimation of parasite DNA rates in mosquitoes. However, analysis of PCR results by household should provide information on the general location of mosquitoes with parasite DNA in a study area.
Figure 3.
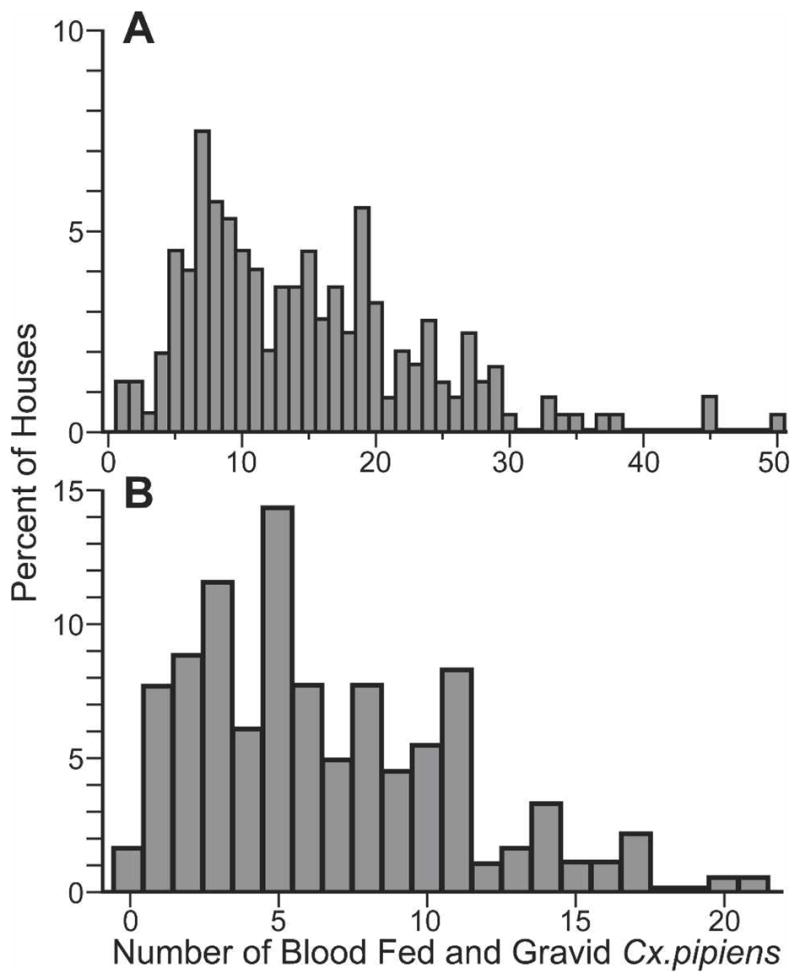
Frequency distributions for the number of houses with different numbers of gravid or blood-fed Culex pipiens captured before treatment in the Giz (A) and Qal (B) study areas.
Impact of MDA on parasite DNA in household mosquito collections (poolwise analysis)
Five rounds of MDA significantly reduced the frequency of positive mosquito pools in both study areas (P ≤ 0.001) for pools with ≥ 5 females (Figure 4) and also for houses with pools of ≥ 10 females (data not shown). Parasite DNA rates in mosquitoes decreased more quickly in Qal (with the low baseline MF prevalence rate) than in Giz.
Figure 4.
