Abstract
The mechanism by which v-Src disrupts connexin (Cx)43 intercellular gap junctional communication (GJC) is not clear. In this study, we determined that Tyr247 (Y247) and the previously identified Tyr265 (Y265) site of Cx43 were the primary phosphorylation targets for activated Src in vitro. We established an in vivo experimental system by stably expressing v-Src and wild-type (wt) Cx43, or Y247F, Y265F, or Y247F/Y265F Cx43 mutants in a Cx43 knockout mouse cell line. Wt and mutant Cx43 localized to the plasma membrane in the absence or presence of v-Src. When coexpressed with v-Src, the Y247F, Y265F, and Y247F/Y265F Cx43 mutants exhibited significantly reduced levels of tyrosine phosphorylation compared with wt Cx43, indicating that Y247 and Y265 were phosphorylation targets of v-Src in vivo. Most importantly, GJC established by the Y247F, Y265F, and Y247F/Y265F Cx43 mutants was resistant to disruption by v-Src. Furthermore, we did not find evidence for a role for mitogen-activated protein kinase in mediating the disruption of GJC by v-Src. We conclude that phosphorylation on Y247 and Y265 of Cx43 is responsible for disrupting GJC in these mammalian cells expressing v-Src.
Keywords: connexin 43; gap junction; Src; phosphorylation; phosphotyrosine
Introduction
Gap junctions are plasma membrane channels between adjacent cells with diameters of ∼1.5–4 nm (Unger et al., 1999). Gap junctions allow the intercellular exchange of molecules <1 kD. Each gap junction channel is composed of two hexamers of connexin (Cx)*molecules provided by each of the adjacent cells. Connexins are a multigene family of membrane-spanning proteins that are highly homologous in their extracellular and transmembrane domains but are more structurally diverse in the cytoplasmic loops and COOH tails (Goodenough et al., 1996).
It has been hypothesized that the disruption of the gap junctional channel contributes to the uncontrolled growth of cancer (Trosko and Ruch, 1998), possibly by interfering with the exchange of growth regulatory signals through gap junction channels (Loewenstein, 1981). Earlier studies demonstrated that concomitant with the disruption of gap junctional communication (GJC) in v-Src transformed cells unique phosphorylation occurred on tyrosine (Tyr or Y) sites in Cx43, a major connexin family member (Crow et al., 1990; Filson et al., 1990). Later investigations showed that Cx43 expressed in Sf-9 insect cells contained phosphotyrosine (pTyr) when coexpressed with v-Src but had no pTyr content in the absence of v-Src (Loo et al., 1995). Importantly, purified activated Src phosphorylated Cx43 on tyrosine in vitro (Loo et al., 1995). These data suggested that the phosphorylation of Cx43 on tyrosine was the result of a direct kinase–substrate interaction between v-Src and Cx43. Furthermore, confocal microscopy studies of Rat-1 v-Src fibroblasts suggested that v-Src and Cx43 partially colocalized in regions of the plasma membrane (Loo et al., 1999). Moreover, Cx43 coimmunoprecipitated with v-Src in in vivo studies (Kanemitsu et al., 1997; Loo et al., 1999). Taken together, these results suggested that v-Src interacts with Cx43 directly in vivo.
Because the SH2 and SH3 domains of v-Src mediate its interactions with substrate proteins (Brown and Cooper, 1996), the role that these domains play in the interaction of v-Src with Cx43 was investigated (Kanemitsu et al., 1997). This study showed that mutation of the proline-rich motif located at P274-P284 of Cx43 but not the motif at P253-P256 decreased both the binding of Cx43 to v-Src and tyrosine phosphorylation of Cx43 in vitro. In addition, mutating Y265 of Cx43 to phenylalanine (Phe or F) but not Y247 or Y267 greatly reduced binding to v-Src and tyrosine phosphorylation of Cx43, indicating that phosphorylation of the Y265 site was important for the interaction of Cx43 with v-Src in vivo (Kanemitsu et al., 1997). Thus, the SH3 and SH2 domains of v-Src appear to mediate the interaction with Cx43, and this may promote the direct phosphorylation of Cx43 on tyrosine.
Two studies using the Xenopus oocyte expression system investigated how phosphorylation of Cx43 at specific tyrosine sites by v-Src affected GJC. In the study of Swenson et al. (1990), comicroinjection of cx43 and v-src RNAs into Xenopus oocytes demonstrated that the expression of v-Src completely disrupted GJC established by the expression of wild-type (wt) Cx43 in the oocytes. However, substituting a nonphosphorylatable Phe residue at the Y265 site greatly reduced tyrosine phosphorylation on Cx43 and rendered the Cx43 channels resistant to disruption by v-Src. These results suggested that phosphorylation of Y265 in Cx43 by v-Src was required for the disruption of GJC. The data strongly supported the concept that v-Src mediated the disruption of GJC via tyrosine phosphorylation of Cx43.
However, a more recent study using the same experimental expression system suggested that the v-Src–mediated closure of Cx43 channels was not attributed to the tyrosine phosphorylation of Cx43 at Y265 but rather to serine phosphorylation in the COOH-terminal domain of Cx43 (Zhou et al., 1999). In this second study, gap junction channels were allowed to form by the injection of cx43 cRNA into Xenopus oocytes before the introduction of the v-src cRNA. Neither the Y265F nor the Y247F Cx43 mutant was found to be resistant to the disruption of GJC induced by v-Src. In addition, the use of inhibitors to block the activation of mitogen-activated protein (MAP) kinase and expression of MAP kinase serine site Cx43 mutants provided results that implicated the MAP kinase pathway and serine phosphorylation of Cx43 in the v-Src–mediated disruption of GJC. However, the effects of these Cx43 mutations and inhibitors of the MAP kinase pathway on Cx43 phosphorylation were not examined in this study.
In the present study, a mammalian cell system was established to express wt or phosphorylation site mutants of Cx43 in the absence or presence of v-Src. We observed that the disruption of GJC by v-Src was inhibited by substituting Phe for Tyr at the Y247 and Y265 sites of Cx43 but was not disrupted by blocking the MAP kinase pathway. This inability of v-Src to disrupt GJC in cells coexpressing the Cx43 mutants was accompanied by a reduction in tyrosine phosphorylation of Cx43. Thus, the results presented here indicate that tyrosine phosphorylation of Cx43 represents a major mechanism that underlies the disruption of GJC in these mammalian cells expressing the v-Src oncoprotein. These data support a model for the processive phosphorylation of Cx43 by v-Src in vivo.
