Abstract
Infection of Indian-origin rhesus macaques by the simian human immunodeficiency virus (SHIV) is considered to be a suitable preclinical model for directly testing efficacy of vaccine candidates based on the HIV-1 envelope. We used this model for prophylactic vaccination with a peptide-cocktail comprised of highly conserved HIV-1 envelope sequences immunogenic/antigenic in macaques and humans. Separate groups of macaques were immunized with the peptide-cocktail by intravenous and subcutaneous routes using autologous dendritic cells (DC) and Freund’s adjuvant, respectively. The vaccine elicited antigen specific IFN-γ-producing cells and T-cell proliferation, but not HIV-neutralizing antibodies. The vaccinated animals also exhibited efficient cross-clade cytolytic activity against target cells expressing envelope proteins corresponding to HIV-1 strains representative of multiple clades that increased after intravenous challenge with pathogenic SHIVKU2. Virus-neutralizing antibodies were either undetectable or present only transiently at low levels in the control as well as vaccinated monkeys after infection. Significant control of plasma viremia leading to undetectable levels was achieved in majority of vaccinated monkeys compared to mock-vaccinated controls. Monkeys vaccinated with the peptide-cocktail using autologous DC, compared to Freund’s adjuvant, and the mock-vaccinated animals, showed significantly higher IFN- γ production, higher levels of vaccine-specific IFN- γ producing CD4+ cells and significant control of plasma viremia. These results support DC-based vaccine delivery and the utility of the conserved HIV-1 envelope peptide-cocktail, capable of priming strong cell-mediated immunity, for potential inclusion in HIV vaccination strategies.
Keywords: Dendritic cells, peptide-vaccine, rhesus macaques, SHIV, cell-mediated immunity
INTRODUCTION
The use of the highly active anti-retroviral treatment (HAART), pioneered approximately a decade ago has made a strong positive impact on the clinical management of the acquired immunodeficiency syndrome (AIDS) caused by the human immunodeficiency virus type 1 (HIV-1) infection. Nevertheless, the problems associated with drug-related toxicity such as, resistance development, and economic constraints on populations that desperately need it, make the development of an effective vaccine to control HIV-1 infection and prevent development of AIDS a high priority.
In terms of HIV-specific humoral immunity, adoptive transfer of the neutralizing antibodies was successful in providing protection against viral challenge in animal models, but only when the quantity of the antibody employed was high and the timing of the adoptive transfer was matched close to the challenge (Baba et al, 2000; Mascola et al, 2003; Robbins et al, 1995; Gauduin et al, 1997; Poignard et al, 1999). Most of the preclinical and clinical development strategies employing monomeric forms of the envelope protein, gp160 or the surface subunit gp120, proved ineffective, mainly against primary patient isolates (Mascola et al, 1996; Barouch and Letvin, 2002). With respect to the HIV-specific cellular immune responses, there is clear evidence from seminal findings in the literature for an association with control of primary HIV-1 infection in humans as well as simian immunodeficiency virus (SIV) infection in macaques, long-term non-progression, and undetectable virus status in certain individuals belonging to high-risk groups (Robinson et al, 1999; Kuroda et al, 1999; McMichael and Rowland, 2001; Hel et al, 2002; Johnson et al, 1997; Amara et al, 2001; Letvin et al, 1997; Barouch et al 2001; Kostense et al,2004; Rinaldo et al, 1995; Rowland et al, 1993;1995). Several recent studies evaluating a variety of HIV/SIV vaccine candidates in primate models revealed protection mediated by virus-specific helper T cells (TH) and CTL (Nilsson et al, 1998; Robinson et al, 1999; Migueles et al, 2002; Hel et al, 2002; Shearer and Clerici, 1998; Clerici et al, 1996). It is important to note that immune pressure exerted by virus-specific neutralizing antibodies as well as CTL has been implicated with the emergence of resistant mutant strains in animal model studies (Barouch et al, 2002; 2003), but studies connecting the CTL responses with escape mutants primarily employed single epitope vaccines or used animals expressing specific MHC alleles that are prone to promote escape mutations (Barouch et al, 2002; 2003). Thus, despite high potential for efficacy by the neutralizing antibodies, it is not clear as to what are the suitable immunogens and how to develop them, while several animal models as well as human studies established the potential of linear sequences from several HIV proteins for priming strong virus-specific cellular immune responses (Mascola et al, 1996; Barouch and Letvin, 2002; Kuroda et al, 1999; McMichael and Rowland, 2001; Hel et al, 2002; Letvin et al, 1997; Robinson et al, 1999; Sastry and Arlinghaus, 1991; Nehete et al, 1993; 1998a; 1998b; 2002; 2005).
Focusing on the potential importance of virus-specific TH and CTL for protective immunity, we selected eight HIV-1 envelope peptides for selective induction of HIV-specific cellular immune responses through a series of studies in multiple animal models (Sastry and Arlinghaus, 1991; Nehete et al, 1993; 1998a; 1998b; 2002; 2005). A comparison of the primary amino acid sequence of each of these envelope peptides with consensus sequences for HIV-1 strains from various clades (HIV sequence Compendium, 2005) revealed a high degree of homology, ranging between 80-100%. Here we report the efficacy of prophylactic vaccination with a cocktail of these eight HIV-1 envelope peptides in the Indian origin rhesus macaques (Macaca mulatta) against pathogenic SHIVKU2 challenge.
Materials and Methods
Animals
A total of 18 adult rhesus macaques (Macaca mulatta) of Indian origin between the ages of 8-17 years, from the specific pathogen-free breeding colony at the Michael E. Keeling Center for Comparative Medicine and Research of The University of Texas MD Anderson Cancer Center, Bastrop TX, were used in the study. The monkeys were maintained in animal facilities fully accredited by the Association for Assessment and Accreditation of Laboratory Animals Care International, and the study was conducted according to National Institute of Health Guidelines on Care and Use of Laboratory Animals.
Peptides
A group of eight HIV-1 envelope peptides were included in the cocktail vaccine. Comparison of the primary amino acid sequences of these peptides to the corresponding consensus sequences in HIV-1 strains from various clades (HIV sequence Compendium, 2000) revealed a high degree of homology (Table 1). The antigenic and immunogenic properties of the HIV-1 envelope peptide-cocktail were reported earlier (Sastry and Arlinghaus, 1991; Nehete et al, 1993; 1998a; 1998b; 2002; 2005; Lekutis and Letvin, 1997; Wahren et al, 1989). The peptides were prepared in the institutional antigen-core facility utilizing FMOC solid phase chemistry on a PTI Symphony Peptide Synthesizer (Protein Technologies Inc., Tucson, AZ) and the purity of the peptides was determined to be >95% by high-pressure liquid chromatography and was validated by mass spectrometry.
TABLE 1.
Peptide sequences in the cocktail vaccine and conservation of primary amino acid sequence among various HIV-1 clades
| Peptide | Amino acid
Sequence |
Conservation among
HIV-1 Clades (A-O) |
|---|---|---|
| 62 (gp41) | 586YLRDQQLLGIWG597 | 84-100% |
| 64 (gp41) | 519FLGFLGAAGSTMGAASLTLTVQARQ543 | 80-100% |
| J44 (gp41) | 549RQQQNNLLRAIEA561 | 77-92% |
| 104 (gp120) | 45VYYGVPVWKEA55 | 99-100% |
| 111 (gp120) | 118LWDQSLKPCVKLT130 | 93-100% |
| 113 (gp120) | 204SVITQACSKVSFE216 | 92-100% |
| 116 (gp120) | 240GTGPCTNVSTVQC252 | 92-100% |
| J47 (gp120) | 486YKVVKIEPL494 | 78-100% |
Processing of blood and serum samples
Peripheral venous blood samples were collected in EDTA or Sodium heparin at different time points pre- and post-immunizations and after challenge with SHIVKU2 (at week 25 with cell-free SHIVku2, 1×104 TCID50). Before the separation of peripheral blood mononuclear cells (PBMC) from the blood samples, plasma was separated and stored immediately at −80°C. The PBMC were prepared from the blood samples by the standard ficoll-hypaque density-gradient centrifugation and used for virus isolation and various immune assays.
Preparation of dendritic cells (DC) from PBMC
DC were prepared from macaque PBMC samples by the standard protocol (O’Doherty et al, 1997; Nehete et al, 2003) involving separation of plastic adherent monocyte population and cytokine-mediated differentiation during 7-day culturing with GM-CSF (1000 units/ml, Sargramostim Leukine, Immunex, Seattle WA) and IL-4 (1000 units/ml, R&D System, Minneapolis, MN) at 37°C. On day 6 of incubation, the cells with dendritic morphology were collected in 1 ml of cytokine medium in 24-well tissue culture plates and pulsed with the peptide mixture consisting of 2ug each of the peptides along with maturation using TNF-α (1000 units/ml) by incubating overnight at 37°C. Subsequently, the mature DC were washed and used either for in vitro functional assays or for immunization of the monkeys. On an average 1-3 ×106 mature DC were obtained using PBMC from 20 ml of blood (1-3% of PBMC), similar to the yields reported in the literature (O’Doherty et al, 1997; Nehete et al, 2003). Phenotypic analyses were performed to ascertain expression of DC surface markers (Lin-, HLA-DR+, CD83+, CD86+ and CD11c+) by flow cytometry. On an average, a dose of 1 × 106 mature DC pulsed with the peptide cocktail was used for each infusion.