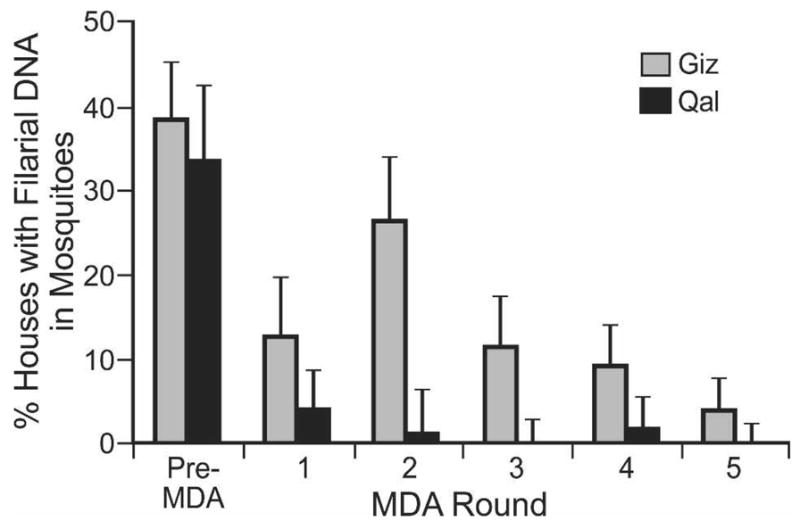
Impact of mass drug administration on the percent of houses with Wuchereria bancrofti DNA in Culex pipiens in the Giz (gray bars) and Qal (black bars) study areas. Data are shown for households with mosquito pool sizes ≥ 5; error bars show 95% confidence limits.
Impact of MDA on filarial DNA rates in mosquitoes (poolwise and PoolScreen analyses)
Table 1 shows parasite DNA rates in mosquitoes in the 2 study areas before and after MDA. This analysis includes all mosquito pools. Baseline infection rates were similar in the 2 areas prior to MDA. Parasite DNA rates in mosquitoes and the percentage of positive pools declined more rapidly after MDA in Qal than in Giz. Five rounds of MDA decreased the parasite DNA rate in Giz mosquitoes by 93.8%, from 3.066% to 0.190%, and the percentage of mosquito pools with parasite DNA decreased by 90.2%. The MF prevalence rate in humans in Giz decreased from 11.5% to 1.2% by membrane filter during this time (89.6% decrease); the post-MDA MF rate by 50-μL thick smear was 0.3%.
Table 1.
Impact of 5 annual rounds of mass drug administration (MDA-0 to MDA-5) of single-dose DEC/Alb on Wuchereria bancrofti DNA rates in Culex pipiens collected in the Giz and Qal study areas
| Mosquitoes
|
|||||||
|---|---|---|---|---|---|---|---|
| PoolScreen mosquito infection estimates
|
|||||||
| Study area | MDA round | No. tested | No. of pools | Mosq./pool ± SD | % Infected pools | % | 95% CI |
| Giz | MDA-0 | 3,270 | 248 | 13.2 ± 5.8 | 33.5 | 3.07 | 2.38–3.88 |
| MDA-1 | 1,446 | 155 | 9.3 ± 5.4 | 15.5 | 1.76 | 1.08–2.68 | |
| MDA-2 | 2,364 | 155 | 15.3 ± 5.1 | 24.5 | 1.84 | 1.25–2.58 | |
| MDA-3 | 2,543 | 174 | 14.6 ± 5.0 | 9.8 | 0.70 | 0.38–1.14 | |
| MDA-4 | 3,970 | 252 | 15.8 ± 4.5 | 7.1 | 0.47 | 0.27–0.77 | |
| MDA-5 | 4,273 | 245 | 17.4 ± 4.3 | 3.3 | 0.19 | 0.08–0.38 | |
| Qal | MDA-0 | 1,197 | 179 | 6.7 ± 4.3 | 24.6 | 4.37 | 3.07–5.99 |
| MDA-1 | 1,832 | 153 | 12.0 ± 5.2 | 3.3 | 0.28 | 0.08–0.66 | |
| MDA-2 | 1,374 | 136 | 10.1 ± 6.4 | 0.7 | 0.07 | 0.00–0.38 | |
| MDA-3 | 2,110 | 151 | 14.0 ± 5.0 | 0.0 | 0.00 | 0.00–0.09 | |
| MDA-4 | 3,730 | 220 | 17.0 ± 3.9 | 1.4 | 0.08 | 0.02–0.24 | |
| MDA-5 | 4,258 | 237 | 18.0 ± 3.7 | 0.0 | 0.00 | 0.00–0.05 | |
Five rounds of MDA decreased the parasite DNA rate in mosquitoes, percent positive mosquito pools, and MF prevalence by 100% in Qal. Indeed, only a few positive mosquito pools or MF carriers were detected in Qal after the second round of MDA.
