Abstract
Phagosomal biogenesis is a fundamental biological process of particular significance for the function of phagocytic and antigen-presenting cells. The precise mechanisms governing maturation of phagosomes into phagolysosomes are not completely understood. Here, we applied the property of pathogenic mycobacteria to cause phagosome maturation arrest in infected macrophages as a tool to dissect critical steps in phagosomal biogenesis. We report the requirement for 3-phosphoinositides and acquisition of Rab5 effector early endosome autoantigen (EEA1) as essential molecular events necessary for phagosomal maturation. Unlike the model phagosomes containing latex beads, which transiently recruited EEA1, mycobacterial phagosomes excluded this regulator of vesicular trafficking that controls membrane tethering and fusion processes within the endosomal pathway and is recruited to endosomal membranes via binding to phosphatidylinositol 3-phosphate (PtdIns[3]P). Inhibitors of phosphatidylinositol 3′(OH)-kinase (PI-3K) activity diminished EEA1 recruitment to newly formed latex bead phagosomes and blocked phagosomal acquisition of late endocytic properties, indicating that generation of PtdIns(3)P plays a role in phagosomal maturation. Microinjection into macrophages of antibodies against EEA1 and the PI-3K hVPS34 reduced acquisition of late endocytic markers by latex bead phagosomes, demonstrating an essential role of these Rab5 effectors in phagosomal biogenesis. The mechanism of EEA1 exclusion from mycobacterial phagosomes was investigated using mycobacterial products. Coating of latex beads with the major mycobacterial cell envelope glycosylated phosphatidylinositol lipoarabinomannan isolated from the virulent Mycobacterium tuberculosis H37Rv, inhibited recruitment of EEA1 to latex bead phagosomes, and diminished their maturation. These findings define the generation of phosphatidylinositol 3-phosphate and EEA1 recruitment as: (a) important regulatory events in phagosomal maturation and (b) critical molecular targets affected by M. tuberculosis. This study also identifies mycobacterial phosphoinositides as products with specialized toxic properties, interfering with discrete trafficking stages in phagosomal maturation.
Keywords: EEA1; endosome; hVPS34; LBPA; LAM
Introduction
Phagosomal maturation is a fundamental biological process governed by vesicular and intracellular membrane and protein trafficking. The details and specific factors involved in phagosome maturation are only now beginning to be understood (Alvarez-Dominguez et al., 1996, 1997; Jahraus et al., 1998; Alvarez-Dominguez and Stahl, 1999; Downey et al., 1999; Bajno et al., 2000; Botelho et al., 2000; Defacque et al., 2000). Since phagosomal characteristics are of significance for the control or survival of intracellular pathogens in mammalian cells (Meresse et al., 1999b), a significant portion of the current knowledge regarding phagosomal maturation comes from the studies of vacuoles containing intracellular pathogens (Russell et al., 1992; Chakraborty et al., 1994; Scidmore et al., 1996; Via et al., 1997; Meresse et al., 1999a). In extreme cases, microbes can parasitize phagocytic cells, such as macrophages, where their intracellular survival depends on escape from the phagosome or more frequently on alterations of the default maturation pathway of phagosomes into the phagolysosome (Meresse et al., 1999b). Mycobacterium tuberculosis is one of the few bacterial pathogens that survive in immune phagocytic cells. The establishment of its productive infectious cycle depends on mycobacterial entry into macrophages (Schorey et al., 1997; Ernst, 1998; Fratazzi et al., 2000) and their subsequent intraphagosomal survival (Armstrong and Hart, 1971; Clemens and Horwitz, 1995). M. tuberculosis phagosomes do not mature into phagolysosomes (Deretic and Fratti, 1999), a phenomenon that has been recognized as a central paradigm of M. tuberculosis pathogenesis, referred to in classical texts as the inhibition of phagosome–lysosome fusion (Armstrong and Hart, 1971). It has been established that M. tuberculosis, the vaccine strain M. tuberculosis variant bovis BCG (Bacillus Calmette-Guérin) (BCG)*, and Mycobacterium avium reside in privileged phagosomal compartments sequestered from the terminal endocytic organelles (Xu et al., 1994; de Chastellier et al., 1995; Clemens and Horwitz, 1995; Deretic and Fratti, 1999). Additional interactions with exogenously added markers (Clemens and Horwitz, 1996; Sturgill-Koszycki et al., 1996) and the biosynthetic secretory pathway (Ullrich et al., 1999) have been implicated in the remodeling of mycobacterial phagosomes. Mycobacterial phagosomes display diminished acidification due to the paucity of H+ATPase (Sturgill-Koszycki et al., 1994), show limited acquisition of late endosomal markers, presence of an immature intermediate form of Cathepsin D (Sturgill-Koszycki et al., 1996), absence of mannose 6-phosphate receptors (Xu et al., 1994), and reduced clearance of plasma membrane markers (Clemens and Horwitz, 1995) and early phagosomal proteins such as coronin (Ferrari et al., 1999; Fratti et al., 2000). However, the exact molecular mechanisms of the inhibition of mycobacterial phagosomal maturation are not known.
Phagosomes are dynamic structures interacting with endosomal (Desjardins et al., 1994) and possibly other compartments (Fratti et al., 2000) in a process of acquisition and removal of membrane and lumenal components as phagosomes mature into phagolysosomes. The trafficking events within the endosomal network are controlled by a subset of small GTPases from the Ras superfamily: (a) Rab5 (Gorvel et al., 1991; Christoforidis et al., 1999a) and Rab7 (Vitelli et al., 1997; Press et al., 1998; Meresse et al., 1999a) control sequential interactions with early and late endosomes; (b) Rab4 (Mohrmann and van der Sluijs, 1999), Rab11 (Ren et al., 1998), and ARF6 (D'Souza-Schorey et al., 1998) control membrane and protein recycling from endosomal compartments to the plasma membrane; and (c) Rab9 regulates trafficking between the late endosome and trans-Golgi network (TGN) (Riederer et al., 1994). The small GTPases Rab5 and Rab7 have also been implicated in the maturation processes of phagosomes containing intracellular pathogens (Mordue and Sibley, 1997; Alvarez-Dominguez and Stahl, 1999; Meresse et al., 1999a; Mott et al., 1999), although their exact role and the processes they control in the context of phagosome biogenesis are not defined.
The arrest in the maturation of Mycobacterium tuberculosis phagosomal compartments (MPCs) has been linked to a block between the Rab5- and Rab7-controlled stages (Via et al., 1997), suggesting that the molecular processes downstream of Rab5 and upstream of Rab7 are compromised on MPCs. Recently, several Rab5 effectors have been identified (Christoforidis et al., 1999a) as important regulators of early endocytic trafficking. Since the early endosome serves as a hub for the endocytic pathway, these effectors are the likely determinants of specificity and directionality within the Rab5-controlled compartments, which may also include newly formed phagosomes (Fratti et al., 2000). Among the best characterized Rab5-interacting partners that play a role in early endosomal trafficking are Rabaptin-5 (Stenmark et al., 1995), tuberin (a Rab5-GTPase–activating protein) (Xiao et al., 1997), Rabex 5 (a Rab5-nucleotide exchange factor) (Horiuchi et al., 1997), early endosomal autoantigen (EEA1; a tethering molecule that couples vesicle docking with soluble N-ethylmaleimide–sensitive factor [NSF] attachment protein [SNAP] receptor [SNARE] priming) (Simonsen et al., 1998), and the phosphatidylinositol 3-kinases (PI 3-K) p85α-p110β and p150-hVPS34 (Christoforidis et al., 1999b). Although Rab5 has been identified on several types of phagosomes, including model phagosomes such as latex bead phagosomes (Desjardins et al., 1994; Via et al., 1997) and parasitophorous vacuoles containing different pathogens (Mordue and Sibley, 1997; Via et al., 1997; Alvarez-Dominguez and Stahl, 1999; Scianimanico et al., 1999), most of the Rab5 effectors have not been studied in the context of phagosomal maturation. In this work, we extended our studies to the effectors of Rab5 and report a critical role of PI-3K and EEA1 in phagosomal maturation. We also present molecular details of M. tuberculosis phagosome maturation arrest associated with trafficking toxin activity of a highly glycosylated phosphatidylinositol of M. tuberculosis, lipoarabinomannan, causing a block in EEA1 recruitment.
Results
Rab5-interacting components on model phagosomes
To address the mechanism of the arrest of MPC maturation (Armstrong and Hart, 1971; Clemens and Horwitz, 1995; Sturgill-Koszycki et al., 1996) between the Rab5- and Rab7-controlled endosomal stages (Via et al., 1997), we investigated the possibility that the arrest of MPCs at a Rab5-controlled stage is linked to alterations in recruitment or function of Rab5-interacting components. We first tested latex bead phagosomal compartments (LBCs) for the presence of Rabaptin-5, EEA1, and tuberin. LBCs were purified from J774 macrophages as described previously (Desjardins et al., 1994; Via et al., 1997) at various times after uptake. When LBCs were probed for Rabaptin-5, this Rab5-GTP–stabilizing protein was not detected on LBCs at the time points tested, despite the presence of Rab5 (Fig. 1 A). Tuberin was also not observed on LBCs. Nevertheless, Rabaptin-5 and tuberin were readily detectable in postnuclear supernatants (PNSs) (Fig. 1 A, lane P) and on endosomal membranes (Fig. 1 A, lane E). These observations suggest that Rabaptin-5 and tuberin are absent or below detection levels on model phagosomes. The apparent absence of Rabaptin-5 from phagosomal membranes can be interpreted in various ways. First, an exclusion of this effector may serve as a means of maintaining the transient nature of interactions between phagosomes and early endosomal organelles. Second, it is possible that Rabaptin-5 is present only on early endosomes during interactions with LBCs. Alternatively, other factors (Gournier et al., 1998) or putative phagosome-specific Rab5 effectors may substitute for Rabaptin-5 function. In contrast to the absence of Rabaptin-5, LBCs isolated at early time points acquired EEA1 with peak association occurring between 10 and 20 min after infection (Fig. 1 A). The presence of EEA1 on LBCs was transient, since it was not detected at very early or late time points. Nevertheless, the dynamic association of EEA1 with LBCs suggested a role for this Rab5 effector in phagosome maturation.
