Abstract
Caloric restriction increases stress resistance and lifespan in Drosophila melanogaster and other species. The roles of individual nutrients in stress resistance and longevity are largely unknown. The vitamin biotin is a potential candidate for mediating these effects, given its known roles in stress signaling and gene regulation by epigenetic mechanisms, i.e., biotinylation of histones. Here, we tested the hypothesis that prolonged culture of Drosophila on biotin-deficient medium increases stress resistance and lifespan. Flies were fed a biotin-deficient diet for multiple generations; controls were fed a biotin-normal diet. In some experiments, a third group of flies was fed a biotin-deficient diet for 12 generations and then switched to control diets for two generations to eliminate potential effects of short-term biotin deficiency. Flies fed a biotin-deficient diet exhibited a 30% increase in lifespan. This increase was associated with enhanced resistance to the DNA-damaging agent hydroxyurea and heat stress. Also, fertility increased significantly compared with biotin-normal controls. Biotinylation of histones was barely detectable in biotin-deprived flies, suggesting that epigenetic events might have contributed to effects of biotin deprivation.
Keywords: Biotin, gene expression, histones, human, stress resistance
Introduction
Environmental factors and genetic traits impact lifespan. For example, stress resistance is known to increase lifespan in organisms ranging from Drosophila to mammals (1). Drosophila melanogaster is frequently used as a model in lifespan studies because of its relatively short lifespan (3 mo) and numerous genetic tools (2). Resistance to several forms of environmental stress has been associated with increased lifespan in Drosophila including oxidative stress, heat, exposure to UV light and heavy metals, and nutrient deprivation. Caloric restriction has also been shown to increase lifespan (3), coinciding with a decreased number of offspring (4, 5).
A fundamental problem associated with previous studies on lifespan extension by caloric restriction in Drosophila is that caloric restriction was achieved by diluting all nutrients present in food (6, 7). Therefore, it was not possible to identify individual nutrients that may account for lifespan extension in Drosophila. Here we overcame this limitation by testing the effects of the deficiency of a single nutrient (the water-soluble vitamin biotin) on stress resistance and lifespan in flies. Biotin was selected as a model based on the following lines of reasoning. First, previous work showed that short-term biotin deficiency (11 d) is associated with altered stress resistance: biotin-deficient male Drosophila had a 40% increase in resistance to oxidative stress as judged by survival times (8). Second, biotin affects signaling pathways that relate to stress resistance and survival pathways (9). For example, biotin deficiency enhances the nuclear translocation of NF-κB and the expression of anti-apoptotic genes in eukaryotes (10). Third, biotin is covalently attached to histones H2A, H3 and H4 (11-13), suggesting that biotin might influence longevity through epigenetic regulation of gene expression patterns. Histone biotinylation is implicated in heterochromatin structures, gene silencing, DNA repair, and mitotic chromatin condensation (14-16). Biotinylation of histones is mediated by holocarboxylase synthetase (HCS)3 (17), which is a chromosomal protein (9). Camporeale et al. (9, 18) demonstrated that HCS deficiency affects gene expression patterns, lifespan, and heat resistance in Drosophila.
Here, we tested the hypothesis that feeding a biotin-deficient diet for more than 10 generations increases stress resistance and lifespan in Drosophila. Specifically, we tested (i) if biotin deprivation is associated with increased lifespan in Drosophila; (ii) whether biotin deficiency is associated with altered fecundity; (iii) whether alterations in lifespan are due to a specific resistance to biotin deficiency or to a global resistance to stress; (iv) whether behavioral changes occur in response to biotin deprivation; and (v) whether changes in biotin-dependent phenotypes are associated with loss of specific histone biotinylation isoforms.
Methods
Drosophila Stocks.
Drosophila melanogaster were obtained from a laboratory-adapted population of a non-selected wild type strain of Drosophila. The laboratory population was originally established from a natural Drosophila population and allowed to adapt to laboratory conditions through a large cage where the population size was typically over 10,000. This large size preserved natural genetic variation.
Drosophila Husbandry.
The flies were fed one of two diets, control or biotin deficient. Fly medium was prepared as described previously (19), and rendered biotin deficient by the addition of 80 g spray dried egg white (EW) per 1 L of diet (8). Raw egg white contains the protein avidin, which has a high affinity for biotin (20). Avidin-bound biotin is unavailable for absorption (8). For this reason, the flies grown on this medium were denoted “biotin deficient” (BD). Because 80 g of egg white contained approximately 56 g of protein, protein-matched control diets were prepared by substituting 56 g of bovine serum albumin for egg white. Variations in amino acid composition between these two proteins are moderate (Supplemental Table S1) (21, 22). In addition, the yeast powder used in the preparation of fly diets contains about 400 g protein/kg powder (23). Given that approximately 1 g of egg white protein or bovine serum albumin were added per 2 g of yeast powder during preparation of diets (8, 19), the variation introduced by egg white protein and serum albumin in regard to amino acid composition appears to be small. The concentration of bioavailable biotin in both diets was confirmed by avidin binding assay (8). The biotin-deficient diet contained 0.22 ± 0.09 nmol/L bioavailable biotin; the control diet contained 32 ± 0.3 nmol/L bioavailable biotin. Dietary biotin was routinely monitored throughout this study. Flies were fed these diets for multiple generations, as described below.
In some experiments, it was important to distinguish between short-term effects of biotin deficiency on gene expression and stress resistance, and effects of adaptation to biotin deficiency over multiple generations on gene expression and stress resistance. Therefore, a third group of flies was fed a biotin-deficient diet for 12 generations and then switched to control diet for two generations. These flies were denoted “F12+2.” Flies were housed in 60 × 130 mm polypropylene bottles at 25°C and kept on a 12 h dark/light cycle. A minimum of 500 parents were kept for each generation. Flies were collected randomly for stress and longevity experiments; each experiment was performed using 25 flies per repeat (25 × 95 mm polystyrene vials) for a total of 4 repeats per diet group unless noted otherwise.