Effect of location in the village on mosquito and human infection rates
Prior studies in Egypt have suggested that filariasis infection rates are higher in peripheral houses (houses that face vacant or agricultural land) than in core houses (houses that are surrounded by other houses).18 Therefore, we compared human infection rates and parasite DNA rates in mosquitoes in peripheral and core houses pre-MDA in Giz and Qal (Table 2). Human infection and parasite DNA rates in mosquitoes were essentially the same in core and peripheral houses in Giz. Human infection rates were higher in peripheral houses in the Qal study area, although only the filarial antigen test difference was statistically significant. The parasite DNA rate in Qal mosquitoes was higher in core houses, but this difference was not statistically significant. Thus, household location (core versus periphery) was not a major risk factor for filariasis in these study areas.
Table 2.
Human infection rates and parasite DNA rates in Culex pipiens from the Giza (Giz) and Qalubiya (Qal) study areas before mass drug administration
| Mosquitoes
|
||||||
|---|---|---|---|---|---|---|
| Residents
|
PoolScreen
|
|||||
| Study area | Location* | No. | ICT card, % Positive | MF filter, % Positive | No. of pools | % Infected (95% CI) |
| Giz | Periphery | 517 | 18.4 | 10.6 | 126 | 3.12 (2.20–4.28) |
| Core | 322 | 18.6 | 12.1 | 83 | 3.76 (2.43–5.50) | |
| P | 0.998 | 0.585 | ||||
| Qal | Periphery | 310 | 18.7 | 3.5 | 64 | 3.48 (1.79–5.94) |
| Core | 193 | 8.3 | 2.1 | 46 | 6.54 (3.39–11.11) | |
| P | 0.005 | 0.356 | ||||
“Periphery” refers to houses that faced vacant land on at least one side. “Core” houses were surrounded by other houses on all sides.
Relationship between filarial DNA in mosquitoes and human filariasis in households
Households with one or more MF carriers before MDA did not have significantly higher filarial DNA rates in mosquitoes than houses without MF carriers (Table 3). Mosquitoes with parasite DNA were sometimes detected in households where none of the tested residents had MF, whereas no PCR-positive mosquitoes were detected in some houses with MF-positive residents. After MDA-5, MF-positive people were detected in 5 houses in Giz, but no filarial DNA was detected in mosquitoes collected in these houses. No MF carriers and no DNA-positive mosquitoes were detected in Qal houses after MDA-5.
Table 3.
Relationship between rates of filarial DNA in Culex pipiens and human infections in Giz and Qal households before mass drug administration
| Mosquitoes
|
||||||||
|---|---|---|---|---|---|---|---|---|
| PoolScreen mosquito infection estimates
|
||||||||
| Study area | Household status* | No. of houses tested | No. tested | No. of pools | Mosq./pool ± SD | % Pools positive for filarial DNA | % | 95% CI |
| Giz | MF-negative | 127 | 1,797 | 138 | 13.0 ± 5.8 | 32.6 | 3.0 | 2.14–4.16 |
| MF-positive | 61 | 948 | 70 | 13.5 ± 5.9 | 41.4 | 3.9 | 2.49–5.72 | |
| Qal | MF-negative | 112 | 772 | 112 | 6.9 ± 4.5 | 27.7 | 4.9 | 3.22–7.15 |
| MF-positive | 14 | 84 | 14 | 6.0 ± 3.8 | 21.4 | 4.3 | 0.83–12.03 | |
“MF-negative” means that no microfilaria carriers were identified in the household sample; “MF-positive” means that at least 1 person tested in the house had microfilaremia.
DISCUSSION
This study was conducted in 2 areas in Egypt in the context of its NPELF. The Qal study area had a low filariasis prevalence rate before the initiation of MDA in September 2000. This area was fairly typical of filariasis-endemic villages in Egypt just prior to the NPELF. In contrast, Giz had very high filariasis endemicity (for Egypt) pre-MDA. This was probably due to a lack of prior systematic treatment in the area, high mosquito densities, and perhaps other uncharacterized local conditions favorable for transmission.