Figure 1.
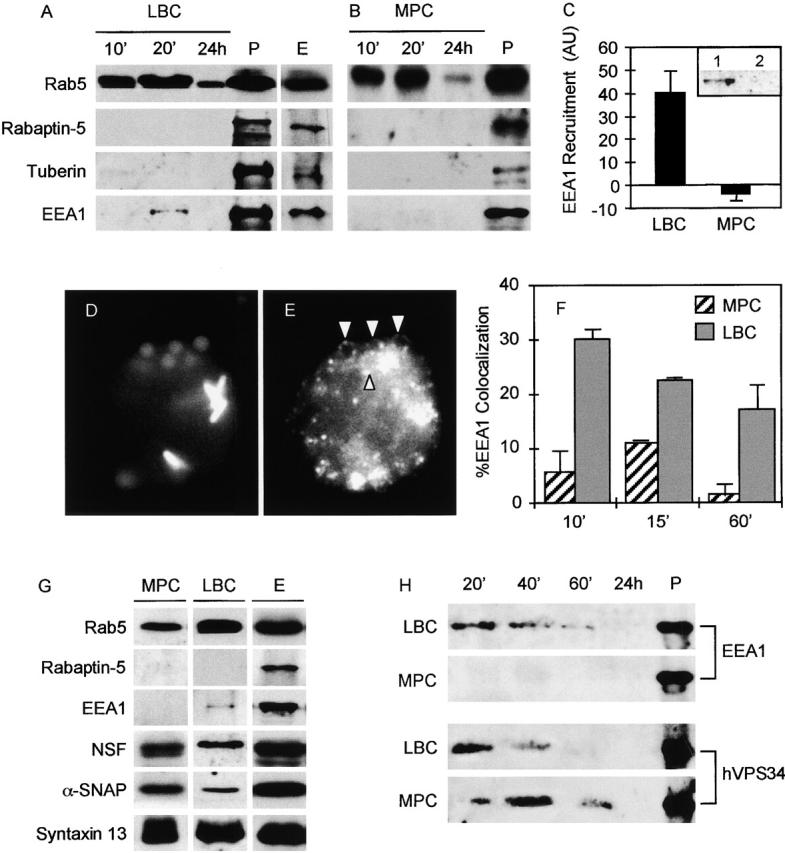
Transient recruitment of Rab5 effector EEA1 to latex bead phagosomes and exclusion from mycobacterial phagosomes. Western blot analysis of purified LBCs (A) and MPCs (B). Phagosomes were purified from macrophages as previously described (Via et al., 1997) after synchronized infections. PNSs (P) and early endosomal membranes (E) were isolated from mock 24-h infections. LBC (5 μg of protein), P (50 μg), and E (50 μg) were probed with antibodies against Rab5, Rabaptin-5, tuberin, and EEA1. Discontinuous membranes were developed simultaneously. (C) Quantitative analysis of EEA1 by Western blots. EEA1 recruitment to LBCs and MPCs after a 20-min infection was quantitated by analyzing relative image intensities using NIH Image 1.61 (n = 3, P = 0.0119; ANOVA). (C, inset) Western blot of an endosomal membrane fraction (lane 1) and an MPC-containing fraction (lane 2) obtained from the same final isopycnic gradient purification step probed with antibody against EEA1. (D and E) J774 cells were coinfected with latex beads and GFP-labeled BCG and processed for immunofluorescence. Synchronization of infection was achieved by centrifugation of particles onto macrophages adherent to coverslips. Cells were processed for immunofluorescence labeling with affinity purified antibody against EEA1 after 10, 15, and 60 min of phagocytosis as described in Materials and methods. (D) GFP fluorescence (mycobacteria) and autofluorescence (latex beads). (E) EEA1 immunofluorescence detection. Arrowheads indicate EEA1 rings surrounding latex beads. (F) Quantitation of EEA1 colocalization with phagosomes (n = 4,317 phagosomes; all samples). The data are means ± SE of three separate experiments. (G) Analysis of the components of the EEA1–macromolecular docking and fusion complex on purified phagosomes. MPC, LBC, and E were isolated from J774 cells after a 20-min infection (MPC and LBC) or mock infection (E) and tested for the presence of Rab5, Rabaptin-5, EEA1, Syntaxin 13, NSF, and α-SNAP. Equivalent amounts of LBC and MPC (5 μg of protein) and E (25 μg of protein) were separated by SDS-PAGE, transferred to Immobilon-P, and probed with corresponding antibodies. Discontinuous membranes were developed simultaneously. (H) Latex bead and mycobacterial phagosomes recruit type III PI-3K hVPS34. LBCs and MPCs were isolated from synchronized infections after 20, 40, and 60 min and 24 h (24 h sample: 1 h phagocytosis, 24-h chase). PNS (P) was isolated from mock 24-h infections. LBC (5 μg of protein), MPC (5 μg of protein), and P (25 μg of protein) were separated by SDS-PAGE, transferred to Immobilon-P membrane, and probed with affinity purified rabbit antibodies against EEA1 and hVPS34.
MPCs do not acquire EEA1
Experiments with synchronized infection and phagosome maturation during early chase periods were also carried out with BCG. After phagocytosis, MPCs were purified as described previously (Via et al., 1997) and membranes probed for Rab5, Rabaptin-5, tuberin, and EEA1. Similarly to LBCs, MPCs did not acquire Rabaptin-5 and tuberin. However, unlike LBCs MPCs did not recruit detectable levels of EEA1 (Fig. 1 B), indicating that MPCs excluded this Rab5 effector. Examination of additional time points did not result in detection of EEA1 on MPCs. As in the case of LBCs, Rabaptin-5, tuberin, and EEA1 were detectable in PNS from macrophages infected with mycobacteria (Fig. 1 B, lane P). Quantitation of Western blots further illustrates the marked difference in EEA1 recruitment between LBCs and MPCs (P = 0.012; ANOVA) (Fig. 1 C). To examine whether the exclusion of EEA1 from MPCs was related to the longer centrifugation required for MPC isolation, other fractions from the same gradients were probed for EEA1. We found that EEA1 was present on pelleted membranes from fractions other than those containing MPCs (Fig. 1 C, inset). This indicates that EEA1 exclusion from MPCs was not due to longer centrifugation periods.
EEA1 recruitment to phagosomes was also investigated by immunofluorescence microscopy (Fig. 1, D–F). Macrophages were coinfected with BCG and latex beads using synchronized phagocytosis. The results of these experiments showed that latex beads recruited EEA1, forming distinct ring structures around LBCs detected by conventional epifluorescence microscopy (Fig. 1 E), whereas EEA1 was excluded from MPCs (Fig. 1 E). At any given time point, only a subpopulation of LBCs stained positive for EEA1 with the dynamics consistent with transient recruitment of this Rab5 effector to the model phagosomes. Quantitative comparisons of EEA1-positive MPCs and LBCs (Fig. 1 F) showed statistically significant differences in EEA1 staining (P = 0.003; ANOVA; P = 0.0246 for 10 min, 0.0018 for 15 min, and 0.0795 for 1 h; ANOVA). It should be noted that the 15 min time point corresponds to the purified phagosomes at 20 min, adjusted for differences in technical manipulations. In morphological analysis, MPCs counted as EEA1-positive by immunofluorescence were allowed to be scored on less stringent requirements, since the LBCs considered as positive for EEA1 were required to display a distinct ring of EEA1 staining, whereas MPCs were counted as EEA1 positive even if only a partial overlap was observed. In very few instances (<2%) was the EEA1 staining of BCG phagosomes complete and uniform as seen with LBCs (unpublished data). Although based on biochemical tests we have not detected EEA1 on BCG phagosomes, some EEA1 staining by immunofluorescence in infected cells is expected. For example, a small fraction of MPCs normally proceeds with maturation into the phagolysosome, a phenomenon reflecting heterogeneity in mycobacterial culture that has been repeatedly described (Armstrong and Hart, 1971; Clemens and Horwitz, 1995; Via et al., 1997). These EEA1-positive mycobacterial phagosomes present in small numbers may be the fraction destined for further maturation into phagolysosomes. By biochemical assays, such a subpopulation of EEA1-positive BCG phagosomes would likely acquire different membrane characteristics and purify away from the main peak defined as the MPC during isopycnic centrifugation (Via et al., 1997), or they could be present in amounts too small for detection by Western blots.
NSF, αSNAP, and Syntaxin 13 are present on both MPCs and LBCs
EEA1 participates in the formation of large oligomeric complexes via interactions with the SNARE Syntaxin 13 and SNARE-priming factors NSF and α-SNAP. These interactions are important for tethering-triggered membrane fusion events (McBride et al., 1999). We next tested whether other constituents of EEA1-associated docking and fusion machinery (Rab5, Rabaptin-5, NSF, αSNAP, and Syntaxin 13) were present on phagosomes (Fig. 1 G). The components of the fusion apparatus Syntaxin 13, NSF, and αSNAP were present on both LBCs and MPCs, indicating that the main difference between MPCs and LBCs is the exclusion of EEA1 from the mycobacterial phagosome, a critical organizing component in the membrane-tethering complex. Although EEA1 does not directly take part in the recruitment of other docking and fusion components, EEA1 is required for oligomerization of the complex and subsequent membrane tethering (McBride et al., 1999). Thus, the absence of EEA1 is likely to affect not only the tethering potential of MPCs but may also have repercussions on the dynamic restructuring of the fusion components on MPCs necessary for efficient docking and downstream membrane fusion events (McBride et al., 1999).