Results
Phosphorylation of Y247 and Y265 of Cx43CT by activated Src in vitro
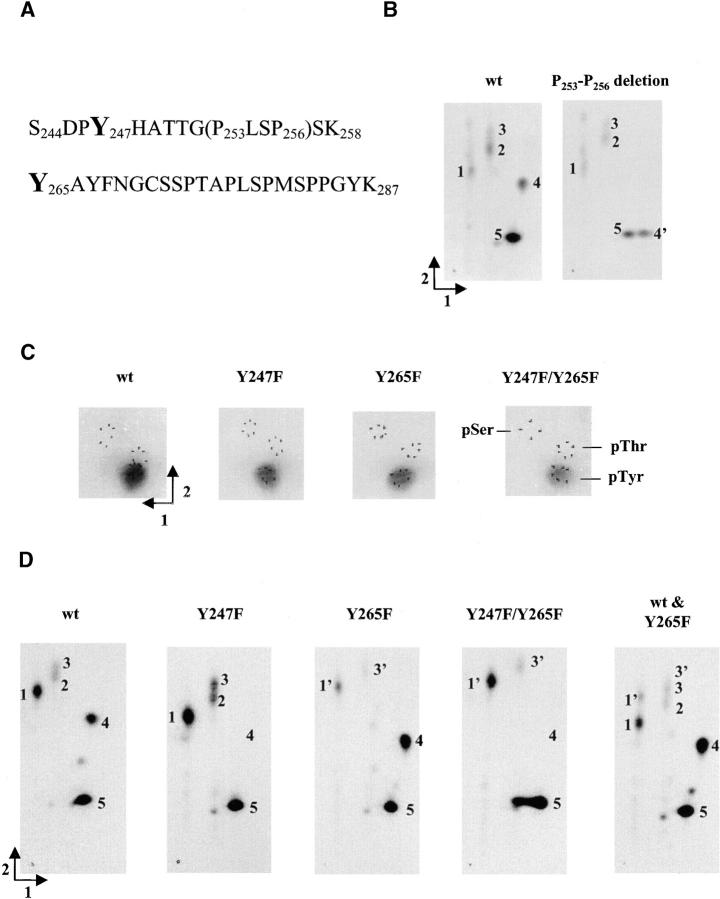
Site-directed mutagenesis indicated that Y265 of Cx43 was a primary Tyr site targeted by v-Src upon coexpression of these proteins in Xenopus oocytes (Swenson et al., 1990). This study did not attempt to identify possible additional tyrosine phosphorylation sites in Cx43. However, the observation of two distinct pTyr-containing phosphopeptides of Cx43 in Rat-1 v-Src cells suggested that v-Src may phosphorylate Cx43 at an additional Tyr site(s) (Kurata and Lau, 1994). Because Y247, like Y265, is in a tyrosine kinase phosphorylation consensus sequence, we examined whether Y247 is another site targeted by activated Src. Glutathione S-transferase (GST) fusion proteins with wt Cx43 COOH-terminal tail (CT) or a deletion mutant that lacks residues P253-P256 (Kanemitsu et al., 1997) of Cx43CT were phosphorylated by activated Src in vitro and subjected to two-dimensional phosphotryptic peptide analysis. Since the P253-P256 deletion is located in tryptic peptide S244-K258 (Fig. 1 A), it was expected to alter the migration of this peptide. Phosphopeptide 4 detected in the map of phosphorylated wt GST–Cx43CT disappeared from the map of the phosphorylated deletion mutant GST–Cx43CT with the concomitant appearance of a slower migrating phosphopeptide, labeled 4′ (Fig. 1 B). Because this was the only difference observed in the peptide maps, we concluded that peptide 4 must be peptide S244-K258, whereas peptide 4′ represents the same peptide with the P253-P256 deletion. This conclusion was supported by a calculation of the relative peptide mobilities, which indicated that the peptide from the deletion mutant would migrate more slowly than the wt peptide in the second chromatographic dimension (Boyle et al., 1991). Because the deletion localizes in a phosphopeptide that contains a single Tyr site (Y247) (Fig. 1 A) and Src phosphorylates GST–Cx43CT only on Tyr (see below), Y247 represents another Tyr site in Cx43 that is phosphorylated by Src in vitro.
Figure 1.
Analysis of the phosphorylation of GST–Cx43CT by activated Src in vitro. (A) Amino acid sequence of two predicted tryptic peptides of Cx43CT (S244-K258 and Y265-K287) that contain the putative pTyr sites (Y247 and Y265) in the phosphorylated GST–Cx43CT fusion protein. Cx43CT fused to GST begins at V236 in the cytoplasmic tail of Cx43. The amino acids deleted in the Cx43CT deletion mutant are bracketed (P253LSP256). (B) Two-dimensional phosphotryptic peptide analysis of GST fusion proteins with wt or the Cx43CT deletion mutation phosphorylated by activated Src. Phosphotryptic peptides were resolved by electrophoresis (dimension 1) followed by ascending chromatography (dimension 2). Major phosphotryptic peptides are numbered. (C) Phosphoamino acid content of wt or site-directed mutant GST–Cx43CT phosphorylated by activated Src. Equal amounts of the GST–Cx43CT substrates were phosphorylated by Src, acid-hydrolyzed, and separated by electrophoresis at pH 1.9 (dimension 1) followed by electrophoresis at pH 3.5 (dimension 2). The migration positions of the unlabeled pSer, pThr, and pTyr standards are indicated. (D) Phosphotryptic peptide maps of wt and mutant GST–Cx43CT phosphorylated by activated Src. Resolved peptides were from one type of GST–Cx43CT or a mixture of wt and the Y265F GST–Cx43CT mutant. Peptides are numbered as in B.
To further examine the Src-mediated phosphorylation of Cx43 at Y247 and Y265, we generated GST–Cx43CT with a Y247F, Y265F, or Y247F/Y265F double Cx43 mutation. Phosphoamino acid analysis established that all GST–Cx43CT substrates phosphorylated by Src contained only pTyr (Fig. 1 C). However, data obtained in one experiment with equal amounts of GST–Cx43CT fusion proteins indicated that the Y247F and Y265F Cx43 mutants appeared to have less pTyr than wt Cx43 (86 and 63%, respectively). The Y247F/Y265F double mutant had an even lower level of pTyr than the single mutants (26% of wt), suggesting that both the Y247 and Y265 sites were required for full phosphorylation of wt Cx43CT by Src in vitro. The detection of pTyr in the Y247F/Y265F mutant suggested the presence of an additional pTyr site in wt Cx43CT induced by Src in vitro.
Two-dimensional phosphotryptic peptide mapping revealed that wt Cx43CT phosphorylated by Src in vitro contained three major phosphopeptides: 1, 4, and 5 (Fig. 1 D). Phosphopeptide 4 disappeared in the Y247F and Y247F/Y265F Cx43CT point mutants, indicating that it contained Y247 (Fig. 1 D). These data confirmed the previous conclusion obtained with the GST–Cx43CT P253-P256 deletion mutant (Fig. 1 B) that Src phosphorylated Cx43CT at Y247 in vitro. Compared with wt Cx43CT, phosphorylation of peptide 1 was reduced in the Y265F Cx43 mutant. This peptide appeared to migrate faster in the second dimension in the Y265F and double Cx43 mutants, possibly due to the substitution of Tyr by the more hydrophobic Phe residue (Fig. 1 D, labeled 1′). Analysis of a mixture of wt and Y265F Cx43CT samples confirmed this difference in migration. Taken together, these data suggested that phosphopeptide 1 contained the Y265 site (Fig. 1 D). Interestingly, phosphopeptide 5 still appeared in the double Cx43CT mutant, supporting the suggestion of another pTyr site in Cx43CT induced by Src in vitro. However, significant phosphorylation at an additional tyrosine site was not observed in full-length Cx43 phosphorylated in vivo (see Discussion).
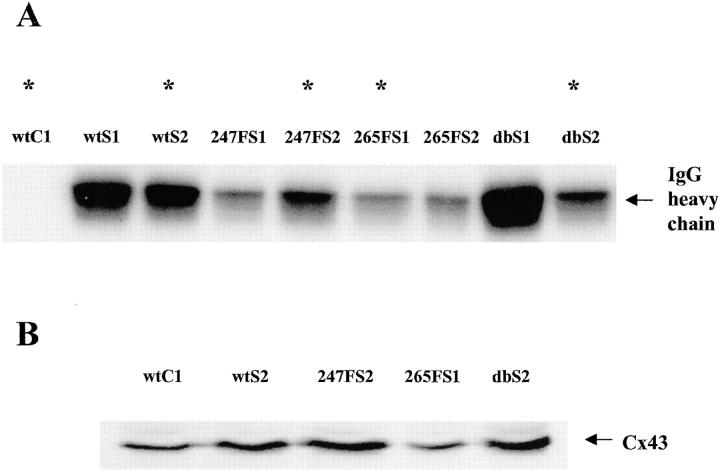
Expression of Cx43 and v-Src in the different cell clones
Wt and mutant Cx43 were expressed in Cx43 knockout (KO) cells and clones with similar levels of GJC were infected with the v-Src retrovirus. To characterize the expression of v-Src in the Cx43 reexpressing cells, an in vitro kinase assay was performed on the morphologically transformed cell clones infected by pLvsrcSH. Equal amounts of antibody were used to immunoprecipitate v-Src from equal amounts of cell lysate, followed by an in vitro kinase assay of immunoprecipitated v-Src. As expected, the negative control wtC1 cells showed no v-Src kinase activity (Fig. 2 A, first lane). All of the other clones exhibited v-Src kinase activity with some differences in the levels of activity. One clone (indicated by an asterisk) from each set of infections by wt or mutant pBABE-cx43 and pLvsrcSH retroviruses with the most similar levels of v-Src kinase activity was chosen for further biochemical analysis. The selected clones were wtS2, 247FS2, 265FS1, and dbS2. The wtC1 clone was used as a negative control.
Figure 2.