We have previously shown that MX is useful for comparing filariasis endemicity levels in different areas.8 Several prior studies have used PoolScreen to estimate filarial DNA rates in culicines. For example, Goodman and co-workers, using 20-μL thick smears,10 found a filarial DNA rate in mosquitoes of 7.2% in an area in Haiti where 11.0% of the people had microfilaremia. A study from Egypt reported a filarial DNA rate in mosquitoes of 8.1% by MX with PoolScreen when the MF prevalence rate by 50-μL smear was 9.5%.17 Lower parasite DNA rates in mosquitoes in the current study are generally consistent with these published results, given that the MF rates in the present study are based on membrane filtration of venous blood rather than thick smear. We were surprised to see equivalent pre-MDA parasite DNA rates in mosquitoes in Giz and Qal because of the difference in MF rates in these areas. Limitation (enhanced ability of Culex mosquitoes to ingest MF at low blood MF levels) may have contributed to this finding.19,20 We doubt that infection rates in Qal were inflated by contamination in the laboratory, because we included negative controls in each PCR run. It is possible that mosquitoes were older (with more prior feedings on average) in Qal than in Giz. A prior study by our group showed that differences in rates of daily mosquito survival and survival to infectivity might explain local differences in filariasis endemicity.21 Because PCR can detect parasite DNA taken up at any time during a mosquito’s life, age could certainly affect parasite DNA rates in mosquitoes.22 We did not assess mosquito parity in this study. In any case, we believe that comparison of parasite DNA rates in selected areas over time (as in this study) is more useful than comparisons between villages at a single point in time.
Prior studies have reported clustering of mosquitoes and human filarial infections at the edges of villages where larval breeding sites are concentrated and where residents are exposed to more mosquito bites.18,23,24 Although the main mosquito breeding sites were in peripheral locations in both of our study sites, we did not observe significantly higher parasite DNA rates in mosquitoes, higher human infection rates, or higher mosquito abundance (data not shown) in peripheral houses in this study.
The Egyptian NPELF achieved high MDA coverage rates, and this had a dramatic impact on MF rates in humans.11 Our study shows that MDA also had a dramatic impact on parasite DNA in mosquitoes whether this is expressed as the percent of mosquitoes with parasite DNA using PoolScreen or as the percent of houses with parasite DNA in mosquitoes. MDA had a more rapid impact on both measures of parasite DNA in mosquitoes in Qal, where MF prevalence rates and counts had already been decreased by treatment prior to initiation of MDA in 2000.
Researchers in Papua New Guinea and Haiti have reported significant declines in W. bancrofti infection rates in mosquitoes by dissection after MDA.10,25 However, this is the first study that has demonstrated dramatic decreases and clearance of filarial DNA in mosquitoes after MDA using MX. Very large numbers of mosquitoes have to be tested to detect low residual filarial infection rates in mosquitoes after MDA and to show the absence of filarial infections with any degree of certainty. MX is superior to dissection for this purpose because of its high sensitivity and its high-throughput capability. For example, we detected a residual parasite DNA rate of 0.19% (CI: 0.076–0.38) in Giz mosquitoes after MDA-5 with 4,273 mosquitoes in 245 pools. Confidence limits for parasite DNA rates in mosquitoes become smaller with larger mosquito samples. However, as with dissection, there are practical limits to the number of mosquitoes that can be captured and tested by PCR.