Effects of PI-3K inhibitors on phagosome maturation
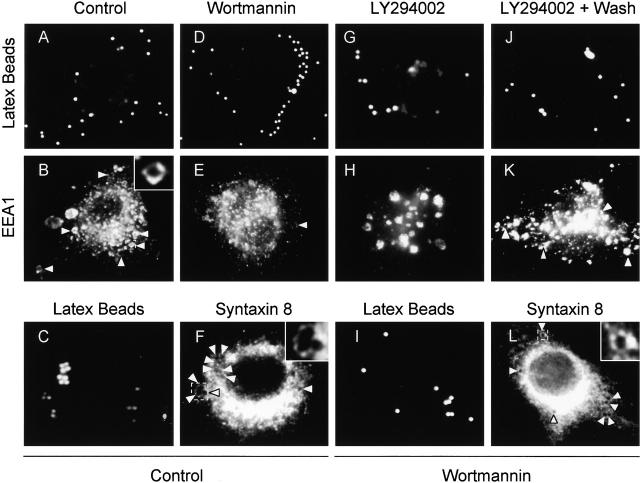
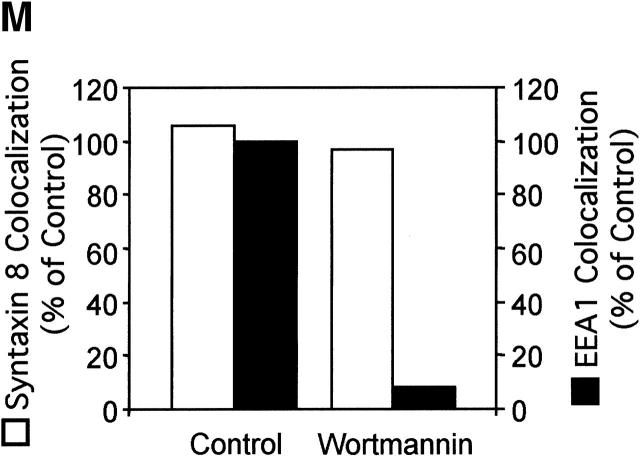
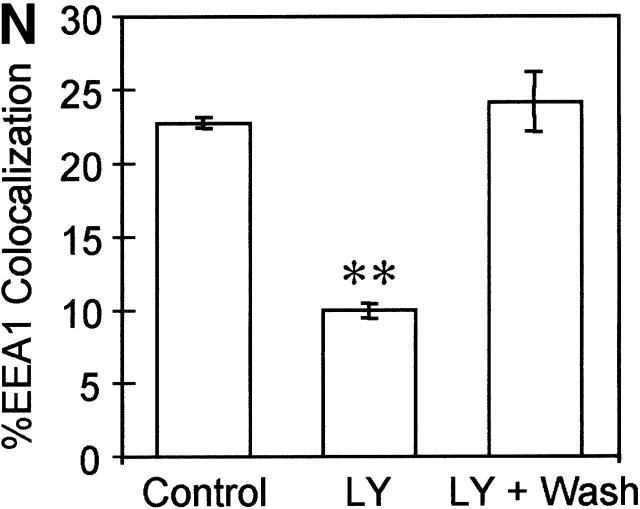
The absence of EEA1 on MPCs could affect directionality or preclude a subset of interactions between mycobacterial phagosomes on one end and early endosomes or vesicles from the TGN (Simonsen et al., 1999). EEA1 is recruited to the endosomes through interactions with Rab5 (Simonsen et al., 1998) and via binding of the EEA1 FYVE domain to phosphatidylinositol 3-phosphate (PtdIns[3]P) on endosomal membranes (Patki et al., 1997). Although PtdIns(3)P and EEA1 have been implicated in the endocytic pathway (Christoforidis et al., 1999a; McBride et al., 1999; Simonsen et al., 1999), the roles of PtdIns(3)P and EEA1 in phagosome maturation have not been investigated. Since PtdIns(3)P production is necessary for efficient recruitment of EEA1 to the membrane (Patki et al., 1997; Christoforidis et al., 1999b), we first tested the effects on phagosome maturation of wortmannin, a drug which inhibits PI-3K activity. Since PI-3K activity is necessary for the final stage of receptor-mediated phagocytosis (Araki et al., 1996), cells were treated with the PI-3K inhibitor only after phagocytosis was allowed to proceed for 10 min. Compared with the untreated controls (Fig. 2, A, B, and M) , latex bead phagosomes in cells treated with wortmannin did not acquire significant amounts of EEA1 (Fig. 2, D, E, and M). Wortmannin treatment under the conditions used did not completely remove EEA1 from all endomembranes, indicating that the effects observed were not due to massive perturbations of the cell. As a control for the intracellular localization of latex beads in these experiments, phagosomes were also stained for the acquisition of Syntaxin 8, an endosomal SNARE that primarily overlaps with Rab5 (Subramaniam et al., 2000). Syntaxin 8 association with latex beads was equal in wortmannin-treated and control macrophages (Fig. 2, C, F, I, L, and M), demonstrating that the decrease in EEA1 cannot be attributed to an inhibition in latex bead uptake by macrophages but represents a reduction in EEA1 recruitment to latex bead phagosomes in cells treated with the drug. Since wortmannin could potentially affect other lipid kinases (Nakanishi et al., 1995), the role of PI-3K activity in the recruitment of EEA1 to phagosomes was verified by using another PI-3K–specific inhibitor LY294002 (Vlahos et al., 1994). Treatment of cells with 200 μM LY94002 applied 10 min after phagocytosis also inhibited the recruitment of EEA1 to latex bead phagosomes, confirming that EEA1 is recruited to model phagosomes (Fig. 2, G, H, and N) in a PtdIns(3)P-dependent manner. The effect of LY294002 on EEA1 recruitment was reversible, since washing away the inhibitor and incubation for an additional 10-min period in fresh medium restored normal EEA1 recruitment (Fig. 2, J, K, and N).
Figure 2.
Inhibition of PI-3K activity diminishes EEA1 recruitment to latex bead phagosomes. Epifluorescence microscopy of latex bead phagosomes in J774 cells is shown. (A, C, D, G, I, and J) Green fluorescence of latex beads. (B, E, H, and K) Upon phagocytosis, cells were fixed, permeabilized, incubated with anti-EEA1 antibody, washed, and incubated with secondary Alexa 568–conjugated antibody. Arrowheads indicate colocalization of latex beads and EEA1 staining. (F and L) Cells were also fixed, permeabilized, and incubated with anti–Syntaxin 8 antibody as a control for latex bead uptake. Arrowheads indicate colocalization of latex beads and Syntaxin 8 staining. Cells containing nascent phagosomes (10 min of phagocytosis) were treated with medium alone (A, B, C, and F), 100 nM wortmannin (D, E, I, and L), exposed continuously to 200 μM LY294002 (20 min) (G and H), or first treated with LY294002 for 10 min, which was then removed by washing, and incubated for an addition 10 min in medium without the inhibitor (J and K) as described in Materials and methods. (M) ▪, Quantitation of EEA1 colocalization with phagosomes (20 min after infection) in wortmannin treated and control cells; □, quantitation of staining with Syntaxin 8 (n = 1,068; all samples). (N) Quantitation of EEA1 colocalization with phagosomes (30 min after infection) in control cells and LY294002-treated cells (n = 1,816 phagosomes). The data are means ± SE of three separate experiments. **P = 0.0003.
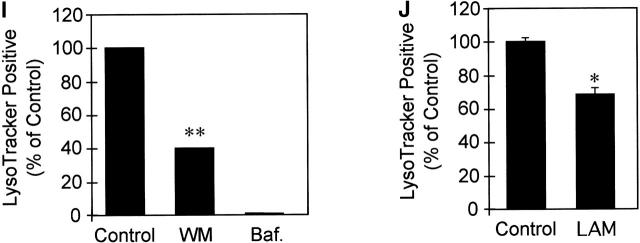
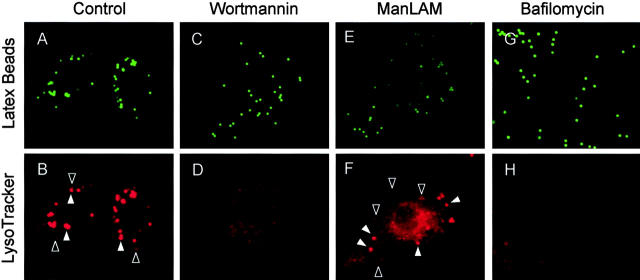
To examine the role of PI-3K activity, and by extension EEA1, in phagosome maturation, we analyzed the effect of wortmannin on phagosome acidification as determined by LysoTracker staining, which serves as a marker of maturing phagosomes (Via et al., 1998; Malik et al., 2000). Phagosomes in cells treated with wortmannin did not acidify properly, suggesting that PI-3K activity is required for phagosome maturation (Fig. 3 , C–D and I). Thus, inhibition of PI-3K activity and exclusion of EEA1 correlates with phagosomal maturation arrest. These observations define specific events in phagosome biogenesis involving PI-3K.
Figure 3.
M. tuberculosis ManLAM and inhibition of PI-3K activity abrogates phagosomal acidification. Epifluorescence microscopy of latex bead phagosomes in J774 cells is shown. Staining with the fixable acidotropic dye LysoTracker was used as a measure of phagosomal acidification and maturation. (A, C, E, and G) Green fluorescence of latex beads. (B, D, F, and H) LysoTracker staining carried out by incubating J774 cells with the dye 2 h before infection and maintained throughout the infection. Phagosomes positive for LysoTracker staining are exemplified by a solid arrowhead. Open arrowheads exemplify phagosomes negative for LysoTracker staining. Cells containing nascent latex bead phagosomes were treated with medium alone (A and B) or 100 nM wortmannin (C and D). (E and F) Cells were also infected with ManLAM-coated latex beads. Cells were also treated with bafilomycin as a control for acidic vesicle staining (G and H). (I) Quantitation of LysoTracker colocalization with phagosomes (20 min after infection) in wortmannin (WM)-treated and control cells; bafilomycin (Baf.) treatment was used as a control for dependence of LysoTracker staining on acidification (n = 651 phagosomes). (J) Quantitation of LysoTracker with phagosomes containing uncoated control beads or ManLAM-coated beads (n = 2,553 phagosomes). The data are means ± SE of three separate experiments. *P < 0.01; **P < 0.001.