ELISPOT assay for detecting antigen-specific IFN-γ producing cells
Freshly prepared PBMC were used for isolating CD4+ and CD8+ cells by positive selection using magnetic beads according to manufacturer instructions (Dynal Inc. Lake Success, NY) and stimulated with peptide-pulsed autologous monocyte-derived DC for the IFN- γ ELISPOT assay as described earlier (Nehete et al, 2003). Peptides from the vaccine cocktail were used either individually or as mixtures along with an unrelated control peptide (from human papillioma virus, HPV) at 10 μg/ml final concentration, and Con A (5 μg/ml) was used as positive control reagent. The cells (1 × 105) were seeded in duplicate wells of 96-well plates (polyvinylidene difluoride backed plates, MAIP S 45, Millipore, Bedford, MA) and incubated in the presence of the various antigens for 36 h at 37°C. Subsequently, the cells were removed and the wells were thoroughly washed with PBS before incubation with 100 μl of biotynylated secondary antibody to IFN-γ (detection antibody) for 3 h at 37°C followed by avidine peroxidase treatment for another 30 minutes. Spots representing individual cells secreting IFN-γ were developed using freshly prepared substrate (0.3 mg/ml of 3-amino-9-ethyl-carbazole) in 0.1 M sodium acetate buffer, containing 0.015% hydrogen peroxide. Plates were washed to stop color development, and the filters were sent to an independent agency (Zellnet Consulting, New Jersy, NJ) where the spots were counted using the KS-ELISPOT automatic system (Carl Zeiss, Inc. Thornwood, NY) for the quantitative analysis of the number of IFN-γ spot forming cells (SFC). The responses in terms of IFN-γ spot forming cells (SFC) for 105 total input CD8+ T cells were determined for individual monkeys after subtracting background values of cells cultured in the medium. The cut off value for determining the positive response in the assay is defined as a minimum of 10 spots that is twice the number observed in cells cultured in the medium.
Cytokine assay
Aliquots of the PBMC (1×105) from the different monkeys were cultured in the presence of the peptide mixture (each peptide at10 μg/ml final concentration), in complete RPMI-1640 medium, in triplicate wells of 96-well U-bottom plates for 36 h at 37°C. Supernatants (100 μl) were removed from each well after centrifuging the plates, and stored frozen at -70°C. At the time of assay, the samples were thawed and assayed for IFN-γ and IL-6, using the BD Cytometric Bead Array (CBA) kit (Beckton Dickinson, San Jose, CA). Culture medium and Con A (5 μg/ml) were used as negative and positive controls respectively. The antibodies used exhibit cross reactivity with rhesus blood cells as reported by the manufacturer.
CTL Assay
Antigen-specific CTL activity was measured from isolated and stimulated PBMC using the standard 6-hr radioactive chromium (51Cr)-release assay as described before (Nehete et al, 2002; 2005). Briefly, isolated PBMC were stimulated for two rounds on days 0 and 7 with paraformaldehyde-fixed autologous B-lymphoid cell lines (B-LCL) that were loaded with the peptide mixture. The target cells for the assay were prepared by infecting separate aliquots of the autologous B-LCL with recombinant vaccinia viruses expressing the gp160 envelope protein corresponding to different geographical clade isolates of HIV-1 and the control vaccinia virus (vSC8), obtained from the AIDS Research and Reference Reagent Program, Rockville, MD. Target cell lysis was measured at various effector-to-target ratios. In some experiments, unlabeled K562 or autologous B-LCL cells were used to reduce the background natural killer cell-mediated lysis. After 6 h incubation, 100 μl of supernatant was collected from each well and the amount of 51Cr released was determined using the γ-counter. To account for the maximum release, the cells were incubated with 5% Triton X-100. Spontaneous release was determined from target cells incubated without added effector cells. The % of specific lysis was calculated by the following formula: % Specific lysis = (experimental release-spontaneous release)/ (maximum release-spontaneous release) × 100.
Viral load determination
Viral copy numbers in EDTA plasma samples from each monkey were monitored by detecting SHIVKU2 RNA using real-time RT-PCR analyses performed at the NIH core facility (run by Dr. Jeff Lifson’s group). The threshold sensitivity of the assay is between 100-300 viral RNA copy-equivalents/ml of plasma, and the inter-assay variation is <25% (coefficient of variation).
Determination of Cell Associated virus
The PBMC collected from the control and vaccinated macaques at three separate time points were assessed for cell-associated virus by infectious center assay (ICA) as described earlier (Nehete et al, 2002; 2005). Briefly, 10-fold dilutions of PBMC (ranging between 1 and 105 cells) were co-cultured with 106 indicator C8166 cells (four wells/per dilution) in 24-well tissue culture plates for 7 days and the cytopathic effect was noted. At the end of the culture period, the cell-free supernatant from each well was used for determining the amount of infectious virus by incubating 10-fold serial dilutions with fresh indicator MT4 cells and the 50% tissue culture infectious dose (TCID50) was calculated by the standard MTT-dye reduction assay.
Neutralizing antibody assay
Plasma samples collected from heparin blood of monkeys at the beginning of the study and at different time points before and after SHIV-challenge were assayed using the protocol described earlier (Nehete et al, 2002; 2005). Briefly, serial two-fold dilutions of heat-inactivated plasma in RPMI were prepared in duplicate wells of 24-well plates and mixed with SHIVKU2 (20 TCID50). The plates were incubated for 2 h at 37°C followed by the addition of 104 indicator MAGI cells (HeLa cells expressing β-galacotosidase and human CD4 obtained from the AIDS Research and Reference Reagent Program, Rockville, MD). Plates were observed for infected cells (blue colored cells expressing β-galacotosidase) 3 days later, and the wells were scored individually. Pre-immunization plasma was used as negative control, and plasma from a SHIV-infected monkey from a previous unrelated study was used as positive control. The neutralization titer was calculated as the plasma dilution at which the numbers of blue cells were equal to that in control wells where cells were infected with virus pretreated with pre-immune plasma or control uninfected monkey plasma. In general titers of <10, obtained with pre-immune serum samples, were considered background values.
Statistical analysis
Data for the viral loads in the monkeys from all three groups (Control-group, FA-group and DC-group) was analyzed using a repeated measures model to evaluate the effects of the two different vaccination strategies, in comparison to the mock-vaccinated control group, using changes in the viral RNA copies at different time points. The data were standardized using logarithm transformation, in order to stabilize the variances, for all subsequent analyses. To determine the significance, the p-values were compared after factoring in the Bonferroni correction for multiple comparisons. Additionally, standard two-sided t-tests with a p-value set for 0.05 were utilized for determining the differences in the amounts of cell-associated virus (using infectious center assay), cytokine producing cells (using ELISPOT assay) and secretion of cytokines (using cytokine bead array method) at a single time point between the control and each of the vaccine groups separately.
RESULTS AND DISCUSSION
Two different immunization strategies were tested to determine the effectiveness of the conserved HIV-1 envelope peptide-cocktail for protection against pathogenic challenge with SHIVKU2 in Indian-origin rhesus macaques (Fig. 1). One group of six monkeys received intravenous infusions of autologous peripheral blood monocyte-derived DC pulsed ex vivo with the peptide-cocktail (2ug of each peptide) three times at monthly intervals (DC-group). Another group of seven monkeys were injected by the subcutaneous route with the peptide-cocktail (100ug of each peptide) emulsified in complete Freund’s adjuvant (CFA) followed by two monthly booster doses of the peptide-cocktail in incomplete Freund’s adjuvant (IFA) and this group was designated as Freund’s adjuvant group (FA-group). A third group of five monkeys were mock vaccinated once with the CFA and two times with autologous DC, both without the peptide-cocktail (Control-group).
Fig. 1.
Immunization and challenge scheme for rhesus monkeys in the three different groups: Control-group, FA-group and DC-group where monkeys were either mock-vaccinated or immunized with the HIV-1 envelope peptide-cocktail delivered to rhesus macaques using either the Freund’s adjuvant or autologous DC, respectively. Adult female rhesus macaques (Macaca mulatta) of Indian-origin between the ages of 8-17 years from the specific pathogen-free breeding colony were uniformly distributed in the three study groups.
Cellular immune responses, in terms of IFN-γ producing CD8+ T cells specific to all the 8 peptides in the vaccine cocktail were observed in 5 of 6 monkeys in the DC-group and 5 of 7 in the FA-group, while the remaining monkeys in each group exhibited responses to a minimum of 5 peptides (Fig. 2A). Further, vaccine-specific secretion of IFN-γ (a typical example for TH1-type cytokines) and IL-6 (a typical example of inflammatory cytokines) by the PBMC in both the FA-, and DC-groups of monkeys was evident at week 16 post-immunization (Fig. 2B). Levels of IL-4, also determined in these samples, did not show values above the background (data not shown). Since this time point is eight weeks after the last immunization, this analysis provided a potential estimate of the vaccine-induced memory response in the vaccinated monkeys. None of the mock-vaccinated control-group monkeys showed IFN-γ producing CD8+ T cells (<10 spot forming cells, SFC) or secretion of IFN-γ or IL-6 (<2pg/ml) in response to in vitro stimulation of the corresponding PBMC by the individual peptides or the peptide-cocktail. However, innate immune responses indicated by proliferation and cytokine production upon stimulation with mitogens like ConA were observed in both the control and vaccinated monkeys at comparable levels (data not shown). The peptide-cocktail also primed HIV-1 envelope-specific cytotoxic T lymphocyte (CTL) responses as shown for a representative monkey from each of the FA-, and DC-group animals (Fig. 2C). Prior to, as well as at several time points post-immunization, serum/plasma samples from the vaccinated monkeys were analyzed for neutralizing antibodies to SHIVKU2 and none were observed (titers <10 in all the monkeys), while a positive control plasma from a SHIVKU2-infected monkey from a previous unrelated study showed neutralizing titer of 160 (data not shown). These results indicate that vaccination with the peptide-cocktail using either autologous DC or Freund’s adjuvant was effective in selectively inducing antigen-specific cell-mediated immunity without anti-viral neutralizing antibody responses, and thus enabled us to specifically test the protective efficacy of HIV-1 envelope peptide-cocktail-specific cellular immune responses against pathogenic SHIVKU2-challenge.