Lifespan.
Lifespan experiments were conducted as described previously (8). Flies were kept on biotin-normal medium while monitoring lifespan.
Stress Experiments.
Resistance to heat stress, starvation, and DNA-damaging agents were evaluated as follows. First, flies were exposed to temperature stress (34°C) as previously described and survival was monitored at timed intervals (9). Second, flies were exposed to the following starvation stresses: (i) no access to food or water (denoted “starved/dehydrated flies”); (ii) access to water through a plastic foam plug that was moistened every 24 h (denoted “starved flies”); and (iii) access to 2% agar/water medium, a starvation medium commonly used in Drosophila experiments (denoted “starved agar flies”); starved agar flies were transferred to fresh vials every 72 h. Fly survival was monitored at timed intervals (2 h for starved/dehydrated, 4 h for starved, 8 h for starved agar flies) until all flies had died. Third, flies were exposed to hydroxyurea in order to evaluate their resistance to DNA damage (24). In these experiments, young adult flies (6 males and 6 females) were fed biotin-deficient or biotin-normal diets containing 5, 7, 9, or 11 mmol/L of hydroxyurea; controls were fed hydroxyurea-free medium (4 repeats/group). Flies were allowed to lay eggs for 48 hours. Parent flies were discarded and progeny were counted upon eclosion.
DNA Microarray.
Candidate genes that mediate stress resistance were identified by using the Drosophila Genome 2.0 Array (Affymetrix, Santa Clara, CA). RNA was extracted from 100 flies (9). Microarray analyses were conducted at the Genomics Core Research Facility, University of Nebraska-Lincoln as described (9). Affymetrix GeneChip operating software 1.4 was used for normalization and analysis of microarray data. In these experiments F12+2 males and females were compared to biotin-normal males and females (controls), respectively. BD flies were not included in microarray analysis because short-term effects of biotin deficiency could have confounded expression data. Expression changes were considered dependent on biotin deprivation if their expression increased by at least 100% or decreased by at least 50% in F12+2 flies compared with biotin-normal controls.
Fertility Studies.
Potential effects of population density and biotin deprivation on fertility were monitored using two different methods: First, six virgin females were mated with six males and allowed to lay eggs for 24 h; the small number of parents resulted in a low population density for both BD and control flies. Parents were removed and offspring was counted upon eclosion. Second, we determined whether the biotin status of parents or the biotin concentration in media during egg and larval development were more crucial for fecundity. In these experiments 90 eggs were collected in 8 replicates (720 total eggs) from BD flies and biotin-normal controls; the eggs were transferred to both BD and control diets in all possible permutations (4 repeats at 90 eggs each): eggs from BD parents to BD diet; BD parents to control diet; control parents to BD diet; and control parents to control diet. Emerging adults were counted and transferred to a fresh vial of the diet from which they had eclosed; offspring of these flies were used to conduct stress experiments with heat and hydroxyurea.
Body Composition.
Total protein levels of BD and control flies were measured by extracting the homogenate of 100 male and 100 female flies with 300 μL of PBS. Samples were centrifuged (10 g, 1 min) and the supernatant was analyzed using the bicinchoninic acid assay (Pierce; Rockford, IL) according to the manufacturer’s procedures. Total lipids were quantified in pools of flies that were homogenized in the absence of PBS (n = 4 repeats at 150 male and female each). Total fat was quantified by using the SafTestTM PerCent Fat kit (MP Biochemicals; Montreal, Canada), according to the manufacturer's instructions.
Behavioral Analysis.
For all behavioral assays, male and female flies were collected as newly eclosed virgin adults and aged five days before assay.
Geotaxis:
A single fly was placed into a 100 × 30 mm vial marked with a line drawn horizontally 8 cm above the surface. The flies were simultaneously tapped to the surface and given 10 s to demonstrate their normal negative geotaxis by migrating against the natural force of gravity. Flies crossing the 8 cm line within 10 s are considered normal (e.g., negatively geotactic).
Righting:
A single fly was aspirated into a 23 × 75 mm polystyrene vial, allowed to recover from aspiration for 30 s, and subjected to a brief, 5-s mechanical shock by vortexing (25). The fly was tapped into a supine position and given 10 s to right from this position. If the fly righted within 10 s, the response was scored as positive. If the fly failed to right within 10 s, the response was scored as negative.
Locomotion:
General activity for adult animals was assessed using a simple locomotor paradigm (26). A single fly was aspirated into a 60 mm petri dish marked with a grid of 1 cm squares, allowed to recover for 30 s, and locomotor activity was observed for the first two (exploratory locomotion) and last two minutes of a 15-min period (basal locomotion). The number of grid lines crossed during each observation period was recorded.
Metabolite Profiles.
Intermediary metabolites were measured as previously described (27). Briefly, 10 flies were homogenized in 400 μL water and to the full volume was added 1.6 mL acetone. Samples were chilled and centrifuged. The supernatant was decanted into vials prepared with eleven stable isotope labeled internal standards: 500 nmol/L d3 creatine; 10 nmol/L d3 methylmalonic acid; 100 nmol/L each of the following 13C3 lactate, 13C3 pyruvate, d3 serine, d5 phenylalanine, 15N2 orotate, d4 sebacic acid, 13C6 glucose, d6 inositol and d5 tryptophan. After 20 μL of triethylamine-trifluoroacetate (custom made liquid salt) was added to the supernatants, the samples were heated to 70°C under a nitrogen stream. As volume was reduced, acetonitrile was added until a constant volume was reached. Pellets were resuspended in methylene chloride and reduced to constant volume. The entire residue was suspended in 150 μL N-methyl-N-trimethylsilyltrifluoracetamide (Sigma), sealed under a teflon septum and heated under nitrogen at 70°C for 1 h. One μL of the soluble portion was injected into an Agilent 5975 gas chromatograph mass spectrometer. A splitless glass insert was used and purge gas was turned off for 1 min. The injection temperature was 250°C. A 30 m DB5 capillary column, film thickness 0.5 μm, ID 0.32 mm was used with a temperature program of: 80−130°C at 4, 130−200°C at 6, and 200−285°C at 12 degrees/min with holds of 1 min at 80°C and 10 min at 285°C for a total run time of 42.5 min. The mass spectrometer, in EI+ mode, scanned from 50−650 atomic mass units every 1.95 sec with 0.05-sec interscan time after a 3.5-min delay. Metabolite concentrations were determined from previously calculated 5-point standard curves.