Appearance of the late endocytic marker lysobisphosphatidic acid on phagosomes is sensitive to PI-3K inhibitors
As another measure of phagosome maturation, we examined the role of PI-3K activity on the acquisition of late endosomal markers. Although LAMP accumulation is a marker used frequently to reveal late endocytic compartments, it may not be an ideal marker for examining PI-3K–dependent events on phagosomes for the following reasons. (a) LAMP delivery to the endocytic network from the TGN is independent of PI-3K activity (Karlsson and Carlsson, 1998), in contrast to several other markers whose sorting to the endocytic pathway is inhibited by wortmannin. (b) LAMP is accumulated by M. avium phagosomes (Sturgill-Koszycki et al., 1994), whereas it is excluded from or is variably present on phagosomes containing M. tuberculosis (Xu et al., 1994; Clemens and Horwitz, 1995) and BCG (Via et al., 1997). (c) Experiments examining purified LBCs from cells treated with wortmannin showed that LAMP acquisition was not affected (unpublished data). Given a low discrimination value of LAMP for PI-3K–dependent events (Karlsson and Carlsson, 1998), we employed another highly specific late endocytic marker, the lipid lysobisphosphatidic acid (LBPA) (Kobayashi et al., 1998, 1999). Unlike LAMP splice variants, which can be detected throughout the endocytic pathway and plasma membrane (Gough and Fambrough, 1997), LBPA is found exclusively in late endosomes (Kobayashi et al., 1998) where it colocalizes with Rab7.
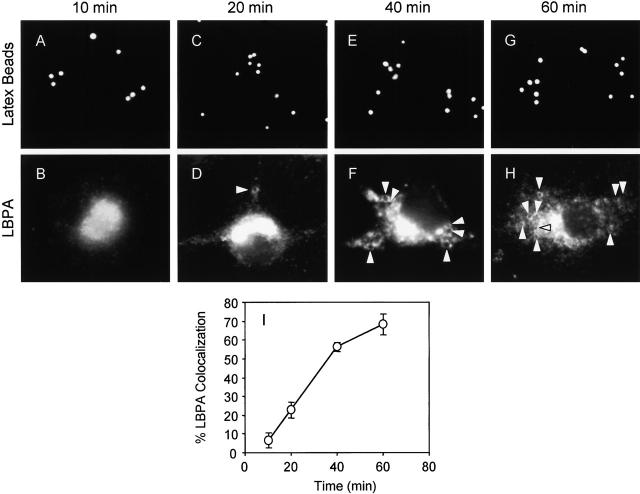
We first examined the kinetics of LBPA staining and found that phagosomes acquired increasing amounts of LBPA over time (Fig. 4) in a manner consistent with phagosome maturation into late endocytic organelles. The accumulation of LBPA by latex bead phagosomes was considerably slower than what is usually seen with LAMP association with phagosomes (Downey et al., 1999; Botelho et al., 2000), suggesting that LBPA accumulation may occur independent or downstream of LAMP acquisition.
Figure 4.
Kinetics of LBPA acquisition by model latex bead phagosomes. J774 cells were infected with latex beads for 10, 20, 40, and 60 min. (A, C, E, and G) Fluorescence of latex beads. (B, D, F, and H) After phagocytosis, cells were fixed, permeabilized, and incubated with anti-LBPA antibody, washed, and incubated with secondary Alexa 568–conjugated antibody. Arrowheads indicate phagosome colocalization with LBPA. (I) Quantitation of LBPA colocalization with phagosomes (n = 1,017 phagosomes). The data are means ± SE of three separate experiments.
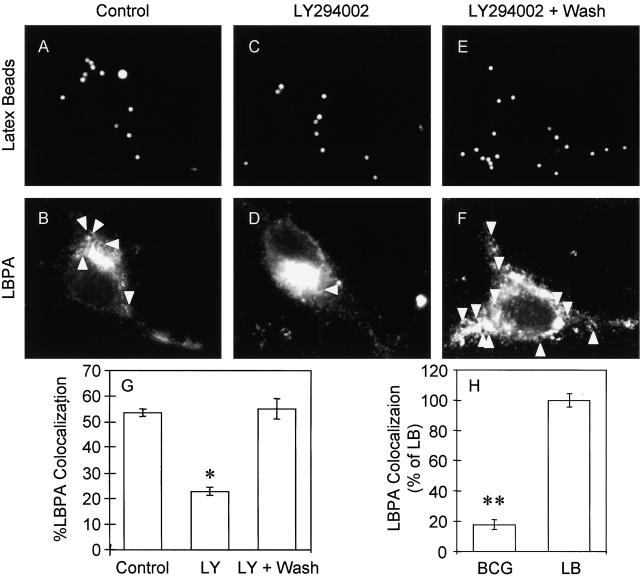
Inhibition of PI-3K activity with LY294002 in cells containing nascent phagosomes (treated after 10 min of uninhibited phagocytosis) reduced the colocalization of latex bead phagosomes with LBPA in treated cells (Fig. 5, C, D, and G) relative to those in untreated cells (Fig. 5, A, B, and G). The effect of LY294002 on LBPA staining was reversible, and when the inhibitor was washed away and cells incubated for an additional 10 min in fresh medium acquisition of LBPA resumed (Fig. 5, E, F, and G). These experiments demonstrate a role of PI-3K activity in the acquisition of late endosomal markers. LBPA accumulation on latex bead phagosomes was also compared with that on mycobacterial phagosomes. Consistent with the mycobacterial phagosome maturation arrest, only 11% of phagosomes containing BCG colocalized with LBPA compared to 65% LBPA positivity among the model latex bead phagosomes (P = 0.0007; ANOVA) (Fig. 5 H). The exclusion of LBPA from the mycobacterial phagosome is consistent with the maturation block involving generation of PtdIns(3)P or Ptd-Ins(3)P-dependent recruitment of downstream effectors.
Figure 5.
Inhibition of PI-3K activity with LY294002 reduces LBPA acquisition by latex bead phagosomes. Epifluorescence microscopy of latex bead phagosomes in J774 cells is shown. (A, C, and E) Fluorescence of latex beads. (B, D, and F) After phagocytosis, cells were fixed, permeabilized, incubated with anti-LBPA antibody, washed, and incubated with secondary Alexa 568–conjugated antibody. Arrowheads indicate colocalization of latex beads and LBPA staining. Cells containing nascent phagosomes (10 min of phagocytosis) were treated with medium alone (A and B), exposed continuously to 200 μM LY294002 (20 min) (C and D), or first treated with LY294002 for 10 min, which was then removed by washing, and incubated for an additional 10 min in medium without the inhibitor (E and F). (G) Quantitation of LBPA colocalization with phagosomes (30 min after infection) in control cells and LY294002-treated cells (n = 1,217 phagosomes). The data are means ± SE of separate experiments. *P = 0.001. (J) Quantitation of LBPA colocalization with latex beads (LB) and BCG phagosomes (30 min after infection) as described in Materials and methods (n = 350 phagosomes). The data are means ± SE of three separate experiments. **P = 0.0007.
Microinjection of anti-hVPS34 antibody inhibits latex bead phagosome maturation
EEA1 binding to early endosomal membranes is contingent upon the modification of lipids by the PI-3K enzyme hVPS34 (Panaretou et al., 1997; Christoforidis et al., 1999b). The PI-3K hVPS34 generates PtdIns(3)P, which binds the FYVE domain of proteins such as EEA1 with high affinity. When purified phagosomes were probed for the presence of hVPS34, we found that both the MPCs and LBCs acquired this kinase, albeit with some differences in the kinetics of the recruitment (Fig. 1 H).
To determine whether hVPS34 recruitment to phagosomal membranes is required for phagosome maturation, hVPS34 was inhibited by microinjecting an isoform-specific inhibitory antibody against this Rab5 effector (Siddhanta et al., 1998). Phagosome maturation was assessed by accumulation of LBPA. When the cells were injected with anti-hVPS34 antibody, latex bead phagosomes manifested a markedly reduced accumulation of LBPA relative to phagosomes in control cells. Although >58% of latex bead phagosomes in the control acquired LBPA at 30 min after phagocytosis (Fig. 6 I and Fig. 7 A), only 15% of phagosomes in cells injected with anti-hVPS34 antibody accumulated LBPA (Fig. 6 J and Fig. 7 A) (P = 0.0001; ANOVA). Microinjecting cells with control rabbit IgG did not affect LBPA accumulation (P = 0.41; ANOVA) (Fig. 6 L and Fig. 7 A). These data demonstrate that hVPS34 plays a role in phagosome maturation into vacuoles with late endosomal characteristics. One possible explanation for the maturation-arresting effect of microinjected anti-hVPS34 antibody is that it may prevent or reduce EEA1 recruitment. To examine this possibility, we tested the effect of anti-hVPS34 antibody on EEA1 recruitment to phagosomes. EEA1 localization to latex bead phagosomes was reduced by 63% in cells injected with anti-hVPS34 relative to uninjected cells or cells injected with control rabbit IgG (P < 0.001; ANOVA) (Fig 7 B).
Figure 6.
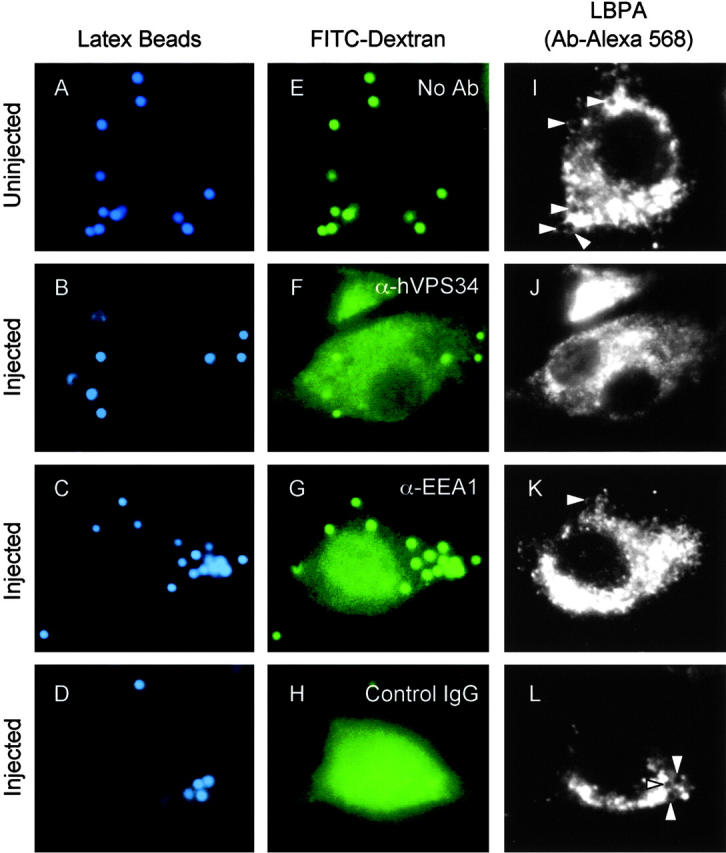
Microinjection of anti-hVPS34 and anti-EEA1 antibodies reduces accumulation of LBPA on latex bead phagosomes. Epifluorescence microscopy of latex bead phagosomes in J774 cells is shown. Control uninjected cells (A, E, and I) or cells injected with anti-hVPS34 antibody (B, F, and J), anti-EEA1 antibody (C, G, and K), or control rabbit IgG (D, H, and L) were infected with latex beads for 30 min and processed for immunofluorescence. (A–D) Latex bead fluorescence (blue pseudocolor). (E–H) FITC-Dextran (Mr 10,000) with anti-hVPS34 antibody (green pseudocolor). (I–L) Cells were fixed, permeabilized, and incubated with anti-LBPA staining. Arrowheads indicate colocalization of latex beads and LBPA.