Fig. 2.
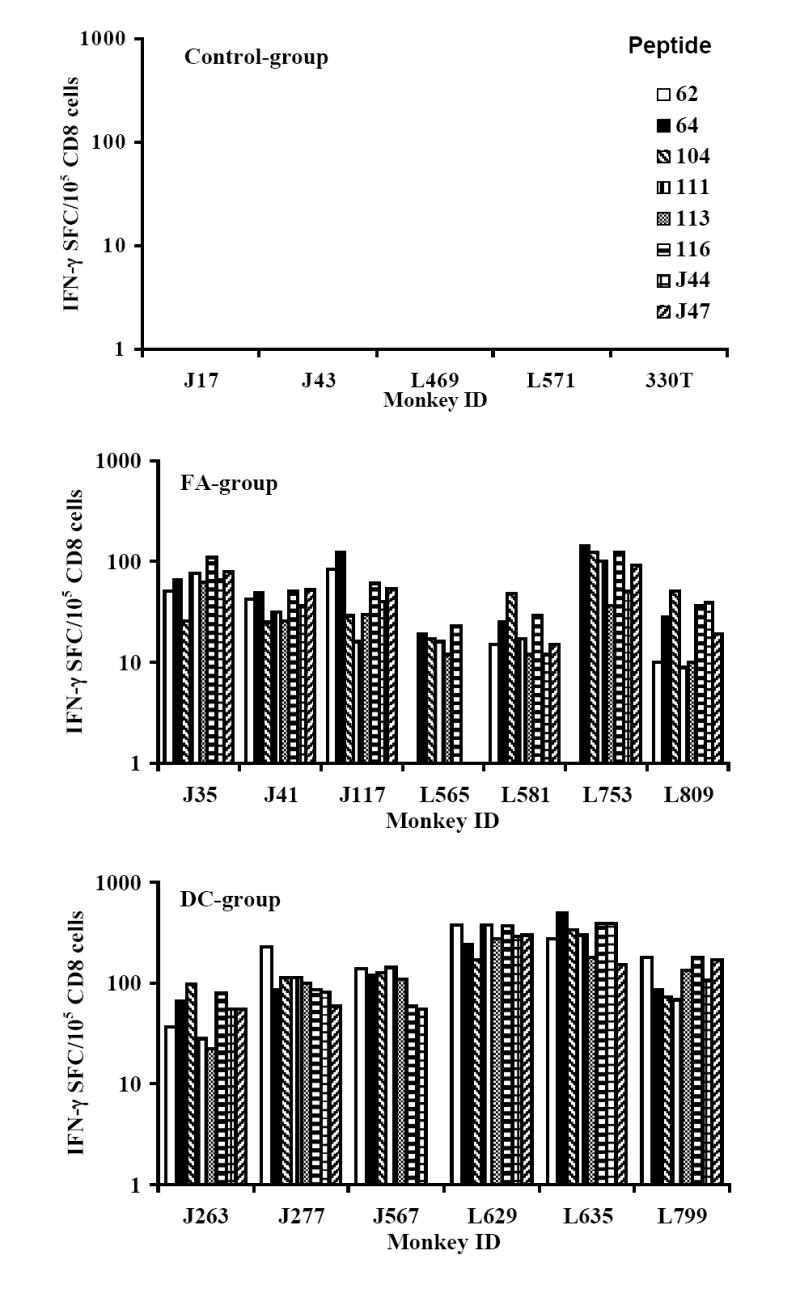
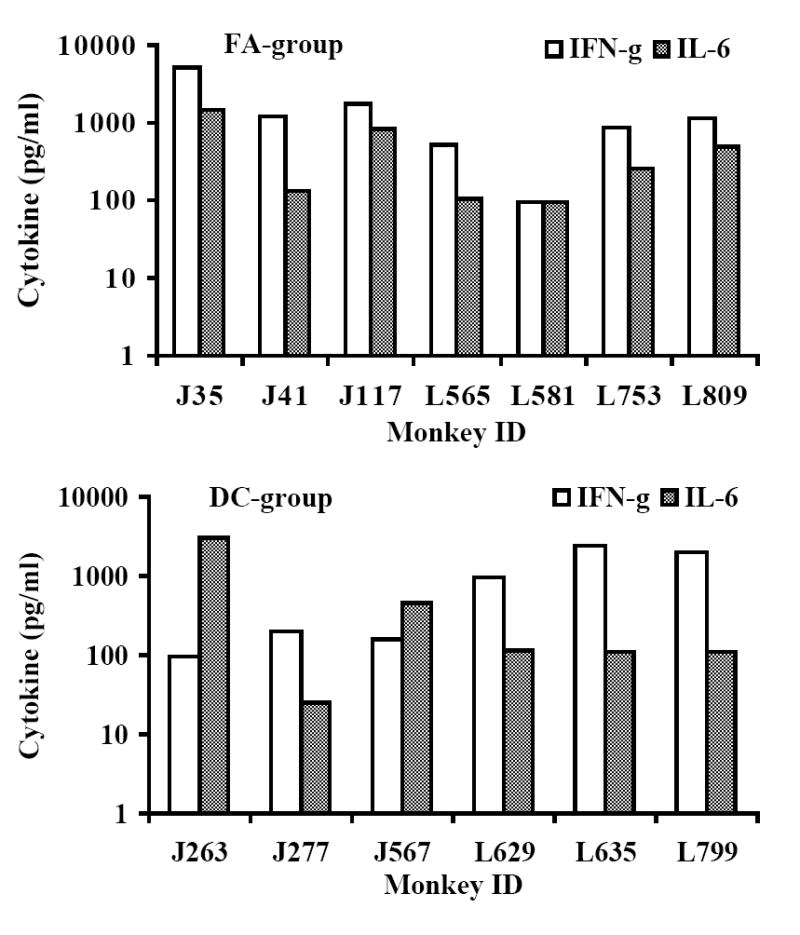
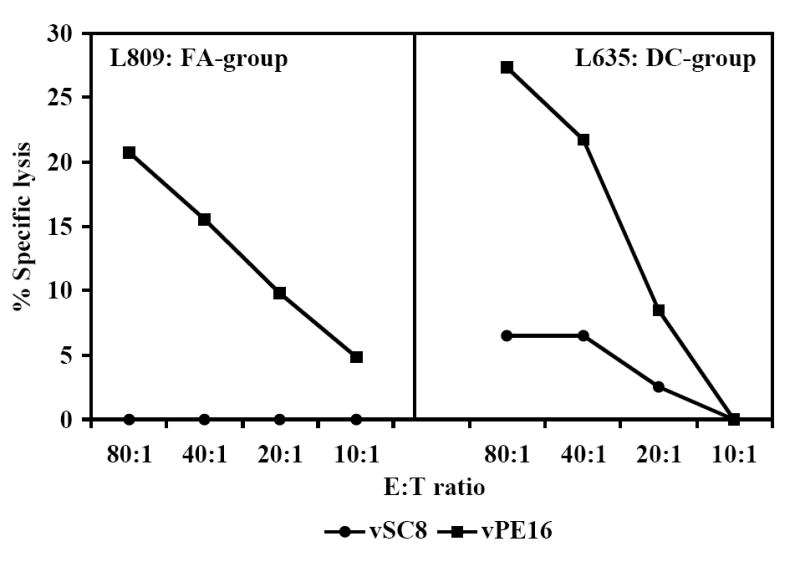
Induction of antigen-specific cellular immune responses by the HIV-1 envelope peptide-cocktail delivered to rhesus macaques using either the Freund’s adjuvant or autologous DC (FA-group and DC-group, respectively). The peak responses in a total of 105 input CD8+ T cells (isolated from the PBMC of the monkeys) in the three different groups in terms of IFN-γ producing cells in response to stimulation for 36 h at 37°C with individual peptides in the peptide-cocktail vaccine were determined by the ELISPOT assay (Fig. 2A). Induction of antigen-specific cytokine production in the monkeys from the FA-group and the DC-group was determined by the CBA assay (Fig. 2B). Monkeys in the Control-group did not show production of either of the cytokines in response to stimulation with the peptide-mix (data not shown). Immunization with the HIV-1 envelope peptide-cocktail primes HIV-1 envelope-specific CTL responses (Fig. 2C). The PBMC obtained from the monkeys in the FA-group and DC-group were stimulated in vitro for 14 days with the mixture of the eight HIV-1 envelope peptides in the peptide-cocktail vaccine (each peptide at 10ug/ml final concentration), and the CTL activity was assayed against autologous B lymphocyte cell lines (B-LCL) infected with recombinant vaccinia virus expressing gp160 from HIV-1IIIB (vPE160), and the control vaccinia virus (vSC8) in the standard 6 h 51Cr-release methodology. Data for a representative monkey each from the FA-group and the DC-group are shown.