Levels of v-Src kinase activity and Cx43 in the cell clones. (A) In vitro v-Src protein kinase activities. The same amounts of anti-Src TBR serum 6-1-4 were reacted with equal amounts of cell lysate from each clone. The ability of v-Src to phosphorylate the heavy chain of IgG in an immune complex kinase assay was examined. Clone wtC1 infected by the pLXSH vector alone served as a negative control. Asterisks indicate the clones chosen for further biochemical analysis. (B) Expression of Cx43. 40 μg of whole cell lysates was resolved by SDS-PAGE and electrotransferred to Immobilon-P membranes.
Immunoblotting analysis was performed to measure the expression of Cx43 in the selected clones (Fig. 2 B). 40 μg of whole cell lysate was loaded for each sample. Approximately equal levels of Cx43 were detected in all cell clones, indicating that the antibody recognized both the wt and the mutated Cx43 proteins and that the substitution of the Phe residue for Tyr did not affect the expression of Cx43 (Fig. 2 B). The antibody recognized the most abundant nonphosphorylated isoform of Cx43, and in some samples a slower migrating phosphorylated isoform was also detected.
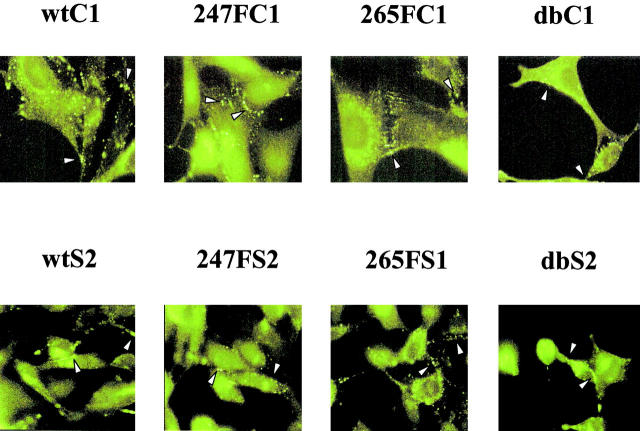
Localization of wt and mutant Cx43 in v-Src–expressing cells
Immunofluorescence microscopy of Cx43 was performed to determine whether the conservative substitution of Phe at the Y247 and/or Y265 site or the expression of v-Src in the cells disrupted the assembly or maintenance of Cx43 gap junction plaques. Cx43 gap junction plaques were detected in the plasma membranes of wtC1 cells and in the 247FC1, 265FC1, and dbC1 cells (Fig. 3 , top, arrowheads). Wt and mutant Cx43 were also localized to the plasma membrane of the clones coexpressing v-Src (Fig. 3, bottom panel). The v-Src–expressing cells were more rounded than the non-Src cells, and some gap junctions were not clearly in focus in a particular focal plane. All of the clones also exhibited Cx43-specific reactions in intracellular locations. These results indicated that the assembly of the Cx43 mutants into gap junction plaques was not largely disrupted by the introduction of phosphorylation site mutations or by the coexpression of v-Src. Thus, wt and mutant Cx43 have the potential to establish functionally active gap junction channels, and no gross loss of gap junctional plaques was apparent in our cells stably expressing v-Src.
Figure 3.
Localization of wt or mutant Cx43 to the plasma membrane. Top, cells lacking v-Src; bottom, cells expressing v-Src. Cells were grown to subconfluence on coverslips before being fixed and permeabilized. Cx43CT368 antiserum was used to detect Cx43. Arrowheads indicate the positions of some Cx43 gap junction plaques.
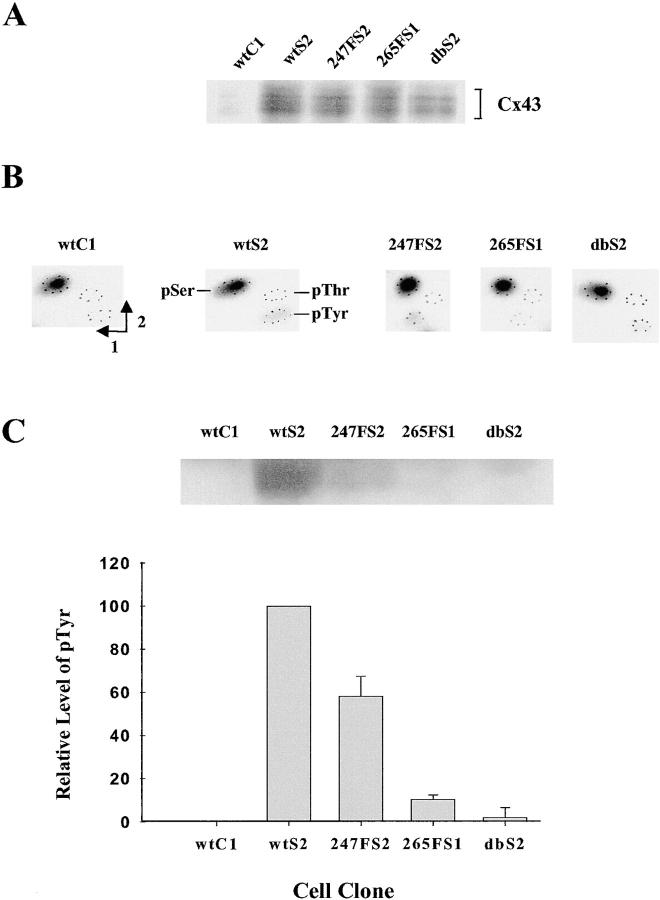
Phosphorylation of wt and mutant Cx43 in v-Src–expressing cells
To examine the phosphorylation of Cx43 in cells expressing Cx43 and v-Src, the cells were metabolically labeled with 32Pi, and Cx43 was immunoprecipitated from the cell lysates with equal amounts of antibody (Fig. 4 A). Phosphorylated Cx43 from the different clones was present as isoforms migrating with different mobilities as observed previously (Crow et al., 1990; Filson et al., 1990; Laird et al., 1995). More importantly, the levels of phosphorylated Cx43 in the v-Src–expressing clones were markedly increased compared with the wtC1 cells that lacked v-Src. Given that the levels of Cx43 were similar among the cell clones (Fig. 2 B), these data suggested that v-Src upregulated the phosphorylation of Cx43, consistent with previous reports (Crow et al., 1990; Filson et al., 1990; Goldberg and Lau, 1993).
Figure 4.
Phosphorylation of wt or mutant Cx43 in v-Src–expressing cells. (A) Immunoprecipitation of Cx43. Confluent cells were metabolically labeled with 32Pi. Cx43 was immunoprecipitated from cell lysates with Cx43CT368 antiserum under conditions of antigen excess. The positions of multiple isoforms of phosphorylated Cx43 are shown by brackets. (B) Phosphoamino acid content of Cx43 immunoprecipitated from the cell clones. Immunoprecipitated 32P- labeled Cx43 was acid hydrolyzed and separated in two dimensions as indicated. (C) pTyr content of Cx43 from the cell clones. Cells were grown to confluence and the same amount of Cx43CT368 antiserum was used to immunoprecipitate Cx43 from cell lysates under conditions of antigen excess. The pTyr content of Cx43 was detected with a pTyr antibody (top). Quantitation of pTyr in Cx43 is shown in the bottom part of the figure. The average relative amounts of pTyr of Cx43 were obtained from scans of the Cx43 images of the top and from a second experiment. The levels of pTyr of Cx43 from wtC1 and wtS2 were normalized to 0 and 100%, respectively.
Phosphoamino acid analysis demonstrated that Cx43 immunoprecipitated from 32P-labeled wtC1 cells, which did not express v-Src, contained only phosphoserine (pSer) (Fig. 4 B). Wt Cx43 from the v-Src expressing clone wtS2 contained pSer and pTyr (Fig. 4 B), suggesting that v-Src targeted wt Cx43 in these cells. However, the levels of pTyr appeared to be reduced in the Y265F, and the double Cx43 mutants coexpressed with v-Src. Finally, the Y247F Cx43 mutant appeared to contain more pTyr than the Y265F Cx43 mutant.