Figure 7.
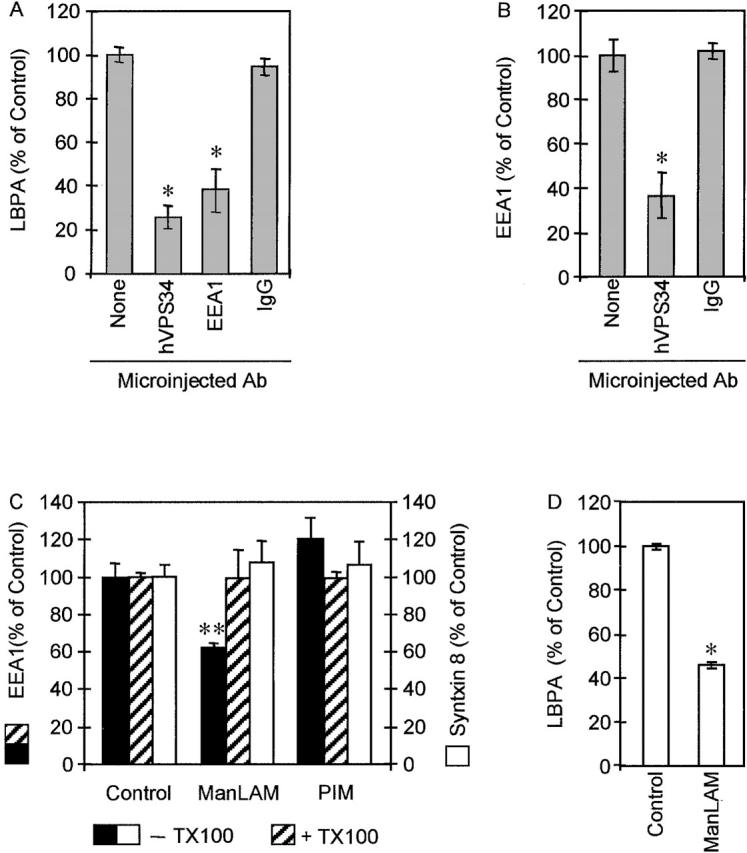
EEA1 exclusion reduces LBPA accumulation on latex beads. (A) Quantitation of LBPA colocalization with latex bead phagosomes (30 min after infection) in cells injected with anti-hVPS34, anti-EEA1, control rabbit IgG, and uninjected control cells (n = 1,347 phagosomes). The data are means ± SE of three separate experiments. *P = 0.0001. (B) Quantitation of EEA1 colocalization with latex bead phagosomes (30 min after infection) in cells injected with anti-hVPS34, control rabbit IgG, and uninjected control cells (n = 601 phagosomes). The data are means ± SE of three separate experiments. *P < 0.001. (C) M. tuberculosis ManLAM but not its precursor PIM reduces EEA1 recruitment to the phagosome. Epifluorescence microscopy analysis of the effect of coating of latex beads with mycobacterial phosphoinositides on EEA1 recruitment. Latex beads were coated with purified M. tuberculosis H37Rv ManLAM or PIM and phagocytosed by J774 cells for 20 min. Quantitation of EEA1 colocalization with phagosomes (n = 1,321 phagosomes). Black bars, control or lipid-coated beads (as indicated) not treated with Triton X-100; hatched bars, control or lipid-coated beads (as indicated) extracted with Triton X-100 before infection. The data are means ± SE of three separate experiments. **P = 0.002. M. tuberculosis glycosylated phosphoinositides do not affect Syntaxin 8 acquisition by latex bead phagosomes. Epifluorescence microscopy analysis of Syntaxin 8 accumulation by phagosomes containing control uncoated latex beads or beads coated with mycobacterial phosphoinositides. Latex beads were coated with ManLAM or PIM and phagocytosed by J774 cells for 20 min. White bars, quantitation of Syntaxin 8 colocalization with phagosomes (n = 432 phagosomes). The data are means ± SE of three separate experiments. (D) M. tuberculosis ManLAM reduces LBPA recruitment to the phagosome. Epifluorescence microscopy analysis of the effect of coating of latex beads with ManLAM on LBPA accumulation. Quantitation of LBPA colocalization with phagosomes after 30 min of phagocytosis (n = 506 phagosomes). The data are means ± SE of three separate experiments. *P = 0.0001.
Microinjection of anti-EEA1 antibody inhibits latex bead phagosome maturation
Next, we tested the role of EEA1 in phagosomal maturation by microinjection of blocking antibodies against this Rab5 effector. In cells injected with the anti-EEA1 antibody, latex bead phagosomes accumulated significantly lower quantities of LBPA relative to phagosomes in uninjected control cells. Although >60% of latex bead phagosomes in control cells acquired LBPA (Fig. 6 I and Fig. 7A), only 23% of phagosomes in cells microinjected with anti-EEA1 antibody accumulated LBPA (Fig. 6 K and Fig. 7 A). Injection of antibodies against another Rab5 effector, Rabaptin-5, did not affect LBPA accumulation by latex bead phagosomes (unpublished data). These data demonstrate that EEA1 plays a critical role in phagosome biogenesis and their maturation into organelles with late endosomal characteristics. Furthermore, these findings show that the exclusion of EEA1 from mycobacterial phagosomes is likely to be the principal cause of the maturation arrest associated with M. tuberculosis phagosomes.
M. tuberculosis lipoarabinomannan inhibits EEA1 recruitment and phagosome acidification
M. tuberculosis produces a variety of lipids that have been implicated in pathogenesis (Brennan and Nikaido, 1995; Cox et al., 1999; Moody et al., 2000). Possibly the most intriguing class of compounds that dominate the lipid-rich mycobacterial cell wall are modified phosphatidyl-myo-inositols, referred to as phosphatidyl-myo-inositol mannosides (Besra and Chatterjee, 1994). A heavily glycosylated form of these phospholipids, mannose-capped lipoarabinomannan (ManLAM), has been shown to interact with host immune cells (Besra and Chatterjee, 1994; Ernst et al., 1998). The exact mode of action of ManLAM and other related mycobacterial phospholipids is not known. Here, we tested the possibility that ManLAM may affect recruitment of EEA1 to the phagosomal membrane based on the following two considerations. (a) PtdIns(3)Ps are essential for the efficient recruitment of EEA1 to endosomal (Patki et al., 1997; Simonsen et al., 1998; Lawe et al., 2000) and as shown here, to phagosomal membranes, and thus chemically analogous bacterial products could interfere with the generation or downstream function of PtdIns(3)P. (b) Recently, Beatty and colleagues (Beatty et al., 2000) demonstrated that mycobacterial lipids, including ManLAM and its precursor phosphatidylinositol mannoside (PIM), are released from phagocytosed mycobacteria and intercalate into several endomembrane compartments in the host cell. Thus, mycobacterial lipids translocate into relevant membranes where they could interfere with EEA1 recruitment. To examine the possibility that mycobacterial PIMs affect phagosomal biogenesis, we coated latex beads with ManLAM or its precursor PIM and tested maturation of phagosomes containing such beads. Purified ManLAM from the virulent M. tuberculosis H37Rv was used to coat latex beads as described previously (Kang and Schlesinger, 1998). Phagosomes containing ManLAM beads recruited less EEA1 relative to control beads (Fig. 7 C). The presence of ManLAM reduced EEA1 colocalization with latex bead phagosomes by 40% (P = 0.002; ANOVA) (Fig. 7 C). This observation indicates that ManLAM interferes with the recruitment of EEA1 to the phagosome. In contrast to the effect seen with ManLAM beads, coating of beads with PIM did not reduce EEA1 recruitment to the phagosome (Fig. 7 C). The effects of ManLAM on EEA1 recruitment could be reversed by extracting lipid-coated beads with Triton X-100 before phagocytosis (Fig. 7 C). The effect of ManLAM on EEA1 recruitment cannot be attributed to alterations in phagocytosis of beads, since ManLAM-coated beads recruited Syntaxin 8 at levels indistinguishable from uncoated control beads (Fig. 7 C). ManLAM-coated beads were also subjected to phagosome acidification analysis and used in LBPA acquisition assays. A reduction in the proportion of acidified phagosomes, visualized by LysoTracker staining, was observed with ManLAM-coated beads relative to uncoated control beads (Fig. 3, E–F and J). Additionally, only 50% of ManLAM-coated beads acquired LBPA (Fig. 7 D). These findings are consistent with a model in which M. tuberculosis glycosylated phosphatidylinositol ManLAM is a factor causing or contributing to the block in EEA1 recruitment. Thus, ManLAM should be considered as an M. tuberculosis toxin, interfering with membrane trafficking and organelle biogenesis contributing to the mycobacterial phagosome maturation arrest.