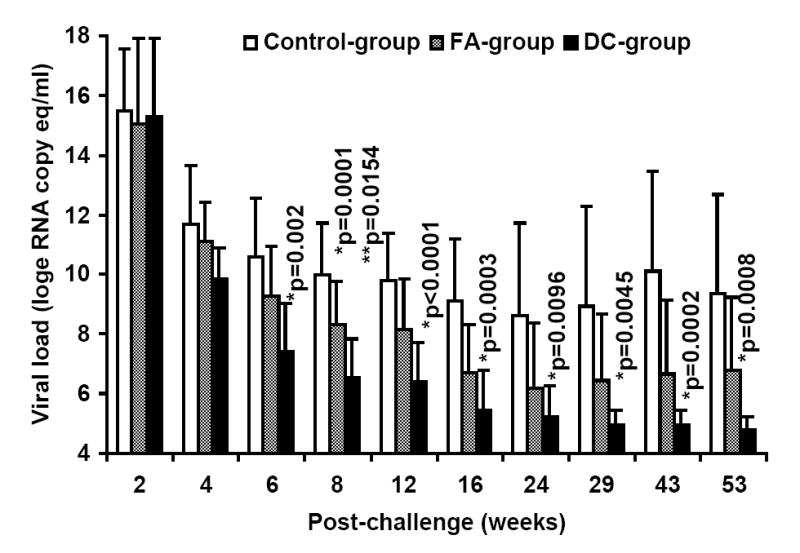
At week-25 post-vaccination, monkeys in the control as well as the two vaccine groups were challenged with SHIVKU2 (104 TCID50) by the intravenous route. All the monkeys became infected as determined by the real-time RT-PCR analysis of viral RNA in the plasma samples (Fig. 3A), and peak virus loads were observed at week-2 post-challenge in both the control and vaccine groups. However, a comparison of mean plasma viral RNA copy numbers for monkeys in the Control-group with those in the two vaccine groups combined over the 53 weeks of follow-up revealed significantly lower viral loads in the vaccinated monkeys at weeks 6, 8, 12, 16 and 43 (Fig. 3B). Coinciding with the significant reduction in viral load in the vaccinated monkeys compared to that in control monkeys strong increase in CTL responses, specific to HIV-1 envelope sequences corresponding to 8 different clade isolates, in addition to HIV-1IIIB, were observed in the vaccinated monkeys at 16 weeks post-challenge as shown for a representative monkey for each group in Fig. 4. Furthermore, as shown in Table 2A, the reduction in viral load past 2 weeks post-challenge in the vaccinated animals (the two vaccine groups combined) was very rapid such that the mean viral load values starting at 24 weeks post-challenge were not significantly different from the pre-challenge values at the baseline which is set at 100 viral RNA copy equivalents/ml of plasma as the lowest sensitivity of the real-time RT-PCR assay used (the p-values for determining significance were compared to 0.001 using a repeated measures model along with the Bonferrroni adjustment for multiple comparisons). On the other hand, the mean viral load values for the Control-group monkeys were significantly different from the baseline even at 53 weeks post-challenge suggesting that, despite some reduction the viral loads did not reach baseline values over one year in these animals. These results indicate establishment of chronic infection in these mock-vaccinated control animals but not the vaccinated animals. Since significant lowering of plasma virus loads was observed in the vaccinated monkeys at the set point around week 8 post-challenge (Fig. 3B), we also analyzed for the productively infected cells between weeks 4-8 by co-culturing serial dilutions of PBMC from the monkeys with indicator cells and observed a significant reduction in the mean TCID50 values for monkeys in both the FA-group and DC-group compared to that in the Control-group (Fig. 5). We also compared the mean plasma viral load in the two vaccine groups separately with the Control-group, and observed that the DC-group, but not the FA-group, showed significantly lower values at the set point between weeks 6-12 as well as at week 43 (Fig. 6A). Furthermore, over the 53-week follow-up the reduction in the mean viral load in the DC-group was most rapid followed by that in the in FA-group and then in the Control-group such that the time points when the mean viral load values did not significantly differ from the respective pre-challenge background values were different for the three groups (Table 2B). For the DC-Vaccine group this time was 12 weeks, and for the FA-Vaccine group it was 24 weeks, while for the Control-group the virus load did not decrease appreciably even at 53 weeks with respect to baseline scores. Together, these results suggest that, while delivery of the peptide-cocktail vaccine using the Freund’s adjuvant and DC-based strategies are both effective in containing the infection by SHIVKU2, only the DC-strategy yielded protective effect in terms of statistically significant lowering of the viral load. Among the six monkeys in the DC-Vaccine group, all except the monkey J263 showed a significant decline in the viral load, reaching to undetectable levels, over the 53 weeks of follow-up indicating this monkey as an outlier in the group (Fig. 3A). Therefore, we analyzed the data for the rest of the five monkeys, with similar viral load profiles, as the DC-group (n=5) over the 53 weeks and observed a highly significant lowering of the viral load starting as early as week 6 and continuing through week 53 post-challenge, compared to that in the Control-group, and also at week 8 when compared to the viral load in the FA-Vaccine group (Fig. 6B).
Fig. 3.
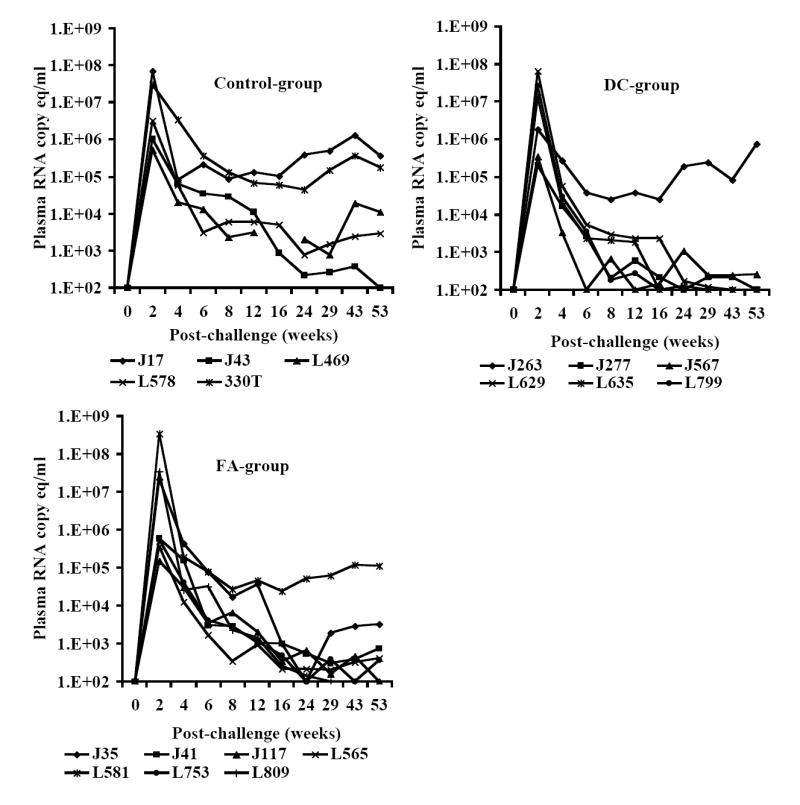
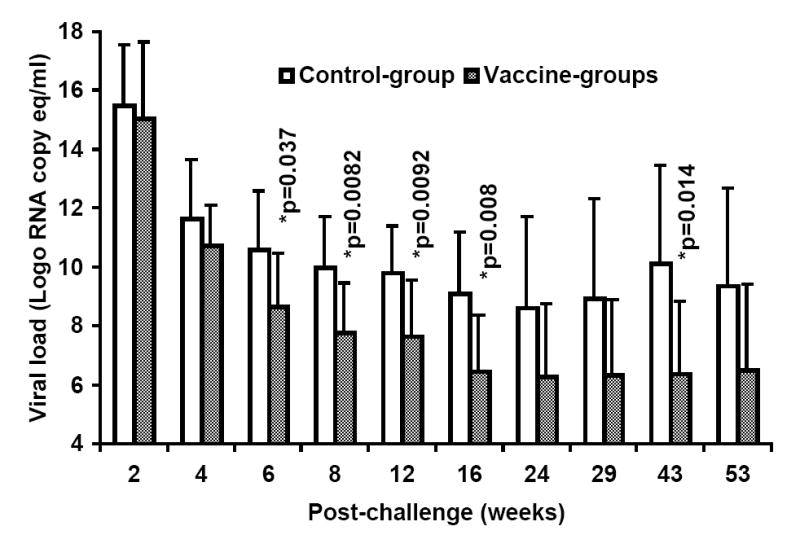
A. Control of SHIVKU2 infection in monkeys vaccinated with the HIV-1 envelope peptide-cocktail. Viral loads in plasma samples from individual monkeys in the Control-group as well as the two vaccine groups (FA-group and DC-group) were determined by real-time RT-PCR methodology at several time points over 53 weeks post-challenge with SHIVKU2 (104 TCID50) by the intravenous route. The peak viral loads were observed in the monkeys in all the three groups at week 2 post-challenge and the relative changes subsequently are different in the different groups with majority of the monkeys in the DC-group group showing lower levels compared to that in the Control-group monkeys. Data in Fig. 3B shows significant differences in the mean plasma viral RNA copy numbers for monkeys in the control-group with those in the FA-, and DC-groups combined over the 53 weeks. Log-transformed average viral load changes show significant reduction in viral load (p<0.001) in the vaccinated monkeys at weeks 6, 8, 12, 16 and 43 post-challenge (*).
Fig. 4.