Protein Biotinylation.
Histone extracts from whole fly homogenates were prepared by using 0.25 mol/L HCl at 4°C overnight as described (28). Denatured proteins were removed by centrifugation and acid-soluble histones in the supernatant were precipitated with trichloracetic acid (1.2 mol/L final concentration). Histones were washed with acetone and dissolved in 8 mol/L urea. Histones were resolved by gel electrophoresis (11) and biotinylated histones were detected using streptavidin-peroxidase as a probe for biotin and two specific antibodies for biotinylated lysine residues, K9 biotinylated H3 (K9BioH3) and K18 biotinylated H3 (K18BioH3) (9, 14).
Biotin also serves as a coenzyme for acetyl-CoA carboxylase α, acetyl-CoA carboxylase β, 3-methylcrontonyl-CoA carboxylase (MCC), propionyl-CoA carboxylase (PCC), and pyruvate carboxylase (PC) (29). We quantified the abundance of biotinylated carboxylases in BD and biotin-normal males and females. Briefly, 100 flies were homogenized as described (8) and proteins (∼100 μg) were resolved using 4−8% Tris-acetate gels (Invitrogen, Carlsbad, CA). Transblots were probed with streptavidin peroxidase (30).
Statistics.
Homogeneity of variances among groups was tested using Bartlett’s test (31). If variances were heterogeneous, data were log-transformed before further statistical analysis. Significance of differences among diet groups or generations were tested by one-way ANOVA. Fisher’s Protected Least Significant Difference procedure was used for posthoc testing (31). For pair-wise comparisons the paired t test was used to determine significance of differences (32). Gender effects were not tested. StatView 5.0.1 (SAS Institute, Cary, NC) was used to perform all calculations. Differences were considered significant if P < 0.01. Data are expressed as mean ± standard deviation.
Results
Biotin Status and Life Span.
Feeding an egg-white diet caused biotin deficiency in Drosophila. When fly extracts were analyzed for biotin, female flies fed the control diet contained significantly more biotin (387 ± 17 fmol biotin/mg protein) compared with females fed the EW diet (2.8 ± 0.3 fmol biotin/mg protein). Likewise, male flies fed the control diet contained significantly more biotin (210 ± 15 fmol biotin/mg protein) compared with males fed the EW diet (4.9 ± 1.4 fmol biotin/mg protein). Biotinylation of carboxylases was also significantly decreased in BD flies compared with controls fed the biotin-normal diet, as determined by streptavidin blotting of cell extracts (Fig. 1). Note that the biotinylated α-chains of MCC and PCC co-migrate as one single band, and that acetyl-CoA carboxylase was barely detectable in any of the treatment groups. F12+2 females and males lived longer than biotin-normal controls (Table 1).
FIGURE 1.
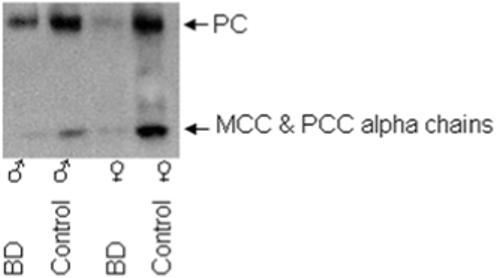
The abundance of biotinylated carboxylases is decreased in flies fed the biotin-deficient diets (BD) compared with flies fed the biotin-normal diet (control). Biotinylated carboxylases were resolved by gel electrophoresis and probed with streptavidin peroxidase. Loading of lanes was normalized by protein concentration.
TABLE 1.
Life span of F12+2 Drosophila melanogaster compared with biotin-normal controls1
| Female | Male | |||
|---|---|---|---|---|
| % Survival | F12+2 | Control | F12+2 | Control |
| Days of Survival2 | ||||
| 50 | 47 ± 5.5a | 40 ± 5.1a | 37 ± 1.8a | 32 ± 2.3a |
| 5 | 75 ± 4.3a | 65 ± 6.7b | 59 ± 1.8a | 52 ± 3.1b |
Controls were fed biotin-normal diets for 14 generations. F12+2 flies were fed biotin-deficient diet for 12 generations and then switched to biotin-normal diets for two generations.
Data are means ± SD.
Values not sharing the same superscript are significantly different from each other for the same sex in the same % survival category; P < 0.01 (n = 4 vials containing 25 flies each). Males were not compared to females.
Values not sharing the same superscript are significantly different from each other for the same sex in the same % survival category; P < 0.01 (n = 4 vials containing 25 flies each). Males were not compared to females.
Stress Resistance.
Biotin-deficient flies exhibited increased stress resistance. BD flies fed an egg-white food for 14 generations exhibited increased resistance to the DNA-damaging agent hydroxyurea compared with biotin-normal and F12+2 flies did not exhibit this increased resistance. When flies laid eggs on a medium containing 0, 5, 7, or 11 mmol/L of hydroxyurea only BD flies developed to adulthood; the total number of adult flies decreased in a dose-dependent manner (Fig. 2). F12+2 flies and biotin-normal controls did not survive to adulthood if exposed to hydroxyurea. The susceptibility of F12+2 flies to hydroxyurea, similar to that observed after only two generations on biotin-deficient food, suggests that hydroxyurea resistance was a result of the biotin-deficient state per se, rather than a stable change in gene expression resulting from biotin deficiency. Both female and male BD flies exhibited a greater 5% survival rate compared with biotin-normal controls if exposed to 34°C after 11 generations of biotin deprivation (Fig. 3, compare BD vs. control). Patterns were similar for 50% survival rates (data not shown). Heat stress resistance was not evident in the second generation of biotin-deprived flies, suggesting that acquisition of stress resistance occurred some time between F2 and F11 (Fig. 3, compare BD F2 vs. control F2, and BD F2 vs. BD F11).