At this stage of the GPELF, when a number of countries have completed 5 or more rounds of MDA, there is heightened interest in endpoints or targets that program managers can use to decide when to stop MDA.26 A recent paper suggested a post-MDA target of 0.5% MF for culicine-transmitted filariasis.27 Based on our experiences with MX in Egypt, we believe it is reasonable for MDA programs to aim for residual parasite DNA rates in mosquitoes with an upper confidence limit of 0.25%. This is a conservative target that might be lower than necessary. In practical terms, this target is met if no more than 3 of 200 pools of 15 fed or gravid mosquitoes are positive by PCR. This proposed target applies to Culex mosquitoes. Other targets will be needed for areas where filariasis is transmitted by Anopheles or Aedes mosquitoes. The parasite DNA rate in Giz mosquitoes was just over our proposed target after MDA-5 (0.38%), when the MF rate in Giz was 1.2% by filter and 0.3% by 50-μL blood smear. Parasite DNA rates met this target in Qal after MDA rounds 3–5, when MF rates in the population were well under 0.5% by filter. The target might also have been achieved in Qal after MDA-2 (when the residual MF rate was 0.6% by filter) if more mosquitoes had been tested. The small number of mosquitoes and pools tested that year limited the power of MX to show that the target had been reached. Of course, low residual rates of parasite DNA in mosquitoes do not necessarily mean that filariasis transmission is ongoing in an area. Prior studies by our group showed that mosquitoes fed on people with very low MF levels sometimes ingested MF but rarely produced infective larvae.28 These mosquitoes would have been scored as positive by PCR. More data are needed on relationships between MF rates and other parameters after MDA to firm up targets for MDA programs.
More work is needed to develop better guidelines for mosquito sampling for monitoring by dissection or MX. A WHO workshop report recommended collection of at least 1,000 mosquitoes (10–50 mosquitoes per house, with 100–250 households) per sentinel site prior to MDA.5 More mosquitoes are needed to detect parasites by MX or dissection when infection rates are reduced by MDA.5,29,30 Our results confirm that large mosquito samples are critical for detecting W. bancrofti infection during late stages of a MDA program. Pool infection rates were higher for larger pools. This emphasizes the advantage of following PoolScreen estimates of DNA rates in mosquitoes over the simpler parameter “percent of positive pools.”
We were able to obtain sufficient samples of resting mosquitoes for MX studies in this research project. However, the mosquito capture method we used is labor intensive and intrusive to village residents; our experienced field teams collected only 3.8 ± 3.5 (median 3) blood-fed or gravid mosquitoes per house-night. This method is probably not practical for large-scale evaluation of national elimination programs. Gravid traps, which capture blood fed culicine mosquitoes in the act of oviposition, might be more practical for conducting MX on a large scale. Although filarial DNA rates in gravid trap mosquitoes tend to be somewhat lower than those in resting mosquitoes (Gad AM and others, unpublished data), we have found that gravid traps are much more efficient than aspiration of resting mosquitoes for collecting large numbers of Cx. pipiens for MX.
Improvements are also needed on technical aspects of MX for it to be practical for use in large-scale filariasis elimination programs. DNA isolation and PCR methods used in this study are labor-intensive and inefficient. New methods for isolation of DNA from mosquitoes and real-time PCR (with better sensitivity and throughput capacity relative to conventional PCR) promise to greatly improve our capacity to test large numbers of mosquitoes.31 Work is also ongoing to further improve the statistical analysis and interpretation of MX data.
We found no significant relationship between filarial infections in humans and filarial DNA in mosquitoes in individual households. Filarial DNA was sometimes detected in mosquitoes collected in houses with no MF carriers and sometimes not detected in houses with MF carriers. Thus, MX was not useful for identification of households with MF carriers in this study. Such households may be more efficiently detected by pooling night blood samples by household for PCR testing in areas where filarial DNA is detected in mosquitoes. Complex blood feeding behavior may have contributed to the discrepancy between MF and MX test results. MF carriers may have been away from their houses when mosquitoes were blood feeding, or they may have not served as a blood source for collected mosquitoes. Positive mosquitoes may have taken blood from microfilaremic visitors in or near houses where they were captured, or they may have ingested MF in prior feedings. They also may have fed on microfilaremic household members who did not have blood collected for MF testing. Endophagic mosquitoes like Cx. pipiens rest indoors to release excess fluid from ingested blood for 6–8 hours after feeding, and they fly away once this process is completed.32,33 However, studies in Egypt and India have shown that blood-fed mosquitoes sometimes move between houses.8,34,35 Therefore, the lack of concordance between the presence of MF carriers and DNA-positive mosquitoes in the same houses is not too surprising.