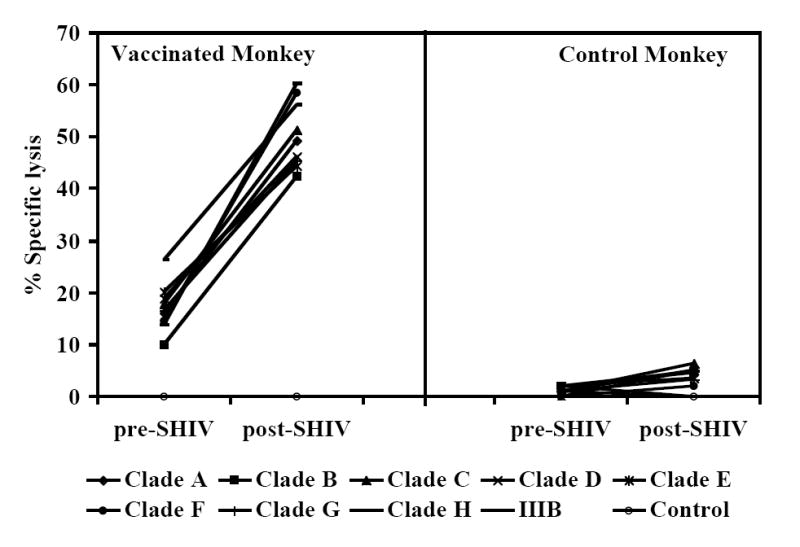
Cross-clade CTL responses in monkeys vaccinated with the HIV-1 envelope peptide-cocktail. Higher CTL responses, specific to HIV-1 envelope sequences corresponding to 8 different clade HIV-1 isolates, in addition to HIV-1IIIB, were observed in the vaccinated monkey compared to that in a control monkey and the responses in the vaccinated monkey increasedsubstantially after SHIVKU2-challenge (post-SHIV) compared to levels prior to challenge (pre-SHIV). The CTL assay was performed as described in Fig. 2C, and the results are shown for the 25:1 effecter to target ratio for a representative monkey from the control and vaccinated groups.
TABLE 2A.
Comparison of the mean plasma viral loads for the monkeys in the two vaccine groups (FA-group and DC-group together) with that in the Control-group monkeys over the 53 weeks post-challenge follow-up*
| Versus | Control-group | Vaccine-groups |
|---|---|---|
| 0 wk | 0 wk | |
| 2 wk | <0.0001* | <0.0001* |
| 4 wk | <0.0001* | <0.0001* |
| 6 wk | <0.0001* | <0.0001* |
| 8 wk | <0.0001* | <0.0001* |
| 12 wk | <0.0001* | <0.0001* |
| 16 wk | <0.0001* | 0.0002* |
| 24 wk | 0.0013 | 0.0122 |
| 29 wk | 0.0017 | 0.0103 |
| 43 wk | <0.0001* | 0.0080 |
| 53 wk | 0.0004* | 0.0135 |
The p-values were compared to 0.001 to determine significance using Bonferroni method for adjusting multiple comparisons
Fig. 5.
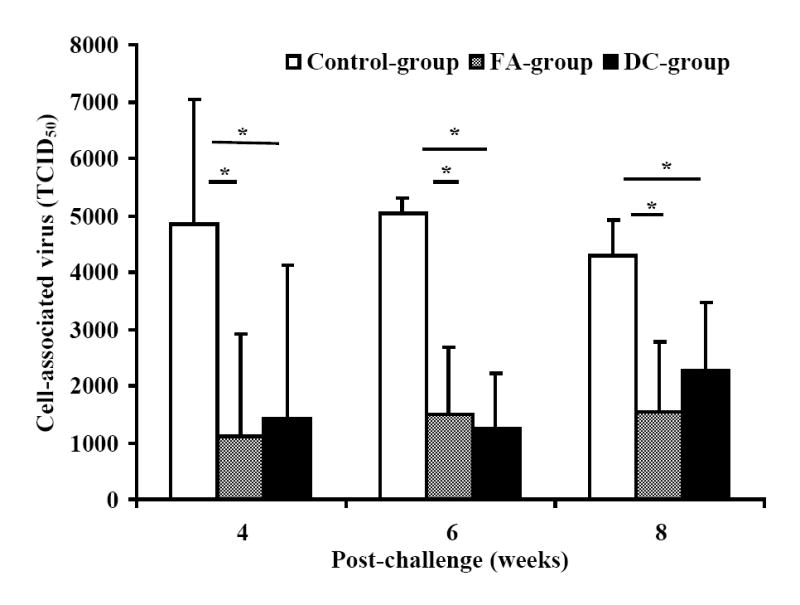
Decrease in cell-associated virus in monkeys vaccinated with the HIV-1 envelope peptide-cocktail. A significant decrease (*, p<0.05) in cell-associated virus at the set point (weeks 4-8) was observed in vaccinated animals (FA-group and DC-group) compared to that in the Control-group. Serial 10-fold dilutions of PBMC (106 cells/ml) from the monkeys in the different groups were co-cultured with indicator C8166 cells for 7 days. At the end of the culture period, the cell-free supernatant from each well was used for determining the amount of infectious virus by incubating 10-fold serial dilutions with fresh indicator MT4 cells and the 50% tissue culture infectious dose (TCID50) was calculated by the standard MTT-dye reduction assay. Results shown are average values for the 50% tissue culture infectious dose (TCID50) for 106 PBMC form monkeys in each group.
Fig. 6.
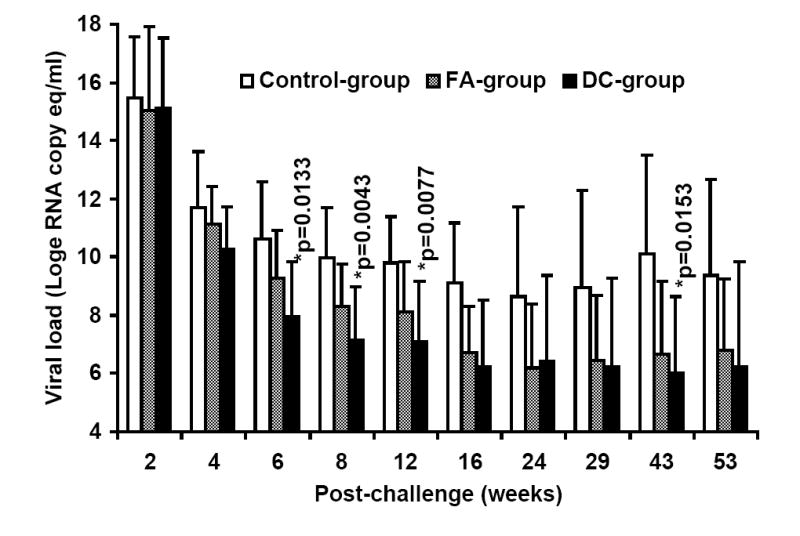
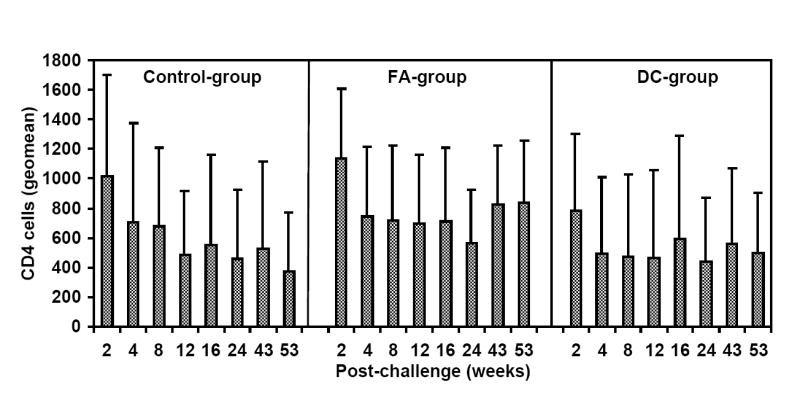
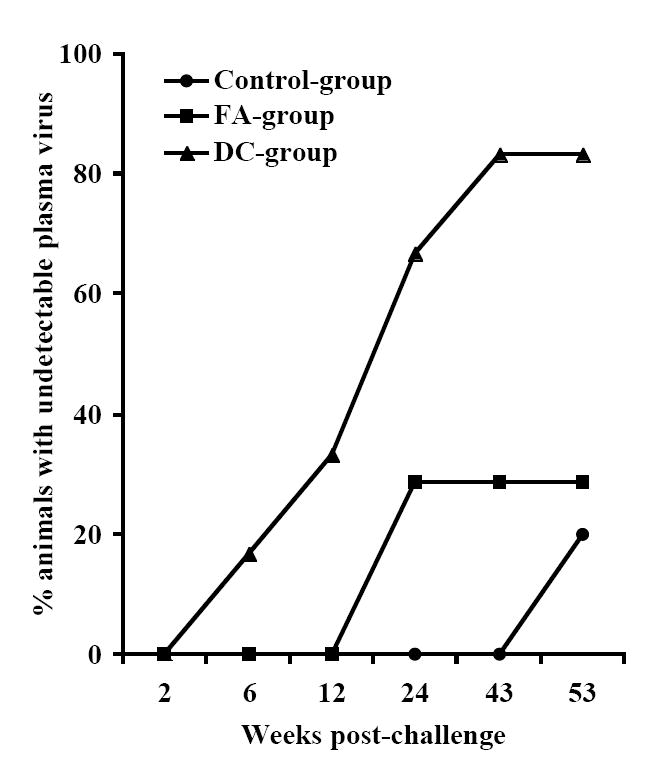
Delivery of the HIV-1 envelope peptide-cocktail vaccine using DC is more effective than the Freund’s adjuvant for significantly lowering the viral load. The mean plasma viral RNA copy numbers for monkeys in the control-group was compared to that in the vaccinated monkeys (the FA-, and DC-groups) over the 53 weeks. The DC-group, but not the FA-group, showed significantly lower mean plasma viral loads (*) between weeks 6-12 and also at week 43 (Fig.6A). Furthermore, as shown in Fig. 6B, the mean viral load in five monkeys in the DC-group as a sub-group, except J263, that showed similar trends in the viral load changes, showed significant reduction in the virus load past week 4 post-challenge compared to that in the Control-group at all time points (*) and also when compared to the FA-group at week 8 (**). Fig. 6C shows geometric mean values for CD4 cells in the blood for the three different groups with steady decline in the Control-group over the 53 weeks follow-up while stabilization of the CD4 cells was observed in the animals from the two vaccine groups. Fig. 6D shows percentages of monkeys with undetectable plasma viral loads (using 300 RNA copy equivalents/ml as the lower cut-off value for the sensitivity of the assay) in the three different groups; Control-group, FA-group and DC-group.