Because it is difficult to obtain quantitative data from phosphoamino acid analysis, immunoblotting analysis using a pTyr antibody was performed to quantify the levels of pTyr in Cx43 isolated from the various clones. Cx43 was immunoprecipitated from lysates of the different clones using the same amount of antibody under conditions of antigen excess. As expected, Cx43 from wtC1 cells did not contain pTyr, whereas Cx43 from wtS2 cells expressing v-Src contained pTyr (Fig. 4 C). In cells coexpressing the Y247F Cx43 mutant and v-Src, a reduced but significant amount of pTyr of Cx43 was observed (∼57% of wt Cx43). However, compared with wt Cx43 the levels of pTyr in the Y265F and the double Cx43, mutants coexpressed with v-Src were greatly reduced to ∼10% and 2%, respectively (Fig. 4 C). These results indicated that v-Src phosphorylated wt Cx43 at both the Y265 and Y247 sites in vivo, confirming the identification of these sites as Src targets in our in vitro studies.
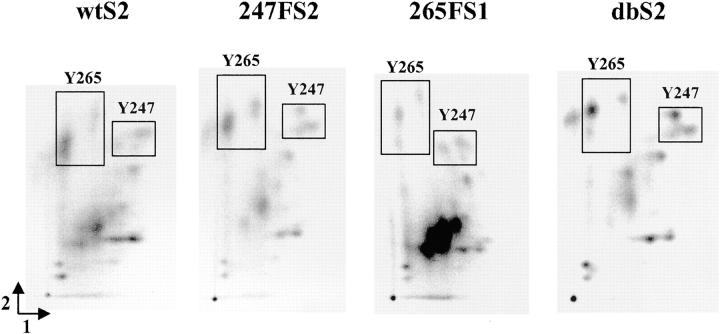
To further characterize Cx43 phosphorylation in vivo, we employed two-dimensional phosphotryptic peptide analysis. The peptide maps obtained were complex due to potential phosphorylation on multiple serine and tyrosine sites (Fig. 5) . Although the maps did not provide additional insight into the mechanism(s) of v-Src–induced Cx43 phosphorylation, some differences were apparent, which were consistent with the mutations introduced in Cx43. Three phosphopeptides migrated in the region of peptide 4 (Fig. 1 D) and were assigned tentatively to the Y247 peptide (Fig. 1D, boxed area). These peptides migrated similarly in the Y247F and Y247F/Y265F Cx43 mutants and had a different arrangement in the wt and Y265F proteins. This difference in migration is consistent with the loss of a tyrosine phosphorylation site and the substitution of the more hydrophobic Phe residue at the Y247 site in the Y247F and Y247F/Y265F mutants. Phosphopeptides ascribed to the Y265 peptide are also boxed. The migration patterns here are more similar in the Y265F and Y247F/Y265F mutants, which have a Phe substitution on the Y265 peptide. These patterns differ from those obtained with the wt and Y247F Cx43 proteins as observed previously in our in vitro studies (Fig. 1 D).
Figure 5.
Two-dimensional phosphotryptic peptide analysis of wt and mutant forms of Cx43 isolated from cells metabolically labeled with 32Pi. Phosphotryptic peptides were resolved by electrophoresis (dimension 1) followed by ascending chromatography (dimension 2). Boxes show phosphopeptides tentatively ascribed to the Y247 or the Y265 containing peptides.
GJC in cells expressing wt and mutant Cx43 and v-Src
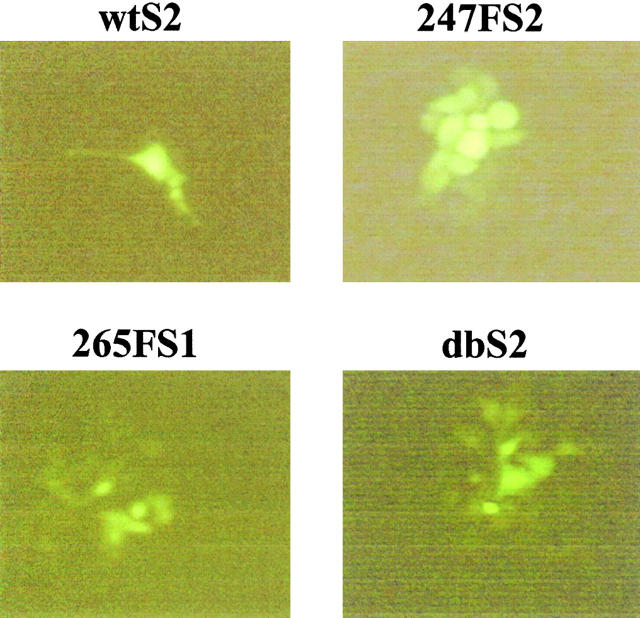
To determine the functional significance of phosphorylation at the Y247 or Y265 site in Cx43, GJC was measured in the clones by the transfer of Lucifer yellow dye 1 min after injection of the dye into the parental cells. To address the possibility of clonal variability, GJC was determined for two independently isolated clones for each experimental group. In the absence of v-Src, the substitution of Phe for Tyr at residues 247 or 265 did not disrupt the ability of Cx43 to establish functional gap junction channels (Table I). However, the expression of v-Src induced a dramatic disruption of dye transfer (∼14-fold reduction in degree of coupling and ∼5-fold change in the incidence of coupling) in cell clones coexpressing wt Cx43 compared with cell clones expressing wt Cx43 without v-Src (Table I, P < 0.01). This observation was consistent with previous results that showed that GJC was disrupted in cells containing the v-Src oncoprotein (Atkinson et al., 1981; Crow et al., 1990; Filson et al., 1990; Kurata and Lau, 1994). Importantly, dye transfer was unperturbed (P > 0.05) by the expression of v-Src in cells expressing the Y247F Cx43 mutant (247FS1 and 247FS2) or the double Cx43 mutant (dbS1 and dbS2). In addition, cells expressing v-Src together with the Y265F Cx43 mutant (265FS1 and 265FS2) exhibited high levels of GJC. Thus, v-Src either failed to disrupt, or induced a low level (∼20%) of disruption of, GJC in the cells coexpressing the Y247F and/or Y265F Cx43 mutants. Furthermore, the incidence of gap junctional coupling in cell clones expressing the Cx43 mutants in the presence or absence of v-Src was 100% in nearly all cases (Table I) in contrast to the cells expressing wt Cx43 and v-Src (18–19% coupled). The dramatic reduction of GJC in the cells expressing wt Cx43 with v-Src but not in the cells expressing Cx43 tyrosine mutants and v-Src, is also illustrated by fluorescent images captured at 4–6 min after the microinjection of single cells (Fig. 6) . These dye transfer results obtained by microinjection of single cells were substantiated by the scrape-loading technique (unpublished data). The observation that the Y247F and Y265F substitutions rendered Cx43 resistant to the disruption of GJC by v-Src strongly suggested that phosphorylation of Cx43 at these specific tyrosine sites represents a key mechanism underlying v-Src's ability to disrupt GJC in these cells.
Table I. Measurement of GJC in cell clones containing wt or mutant cx43 genes with or without the v-src gene.
| Cell clone | wt or mutant cx43 | v-src | GJC | Incidence of coupling |
|---|---|---|---|---|
| % | ||||
| wtC1 | wt | − | 13.1 ± 0.5 (24) | 100 |
| wtC2 | wt | − | 13.9 ± 0.9 (23) | 100 |
| wtS1 | wt | + | 1.3 ± 0.2 (53) | 19 |
| wtS2 | wt | + | 1.0 ± 0.2 (45) | 18 |
| 247FC1 | Y247F | − | 10.3 ± 0.5 (15) | 100 |
| 247FS1 | Y247F | + | 10.4 ± 0.8 (45) | 98 |
| 247FS2 | Y247F | + | 11.8 ± 0.4 (43) | 100 |
| 265FC1 | Y265F | − | 10.7 ± 0.8 (17) | 100 |
| 265FC2 | Y265F | − | 12.5 ± 0.5 (32) | 100 |
| 265FS1 | Y265F | + | 9.0 ± 0.6 (39) | 97 |
| 265FS2 | Y265F | + | 8.6 ± 0.6 (31) | 100 |
| dbC1 | Y247F/Y265F | − | 11.3 ± 0.6 (28) | 100 |
| dbC2 | Y247F/Y265F | − | 9.8 ± 0.6 (42) | 95 |
| dbS1 | Y247F/Y265F | + | 12.7 ± 0.7 (39) | 100 |
| dbS2 | Y247F/Y265F | + | 9.6 ± 0.6 (37) | 100 |
The nomenclature of the cell clones, the different cx43 genes, and whether the v-src gene is expressed are indicated. GJC values were measured by transfer of Lucifer yellow dye following the microinjection of single cells. The number of adjacent cells that become fluorescent (± SEM) was counted 1 min after microinjection. The total numbers of cells injected for each clone are indicated in parentheses. The percentages of injected cells that transferred dye to more than one neighboring cell are recorded as the incidence of coupling. Except for the wt cells (P < 0.01), there was no significant difference in the values of GJC between the v-Src containing clones and the non–v-Src–containing clones (P > 0.05 in a two-tailed Student's t test).