FIGURE 2.
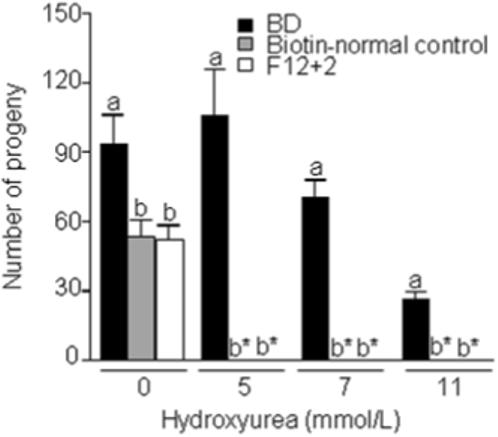
Flies maintained on biotin-deficient diets (BD) exhibit an increased survival in response to hydroxyurea stress. Flies were maintained on the following biotin-defined diets for 14 generations (1) BD; (2) biotin-normal control diet; and (3) F12+2. Flies were mated in the presence of up to 11 mmol/L hydroxyurea and eclosed flies were counted. a,b,cValues not sharing the same superscript are significantly different for the same concentration of hydroxyurea (P < 0.01; n = 4 vials). *No live flies eclosed.
FIGURE 3.
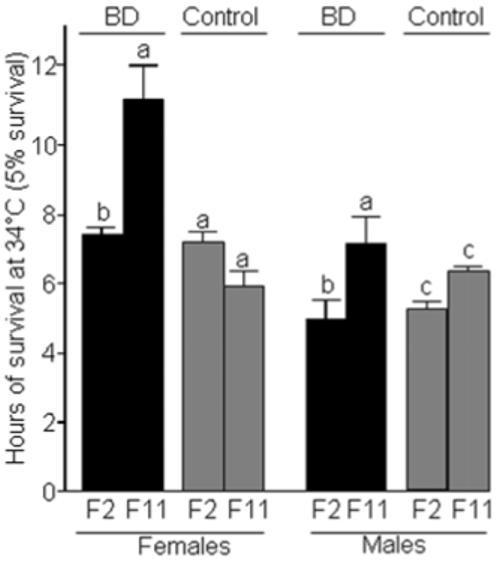
Flies fed a biotin-deficient diet (BD) exhibit a greater resistance to heat stress (34°C) compared with biotin-normal controls. Five percent survival rates after 2 and 11 generations of feeding biotin-defined diets are depicted. Values are means ± SD. a,b,cValues not sharing the same superscript are significantly different within the same gender and diet group (P < 0.01; n = 4 vials, containing 25 adults each). Statistical comparisons were performed within each gender.
Effects of biotin deprivation on starvation resistance were different compared with heat stress and hydroxyurea. For example, agar-starved biotin-normal female flies exhibited a significantly longer lifespan (157 ± 2.8 h) compared with BD female flies (128 ± 2.2 h); agar-starved biotin-normal males exhibit a significantly longer lifespan (161 ± 9.3 h) compared with BD males (130 ± 8.6 h). A similar trend was observed in the starved group (receiving water but no food; data not shown). In contrast, flies from all groups died within 24 h if they were both starved and dehydrated (data not shown). Note that both sexes followed the same trend in regard to effects of biotin deprivation.
Behavioral Changes.
Biotin-deficient flies were significantly more active than biotin-normal controls. Results from the locomotion studies showed that BD males and females displayed increased locomotion relative to biotin-normal, gender-matched controls (Fig. 4). Biotin-deficient flies display normal righting and geotactic abilities, suggesting that the increased locomotor activity in biotin-deficient flies is a specific response.
FIGURE 4.
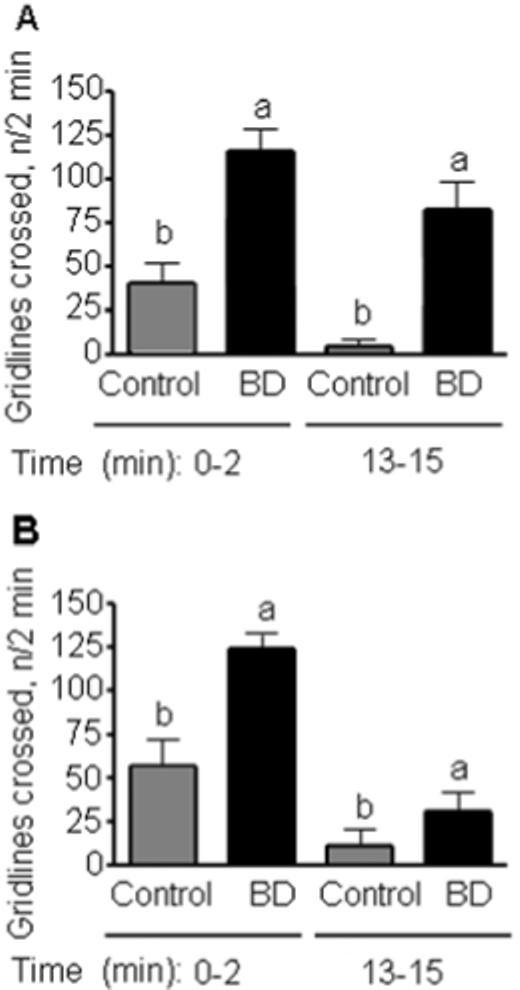
Female (panel A) and male (B) flies fed biotin-deficient diets (BD) are significantly more active than the biotin-normal gender-matched controls. Locomotion studies were performed by monitoring the number of 1 cm grid lines crossed in a two minute period. 0−2, exploratory locomotion; 13−15, basal locomotion. a,bValues not sharing the same superscript are significantly different for the same time period (P < 0.01; n = 4 vials).