TABLE 2B.
Comparison of the changes in the mean plasma viral loads for the monkeys in the three different experimental groups over the 53 weeks post-challenge follow-up*
| Versus | Control-group | FA-group | DC-group |
|---|---|---|---|
| 0 wk | 0 wk | 0 wk | |
| 2 wk | <0.0001* | <0.0001* | <0.0001* |
| 4 wk | <0.0001* | <0.0001* | <0.0001* |
| 6 wk | <0.0001* | <0.0001* | <0.0001* |
| 8 wk | <0.0001* | <0.0001* | 0.0003* |
| 12 wk | <0.0001* | <0.0001* | 0.0017 |
| 16 wk | <0.0001* | 0.0003* | 0.0533 |
| 24 wk | 0.0013 | 0.0442 | 0.1070 |
| 29 wk | 0.0017 | 0.0180 | 0.1582 |
| 43 wk | <0.0001* | 0.0180 | 0.1550 |
| 53 wk | 0.0005* | 0.0120 | 0.2197 |
The p-values were compared to 0.001 to determine significance using Bonferroni method for adjusting multiple comparisons
Infection by SHIVKU2 resulted in some degree of depletion of CD4 cells in both control and vaccinated monkeys, and the overall changes in absolute CD4 cell numbers, measured as geometric mean, were more pronounced in the control-group than the vaccinated animals, but the differences did not reach statistical significance (Fig. 6C). Based on a 300 viral RNA copy eq/ml value as the lower limit of sensitivity for the real-time RT-PCR assay the plasma virus was undetectable in one of the DC-group macaques (L799) as early as week-8, and in 5 of 6 monkeys (83%) by week-43 post-challenge (Fig. 6D). On the other hand, at the completion of the study (week 53) 4 of the 5 monkeys in the Control-group and 5 of the 7 in the FA-group were positive for plasma virus loads (measured as >300 viral RNA copies/ml of the plasma). Similar results were obtained in assays determining the infected cells measured by co-culturing of PBMC with indicator cell line (data not shown). These results strongly suggest that vaccination of monkeys with HIV-1 envelope peptide-cocktail-pulsed DC was effective in controlling the virus compared to mock-vaccinated Control-group animals as well as those vaccinated with the peptide-cocktail using Freund’s adjuvant (FA-group).
For determining the potential correlates of the observed viral control in the animals vaccinated with the peptide-cocktail, we analyzed the plasma and PBMC samples for antigen-specific humoral and cellular immune responses post-challenge. Low levels of neutralizing antibody responses against the challenge virus SHIVKU2 (titers ≤1:40) were observed in four and three monkeys each in the FA-group and DC-group animals, respectively at around week 1 post-challenge but did not persist beyond this time point (data not shown). Similarly, the Control-group monkeys with persistent viremia and significantly higher viral loads than the vaccinated animals, also showed low levels of neutralizing antibodies (titers ≤1:40) through week-20 post-challenge (data not shown) similar to earlier reports for this virus in rhesus macaques (Singh et al 2005, Jog et al 1998). Thus, low to undetectable levels of neutralizing antibodies were observed post-challenge in all the monkeys with no significant differences between the different groups. These results, together with the observation of lack of induction of virus neutralizing antibodies in any of the vaccinated monkeys (in both FA-, and DC-groups) prior to challenge, suggest that the observed viral control in macaques vaccinated with the HIV-1 envelope peptide-cocktail was independent of anti-HIV humoral immunity.
Next, we analyzed the peptide-cocktail-specific IFN-γ producing CD4+ and CD8+ T cells. The viral load set point at week-8 post-challenge was used as a reference point to compare responses between the early and late time points (8 and 24 weeks post-challenge, respectively). There was a reduction in the number of IFN-γ producing CD4+ T cells at week 24 as compared to that at week 8 in macaques in the Control-group that coincided with a rebounding trend for the mean viral load (Fig. 7A). In the FA-group monkeys where the plasma viral loads did not show significant decrease compared to that in the Control-group, the number of IFN-γ producing CD4+ T cells decreased at week 24 compared that at week 8. On the other hand, the number of IFN-γ producing CD4+ T cells increased between weeks 8 and 24 in the DC-group macaques (Fig. 7A). We also determined the levels of IL-6 and IFN-γ produced by PBMC stimulated with the vaccine peptide-cocktail in the monkeys at week 24 post-challenge and observed production of IL-6, but not IFN-γ, in majority of monkeys in the Control-group (Fig. 7B). While monkeys in the FA-group as well as DC-group showed production of both IL-6 and IFN-γ on an average, these two vaccine group monkeys exhibited significantly higher amounts of IFN-γ secretion in response to peptide cocktail stimulation compared to that in mock-vaccinated Control-group monkeys (Fig. 7C). Furthermore, IFN-γ secretion by the DC-group monkeys was significantly higher than that by the FA-group monkeys. Production of IL-6 in response to peptide cocktail stimulation was not significantly different between the three groups of animals. These results suggesting potential association between vaccine-specific TH1-type cytokine response (IFN-γ production) and viral control in the DC-group monkeys are in agreement with previous human studies that showed a correlation of cellular immune responses indicated by TH1-type rather than TH2-type cytokine profile with the control of disease progression in HIV infected individuals (Shearer and Clerici, 1998; Clerici et al, 1996).
Fig. 7.
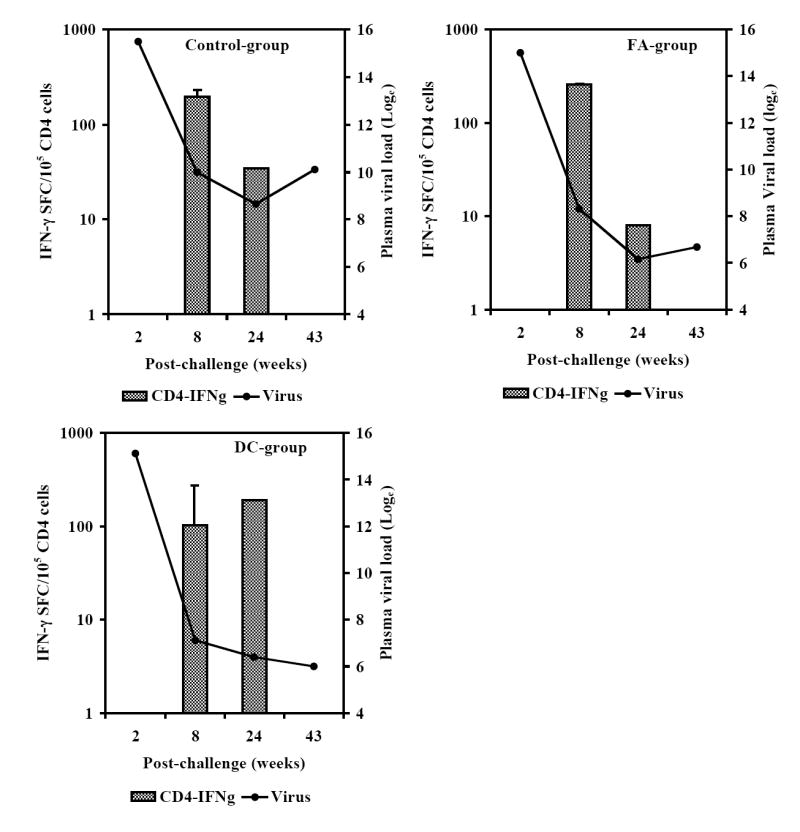
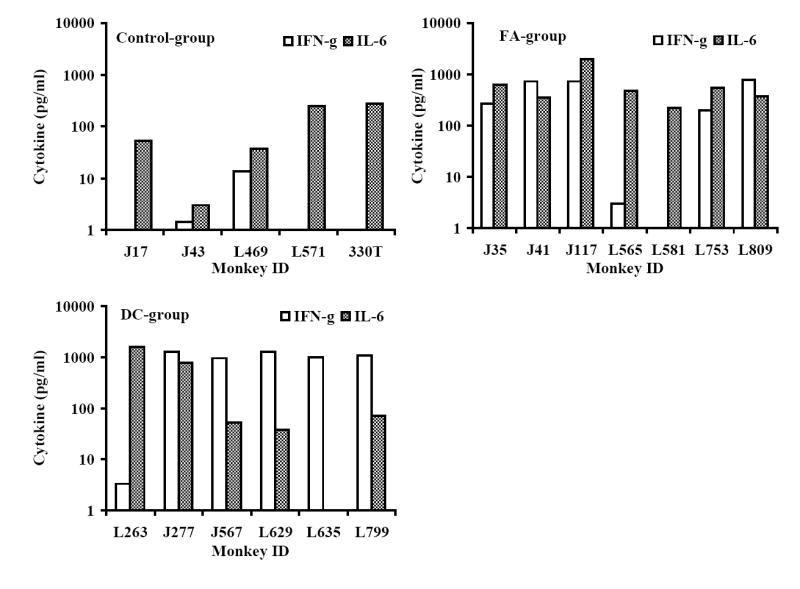
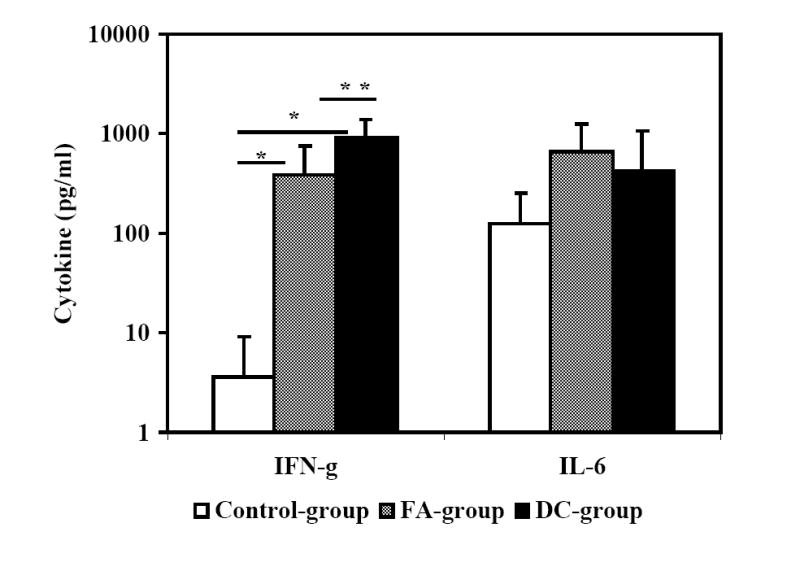
Monkeys immunized with autologous DC pulsed with the HIV-1 envelope peptide-cocktail exhibit strong TH1 responses and control of viremia. The numbers of IFN-γ-producing cells in a total of 105 input CD4+ T cells (isolated from the PBMC), in terms of IFN-γ spot forming cells (SFC) were analyzed by the ELISPOT assay at the early and late time points (8 and 24 weeks post-challenge, respectively) as described in the methods section. Fig. 7A shows the average numbers of IFN-γ-producing CD4+ T cells showed a steep decrease between weeks 8 and 24 time points for the monkeys in the Control-group and FA-group where significant drop in viral loads were not observed, but they increased over the same period in the DC-group where the viral loads decreased significantly. Fig. 7B shows the levels of IFN-γ and IL-6 produced by PBMC collected at week 24 post-challenge from the individual monkeys in the three different groups in response to stimulation with the mixture of the peptides in the vaccine-cocktail using the Cytometric Bead Array assay as described in the methods section. Monkeys in the control group predominantly showed production of IL-6 but not IFN-γ. Fig. 7C shows comparison of average values for the levels of IFN-γ and IL-6 produced, in response to stimulation with the mixture of the peptides in the vaccine-cocktail, in the three different groups. Significantly higher amount of IFN-γ production (p<0.05) was observed for monkeys in the FA-group and DC-group compared to that in the Control-group (*), as well as significantly higher amounts in the DC-group compared to the FA-group (**).