Figure 6.
Fluorescent images from microinjection of the v-Src– expressing cell clones. Fluorescent images were captured and recorded at 4–6 min after microinjections of single cells with Lucifer yellow dye.
Role of increased serine phosphorylation of Cx43 induced by v-Src
In this study, we demonstrated that phosphorylation of Cx43 at Y247 and Y265 was primarily responsible for the disruption of GJC by v-Src. However, Zhou et al. (1999) reported that the ability of v-Src to induce cell uncoupling was attenuated by treating cells with the MAP kinase kinase (MEK) inhibitor, PD98059, to block MAP kinase activation. This suggested that serine phosphorylation of Cx43, perhaps by activated MAP kinase, may have contributed to the disruption of GJC by v-Src. We also used the MEK inhibitor to examine the possibility that serine phosphorylation of Cx43 might be involved in the downregulation of GJC by v-Src in mammalian cells.
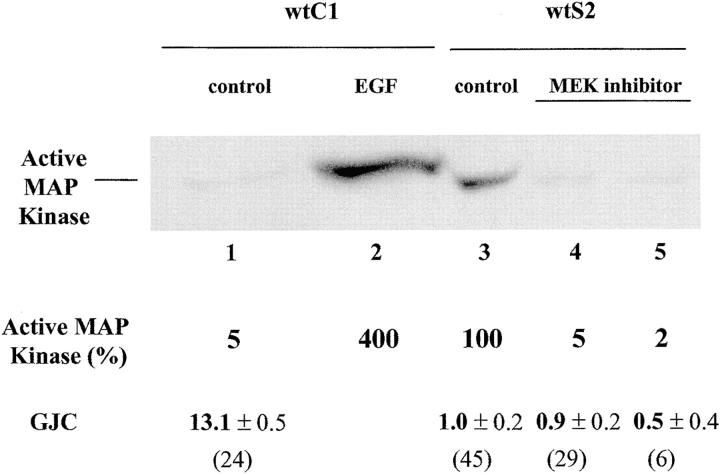
WtS2 cells were treated with 100 μM MEK inhibitor for 1 h. Interestingly, this treatment failed to restore GJC. Untreated wtS2 cells communicated to an average of 1.0 neighboring cell compared with an average of 0.9 adjacent cells after treatment (Fig. 7 , lanes 3 and 4). The wtC1 control cells, expressing wt Cx43 without v-Src, communicated to an average of 13.1 adjacent cells (Fig. 7, lane 1). To confirm that the MEK inhibitor treatment inhibited MAP kinase activation, the levels of active MAP kinase were determined from equivalent amounts of whole cell lysate by immunoblotting with an antibody recognizing the phosphorylated, activated form of MAP kinase. As a positive control, wtC1 cells treated with EGF showed increased levels of active MAP kinase compared with the untreated cells (Fig. 7, lanes 2 and 1, respectively). Activated MAP kinase was detected in the wtS2 cells treated with DMSO alone; however, treatment of these cells with the MEK inhibitor reduced activated MAP kinase to ∼5% of the untreated wtS2 cells. Fig. 7, lane 5, represents a single plate of wtS2 cells that communicated to an average of 0.5 neighboring cells after treatment with the MEK inhibitor. Immunoblotting the cell lysate from this single plate demonstrated that MAP kinase was reduced markedly to ∼2% of the untreated wtS2 cells. In addition, we determined that the wtS2 cells treated with 100 μM MEK inhibitor for 4 h communicated to an average of 1.0 ± 0.2 (n = 20) neighboring cells and also showed significantly reduced levels of activated MAP kinase (unpublished data). Taken together, these observations strongly suggested that activated MAP kinase did not play a role in the disruption of GJC induced by v-Src in our cells.
Figure 7.
The role of MAP kinase in the disruption of GJC by v-Src. Cells were untreated or treated with EGF or the MEK inhibitor before being lysed. The same amount of whole cell lysate was loaded for each sample. Activated MAP kinase was detected with an antibody recognizing phosphorylated MAP kinase. Lane 1, untreated wtC1; lane 2, wtC1 treated with 100 ng/ml EGF for 2 min; lane 3, untreated wtS2; and lanes 4 and 5, duplicate plates of wtS2 treated with 100 μM MEK inhibitor for 1 h. The same membrane used to detect active MAP kinase was stripped and reprobed with an antibody recognizing all isoforms of p42 MAP kinase. The ratios of active MAP kinase to total MAP kinase were determined and normalized to the untreated wtS2 cells set as 100%. Average GJC values obtained from multiple plates are reported for lanes 1, 3, and 4, and the GJC value from a single plate that was also used to measure the level of activated MAP kinase is shown for lane 5. The number of injections contributing to the determination of GJC is shown in brackets. Active MAP kinase was reduced to 5 and 2% of the starting level by the 1 h treatment with MEK inhibitor as shown in lanes 4 and 5, respectively.
Discussion
We have been interested in identifying molecular mechanisms that regulate Cx43-mediated GJC and how GJC is regulated by tyrosine protein kinases such as the EGF receptor and v-Src. The regulation of GJC is of particular interest due to the putative role of connexins in the regulation of cell growth. The expression of v-Src in mammalian cells results in cell transformation, tyrosine phosphorylation on numerous proteins, and a dramatic reduction in GJC. In this study, both in vitro and in vivo experimental approaches were employed to study the molecular mechanisms responsible for the disruption of GJC by v-Src. Fusion proteins containing wt or mutant Cx43CT were phosphorylated in vitro by activated Src to characterize phosphorylation of Cx43CT. More importantly, wt Cx43 and Y247F, Y265F, and Y247F/Y265F Cx43 mutants were expressed in a Cx43 KO cell line and cell clones that stably expressed membrane-localized Cx43, and established GJC were selected for further study. These cells were infected with a retroviral construct containing the v-src gene and the effects of v-Src on Cx43 membrane localization, protein phosphorylation, and GJC were examined.
Identification of Y265 and Y247 as Cx43 sites targeted by v-Src
The results presented in this study not only confirmed previous reports that Y265 of Cx43 is a primary site phosphorylated by v-Src (Swenson et al., 1990; Kanemitsu et al., 1997) but also identified Y247 as an additional major site of Cx43 targeted by activated Src in vitro and in intact cells. These conclusions were based upon several observations. First, in an earlier study we found evidence for a second pTyr site on Cx43 isolated from Rat-1 v-Src cells (Kurata and Lau, 1994). A potential consensus tyrosine target site for Src was identified at Y247 of Cx43. In the present study, phosphorylation of a mutant Cx43CT lacking amino acids P253-P256 resulted in a clear shift in the migration of a major phosphotryptic peptide (peptide 4, S244–K258), which contains a single tyrosine residue, Y247 (Fig. 1, A and B). Since activated Src phosphorylated GST–Cx43CT only on tyrosine (Fig. 1 C), these data strongly indicated that Y247 was a major site phosphorylated by activated Src in vitro. Second, Cx43CT phosphorylated by activated Src showed reduced amounts of pTyr (Fig. 1 C) and the complete loss or a shift in the migration of specific peptides (Fig. 1 D) when Y247 and/or Y265 were mutated to nonphosphorylatable Phe. These data supported the concept that activated Src phosphorylated Y247 and Y265 in vitro.