Organic Acids.
Prolonged maintenance of flies on biotin-defined diets was associated with substantial changes in metabolite profiles. For example, the level of 3-hydroxyisovaleric acid was greater in extracts from BD flies (1.9 ± 0.5 μmol/L) compared with biotin-normal flies (0.1 ± 0.04 μmol/L). 3-hydroxyisovaleric acid is a well-known marker for biotin deficiency (33). Likewise, lactate was also significantly increased in extracts from BD flies (94 ± 16 μmol/L) compared with biotin-normal flies (14 ± 5.5 μmol/L). This can be attributed to the decreased abundance of biotinylated pyruvate carboxylases (Fig. 1) in BD flies (see Discussion).
Body Composition.
The weight of biotin-deficient female flies was significantly higher (1.7 ± 0.05 mg/fly) than biotin-normal females (1.3 ± 0.01 mg/fly), as was the weight of biotin-deficient males (0.9 ± 0.03 mg/fly) compared to biotin-normal males (0.8 ± 0.01 mg/fly) (P < 0.01; n = 3 repeats at 150 flies each). Body composition analysis revealed that BD females had 13 ± 3% less fat than biotin-normal females, and that BD males had 21 ± 4% less fat than biotin-normal males. In contrast, BD females contained 0.07 ± 0.02 mg of protein more per fly compared with biotin-normal females, and BD males contained 0.04 ± 0.01 mg of protein more compared with biotin-normal males (P < 0.01; n = 4 repeats at 150 flies each).
Effects of Population Density on Fertility and Stress Resistance.
Flies maintained on biotin-deficient medium exhibited increased fertility. While evaluating the hydroxyurea stress experiment, we observed a greater number of BD adults eclosed from hydroxyurea-free controls compared with biotin-normal controls (Fig. 2). In order to ensure that population density was not a confounder in the hydroxyurea stress experiment, we conducted a population-controlled experiment. Greatest survival to adulthood was observed when eggs from BD parents were transferred to BD diet or control diet (Fig. 5), confirming the above findings. Survival to adulthood decreased significantly if eggs were obtained from biotin-normal parents.
FIGURE 5.
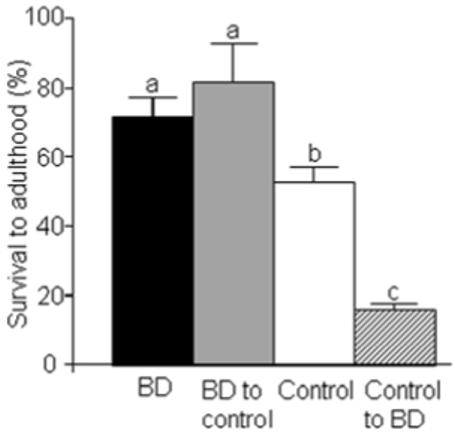
Flies fed biotin-deficient diets (BD) for 30 generations have a higher survival to adulthood rate than biotin-normal controls if controlled for population density. Eggs were collected from both BD flies and biotin-normal controls; the eggs were transferred to both BD and control diets in all possible permutations: eggs from BD parents to BD diet (denoted “BD”); BD parents to control diet (“BD to control”), control parents to BD diet (“control to BD”), and control parents to control diet (“control”). Live adults were counted. Data are means ± SD. a,b,cValues not sharing the same superscript are significantly different (P < 0.01; n = 4 vials).
In flies exposed to hydroxyurea, survival to adulthood was greatest in flies from BD parents that were transferred to BD diets (Fig. 6). If eggs from biotin-normal parents were transferred to BD diet, only a few flies survived to adulthood, but the number was not significantly greater than the other two population-controlled groups. This suggests that the increased stress resistance observed in the BD flies can be acquired after two generations of biotin deficiency. Likewise this resistance can be lost within two generations, based on the lack of eclosion for the progeny of BD parents raised on control medium.
FIGURE 6.
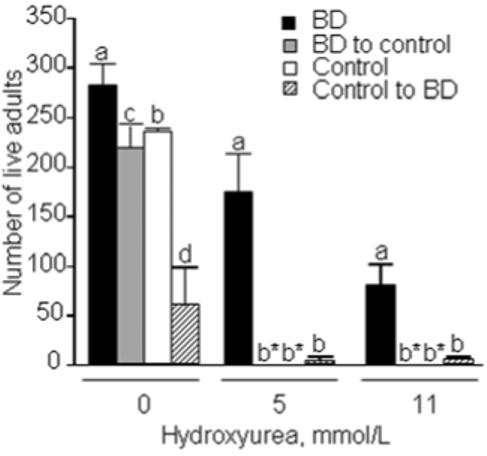
Flies fed a biotin-deficient diet (BD) have greater rates of survival to adulthood in the presence of hydroxyurea compared with biotin-normal controls. In this population-controlled study flies were mated in the presence of up to 11 mmol/L hydroxyurea and eclosed flies were counted. The following groups of flies were used: eggs from BD parents transferred to BD diet (denoted “BD”); eggs from BD parents transferred to control diet (“BD to control”), eggs from control parents transferred to BD diet (“control to BD”), and eggs from control parents transferred to control diet (“control”). Data are means ± SD. a,b,cValues not sharing the same superscript are significantly different for the same concentration of hydroxyurea (P < 0.01; n = 4 vials). *No live flies eclosed.
Stable Transcriptional Changes Associated with Stress Resistance.