Results from the present investigation suggest the comparative superiority of DC-based antigen delivery over the standard animal adjuvant (Freund’s) for protective efficacy of antigen-specific cell-mediated immunity against SHIV-challenge in the non-human primate model for HIV-AIDS. These data are in line with reports in the literature that demonstrated efficiency of the antigen-presenting function of DC and their potency to deliver peptide-based vaccines corresponding to tumor antigens (Banchereau et al, 2003; Lu et al, 2003; Steiman et al, 2003; Pope, 2003; Thurner et al, 1999; Inaba et al, 1990). In general, effectiveness of DC against viral infections, in particular for the immunodeficiency viruses, has not been well established. One report described therapeutic vaccination against SIV in rhesus macaques of Chinese-origin using autologous DC to deliver a chemically inactivated virus and showed effectiveness in significantly reducing viral load by priming both cellular and humoral immune responses (Lu et al, 2003). In the present study, we tested a synthetic peptide-cocktail derived from highly conserved sequences in the HIV-1 envelope protein as a prophylactic vaccine in rhesus macaques of Indian-origin against challenge with SHIVKU2 that expresses the HIV-1 envelope proteins and thus vaccine-induced immune responses controlling the infection are directed against the HIV portion of the challenge virus. Since these HIV-1 envelope peptides encompass human TH and CTL epitopes, as shown in our previous studies and also literature reports (Robinson et al, 1999; Migueles et al, 2002; Hel et al, 2002; Barouch et al, 2002; Nehete et al, 2002; 2005; Joag et al, 1998; Hel et al, 2001), the protective efficacy observed in this model should be of practical value for potentially including this peptide-cocktail in future clinical testing. Also, the effectiveness of the HIV-1 envelope peptide-cocktail in controlling chronic infection by selectively priming cellular immune responses without virus-neutralizing antibodies provides further support for the recognized protective role of antigen-specific cell-mediated immunity.
Despite the general consensus in the scientific community that the HIV-1 envelope protein is the most important immunogen for inducing virus-neutralizing antibodies, an ideal candidate for this is still unavailable (reviewed in Srivastava et al 2005). On the other hand, suppression of CD4+ T cell responses by the HIV-1 envelope protein, involving several mechanisms including inhibition of proliferation and induction of anergy, was reported (Kawamura et al 2003, Masci et al 2003, Fernando et al 2007). Given these constraints for using the envelope protein as an immunogen for HIV vaccine development, we propose that the efficiency of the conserved peptide-cocktail observed in our studies for protection against chronic infection in the SHIV-rhesus model bodes well for the inclusion of the peptide-cocktail in a multi-component HIV vaccine formulation to enrich for virus-specific cellular immune responses.
Acknowledgments
We thank Dr. Opendra (Bill) Narayan for providing tittered stocks of SHIVKU2. This work was supported in part by NIAID grants AI 42694 and 46969 (K.J.S.). All the cell culture media were produced by the Central Media lab and all the synthetic peptides were prepared in the Synthetic Antigen Core Facility, both supported by funds from NIH grant CA 16672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, Montefiori DC, Herndon GS, Wyatt MA, Candido NL, Kozyr PL, Earl JM, Smith HL, Ma BD, Grimm ML, Hulsey J Miller, McClure HM, McNicholl JM, Moss B, Robinson HL. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science. 2001;92:69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- Baba TW, Liska V, Lehmann RH, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou T, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nature Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Paczesny S, Blanco P, Bennett L, Pascual V, fay J, Palucka AK. Dendritic cells: controllers of the immune system and a new promise for immunotherapy. Ann N Y Acad Sci. 2003;987:180–187. doi: 10.1111/j.1749-6632.2003.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Fu TM, Montefiori DC, Lewis MG, Shiver JW, Letvin NL. Vaccine-elicited immune responses prevent clinical AIDS in SHIV (89.6P)-infected rhesus monkeys. Immunol Lett. 2001;79:57–61. doi: 10.1016/s0165-2478(01)00266-8. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Kunstaman J, Juroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gotgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415:335–39. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- Barouch DH, Kunstman J, glowczwsi J, Kunstman KJ, Egan MA, Peyerl FW, Santra S, Kuroda MJ, Schmitz JE, Beaudry K, Krivulka GR, Lifton MA, Gorgone DA, Wolinkey SM, Letvin NL. Viral escape from dominant simian immunodeficiency virus epitope-specific cytotoxic T lymphocytes in DNA-vaccinated rhesus monkeys. J Virol. 2003;77:7367–75. doi: 10.1128/JVI.77.13.7367-7375.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Letvin NL. Viral evolution and challenges in the development of HIV vaccines. Vaccine. 2002;20:A66–8. doi: 10.1016/s0264-410x(02)00390-0. [DOI] [PubMed] [Google Scholar]
- Clerici M, Balotta C, Meroni L, Ferrario E, Riva C, Trabattoni D, Ridolfo A, Villa M, Shearer GM, Moroni M, Galli M. Type 1 cytokine production and low prevalence of viral isolation correlate with long-term nonprogression in HIV infection. AIDS Res Hum Retroviruses. 1996;12:1053–61. doi: 10.1089/aid.1996.12.1053. [DOI] [PubMed] [Google Scholar]
- Fernando K, Hu H, Ni H, Hoxie JA, Weissman D. Vaccine-delivered HIV envelope inhibits CD4(+) T-cell activation, a mechanism for poor vaccine responses. Blood. 2007;109:2538–44. doi: 10.1182/blood-2006-08-038661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauduin MC, Raymond P, Weir C, Barbas F, Burton DR, Koup RA. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Tryniszewska E, Tsai WP, Parks RW, Montefiori DC, Felber BK, Tartaglia J, Pavlakis GN, Franchini G. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J Immunol. 2002;169:4778–87. doi: 10.4049/jimmunol.169.9.4778. [DOI] [PubMed] [Google Scholar]
- Hel Z, Nacsa J, Kelsall B, Tsai WP, Letvin N, Parks RW, Tryniszewska E, Picker L, Lewis MG, Edghill-Smith Y, Moniuszko M, Pal R, Stevceva L, Altman JD, Allen TM, Watkin D, Torres JV, Berzofsky JA, Belyako IM, Strober W, Franchini G. Impairment of Gag-specific CD8 (+) T-cell function in mucosal and systemic compartments of simian immunodeficiency virus mac251- and simian-human immunodeficiency virus KU2-infected macaques. J Virol. 2001;75:11483–95. doi: 10.1128/JVI.75.23.11483-11495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner T, Foley B, Hahn B, Marx P, McCutchan F, Mellora J, Wolinsky S, Kober B, editors. HIV sequence Compendium. Theoretical Biology and Biophysics. Los Alamos. New Mexico, USA: 2005. [Google Scholar]
- Inaba K, Metlay JP, Crowley MT, Steinman RM. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J Exp Med. 1990;172:631–40. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Liu ZQ, Stephens EB, Smith MS, Kumar A, Li Z, Wang C, Sheffer D, Jia F, Foresman L, Adany I, Lifson J, McClure HM, Narayan O. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J Virol. 1998;72:9069–78. doi: 10.1128/jvi.72.11.9069-9078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RP, Glickman RL, Yang JQ, Kaur A, Dion JT, Mulligan MJ, Desrosiers RC. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71:7711–7718. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Gatanaga H, Borris DL, Connors M, Mitsuya H, Blauvelt A. Decreased Stimulation of CD4+ T Cell Proliferation and IL-2 Production by Highly Enriched Populations of HIV-Infected Dendritic Cells. J Immunol. 2003;170:4260–66. doi: 10.4049/jimmunol.170.8.4260. [DOI] [PubMed] [Google Scholar]