Third and most significantly, the Cx43 double mutant coexpressed with v-Src in mammalian cells showed a marked loss of pTyr (∼50-fold) compared with wt Cx43 (Fig. 4 C), suggesting that blocking phosphorylation at the Y247 and Y265 sites eliminated almost all tyrosine phosphorylation on Cx43 in mammalian cells coexpressing v-Src. This suggested that very little tyrosine phosphorylation occurred at other tyrosine sites in Cx43 in vivo, at least in the absence of phosphorylation at the Y265 site. The Y247F Cx43 mutant exhibited a significant amount of pTyr (Fig. 4 B), which represented about 57% of that of wt Cx43 (Fig. 4 C). Phosphorylated Y265 presumably accounted for most of the pTyr in this mutant. Interestingly, the pTyr content of the Y265F Cx43 mutant was also significantly reduced to about 10% of that of wt Cx43 (Fig. 4 C). This suggested that the phosphorylation of Y247 by v-Src was largely dependent upon the prior or concomitant phosphorylation of the Y265 site.
The role of pTyr sites in Cx43 in the disruption of GJC induced by v-Src
Previous studies have indicated a strong correlation between the disruption of GJC and the phosphorylation of Cx43 on tyrosine in cells expressing v-Src (Crow et al., 1990; Filson et al., 1990). Moreover, the specific phosphorylation of Y265 in Cx43 was implicated in the ability of v-Src to disrupt gap junctional conductance in Xenopus oocytes (Swenson et al., 1990). The experimental system developed in this study enabled us to not only directly determine whether phosphorylation at Y247 and/or Y265 of Cx43 was responsible for the downregulation of GJC by v-Src but also provided novel insights into the relative contributions of these two sites to this effect.
These experiments were possible because the Y247F and Y265F mutations did not appear to interfere with the localization of Cx43 to the plasma membrane as determined by immunofluorescence microscopy (Fig. 3). The Cx43 mutants established functional gap junctions that transferred dye at levels similar to the wt Cx43 channels (Table I). However, we found that gap junctions established by the double Cx43 mutant were completely resistant to v-Src in these mammalian cells (Table I). This functional difference correlated with a marked reduction in the pTyr content of the mutant Cx43 (Fig. 4 C). The persistence of GJC in the double Cx43 mutant clones was not due to lower levels of v-Src tyrosine kinase activity. In fact, clone dbS1 expressing the double Cx43 mutant had the highest level of v-Src kinase activity of the clones examined (Fig. 2 A), yet it did not show inhibition of GJC (Table I and Fig. 6). Thus, it was likely that the substitution of Phe for Tyr at the Y247 and Y265 sites of Cx43 rendered the cells resistant to the disruption of GJC by v-Src. Combined with the data that v-Src induced little or no disruption of GJC in cells coexpressing the Y247F or Y265F Cx43 mutants, these observations strongly suggested that v-Src disrupts Cx43-mediated GJC through phosphorylation at the Y247 and Y265 sites.
Measurement of dye transfer in cells coexpressing the single tyrosine site mutants and v-Src offered intriguing insights into the possible differential roles for these sites in the regulation of Cx43 function. The level of GJC for the cells expressing the Y247F Cx43 mutant was unperturbed by v-Src (Table I), even though these cells contained a significant level of pTyr on Cx43 (∼57% of wt Cx43) (Fig. 4 C). This phosphorylation presumably occurred primarily at the Y265 site, since the double Cx43 mutant lost ∼98% of the pTyr content as compared with wt Cx43. Yet, phosphorylation at the Y265 site alone in the Y247F Cx43 mutant was not sufficient to disrupt GJC. The markedly reduced pTyr content (∼10%) of the Y265F Cx43 mutant suggested that phosphorylation of Y265 by v-Src may be required for efficient phosphorylation of Cx43 at the Y247 site in vivo. This result differs from that obtained in our in vitro studies with the GST–Cx43CT fusion protein and may be due to the differences in the protein substrates (GST–Cx43CT versus full-length Cx43) and the lack of competing substrates for v-Src in the in vitro system. Nevertheless, the data obtained from our in vivo studies suggested that phosphorylation of Y247 rather than Y265 is the critical molecular event that is directly responsible for the disruption of gap junctions by v-Src. Taken together with the binding studies of Cx43 and v-Src (Kanemitsu et al., 1997), these data suggested that phosphorylation of Y265 in wt Cx43 may be essential to stabilize the interaction of these two proteins and to permit efficient phosphorylation of Y247. The subsequent phosphorylation at Y247 may be directly involved in the actual closure of gap junctions.
This hypothesis is consistent with the observation that the Y265F Cx43 mutant expressed in either Xenopus oocytes (Swenson et al., 1990) or mammalian fibroblasts (Table I) was largely resistant to the disruption of GJC by v-Src. Tyrosine phosphorylation of this mutant was reduced in the Xenopus oocytes, but the extent of reduction was not quantified. We found that phosphorylation of the Y265F Cx43 mutant on tyrosine in fibroblasts was reduced markedly to ∼10% of the level of wt Cx43 (Fig. 4 C). This indicated that phosphorylation of the wt Y247 site by v-Src was largely blocked in the Y265F Cx43 mutant. Thus, the resistance of the Y265F Cx43 mutant to v-Src may result from the inefficient phosphorylation of Y247 because of reduced stability of the interaction of Cx43 and v-Src in the absence of a phosphorylated Y265 residue.
Our data differ from the results of a second study in the Xenopus expression system (Zhou et al., 1999), which reported that GJC established by either a Y265F or Y247F Cx43 mutant was sensitive to disruption by v-Src. The authors concluded that Tyr phosphorylation was not involved in the disruption of GJC in the v-Src–expressing oocytes. The fundamental reason(s) for these experimental differences is unclear. However, it is important to note that our data strongly corroborate and extend the original work of Swenson et al. (1990), which was performed in the Xenopus oocyte system and was the first demonstration of the requirement for phosphorylation of Tyr265 in v-Src's ability to disrupt GJC. In addition, our results are in agreement with two recently published papers focused on the effects of kinase-activated c-Src on the Y265F Cx43 mutant (Giepmans et al., 2001; Toyofuku et al., 2001). These latter studies demonstrated that activated c-Src not only interacted with Cx43 but also phosphorylated Cx43 at Y265 in mammalian cells and significantly reduced GJC. Thus, four independent reports consistently indicated the importance of tyrosine phosphorylation in Src's effects on Cx43 function. This conclusion is also consistent with Src's ability to interact directly with Cx43 in vivo (Kanemitsu et al., 1997; Loo et al., 1999) and to phosphorylate Cx43 directly in in vitro kinase reactions (Loo et al., 1995; Giepmans et al., 2001).
To explain the differences between their data and those of Swenson et al. (1990) in the oocyte system, Zhou et al. (1999) speculated that their experiments focused on the effects of v-Src on the gating of preestablished Cx43 channels. The cRNA for v-src was injected ∼16 h after the injection of the cRNA for Cx43 in their study, and they speculated that the effects were not occurring through a nonspecific effect on Cx43 biosynthesis or assembly as may have been the case in Swenson et al. (1990). However, this suggestion was not demonstrated experimentally. In the studies of Zhou et al. (1999), the various Cx43 cRNAs were injected at levels from 0.5–8 ng/oocyte to obtain “comparable coupling levels,” whereas the cRNA for v-Src remained constant at 7 ng/oocyte. It is not clear whether there may have been differences in the assembly or maintenance of the Cx43 channels that were formed by the different Cx43 mutants in the oocyte system. Thus, the fundamental difference between the results presented by Zhou et al. (1999) and Swenson et al. (1990) was not clearly established. However, we did not detect large differences in either the level or the plasma membrane localization of wt or mutant Cx43 in the absence or presence of v-Src expressed in our mammalian cells. Cx43 was localized to the plasma membrane in gap junctional plaques in the v-Src–expressing cells (Fig. 3) and the levels of Cx43 protein did not appear to be reduced on Western blots (Fig. 2 B). Similar results demonstrating a lack of effect of v-Src on Cx43 localization have been reported in previous studies (Atkinson and Sheridan, 1985; Crow et al., 1990; Loo et al., 1999). Zhou et al. (1999) also did not detect obvious effects on the distribution of gap junction plaques in the normal rat kidney (NRK) cells after the shift to the permissive temperature in the time examined.