Discussion
This report demonstrates a critical role in phagosomal maturation for PtdIns(3)P and the Rab5 effectors hVPS34 and EEA1. Our study also identifies these processes and factors as molecular targets for the intracellular pathogen M. tuberculosis in the context of mycobacterial phagosome maturation arrest. The findings presented also show a transient but functionally important recruitment to newly formed phagosomes of the early endosomal membrane-tethering molecule EEA1. The presence of EEA1 on phagosomes is a requisite for the subsequent acquisition of late endocytic characteristics. Since EEA1 has been implicated in trafficking between the TGN and endosomes (Simonsen et al., 1999) and in homotypic fusion within early endocytic compartments (Simonsen et al., 1998), the exclusion of this Rab5 effector from mycobacterial phagosomes is responsible for the block in membrane fusion events required for an orderly acquisition of late endosomal lipids, membrane proteins, and lumenal content.
The inhibition of M. tuberculosis phagosome maturation has been associated previously with abnormal patterns of endosomal Rabs characterized by the accumulation of Rab5 and exclusion of Rab7 (Via et al., 1997). Recent experiments with overexpressed Rab7 in HeLa cells (Clemens et al., 2000) are consistent with our observations that the maturation block is closer to Rab5. In this work, the maturation block has been further narrowed down to the recruitment of EEA1, a major Rab5 effector associated with vesicle tethering and SNARE docking in the process of membrane fusion (Simonsen et al., 1998; Christoforidis et al., 1999a; McBride et al., 1999). The mycobacterial phagosome is arrested at a phase involving hVPS34 recruitment, its activation, or accessibility of its enzymatic products as manifested by the inefficient acquisition of EEA1. The block may involve interference with the generation or accessibility of Ptd-Ins(3)P for binding of the FYVE domain of EEA1 (Stenmark et al., 1996; McBride et al., 1999; Lawe et al., 2000; Nielsen et al., 2000). As shown here, the modified M. tuberculosis phosphatidylinositol molecule ManLAM is at least partially responsible for this block.
Regardless of the absence of Rabaptin-5 (known to stabilize Rab5 in its active conformation) (Stenmark et al., 1995; Rybin et al., 1996) and an apparent lack of its role in phagosome maturation as measured by LBPA acquisition, LBCs are capable of transiently recruiting EEA1. This suggests that Rab5 is functional and active on these membranes for significant periods of time. Additional elements of the oligomeric complexes organized by EEA1 into microdomains (McBride et al., 1999) and acting in membrane tethering and fusion, such as Syntaxin 13, NSF, and αSNAP, are also present on LBCs and MPCs. Based on the central role of EEA1 in oligomerization and tethering-associated priming of SNAREs, it is unlikely that MPCs undergo efficient formation of productive docking complexes containing Syntaxin 13, a SNARE implicated both in the early endosomal homotypic fusion (McBride et al., 1999) and endosomal recycling (Prekeris et al., 1999; Trischler et al., 1999). Other alterations associated with EEA1 exclusion from MPCs are likely to include defective trafficking between the TGN and mycobacterial phagosomes. In addition to interactions with the endosomal SNARE Syntaxin 13, EEA1 also interacts with Syntaxin 6, a TGN SNARE (Simonsen et al., 1999). EEA1 and Syntaxin 6 colocalize, a phenomenon that has been implicated in TGN endosomal trafficking (Simonsen et al., 1999). We have found recently that Syntaxin 6 is present on LBCs but absent from MPCs (unpublished data), an observation that complements the finding that MPCs lack EEA1. In a model that follows from these observations, the inability to interact with the TGN leads to a defect in fusion with H+ATPase-containing vesicles, thus explaining the exclusion of this pump from mycobacterial phagosomes (Sturgill-Koszycki et al., 1994) resulting in their defective acidification.
The significance of the exclusion of EEA1 in the context of tuberculosis as a potent infectious disease is underscored by the transient but nearly invariable recruitment of EEA1 to phagosomes harboring several other pathogens (Pizarro-Cerda et al., 1998; Meresse et al., 1999a; Scianimanico et al., 1999; Steele-Mortimer et al., 1999). However, exclusion of EEA1 may not be unique to mycobacteria, since the phagosomes containing human granulocytic Ehrlichiosis agent do not stain with EEA1 antibodies (Mott et al., 1999), although this observation has not been kinetically evaluated. Significantly, an inverse relationship between EEA1 recruitment and the ability for subsequent Rab7 exclusion is apparent from the data shown in a recent analysis of Leishmania donovani phagosomes (Scianimanico et al., 1999). A lipophosphoglycan mutant of L. donovani shows enhanced EEA1 recruitment relative to the wild-type pathogen followed by strong Rab7 labeling versus the normally poor Rab7 staining of phagosomes containing fully virulent leishmaniae. A further implication of these observations is that phospholipid products of a pathogen (that is, leishmanial lipophosphoglycan) may affect phagosomal trafficking. Here, we provide the first direct demonstration of such activities, showing that the bacterial phospholipid ManLAM interferes with host organelle biogenesis. Thus, ManLAM should be considered as a bacterial toxin-targeting membrane trafficking processes.
The nature of the inhibitory activity of ManLAM is not known at present. Since hVPS34 is recruited to mycobacterial phagosomes, one possibility is that ManLAM inhibits its activity. In vitro studies using purified recombinant hVPS34 (Siddhanta et al., 1998) and ManLAM showed only mild (32%, P < 0.001) PI-3K inhibitory activity in the test tube assay. We believe that this alone is not sufficient to explain the phenomena observed. Furthermore, although coating of beads with ManLAM had a significant effect on phagosomal maturation this did not fully mirror the block in acidification seen in mycobacterial phagosomes or latex bead phagosomes in wortmannin-treated cells. Thus, it is possible that ManLAM is one of several mycobacterial lipids or additional products that may affect EEA1 recruitment and phagosome maturation. Alternatively, the inhibitory effect of ManLAM observed here with the beads could be augmented in mycobacterial phagosomes containing viable bacilli. With >250 lipid metabolism genes in the M. tuberculosis genome (Cole et al., 1998) directing the synthesis of polyketides, sulfatides, and phosphatidyl-myo-inositol derivatives, we predict that several of these factors may interfere with host-trafficking machinery in synergy with ManLAM. These inhibitors of EEA1 recruitment underlie the arrest of M. tuberculosis phagosome maturation, thus ensuring long term survival of the tubercle bacillus in host macrophages.
Materials and methods
Cell and bacterial culture conditions
The murine macrophage-like cell line J774 was maintained in DME supplemented with 4 mM l-glutamine and 5% FBS. BCG harboring phsp60-gfp was grown in 7H9 broth and homogenized to generate a single cell suspension (Via et al., 1998).
Phagosome and endosome purification
Synchronization of latex beads and BCG phagocytosis was achieved by cooling at 4°C to inhibit uptake. Latex beads or BCG were added and allowed to settle at 4°C. Samples were warmed and incubated at 37°C for various periods of time, and phagosomes were isolated as described previously (Via et al., 1997). Cells were lysed by passing through 22-gauge needles connected to a two-syringe apparatus. PNSs were generated by centrifugation at 200 g for 6 min, and then sedimented by centrifugation through a sucrose step gradient of 8.5, 15, and 50% sucrose (wt/wt) at 1,000 g for 45 min. The sediment collected at the 15/50% interface was then loaded on a linear 32–53% sucrose gradient (wt/wt) and centrifuged for 15 h at 100,000 g for isopycnic separation of organelles. After separation, the gradient was fractionated and preparations tested for separation from other subcellular compartments as described previously (Via et al., 1997). MPCs were pelleted from individual fractions at 250,000 g for 40 min. For latex bead phagosome purification, cells were infected with latex beads diluted in DME and incubated as indicated above. LBCs were isolated from PNS by flotation as described by Desjardins et al. (1994). An early endosomal-enriched fraction of membranes was prepared as described by Gruenberg et al. (1989).
Treatment with inhibitors
The proton ATPase (H+ATPase) was inhibited in cells by treating with bafilomycin A (25 nM) for 2 h at 37°C. Bafilomycin treatment preceded infection and remained throughout the experiment. In other experiments, cellular PI-3K activity was inhibited by treating cells with 100 nM wortmannin 10 min after infection and incubated with the inhibitor for 10 min before processing. Cells were also treated with 200 μM LY294002 10 min after infection and incubated with the inhibitor for 20 min before processing or incubated with the inhibitor for 10 min before removing, washing the cells, and incubating with fresh medium for an additional 10 min before processing.
Preparation of mycobacterial lipid-coated latex beads
Lipid-coated beads were prepared as described previously (Kang and Schlesinger, 1998). In brief, 2 × 109 polystyrene beads were washed in 50 mM NaHCO3, pH 9.6, and incubated for 2 h at 37°C in the presence or absence of 50 μg of M. tuberculosis H37Rv ManLAM or its precursor PIM (provided by J. Belisle, Colorado State University, Fort Collins, CO) in NaHCO3. Coated beads were washed twice and incubated for 1 h at 37°C in PBS with 5% BSA followed by washes in PBS with 0.5% BSA. Beads were resuspended in PBS with 0.5% BSA and stored at 4°C until used. A portion of control and ManLAM- and PIM-coated beads were extracted with Triton X-100 for 1 h on ice.
Microinjection of anti-EEA1 and anti-hVPS34 antibody
J774 cells grown on 25-mm glass coverslips were washed with PBS and transferred to a perfusion chamber (Harvard Apparatus) in Hepes, pH 7.4, set at a constant temperature of 37°C. Microinjections were performed using an Eppendorf transjector and Eppendorf injectman systems. Antibody was mixed with FITC-Dextran Mr 10,000 (1 mg/ml) to give a final antibody concentration of 1–4 mg/ml. Microinjections were carried out at a pressure of 40 hPa (0.58 pounds per square inch) for 0.2 s using Eppendorf femtotips II. Cells were allowed to recover for 2 h before infection and immunofluorescence processing.
Epifluorescence and laser scanning confocal microscopy
J774 cells were infected with latex beads or BCG. Synchronization of infection was achieved by centrifugation of particles onto macrophages adherent to coverslips. Macrophages were fixed with 3.7% paraformaldehyde followed by membrane permeabilization using 0.2% saponin. Permeabilized cells were incubated with primary followed by secondary Alexa 568–conjugated antibody (Molecular Probes). LysoTracker DND-99 staining was carried out as described (Via et al., 1998). Epifluorescence microscopy was performed using an IX70 Olympus microscope. Images were collected and processed using LSR Esprit and Adobe Photoshop® software.