DNA microarray studies were used to identify candidate genes for mediating stress resistance and increased lifespan in biotin-deprived flies. Here, we compared transcription profiles in F12+2 flies (rather than BD flies) with biotin-normal controls. F12+2 flies were used as the treatment groups to ensure that effects of biotin deprivation on gene expression were not due to ongoing biotin deficiency but rather to the acquisition of stable traits. The expression of 483 genes in females (Supplemental Table S2) and 95 genes in males (Supplemental Table S3) increases by at least 100% in F12+2 flies compared with controls. The expression of 426 genes in females (Supplemental Table S4) and 76 genes in males (Supplemental Table S5) decreased by at least 50% in F12+2 flies compared with controls. Among the most obvious clusters of biotin-dependent genes were those involved in transport, transcription regulation, protein binding, and DNA/nucleotide binding in females (Table 2), and transport, protein binding, and transferase activity in males (Table 3).
TABLE 2.
Clusters of genes with increased and decreased expression in female F12+21 Drosophila melanogaster compared with biotin-normal controls
| mRNA abundance (F12+2 vs. control) | ||
|---|---|---|
| Gene ontology category | Increased | Decreased |
| n | ||
| Biological process | ||
| Signal transduction | 26 | 10 |
| Cell cycle | 0 | 37 |
| Cell death | 8 | 1 |
| Catabolism | 8 | 8 |
| Defense response | 30 | 6 |
| Transport | 65 | 25 |
| Nucleic acid metabolism | 4 | 22 |
| Regulation of transcription | 11 | 59 |
| Morphogenesis | 11 | 11 |
| Molecular function | ||
| Protein binding | 101 | 124 |
| DNA/nucleotide binding | 27 | 112 |
| Kinase | 15 | 22 |
| Transferase | 28 | 32 |
F12+2 flies were fed biotin-deficient diets for 12 generations and then switched to biotin-normal diets for two generations.
TABLE 3.
Clusters of genes with increased and decreased expression in male F12+21 Drosophila melanogaster compared with biotin-normal controls
| mRNA abundance (F12+2 vs. control) | ||
|---|---|---|
| Gene ontology category | Increased | Decreased |
| n | ||
| Biological process | ||
| Signal transduction | 2 | 5 |
| Catabolism | 8 | 3 |
| Defense response | 9 | 6 |
| Transport | 13 | 8 |
| Regulation of transcription | 3 | 3 |
| Molecular function | ||
| Protein binding | 20 | 14 |
| DNA/nucleotide binding | 1 | 4 |
| Kinase | 5 | 5 |
| Transferase | 11 | 4 |
F12+2 flies were fed biotin-deficient diets for 12 generations and then switched to biotin-normal diets for two generations.
Histone Biotinylation Patterns.
Effects of prolonged starvation for biotin on gene expression patterns might have been caused by decreased biotinylation of histones. When biotin in histones was probed with streptavidin-peroxidase, the signal intensity was lower in BD flies compared with biotin-normal controls (data not shown). Next, we tested whether long-term biotin deficiency affected biotinylation of specific lysine residues in histones. Biotinylated H1 and H3 are more abundant than biotinylated H2A, H2B, and H4 in Drosophila (8, 9). Here, we focused on K9BioH3 and K18BioH3, for which site-specific antibodies are available (9, 13). K9BioH3 (Fig. 7A) and K18BioH3 (Fig. 7B) were substantially decreased in BD male flies compared with biotin-normal controls; patterns were similar in females (data not shown).
FIGURE 7.
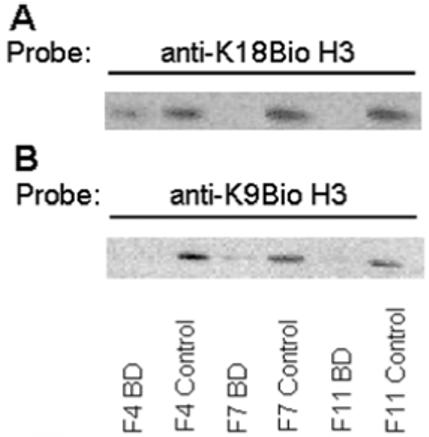
Flies fed biotin-deficient diets (BD) exhibit a decreased abundance of biotinylated histones compared with biotin-normal controls. Panel A: Transblots probed with anti-K18BioH3. Panel B: Transblots probed with anti-K9BioH3. Histone loading was normalized by protein concentration and by using an antibody to the C-terminal tail in histone H3. Note that all lanes shown were from the same blot, but the order of lanes was electronically re-arranged to facilitate comparisons.
Discussion
This study demonstrates that maintaining flies on biotin-defined medium for multiple generations leads to an increase in stress resistance and longevity. Specifically, prolonged biotin starvation is associated with (1) an increase in longevity, (2) an increase in resistance to hydroxyurea stress, (3) an increase in heat resistance, (4) an increase in fertility, (5) an increase in 3-hydroxyisovaleric acid and lactate, (6) a decrease in biotinylated carboxylases, and (7) a decrease in biotinylated histones. The increase in global resistance is due to the removal of one nutrient, namely biotin, over multiple generations. This is the first report to show that a single nutrient affects stress resistance, as previous studies used multiple nutrient deprivation to conduct resistance experiments. Notwithstanding the fact that some of the above effects of biotin deficiency might be considered beneficial, the increased levels of 3-hydroxyisovaleric acid and lactate are consistent with the theory that biotin is an essential nutrient for Drosophila (29, 34).
We hypothesize that effects of biotin deprivation on stress resistance and lifespan might have been caused by epigenetic events. The rationale for our hypothesis is that the biotin-deficient flies that originally exhibited increased stress resistance and longevity did not maintain this resistance during the population density studies, thereby ruling out a genetic effect. Instead, they began to gradually lose their resistance as documented in Fig. 4, which suggests that metastable patterns of differential gene expression may be playing a key role. Further analysis suggests a role for differential histone biotinylation. Previous studies have shown that short-term effects of biotin deficiency did not affect histone biotinylation (8). However, our studies show that BD flies have significant decreases in histone biotinylation and carboxylase biotinylation after 11 generations of biotin deficiency compared with biotin-normal controls (Figs. 1 and 7). These results indicate that long-term biotin deprivation leads to modifications in nuclear and cytoplasmic proteins, though the respective contributions of these modifications to the stress resistance and longevity phenotypes are still unknown. On the other hand, the reported effects could also be attributed to a gene-by-environment interaction. Therefore, when the biotin-deficient flies are exposed to an environment with ample biotin, the increased stress resistance and longevity disappear.