Xenopus oocytes and mammalian cells clearly represent two different expression systems. Differences between these two experimental systems in terms of connexin expression and channel formation have been observed previously. In contrast to HeLa cells, the expression of rat Cx46 and the formation of functional channels in oocytes required sufficient extracellular Ca2+ to prevent hemichannels from opening at the resting potential and depolarizing the cells (Paul et al., 1991; Willecke and Haubrich, 1996). Moreover, murine Cx31 formed functional channels in HeLa cells but not in oocytes. It is also possible that posttranslational processing and other modifications (i.e., phosphorylation) are substantively different between the two expression systems (Willecke and Haubrich, 1996). In this study, the Cx43-deficient fibroblast cell line was selected as the recipient cell for mutant Cx43 because it normally expresses Cx43 and represents the cell type typically targeted by the Rous sarcoma virus harboring the src oncogene. The expression of nonconditionally activated v-Src and Cx43 in fibroblasts occurs in a constitutive fashion, unlike the transient expression of proteins from microinjected RNAs in Xenopus oocytes. Thus, the levels of Cx43 and v-Src, the v-Src kinase activity, and the experimental temperature are unperturbed in this system, unlike the experimental conditions in the Xenopus oocyte and temperature-sensitive NRK cell systems employed by Zhou et al. (1999).
MAP kinase is not involved in the disruption of GJC induced by v-Src
In addition to unique phosphorylation on tyrosine, an apparent increased phosphorylation of Cx43 on serine was observed in fibroblasts transformed by v-Src (Filson et al., 1990; Kurata and Lau, 1994). Zhou et al. (1999) suggested that phosphorylation of Cx43 on serine, possibly through activation of the MAP kinase pathway, was responsible for the disruption of GJC by v-Src. This conclusion was based on the observation that the MEK inhibitor, PD98059, blocked the disruption of dye transfer between NRK cells after activation of a temperature-sensitive v-Src by a shift to the permissive temperature (Zhou et al., 1999). However, the disruption of dye transfer was not completely blocked by the MEK inhibitor (∼50%), and the effects of this treatment on MAP kinase activation were not demonstrated. Treatment with the MEK inhibitor appeared to upregulate dye transfer in the NRK cells at the restrictive temperature (Zhou et al., 1999). Since the effects of the inhibitor on MAP kinase or v-Src kinase activity and on the levels of pTyr in Cx43 were not shown for the NRK cells after the shift to the permissive temperature, it is difficult to ascertain how changing temperature, increasing Src activity, and changes in MAP kinase activity may have affected Cx43 phosphorylation and impacted GJC.
In our mammalian cell system, treatment of wtS2 cells with the MEK inhibitor dramatically reduced the level of activated MAP kinase to ∼5% of that of the untreated v-Src cells (Fig. 7). However, this treatment failed to restore GJC in the wtS2 cells (Fig. 7). Treating the cells with the MEK inhibitor for a longer period of time (4 h) also did not increase GJC. The lack of effect of the MEK inhibitor on GJC and the observation that the double tyrosine Cx43 mutant was completely resistant to v-Src–induced disruption of GJC did not support a significant role for MAP kinase in mediating the disruption of GJC in our cell system. Indeed, our previous studies in the KO cells expressing wt Cx43 demonstrated that EGF treatment, which induced an ∼4-fold greater level of active MAP kinase than was observed in the wtS2 cells expressing Src (see Fig. 7), initiated only a modest fall in GJC (∼40%) (Warn-Cramer et al., 1998). This fall in GJC was blocked by pretreatment with the MEK inhibitor. These results further indicated that MAP kinase was not a likely candidate for the dramatic disruption of Cx43 GJC induced by v-Src in these cells.
The reason(s) for the differences in the experimental results obtained in these different studies is unclear. However, the data presented in this report have clearly demonstrated that preventing tyrosine phosphorylation on Cx43 prevented the disruption of GJC in our mammalian cells transformed by v-Src. Although our data did not support a role for MAP kinase in the disruption of GJC in the v-Src–expressing cells, we cannot exclude the possibility that MAP kinase may play a role in other cell systems or under some experimental conditions.
A model for the interaction of Cx43 with v-Src in the disruption of GJC
The results obtained in this study indicated that substitution of Phe for Tyr at Y247 or Y265 of Cx43 prevented the disruption of GJC by v-Src in a mammalian cell system. Furthermore, although the MEK inhibitor, PD98059, markedly inhibited the activation of MAP kinase in the v-Src cells, it did not block the disruption of GJC. Thus, the simplest explanation for these observations is that v-Src disrupts gap junctions through phosphorylation at the Y247 and Y265 sites of Cx43 and not through MAP kinase–mediated serine phosphorylation in these cells.
In our study, the Y265 and Y247 sites both appeared to be phosphorylated in the mammalian cells that coexpressed wt Cx43 and constitutively active v-Src. However, the roles for phosphorylation at the Y247 and Y265 sites of Cx43 in the regulation of GJC by v-Src appear to be different. Our data support the hypothesis that phosphorylation of Cx43 at Y265 by v-Src is required for efficient phosphorylation at Y247. The phosphorylated Y265 site of Cx43 but not the Y247 or Y267 sites was shown to be required for stable binding of Cx43 to the SH2 domain of v-Src in vitro and in vivo (Kanemitsu et al., 1997). Also, the SH3 domain of v-Src was shown to bind to the proline-rich motif located at P274-P284 of Cx43 (Kanemitsu et al., 1997).
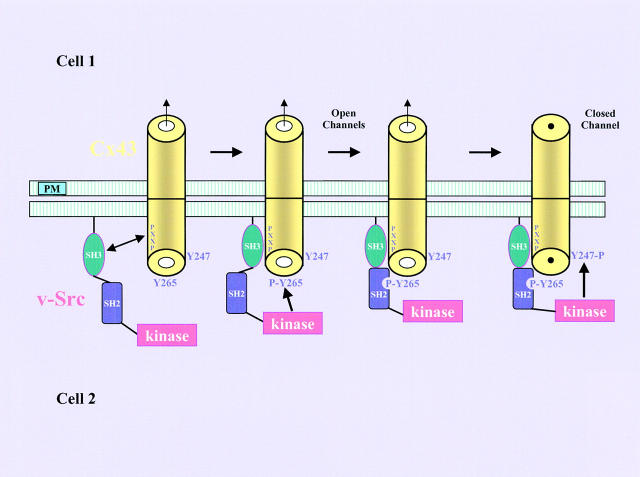
These observations suggest a working model for how v-Src interacts with and phosphorylates Cx43 on tyrosine and disrupts GJC (Fig. 8) . This model is a modification of the one proposed by Kanemitsu et al. (1997). The binding of Cx43 to v-Src is initiated by the interaction of the P274-P284 proline-rich region of Cx43 with the SH3 domain of v-Src. This interaction leads to phosphorylation of Cx43 at the Y265 site by the kinase domain of v-Src. Phosphorylation at Y265 provides a binding site for the SH2 domain of v-Src, which stabilizes the binding of Cx43 to v-Src. In our revised model, the kinase domain of v-Src can now efficiently phosphorylate Cx43 at the Y247 site, which may represent the key molecular event triggering the closure of the Cx43 channel by v-Src.
Figure 8.
A model for the interaction of v-Src with Cx43. In this model, the binding of Cx43 to v-Src is dependent upon SH3 and SH2 domain interactions, which are important for v-Src–induced phosphorylation of Cx43 at the Y265 site and the subsequent phosphorylation at the Y247 site, leading to closure of the Cx43 channel (details described in Discussion). PXXP denotes the proline-rich sequence of Cx43 P274–P284 that interacts with the SH3 domain of v-Src. For simplicity, gap junction channels are represented as cylinders. PM stands for plasma membrane.
This pattern of sequential SH3 and SH2 domain-binding reactions in the phosphorylation of Src substrates has been reported previously for the pSer-containing protein p110 (Kanner et al., 1991), the RNA-binding protein p68 (Taylor and Shalloway, 1994), and the p130Cas-related protein Sin (Taylor and Shalloway, 1994; Alexandropoulos and Baltimore, 1996). In addition, cytoplasmic tyrosine kinases such as v-Src have been found to preferentially phosphorylate peptides that are recognized by their own SH2 domains (Songyang et al., 1995). Moreover, Src family kinases have been demonstrated to phosphorylate substrates at multiple Tyr sites by a processive mechanism (Scott and Miller, 2000), consistent with that proposed for v-Src and Cx43 in our model.