In conclusion, this study has provided interesting data on the use of MX in the context of filariasis elimination programs. Our data show that MX is a powerful tool for assessing the impact of MDA, and we have suggested a MX-based target that might be used with other targets such as MF rate for filariasis elimination programs in areas where filariasis is transmitted by Culex mosquitoes. We have also highlighted limitations of current methods and suggested changes that may make MX more practical for large-scale use.
Acknowledgments
We acknowledge the contributions of the laboratory and field technical staff of the Research and Training Center on Vectors of Diseases. This project would not have been possible without their dedication and effort in activities such as late-night mosquito collection and careful laboratory work over a period of many years.
Financial support: This work was supported by NIH grants AI-35855 and AI-65715.
Footnotes
Publisher's Disclaimer: Disclaimer: The filiarial card test used in this study uses reagents licensed from Barnes–Jewish Hospital, an affiliation of G.J. Weil. All royalties from the sales of this test are donated to the Barnes–Jewish Hospital Foundation, a registered not-for-profit organization. This statement is made in the interest of full disclosure and not because the authors consider this to be a conflict of interest.
References
- 1.World Health Organization. Global program to eliminate lymphatic filariasis. Wkly Epidemiol Rec. 2006;81:221–232. [PubMed] [Google Scholar]
- 2.World Health Organization. Elimination of lymphatic filariasis as a public health problem—resolution of the executive board of the WHO. 50th World Health Assembly; May 6, 1997; Geneva, Switzerland. Geneva: WHO; 1997. p. WHA 50.29. [Google Scholar]
- 3.Ottesen EA, Ismail MM, Horton J. The role of albendazole in programmes to eliminate lymphatic filariasis. Parasitol Today. 1999;15:382–386. doi: 10.1016/s0169-4758(99)01486-6. [DOI] [PubMed] [Google Scholar]
- 4.Gad AM, Riad IM, Farid HA. Host-feeding patterns of Culex pipiens and Culex antennatus (Diptera: Culicidae) from a village in Sharqiya Governorate, Egypt. J Med Entomol. 1995;32:573–577. doi: 10.1093/jmedent/32.5.573. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Defining the roles of vector control and xenomonitoring in the global programme to eliminate lymphatic filariasis; Report of the informal consultation WHO/HQ; Geneva. 29–31 January 2002; Geneva: WHO; 2002. p. 42. WHO/CDS/CPE/PVC/2002.3. [Google Scholar]
- 6.World Health Organization. Report on the mid-term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the Global Programme to Eliminate Lymphatic Filariasis. Wkly Epidemiol Rec. 2004;79:358–365. [PubMed] [Google Scholar]
- 7.Bockarie MJ, Alexander ND, Hyun P, Dimber Z, Bockarie F, Ibam E, Alpers MP, Kazura JW. Randomised community-based trial of annual single-dose diethylcarbamazine with or without ivermectin against Wuchereria bancrofti infection in human beings and mosquitoes. Lancet. 1998;351:162–168. doi: 10.1016/S0140-6736(97)07081-5. [DOI] [PubMed] [Google Scholar]
- 8.Farid HA, Hammad RE, Hassan MM, Morsy ZS, Kamal IH, Weil GJ, Ramzy RMR. Detection of Wuchereria bancrofti in mosquitoes by the polymerase chain reaction: a potentially useful tool for large-scale control programmes. Trans R Soc Trop Med Hyg. 2001;95:29–32. doi: 10.1016/s0035-9203(01)90322-0. [DOI] [PubMed] [Google Scholar]