Posttranslational modifications of histones play important roles in chromatin structure and genomic stability. Histone biotinylation is mediated by HCS (17) and perhaps biotinidase (BTD) in humans (35). Evidence suggests that BTD may mediate debiotinylation of histones (36) in addition to acting as a histone biotinyl transferase (35). For example, biotinylation of histones is decreased in HCS-deficient human fibroblasts (13, 17). Camporeale et al. showed that HCS is a chromosomal protein in Drosophila and is a direct effector of histone biotinylation in chromatin (9), which is consistent with our findings here that BD flies have decreased biotinylated histones. The data also support findings that reduced histone biotinylation, and not reduced biotinylated carboxylases, to be associated with stress resistance (18).
Supplementary Material
SUPPLEMENTAL TABLE S1
Amino acid composition of dried chicken egg white protein and bovine serum albumin
SUPPLEMENTAL TABLE S2
Genes with increased expression in F12+2 female Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S3
Genes with increased expression in F12+2 male Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S4
Genes with decreased expression in F12+2 female Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S5
Genes with decreased expression in F12+2 male Drosophila melanogaster compared with biotin-normal controls
Acknowledgments
1 A contribution of the University of Nebraska Agricultural Research Division, supported in part by funds provided through the Hatch Act. Additional support was provided by NIH grants DK 063945 and ES015206, USDA grant 2006−35200−01540, NSF EPSCoR grant EPS-0346476, and by NSF MCB 6552870.
Glossary
The abbreviations used are:
- BD
biotin deficient
- BTD
biotinidase
- EW
egg white
- F
generation
- F12+2
flies were fed biotin-deficient diets for 12 generations before being transferred to control diets for two generations
- HCS
holocarboxylase synthetase
- K9BioH3, K
lysine; K9-biotinylated histone H3
- K18BioH3
K18-biotinylated histone H3
- MCC
3-methylcrotonyl-CoA carboxylase
- PC
pyruvate carboxylase
- PCC
propionyl-CoA carboxylase
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that has been accepted for publication in (journal name), copyright © American Society for Nutrition (ASN). This manuscript may not be duplicated or reproduced, other than for personal use or within the rule of ‘Fair Use of Copyrighted Materials’ (section 107, Title 17, US Code) without permission of the copyright owner, the ASN. The final copyedited article, which is the version of record, can be found at http://pubs.nutrition.org/. The ASN disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Supplemental Tables S1, S2, S3 and S4, and S5 are available as Online Supporting Material with the online posting of this paper at http://jn.nutrition.org.
LITERATURE CITED
- 1.Salmon AB, Murakami S, Bartke A, Kopchick J, Yasumura K, Miller RA. Fibroblast cell lines from young adult mice of long-lived mutant strains are resistant to multiple forms of stress. Am J Physiol Endocrinol Metabol. 2005;289:E23–9. doi: 10.1152/ajpendo.00575.2004. [DOI] [PubMed] [Google Scholar]
- 2.Helfand SL, Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu Rev Genet. 2003;37:329–48. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 3.Norry FM, Loeschcke V. Heat-induced expression of a molecular chaperone decreases by selecting for long-lived individuals. Experimental gerontology. 2003;38:673–81. doi: 10.1016/s0531-5565(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 4.Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. J Evol Biol. 1993;6:171–93. [Google Scholar]
- 5.Phelan JP, Rose MR. Caloric Restriction Increases Longevity Substantially only When the Reaction Norm is Steep. Biogerontology. 2006:161–4. doi: 10.1007/s10522-006-9005-2. [DOI] [PubMed] [Google Scholar]
- 6.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS biology. 2005;3:1305–11. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci USA. 2005;102:13289–94. doi: 10.1073/pnas.0501914102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landenberger A, Kabil H, Harshman LG, Zempleni J. Biotin deficiency decreases life span and fertility but increases stress resistance in Drosophila melanogaster. J Nutr Biochem. 2004;15:591–600. doi: 10.1016/j.jnutbio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Camporeale G, Giordano E, Rendina R, Zempleni J, Eissenberg JC. Drosophila holocarboxylase synthetase is a chromosomal protein required for normal histone biotinylation, gene transcription patterns, lifespan and heat tolerance. J Nutr. 2006;136:2735–42. doi: 10.1093/jn/136.11.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Melendez R, Schwab LD, Zempleni J. Jurkat cells respond to biotin deficiency with increased nuclear translocation of NF-κB, mediating cell survival. Int J Vitam Nutr Res. 2004;74:209–16. doi: 10.1024/0300-9831.74.3.209. [DOI] [PubMed] [Google Scholar]
- 11.Camporeale G, Shubert EE, Sarath G, Cerny R, Zempleni J. K8 and K12 are biotinylated in human histone H4. Eur J Biochem. 2004;271:2257–63. doi: 10.1111/j.1432-1033.2004.04167.x. [DOI] [PubMed] [Google Scholar]
- 12.Chew YC, Camporeale G, Kothapalli N, Sarath G, Zempleni J. Lysine residues in N- and C-terminal regions of human histone H2A are targets for biotinylation by biotinidase. J Nutr Biochem. 2006;17:225–33. doi: 10.1016/j.jnutbio.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobza K, Camporeale G, Rueckert B, Kueh A, Griffin JB, Sarath G, Zempleni J. K4, K9, and K18 in human histone H3 are targets for biotinylation by biotinidase. FEBS J. 2005;272:4249–59. doi: 10.1111/j.1742-4658.2005.04839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley JS, Griffin JB, Zempleni J. Biotinylation of histones in human cells: effects of cell proliferation. Eur J Biochem. 2001;268:5424–9. doi: 10.1046/j.0014-2956.2001.02481.x. [DOI] [PubMed] [Google Scholar]