Colocalizations of endocytic markers with phagosomes were determined using methods of unbiased counting. Phagosomes considered to colocalize with any given endocytic marker were required to meet strict morphological characteristics. Latex bead phagosomes in treated and control categories were required to colocalize with a complete ring of antibody staining as established by others (Botelho et al., 2000), whereas BCG were held to less stringent requirements. Partial colocalization of mycobacterial phagosomes were considered positive for colocalization. This ensures that differences reported between latex bead and mycobacterial phagosomes were impartial to morphological differences between phagosome. Final levels of colocalization were determined by evaluating ≥100 phagosomes from at least five random fields from four monolayers for each experimental condition or time point.
Western blot analysis and antibody sources
Equal amounts of MPCs and LBCs (by protein) were separated by 12.5% SDS-PAGE and transferred to Immobilon-P membranes. Bound antibodies were visualized using the ECL Western blotting system (Dupont). When comparing MPCs and LBCs on discontinuous filters, antibody incubations ECL reactions were performed simultaneously. Membranes were exposed to film simultaneously, and identical exposure times were used. Antibodies used for Western blots and immunofluorescence experiments were against EEA1 (Patki et al., 1997), LBPA (Kobayashi et al., 1998), NSF (provided by S. Whiteheart, University of Kentucky, Lexington, KY), Rab5 (provided by L. Huber, Research Institute of Molecular Pathology, Vienna, Austria), Rabaptin-5 (Transduction Labs), αSNAP (StressGen Biotechnologies), Syntaxin 8 (provided by W. Hong, Institute of Molecular and Cell Biology, Singapore), Syntaxin 13 (provided by R. Prekeris and S. Scheller, Stanford University, Palo Alto, CA), tuberin (Santa Cruz Biotechnology, Inc.), and hVPS34 (Siddhanta et al., 1998).
Statistical analysis
All statistical analyses were calculated using Fisher's protected LSD post hoc test (ANOVA) (SuperANOVA 1.11; Abacus Concepts, Inc.). P values of ≤0.05 were considered significant.
Acknowledgments
We thank W. Hong, L. Huber, R. Prekeris, R. Scheller, and S. Whiteheart for antibodies, and J. Belisle for purified M. tuberculosis H37Rv ManLAM.
This work was supported by National Institutes of Health Grant AI45148 to V. Deretic.
Footnotes
Abbreviations used in this paper: BCG, Mycobacterium tuberculosis variant bovis BCG (Baccillus Calmette-Guérin); EEA1, early endosome autoantigen; LBPA, lysobisphosphatidic acid; LBC, latex bead phagosomal compartment; ManLAM, mannose-capped lipoarabinomannan; MPC, Mycobacterium tuberculosis phagosomal compartment; NSF, N-ethylmaleimide–sensitive factor; PIM, phosphatidylinositol mannoside; PI-3K, phosphatidylinositol 3′(OH)-kinase; PNS, postnuclear supernatant; Ptd-Ins(3)P, phosphatidylinositol 3-phosphate; SNAP, soluble NSF attachment protein; SNARE, SNAP receptors; TGN, trans-Golgi network.
References
- Alvarez-Dominguez, C., and P.D. Stahl. 1999. Increased expression of Rab5a correlates directly with accelerated maturation of Listeria monocytogenes phagosomes. J. Biol. Chem. 274:11459–11462. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dominguez, C., A.M. Barbieri, W. Beron, A. Wandinger-Ness, and P.D. Stahl. 1996. Phagocytosed live Listeria monocytogenes influences Rab5-regulated in vitro phagosome-endosome fusion. J. Biol. Chem. 271:13834–13843. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dominguez, C., R. Roberts, and P.D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731–743. [DOI] [PubMed] [Google Scholar]
- Araki, N., M.T. Johnson, and J.A. Swanson. 1996. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135:1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, J.A., and P.D. Hart. 1971. Response of cultured macrophages to M. tuberculosis with observations of fusion of lysosomes with phagosomes. J. Exp. Med. 134:713–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno, L., X.R. Peng, A.D. Schreiber, H.P. Moore, W.S. Trimble, and S. Grinstein. 2000. Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty, W.L., E.R. Rhoades, H.-J. Ullrich, D. Chatterjee, J.E. Heuser, and D.G. Russell. 2000. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic. 1:235–247. [DOI] [PubMed] [Google Scholar]
- Besra, G.S., and D. Chatterjee. 1994. Lipids and carbohydrates of Mycobacterium tuberculosis In Tuberculosis: Pathogenesis, Protection, and Control. B.R. Bloom, editor. ASM Press, Washington, DC. 285–306.
- Botelho, R.J., D.J. Hackam, A.D. Schreiber, and S. Grinstein. 2000. Role of COPI in phagosome maturation. J. Biol. Chem. 275:15717–15727. [DOI] [PubMed] [Google Scholar]
- Brennan, P.J., and H. Nikaido. 1995. The envelope of mycobacteria. Annu. Rev. Biochem. 64:29–63. [DOI] [PubMed] [Google Scholar]
- Chakraborty, P., S. Sturgill-Koszycki, and D.G. Russell. 1994. Isolation and characterization of pathogen-containing phagosomes. Methods Cell Biol. 45:261–276. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., H.M. McBride, R.D. Burgoyne, and M. Zerial. 1999. a. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 397:621–625. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., M. Miaczynska, K. Ashman, M. Wilm, L. Zhao, S.C. Yip, M.D. Waterfield, J.M. Backer, and M. Zerial. 1999. b. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1:249–252. [DOI] [PubMed] [Google Scholar]
- Clemens, D.L., and M.A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, D.L., and M.A. Horwitz. 1996. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens, D.L., B.Y. Lee, and M.A. Horwitz. 2000. Mycobacterium tuberculosis and Legionella pneumophila phagosomes exhibit arrested maturation despite acquisition of Rab7. Infect. Immun. 68:5154–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole, S.T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S.V. Gordon, K. Eiglmeier, S. Gas, C.E. Barry III, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 393:537–544 (erratum published 396:190). [DOI] [PubMed]
- Cox, J.S., B. Chen, M. McNeil, and W.R. Jacobs, Jr. 1999. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 402:79–83. [DOI] [PubMed] [Google Scholar]
- de Chastellier, C., T. Lang, and L. Thilo. 1995. Phagocytic processing of the macrophage endoparasite, Mycobacterium avium, in comparison to phagosomes which contain Bacillus subtilis or latex beads. Eur. J. Cell Biol. 68:167–182. [PubMed] [Google Scholar]
- Defacque, H., M. Egeberg, A. Habermann, M. Diakonova, C. Roy, P. Mangeat, W. Voelter, G. Marriott, J. Pfannstiel, H. Faulstich, et al. 2000. Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J. 19:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic, V., and R.A. Fratti. 1999. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31:1603–1609. [DOI] [PubMed] [Google Scholar]
- Desjardins, M., L.A. Huber, R.G.F. Parton, and G. Griffiths. 1994. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with endocytic apparatus. J. Cell Biol. 124:677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey, G.P., R.J. Botelho, J.R. Butler, Y. Moltyaner, P. Chien, A.D. Schreiber, and S. Grinstein. 1999. Phagosomal maturation, acidification, and inhibition of bacterial growth in nonphagocytic cells transfected with FcgammaRIIA receptors. J. Biol. Chem. 274:28436–28444. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey, C., E. van Donselaar, V.W. Hsu, C. Yang, P.D. Stahl, and P.J. Peters. 1998. ARF6 targets recycling vesicles to the plasma membrane: insights from an ultrastructural investigation. J. Cell Biol. 140:603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, J.D. 1998. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66:1277–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst, W.A., J. Maher, S. Cho, K.R. Niazi, D. Chatterjee, D.B. Moody, G.S. Besra, Y. Watanabe, P.E. Jensen, S.A. Porcelli, et al. 1998. Molecular interaction of CD1b with lipoglycan antigens. Immunity. 8:331–340. [DOI] [PubMed] [Google Scholar]
- Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell. 97:435–447. [DOI] [PubMed] [Google Scholar]
- Fratazzi, C., N. Manjunath, R.D. Arbeit, C. Carini, T.A. Gerken, B. Ardman, E. Remold-O'Donnell, and H.G. Remold. 2000. A macrophage invasion mechanism for mycobacteria implicating the extracellular domain of CD43. J. Exp. Med. 192:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti, R.A., I. Vergne, J. Chua, J. Skidmore, and V. Deretic. 2000. Regulators of membrane trafficking and Mycobacterium tuberculosis phagosome maturation block. Electrophoresis. 21:3378–3385. [DOI] [PubMed] [Google Scholar]
- Gorvel, J.P., P. Chavrier, M. Zerial, and J. Gruenberg. 1991. rab5 controls early endosome fusion in vitro. Cell. 64:915–925. [DOI] [PubMed] [Google Scholar]
- Gough, N.R., and D.M. Fambrough. 1997. Different steady state subcellular distributions of the three splice variants of lysosome-associated membrane protein LAMP-2 are determined largely by the COOH-terminal amino acid residue. J. Cell Biol. 137:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gournier, H., H. Stenmark, V. Rybin, R. Lippe, and M. Zerial. 1998. Two distinct effectors of the small GTPase Rab5 cooperate in endocytic membrane fusion. EMBO J. 17:1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg, J., G. Griffiths, and K.E. Howell. 1989. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J. Cell Biol. 108:1301–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, H., R. Lippe, H.M. McBride, M. Rubino, P. Woodman, H. Stenmark, V. Rybin, M. Wilm, K. Ashman, M. Mann, et al. 1997. A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell. 90:1149–1159. [DOI] [PubMed] [Google Scholar]