- 9.Williams SA, Laney SJ, Biewert L, Saunders LJ, Boakye DA, Fischer P, Goodman DS, Helmy H, Hoti SL, Lammie PJ, Plichart C, Ramzy R, Ottesen EA. The mosquito PCR project: to develop a rapid assessment tool for the detection of Wuchereria bancrofti infected mosquitoes. Ann Trop Med Parasitol. 2002;96:S41–S46. doi: 10.1179/000349802125002356. [DOI] [PubMed] [Google Scholar]
- 10.Goodman DS, Orelus JN, Roberts JM, Lammie PJ, Streit TG. PCR and mosquito dissection as tools to monitor filarial infection levels following mass treatment. Filaria J. 2003;2:11. doi: 10.1186/1475-2883-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramzy RMR, El Setouhy M, Helmy H, Ahmed ES, Abd Elaziz KM, Farid HA, Shannon WD, Weil GJ. Effect of yearly mass drug administration with diethylcarbamazine and albendazole on bancroftian filariasis in Egypt: a comprehensive assessment. Lancet. 2006;367:992–999. doi: 10.1016/S0140-6736(06)68426-2. [DOI] [PubMed] [Google Scholar]
- 12.Weil GJ, Ramzy RM, El Setouhy M, Kandil AM, Ahmed ES, Faris R. A longitudinal study of bancroftian filariasis in the Nile Delta of Egypt: baseline data and one-year follow-up. Am J Trop Med Hyg. 1999;61:53–58. doi: 10.4269/ajtmh.1999.61.53. [DOI] [PubMed] [Google Scholar]
- 13.Ramzy RMR, El Setouhy M, Helmy H, Kandil AM, Ahmed ES, Farid HA, Faris R, Weil GJ. The impact of single-dose diethylcarbamazine treatment of bancroftian filariasis in a low-endemicity setting in Egypt. Am J Trop Med Hyg. 2002;6:196–200. doi: 10.4269/ajtmh.2002.67.196. [DOI] [PubMed] [Google Scholar]
- 14.Dean AG, Dean JA, Coulombier D, Burton AH, Brendel KA, Smith DC, Dicker RC, Sullivan KM, Fagan RF. EpiInfo, Version 6: A Word Processing, Database, and Statistics Program for Epidemiology on Microcomputers. Atlanta, GA: Centers for Disease Control and Prevention; 1994. [Google Scholar]
- 15.Ramzy RMR, Helmy H, El-Leethy AST, Kandil AM, Ahmed ES, Weil GJ, Faris R. Field evaluation of a rapid-format kit for diagnosis of bancroftian filariasis in Egypt. East Mediterr Health J. 1999;5:880–887. [PubMed] [Google Scholar]
- 16.Zhong M, McCarthy J, Bierwert L, Lizotte MR, Chanteau S, Nutman T, Ottesen EA, Williams SA. A PCR assay for detection of the parasite Wuchereria bancrofti in human blood samples. Am J Trop Med Hyg. 1996;54:357–363. doi: 10.4269/ajtmh.1996.54.357. [DOI] [PubMed] [Google Scholar]
- 17.Helmy H, Fischer P, Farid HA, Bradley MH, Ramzy RMR. Test strip detection of Wuchereria bancrofti amplified DNA in wild-caught Culex pipiens and estimation of infection rate by a PoolScreen algorithm. Trop Med Int Health. 2004;9:158–163. doi: 10.1046/j.1365-3156.2003.01155.x. [DOI] [PubMed] [Google Scholar]
- 18.Gad AM, Feinsod FM, Soliman BA, Nelson GO, Gibbs PH, Shoukry A. Exposure variables in bancroftian filariasis in the Nile Delta. J Egypt Soc Parasitol. 1994;24:439–455. [PubMed] [Google Scholar]
- 19.Southgate B. The significance of low density microfilaremia in the transmission of lymphatic filarial parasites. J Trop Med Hyg. 1992;95:79–86. [PubMed] [Google Scholar]
- 20.Stolk W, Oortmarssen GV, Subramanian S, Das P, Borsboom G, Habbema J, Vlas SD. Assessing density dependence in the transmission of lymphatic filariasis: uptake and development of Wuchereria bancrofti microfilariae in the vector mosquitoes. Med Vet Entomol. 2004;18:57–60. doi: 10.1111/j.0269-283x.2004.0470.x. [DOI] [PubMed] [Google Scholar]
- 21.Farid HA, Morsy ZS, Hassan AN, Hammad RE, Faris R, Kandil AM, Ahmed ES, Weil GJ. The impact of environmental and entomological factors on intervillage filarial focality in the Nile Delta. J Egypt Soc Parasitol. 2000;30:469–485. [PubMed] [Google Scholar]