- Kostense S, Koudstaal W, Sprangers M, Weverling GJ, Penders G, Helmus N, Vogels R, Bakker M, Berkhout B, Havenga M, Goudsmit J. Adenovirus types 5 and 35 seroprevalence in AIDS risk groups supports type 35 as a vaccine vector. AIDS. 2004;18:1213–1216. doi: 10.1097/00002030-200405210-00019. [DOI] [PubMed] [Google Scholar]
- Kuroda MJ, Schmitz JE, Charini WA, Nickerson CE, Lifton MA, Lord CI, Forman MA, Letvin NL. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J Immunol. 1999;162:5127–5133. [PubMed] [Google Scholar]
- Lekutis C, Letvin NL. HIV-1 envelope-specific CD4+ T helper cells from simian/human immunodeficiency virus-infected rhesus monkeys recognize epitopes restricted by MHC class II DRB1*0406 and DRB*W201 molecules. J Immunol. 1997;159:2049–57. [PubMed] [Google Scholar]
- Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies MH, Lekutis C, Alroy M, Freed DC, Lord CI, Handt LK, Liu MA, J Shiver W. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci USA. 1997;94:9378–9383. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Wu X, Lu Y, Guo W, Andrieu JM. Therapeutic dendritic-cell vaccine for simian AIDS. Nat Med. 2003;9:27–32. doi: 10.1038/nm806. [DOI] [PubMed] [Google Scholar]
- Masci AM, Galgani M, Cassano S, Simone SD, Gallo A, Rosa VD, Zappacosta S, Racioppi L. HIV-1 gp120 induces anergy in naive T lymphocytes through CD4-independent protein kinase-A-mediated signaling. J Leukoc Biol. 2003;74:1117–24. doi: 10.1189/jlb.0503239. [DOI] [PubMed] [Google Scholar]
- Mascola JR, Lewis MG, VanCott TC, Stiegler G, Katinger H, Seaman M, Beaudry K, Barouch DH, Korioth-Schmitz B, Kivulka G, Sambor A, Welcher B, Douek DC, Montefiori DC, Shiver JW, Poignard P, Burton DR, Letvin NL. Defining the protective antibody response for HIV-1 Cellular immunity elecited by human immunodeficiency virus type 1/simian immunodeficiency virus DNA vaccination does not augment the sterile protection afforded by passive infusion of neutralizing antibodies. Curr Mol Med. 2003;3:209–216. doi: 10.1128/JVI.77.19.10348-10356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Synder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, Keefer MC, McElrah MJ, Walker MC, Wagner KF, McNeil JG, McCutchan FE, Burke DS. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173:340–348. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3:1061–68. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Chitta S, Hossain MM, Hill L, Bernaky BJ, Baze W, Arlighaus RB, Sastry KJ. Protection against chronic infection and AIDS by HIV envelope peptide-cocktail vaccine in a pathogenic SHIV-rhesus model. Vaccine. 2002;20:813–25. doi: 10.1016/s0264-410x(01)00408-x. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Gambhira R, Nehete BP, Sastry KJ. Dendritic cells enhance detection of antigen-specific cellular immune responses by lymphocytes from rhesus macaques immunized with an HIV envelope peptide cocktail vaccine. J Med Primatol. 2003;32:67–73. doi: 10.1034/j.1600-0684.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Johnson PC, Murthy KK, Schpiro SJ, Sastry KJ. A synthetic peptide from the first conserved region in the envelope protein gp160 is a strong T-cell epitope in HIV-infected chimpanzees and humans. Viral Immunol. 1998a;11:147–58. doi: 10.1089/vim.1998.11.147. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Lewis DE, Tang DN, Pollack MS, Sastry KJ. Presence of HLA-C-restricted cytotoxic T-lymphocyte responses in long-term nonprogressors infected with human immunodeficiency virus. Viral Immunol. 1998b;11:119–29. doi: 10.1089/vim.1998.11.119. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Nehete BN, Manuri P, Hill L, Palmer JL, Sastry KJ. Protection by dendritic cells-based HIV synthetic peptide cocktail vaccine: Preclinical studies in the SHIV-rhesus model. Vaccine. 2005;23:2154–59. doi: 10.1016/j.vaccine.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Nehete PN, Satterfield WC, Matherne CM, Arlinghaus RB, Sastry KJ. Induction of human immunodeficiency virus-specific T cell responses in rhesus monkeys by synthetic peptides from gp160. AIDS Res Hum Retroviruses. 1993;9:235–40. doi: 10.1089/aid.1993.9.235. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Makitalo B, Thorstensson R, Norley S, Binninger-Schinzel D, Cranage M, Rud E, Biberfeld G, Putkonen P. Live attenuated simian immunodeficiency virus (SIV)mac in macaques can induce protection against mucosal infection with SIVsm. AIDS. 1998;12:2261–70. doi: 10.1097/00002030-199817000-00006. [DOI] [PubMed] [Google Scholar]
- O’Doherty U, Ignatius R, Bhardwaj N, Pope M. Generation of monocytederived dendritic cells from precursors in rhesus macaque blood. J Immunol Methods. 1997;207:185–94. doi: 10.1016/s0022-1759(97)00119-1. [DOI] [PubMed] [Google Scholar]
- Poignard P, Sabbe R, Picchio GR, Wang M, Gulizia RJ, Katinger H, Parren P, Mosier DE, Burton DR. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999;10:431–438. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- Pope M. Dendritic cells as a conduit to improve HIV vaccines. Curr Mol Med. 2003;3:229–42. doi: 10.2174/1566524033479870. [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Huang XL, Fan ZF, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T lymphocyte activity and low viral load are associated with lack of disease in HIV-1 infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- Robinson HL, Montefiori DC, Johnson RP, Manson KH, Kalish ML, Lifson JD, Rizvi TA, Lu S, Hu SL, Mazzara GP, Panicali DL, Herndon JG, Glickman R, Candido MA, Lydy SL, Wyand MS, McClure HM. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat Med. 1999;5:26–34. doi: 10.1038/8406. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones SL, Douglas N. HIV-specific CTL activity in an HIV-exposed but uninfected infant. Lancet. 1993;341:860–861. doi: 10.1016/0140-6736(93)93063-7. [DOI] [PubMed] [Google Scholar]
- Rowland-Jones SL, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T, Takiguchi M, Schultz T, McMichael A, Whittle H. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- Sastry KJ, Arlinghaus RB. Identification of T-cell epitopes without B-cell activity in the first and second conserved regions of the HIV Env protein. AIDS. 1991;5:699–07. doi: 10.1097/00002030-199106000-00009. [DOI] [PubMed] [Google Scholar]
- Shearer GM, Clerici M. Cytokine profiles in HIV type 1 disease and protection. AIDS Res Hum Retroviruses. 1998;14(Suppl 2):S149–152. [PubMed] [Google Scholar]
- Singh DK, Liu Z, Sheffer D, Mackay GA, Smith M, Dhillon S, Hegde R, Jia F, Adany I, Narayan O. A noninfectious simian/human immunodeficiency virus DNA vaccine that protects macaques against AIDS. J Virol. 2005;79:3419–28. doi: 10.1128/JVI.79.6.3419-3428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava IK, Ulmer JB, Barnett SW. Role of neutralizing antibodies in protective immunity against HIV. Human Vaccines. 2005;1:45–60. doi: 10.4161/hv.1.2.1764. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Granelli-Piperno A, Pope M, Trumpftheller C, Ignatius R, Arrode G, Racz P, Tenner-Racz K. The interaction of immunodeficiency viruses with dendritic cells. Curr Top Microbiol Immunol. 2003;276:1–30. doi: 10.1007/978-3-662-06508-2_1. [DOI] [PubMed] [Google Scholar]
- Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steiman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–78. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren B, Rosen J, Sandstrom E, Mathiesen T, Modrow S, Wigzell H. HIV-1 peptides induce a proliferative response in lymphocytes from infected persons. J Acquir Immune Defic Syndr. 1989;2:448–56. [PubMed] [Google Scholar]