- Jahraus, A., T.E. Tjelle, T. Berg, A. Habermann, B. Storrie, O. Ulrich, and G. Griffiths. 1998. In vitro fusion of phagosomes with different endocytic organelles from J774 macrophages. J. Biol. Chem. 273:30379–30390. [DOI] [PubMed] [Google Scholar]
- Kang, B.K., and L.S. Schlesinger. 1998. Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66:2769–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson, K., and S.R. Carlsson. 1998. Sorting of lysosomal membrane glycoproteins lamp-1 and lamp-2 into vesicles distinct from mannose 6-phosphate receptor/gamma-adaptin vesicles at the trans-Golgi network. J. Biol. Chem. 273:18966–18973. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., E. Stang, K.S. Fang, P. de Moerloose, R.G. Parton, and J. Gruenberg. 1998. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 392:193–197. [DOI] [PubMed] [Google Scholar]
- Kobayashi, T., M.H. Beuchat, M. Lindsay, S. Frias, R.D. Palmiter, H. Sakuraba, R.G. Parton, and J. Gruenberg. 1999. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell Biol. 1:113–118. [DOI] [PubMed] [Google Scholar]
- Lawe, D.C., V. Patki, R. Heller-Harrison, D. Lambright, and S. Corvera. 2000. The FYVE domain of early endosome antigen 1 is required for both phosphatidylinositol 3-phosphate and rab5 binding. Critical role of this dual interaction for endosomal localization. J. Biol. Chem. 275:3699–3705. [DOI] [PubMed] [Google Scholar]
- Malik, Z.A., G.M. Denning, and D.J. Kusner. 2000. Inhibition of Ca(2+) signaling by Mycobacterium tuberculosis is associated with reduced phagosome-lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride, H.M., V. Rybin, C. Murphy, A. Giner, R. Teasdale, and M. Zerial. 1999. Oligomeric complexes link Rab5 effectors with NSF and drive membrane fusion via interactions between EEA1 and syntaxin 13. Cell. 98:377–386. [DOI] [PubMed] [Google Scholar]
- Meresse, S., O. Steele-Mortimer, B.B. Finlay, and J.P. Gorvel. 1999. a. The rab7 GTPase controls the maturation of Salmonella typhimurium-containing vacuoles in HeLa cells. EMBO J. 18:4394–4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meresse, S., O. Steele-Mortimer, E. Moreno, M. Desjardins, B. Finlay, and J.P. Gorvel. 1999. b. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat. Cell Biol. 1:E183–E188. [DOI] [PubMed] [Google Scholar]
- Mohrmann, K., and P. van der Sluijs. 1999. Regulation of membrane transport through the endocytic pathway by rabGTPases. Mol. Membr. Biol. 16:81–87. [DOI] [PubMed] [Google Scholar]
- Moody, D.B., T. Ulrichs, W. Muhlecker, D.C. Young, S.S. Gurcha, E. Grant, J.P. Rosat, M.B. Brenner, C.E. Costello, G.S. Besra, et al. 2000. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 404:884–888. [DOI] [PubMed] [Google Scholar]
- Mordue, D.G., and L.D. Sibley. 1997. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 159:4452–4459. [PubMed] [Google Scholar]
- Mott, J., R.E. Barnewall, and Y. Rikihisa. 1999. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect. Immun. 67:1368–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi, S., K.J. Catt, and T. Balla. 1995. A wortmannin-sensitive phosphatidylinositol 4-kinase that regulates hormone-sensitive pools of inositolphospholipids. Proc. Natl. Acad. Sci. USA. 92:5317–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, E., S. Christoforidis, S. Uttenweiler-Joseph, M. Miaczynska, F. Dewitte, M. Wilm, B. Hoflack, and M. Zerial. 2000. Rabenosyn-5, a novel Rab5 effector, is complexed with hVPS45 and recruited to endosomes through a FYVE finger domain. J. Cell Biol. 151:601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaretou, C., J. Domin, S. Cockcroft, and M.D. Waterfield. 1997. Characterization of p150, an adaptor protein for the human phosphatidylinositol (Ptd-Ins) 3-kinase. Substrate presentation by phosphatidylinositol transfer protein to the p150.Ptdins 3-kinase complex. J. Biol. Chem. 272:2477–2485. [DOI] [PubMed] [Google Scholar]
- Patki, V., J. Virbasius, W.S. Lane, B.H. Toh, H.S. Shpetner, and S. Corvera. 1997. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA. 94:7326–7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro-Cerda, J., S. Meresse, R.G. Parton, G. van der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J.P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66:5711–5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris, R., B. Yang, V. Oorschot, J. Klumperman, and R.H. Scheller. 1999. Differential roles of syntaxin 7 and syntaxin 8 in endosomal trafficking. Mol. Biol. Cell. 10:3891–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press, B., Y. Feng, B. Hoflack, and A. Wandinger-Ness. 1998. Mutant Rab7 causes the accumulation of cathepsin D and cation-independent mannose 6-phosphate receptor in an early endocytic compartment. J. Cell Biol. 140:1075–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, M., G. Xu, J. Zeng, C. De Lemos-Chiarandini, M. Adesnik, and D.D. Sabatini. 1998. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc. Natl. Acad. Sci. USA. 95:6187–6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer, M.A., T. Soldati, A.D. Shapiro, J. Lin, and S.R. Pfeffer. 1994. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J. Cell Biol. 125:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.G., S. Xu, and P. Chakraborty. 1992. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J. Cell Sci. 103:1193–1210. [DOI] [PubMed] [Google Scholar]
- Rybin, V., O. Ullrich, M. Rubino, K. Alexandrov, I. Simon, M.C. Seabra, R. Goody, and M. Zerial. 1996. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature. 383:266–269. [DOI] [PubMed] [Google Scholar]
- Schorey, J.S., M.C. Carroll, and E.J. Brown. 1997. A macrophage invasion mechanism of pathogenic mycobacteria. Science. 277:1091–1093. [DOI] [PubMed] [Google Scholar]
- Scianimanico, S., M. Desrosiers, J.-F. Dermine, S. Meresse, A. Descoteaux, and M. Desjardins. 1999. Impaired recruitment of the small GTPase rab7 correlates with the inhibition of phagosome maturation by Leishmania donovani promastigotes. Cell Microbiol. 1:19–32. [DOI] [PubMed] [Google Scholar]
- Scidmore, M.A., E.R. Fischer, and T. Hackstadt. 1996. Sphingolipids and glycoproteins are differentially trafficked to the Chlamydia trachomatis inclusion. J. Cell Biol. 134:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddhanta, U., J. McIlroy, A. Shah, Y. Zhang, and J.M. Backer. 1998. Distinct roles for the p110alpha and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol. 143:1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., R. Lippe, S. Christoforidis, J.M. Gaullier, A. Brech, J. Callaghan, B.H. Toh, C. Murphy, M. Zerial, and H. Stenmark. 1998. EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature. 394:494–498. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., J.M. Gaullier, A. D'Arrigo, and H. Stenmark. 1999. The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 274:28857–28860. [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer, O., S. Meresse, J.-P. Gorvel, B.-H. Toh, and B.B. Finlay. 1999. Biogenesis of Salmonella typhimurium-containing vacuoles in epithelial cells involves interactions with the early endocytic pathway. Cell Microbiol. 1:33–49. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., G. Vitale, O. Ullrich, and M. Zerial. 1995. Rabaptin-5 is a direct effector of the small GTPase Rab5 in endocytic membrane fusion. Cell. 83:423–432. [DOI] [PubMed] [Google Scholar]
- Stenmark, H., R. Aasland, B.H. Toh, and A. D'Arrigo. 1996. Endosomal localization of the autoantigen EEA1 is mediated by a zinc-binding FYVE finger. J. Biol. Chem. 271:24048–24054. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S., P.H. Schlesinger, P. Chakraborty, P.L. Haddix, H.L. Collins, A.K. Fok, R.D. Allen, S.L. Gluck, J. Heuser, and D.G. Russell. 1994. Lack of acidification in mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 263:678–681 (erratum published 263:1359). [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S., U.E. Schaible, and D.G. Russell. 1996. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- Subramaniam, V.N., E. Loh, H. Horstmann, A. Habermann, Y. Xu, J. Coe, G. Griffiths, and W. Hong. 2000. Preferential association of syntaxin 8 with the early endosome. J. Cell Sci. 113:997–1008. [DOI] [PubMed] [Google Scholar]
- Trischler, M., W. Stoorvogel, and O. Ullrich. 1999. Biochemical analysis of distinct Rab5- and Rab11-positive endosomes along the transferrin pathway. J. Cell Sci. 112:4773–4783. [DOI] [PubMed] [Google Scholar]
- Ullrich, H.J., W.L. Beatty, and D.G. Russell. 1999. Direct delivery of procathepsin D to phagosomes: implications for phagosome biogenesis and parasitism by mycobacterium. Eur. J. Cell Biol. 78:739–748. [DOI] [PubMed] [Google Scholar]
- Via, L.E., D. Deretic, R.J. Ulmer, N.S. Hibler, L.A. Huber, and V. Deretic. 1997. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272:13326–13331. [DOI] [PubMed] [Google Scholar]
- Via, L.E., R.A. Fratti, M. McFalone, E. Pagan-Ramos, D. Deretic, and V. Deretic. 1998. Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111:897–905. [DOI] [PubMed] [Google Scholar]
- Vitelli, R., M. Santillo, D. Lattero, M. Chiariello, M. Bifulco, C.B. Bruni, and C. Bucci. 1997. Role of the small GTPase Rab7 in the late endocytic pathway. J. Biol. Chem. 272:4391–4397. [DOI] [PubMed] [Google Scholar]
- Vlahos, C.J., W.F. Matter, K.Y. Hui, and R.F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241–5248. [PubMed] [Google Scholar]
- Xiao, G.H., F. Shoarinejad, F. Jin, E.A. Golemis, and R.S. Yeung. 1997. The tuberous sclerosis 2 gene product, tuberin, functions as a Rab5 GTPase activating protein (GAP) in modulating endocytosis. J. Biol. Chem. 272:6097–6100. [DOI] [PubMed] [Google Scholar]
- Xu, S., A. Cooper, S. Sturgill-Koszycki, T. van Heyningen, D. Chatterjee, I. Orme, P. Allen, and D.G. Russell. 1994. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J. Immunol. 153:2568–2578. [PubMed] [Google Scholar]