- 22.Fischer PU, Ericson SM, Fischer K, Fuchs JF, Rao RU, Christensen BM, Weil GJ. Persistence of Brugia malayi DNA in vector and non-vector mosquitoes: implications for xenomonitoring and transmission monitoring of lymphatic filariasis. Am J Trop Med Hyg. 2007;76:502–507. [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro JM, Seulu F, Abose T, Kidane G, Teklehaimanot A. Temporal and spatial distribution of anopheline mosquitoes in an Ethiopian village: implications for malaria control strategies. Bull World Health Organ. 1996;74:299–305. [PMC free article] [PubMed] [Google Scholar]
- 24.Van Der Hoek W, Konradsen F, Amerasinghe PH, Perera D, Piyaratne MK, Amerasinghe FP. Towards a risk map of malaria for Sri Lanka: the importance of house location relative to vector breeding sites. Int J Epidemiol. 2003;32:280–285. doi: 10.1093/ije/dyg055. [DOI] [PubMed] [Google Scholar]
- 25.Bockarie MJ, Tisch DJ, Kastens W, Alexander ND, Dimber Z, Bockarie F, Ibam E, Alpers MP, Kazura JW. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–1848. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 26.Weil GJ, Ramzy RMR. Diagnostic tools for filariasis elimination programmes. Trends Parasitol. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Michael E, Malecela-Lazaro MN, Kabali C, Snow LC, Kazura JW. Mathematical models and lymphatic filariasis control: endpoints and optimal interventions. Trends Parasitol. 2006;22:226–233. doi: 10.1016/j.pt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Farid HA, Kamal SA, Weil GJ, Adham FK, Ramzy RMR. Filariasis elimination in Egypt: impact of low microfilaraemics as sources of infection for mosquitoes. East Mediterr Health J. 2003;9:863–872. [PubMed] [Google Scholar]
- 29.Burkot T, Ichimori K. The PacELF programme: will mass administration be enough? Trends Parasitol. 2002;18:109–115. doi: 10.1016/s1471-4922(01)02221-8. [DOI] [PubMed] [Google Scholar]
- 30.Lammie PJ, Weil G, Noordin R, Kaliraj P, Steel C, Goodman D, Lakshmikanthan VB, Ottesen E. Recombinant antigen-based antibody assays for the diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria J. 2004;3:9. doi: 10.1186/1475-2883-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao RU, Atkinson LJ, Ramzy RMR, Helmy H, Farid HA, Bockarie MJ, Susapu M, Laney SJ, Williams SA, Weil GJ. A real-time PCR-based assay for detection of Wuchereria bancrofti DNA in blood and mosquitoes. J Trop Med Hyg. 2006;74:826–832. [PMC free article] [PubMed] [Google Scholar]
- 32.Chadee DD, Beier JC. Natural variation in blood-feeding kinetics of four mosquito vectors. J Vector Ecol. 1996;21:150–155. [Google Scholar]
- 33.Chadee DD, Williams SA, Ottesen EA. Xenomonitoring of Culex quinquefasciatus mosquitoes as a guide for detecting the presence or absence of lymphatic filariasis: a preliminary protocol for mosquito sampling. Ann Trop Med Parasitol. 2002;96:S47–S53. doi: 10.1179/000349802125002365. [DOI] [PubMed] [Google Scholar]
- 34.Ramzy RMR, Farid HA, Kamal IH, Ibrahim GH, Morsy ZS, Faris R, Weil GJ, Williams SA, Gad AM. A polymerase chain reaction-based assay for detection of Wuchereria bancrofti in human blood and Culex pipiens. Trans R Soc Trop Med Hyg. 1997;91:156–160. doi: 10.1016/s0035-9203(97)90205-4. [DOI] [PubMed] [Google Scholar]
- 35.Michael E, Ramaiah KD, Hoti SL, Barker G, Paul MR, Yuvaraj J, Das PK, Molyneux DH, Zagaria N. Lymphatic filariasis elimination: progress in global programme development. Ann Trop Med Parasitol. 2002;96:S15–S40. doi: 10.1179/000349802125002374. [DOI] [PubMed] [Google Scholar]