- 15.Kothapalli N, Camporeale G, Kueh A, Chew YC, Oommen AM, Griffin JB, Sarath G, Zempleni J. Biological functions of biotinylated histones. J Nutr Biochem. 2005;16:446–8. doi: 10.1016/j.jnutbio.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camporeale G, Oommen AM, Griffin JB, Sarath G, Zempleni J. K12-biotinylated histone H4 marks heterochromatin in human lymphoblastoma cells. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2006.12.014. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narang MA, Dumas R, Ayer LM, Gravel RA. Reduced histone biotinylation in multiple carboxylase deficiency patients: a nuclear role for holocarboxylase synthetase. Hum Mol Genet. 2004;13:15–23. doi: 10.1093/hmg/ddh006. [DOI] [PubMed] [Google Scholar]
- 18.Camporeale G, Zempleni J, Eissenberg JC. Susceptibility to heat stress and aberrant gene expression patterns in holocarboxylase synthetase-deficient Drosophila melanogaster are caused by decreased biotinylation of histones, not of carboxylases. J Nutr. 2007;137:885–9. doi: 10.1093/jn/137.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman T, Partridge L. Female fitness in Drosophila melanogaster: an interaction between the effect of nutrition and of encounter rate with males. Proc R Soc Lond B Biol Sci. 1996;263:755–9. doi: 10.1098/rspb.1996.0113. [DOI] [PubMed] [Google Scholar]
- 20.Green NM. Avidin. Adv Protein Chem. 1975;29:85–133. doi: 10.1016/s0065-3233(08)60411-8. [DOI] [PubMed] [Google Scholar]
- 21.Posati LP, Orr ML. Composition of foods: dairy and egg products, raw-processed-prepared. Composition of foods: dairy and egg products, raw-processed-prepared 1976 [cited 6/12/2007] http://albumen.stanford.edu/library/c20/posati.html Available from:
- 22.Stein WH, Moore S. Amino acid composition of β-lactoglobulin and bovine serum albumin. J Biol Chem. 1949;178:79–91. [PubMed] [Google Scholar]
- 23.Ingledew WM, Langille LA, Menegazzi GS, Mok MH. Spent brewers yeast - analysis, improvement, and heat processing considerations. 1977 [cited 2007 6/12/2007] http://www.mbaa.com/TechQuarterly/Abstracts/1977/tq77ab06.htm Available from:
- 24.Radcliffe CM, Silva EA, Campbell SD. A method for assaying the sensitivity of Drosophila replication checkpoint mutants to anti-cancer and DNA-damaging drugs. Genome. 2002;45:881–9. doi: 10.1139/g02-051. [DOI] [PubMed] [Google Scholar]
- 25.Pavlidis P, Tanouye MA. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J Neurosci. 1995;15:5810–9. doi: 10.1523/JNEUROSCI.15-08-05810.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neckameyer WS, Cooper RL. GABA transporters in Drosophila melanogaster: molecular cloning, behavior, and physiology. Invert Neurosci. 1998;3:279–94. doi: 10.1007/BF02577688. [DOI] [PubMed] [Google Scholar]
- 27.Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991;562:125–38. doi: 10.1016/0378-4347(91)80571-s. [DOI] [PubMed] [Google Scholar]
- 28.Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA. 2002;99:838–43. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camporeale G, Zempleni J. Biotin. In: Bowman BA, Russell RM, editors. Present Knowledge in Nutrition. 9th ed International Life Sciences Institute; Washington, D.C.: 2006. pp. 314–26. [Google Scholar]
- 30.Manthey KC, Griffin JB, Zempleni J. Biotin supply affects expression of biotin transporters, biotinylation of carboxylases, and metabolism of interleukin-2 in Jurkat cells. J Nutr. 2002;132:887–92. doi: 10.1093/jn/132.5.887. [DOI] [PubMed] [Google Scholar]
- 31.SAS Institute . StatView Reference. 3rd ed SAS Publishing; Cary, NC: 1999. [Google Scholar]
- 32.Griffin JB, Zempleni J. Biotin deficiency stimulates survival pathways in human lymphoma cells exposed to antineoplastic drugs. J Nutr Biochem. 2005;16:96–103. doi: 10.1016/j.jnutbio.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Mock DM, Henrich CL, Carnell N, Mock NI. Indicators of marginal biotin deficiency and repletion in humans: validation of 3-hydroxyisovaleric acid excretion and a leucine challenge. Am J Clin Nutr. 2002;76:1061–8. doi: 10.1093/ajcn/76.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mock N, Malik M, Stumbo P, Bishop W, Mock D. Increased urinary excretion of 3-hydroxyisovaleric acid and decreased urinary excretion of biotin are sensitive early indicators of decreased status in experimental biotin deficiency. Am J Clin Nutr. 1997;65:951–8. doi: 10.1093/ajcn/65.4.951. [DOI] [PubMed] [Google Scholar]
- 35.Hymes J, Fleischhauer K, Wolf B. Biotinylation of histones by human serum biotinidase: assessment of biotinyl-transferase activity in sera from normal individuals and children with biotinidase deficiency. Biochem Mol Med. 1995;56:76–83. doi: 10.1006/bmme.1995.1059. [DOI] [PubMed] [Google Scholar]
- 36.Ballard TD, Wolff J, Griffin JB, Stanley JS, van Calcar S, Zempleni J. Biotinidase catalyzes debiotinylation of histones. Eur J Nutr. 2002;41:78–84. doi: 10.1007/s003940200011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL TABLE S1
Amino acid composition of dried chicken egg white protein and bovine serum albumin
SUPPLEMENTAL TABLE S2
Genes with increased expression in F12+2 female Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S3
Genes with increased expression in F12+2 male Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S4
Genes with decreased expression in F12+2 female Drosophila melanogaster compared with biotin-normal controls
SUPPLEMENTAL TABLE S5
Genes with decreased expression in F12+2 male Drosophila melanogaster compared with biotin-normal controls