How does phosphorylation of the Y247 site of Cx43 promote disruption of GJC? One possibility is that phosphorylation of Y247 by v-Src alters the conformation of the Cx43 gap junction channel and leads to closure of the channels. However, the ability of the CT expressed separately from the channel-forming portion of Cx43 to participate in Src- or pH-induced channel closure suggests that this mechanism is unlikely (Morley et al., 1996; Zhou et al., 1999). Another possibility is that the addition of a negatively charged phosphoryl group at Y247 may permit the cytoplasmic tail of Cx43 to interact with positively charged amino acids of Cx43 (for example, residues in the cytoplasmic loop), leading to blockage of the channel. Finally, phosphorylated Y247 may represent a potential binding site, which subsequently recruits an unknown SH2- or PTB-containing protein to Cx43 that promotes channel closure. This model, for Src-induced disruption of GJC by protein–protein interactions and sequential phosphorylation events that lead to the disruption of GJC, will help guide further studies of the regulation of GJC by phosphorylation. A challenge for future studies will be to sort through the various possible mechanisms triggered by tyrosine phosphorylation of Cx43 that ultimately lead to the closure of the Cx43 channel.
Materials and methods
Cx43 and v-Src constructs
The rat cx43 gene (Beyer et al., 1987) encoding a Phe substitution at the Y247 site (Y247F or 247F) was prepared from a full-length cx43 gene using the Chameleon site-directed mutagenesis kit (Stratagene). A full-length cx43 gene containing the Y265F (or 265F) mutation in a SP64t vector was provided by Dr. David Paul (Harvard University, Cambridge, Massachusetts) and was used to generate the cx43 mutant encoding the double site mutant (Y247F/Y265F or db) by a recombinant PCR approach (Higuchi, 1989). The mutated cx43 genes were subcloned into the BamHI/EcoRI sites of the Bluescript (Bssk-) vector and sequenced to confirm the mutations and establish the fidelity of the rest of the gene. The full-length cx43 genes were excised from their original vectors and inserted into the pBABE retroviral vector (Morgenstern and Land, 1990).
Full-length wt or mutated cx43 genes were used to generate DNAs encoding GST fusion proteins containing the CT (amino acids 236–382) of Cx43 (GST–Cx43CT) by PCR amplification. The PCR products were subcloned into the BamHI/EcoRI sites of the pGEX-KG expression vector (Loo et al., 1995), and fidelity of the cx43 genes was confirmed by DNA sequencing.
A pMvsrc plasmid containing a v-src gene was provided by Dr. Tona Gilmer (Glaxo). The full-length v-src fragment was PCR amplified from the pMvsrc clone, subcloned into the XhoI/BamHI sites of the pLXSH retroviral vector (Miller et al., 1993), and sequenced to confirm the authentic v-src sequences.
Expression, purification, and phosphorylation of GST–Cx43CT fusion proteins
GST–Cx43CT fusion proteins were induced in Escherichia coli by treatment with 2 mM IPTG for 2 h at 37°C. Bacterial cells were lysed and the fusion proteins purified as described (Loo et al., 1995). Purified fusion proteins were quantified by the Bio-Rad Laboratories protein assay and phosphorylated by activated Src (Upstate Biotechnology) in a kinase buffer (Loo et al., 1995) containing 10 μCi of [γ-32P]ATP (NEN Life Science Products) for 10 min at 30°C. The reaction was stopped by the addition of 4× SDS sample buffer, and the samples were boiled and resolved by SDS-PAGE.
Generation of Cx43 KO cells reexpressing wt or mutant Cx43 and coexpressing v-Src
pBABE-cx43 DNAs were transfected into the PE501 packaging cell line (Miller et al., 1993) using Lipofectamine (GIBCO BRL). Retroviral supernatants from the transfected cells were harvested, filtered, and used to infect the PA317 packaging cell line with 8 μg/ml polybrene as described previously (Martyn et al., 1997; Warn-Cramer et al., 1998). The filtered retroviral supernatants of the infected PA317 cells were used to infect the Cx43 KO cells (clone 23-3). Infected cells were selected with a medium containing 6–10 μg/ml of puromycin and subcloned by limiting dilution.
The pLvsrcSH or control pLXSH vector DNA was transiently transfected into PA317 cells. Filtered retroviruses from the transfection were used to infect PE501 cells. Filtered retroviruses from the infected PE501 cells were used to infect Cx43 KO cells reexpressing wt or mutant Cx43. Stably infected pLvsrcSH or pLXSH control cells were selected with medium containing 200 U/ml of hygromycin. Single clones were isolated using small squares (∼10 mm2) of Whatman paper (Domann and Martinez, 1995) and recloned by limiting dilution. Different pLvsrcSH-infected clones exhibited varying degrees of morphological transformation. Two transformed clones from each set of infections with wt or mutant cx43 and pLvsrcSH were selected for further study (Table I). As controls, at least one clone was also selected from each set of infections with wt or mutant cx43 retroviruses and pLXSH.
Immunofluorescence microscopy of Cx43
The subcellular localization of Cx43 was established in subconfluent cells grown on glass coverslips. Cells were fixed in 100% methanol at −20°C, permeabilized with 1% Triton X-100 for 5 min, and incubated with a rabbit antiserum directed against peptide 368–382 of Cx43 (Cx43CT368 antiserum) at 1:1,000 dilution with 1% BSA in PBS. The Cx43 immune complexes were detected with Alexa Fluor 488–conjugated goat anti–rabbit antibody (Molecular Probes). The fluorescent images were visualized using a ZEISS Axioplane Universal microscope equipped with epifluorescence and captured with a computer using Mocha software (Jandel Scientific).
Cell culture and metabolic 32Pi labeling
Cells were cultured in DME with 10% FCS (Summit Biotechnology) in a humidified 5% CO2 incubator at 37°C. In studies using the MEK inhibitor, PD98059 (New England Biolabs, Inc.), cells were pretreated for 1 h at 37°C with 100 μM PD98059 in DMSO or DMSO alone (0.1% vol/vol) as a control. For studies of Cx43 phosphorylation, confluent monolayers were metabolically labeled with 32Pi (NEX-053; NEN Life Science Products; DuPont) at 1 mCi/ml in phosphate-deficient medium with 4% calf serum for 2.5 h at 37°C followed by washing with PBS/160 μM Na3VO4/10 mM NaF.
Immunoprecipitation and phosphoamino acid analysis of Cx43
Metabolically labeled or unlabeled cells were solubilized in Ripa buffer (Crow et al., 1990; Warn-Cramer et al., 1998). The lysates were clarified by ultracentrifugation at 436,000 g for 20 min at 4°C followed by incubation with the Cx43CT368 antiserum under conditions of antigen excess. Immune complexes were precipitated with activated protein A–Staphylococcus aureus and resolved by SDS-PAGE. The gels were dried and autoradiographed using Eastman Kodak Co. X-OMAT XAR-5 films at −70°C.
For phosphoamino acid analysis, 32P-labeled proteins were electrotransferred from the unfixed gel to an Immobilon-P membrane (Millipore). Membrane pieces containing the desired Cx43 protein bands were excised and acid hydrolyzed as previously described (Loo et al., 1995). Hydrolyzed samples were mixed with nonradioactive phosphoamino standards (pSer, phosophpthreonine [pThr], and pTyr) and separated on TLC plates at pH 1.9 and 3.5 (Kamps and Sefton, 1989; Crow et al., 1992; Warn-Cramer et al., 2000). The plates were exposed to Biomax ML films with Biomax intensifying screens (Eastman Kodak Co.) at −70°C. The relative amounts of pTyr of Cx43 were quantified by measuring the density times the area of the pTyr spots using the Quantity One software provided with the Fluor-S MultiImager (Bio-Rad Laboratories).
Two-dimensional phosphotryptic peptide analysis of GST–Cx43CT fusion proteins phosphorylated by activated Src and Cx43 isolated from v-Src–expressing cells
GST–Cx43CT phosphorylated by activated Src in vitro with [γ-32P]ATP or full-length Cx43 isolated from 32P-labeled cells expressing v-Src was resolved by SDS-PAGE and autoradiographed. Gel pieces containing the phosphorylated Cx43 were excised from the wet unfixed gel and finely ground. Phosphorylated Cx43 was eluted from the gel pieces as described (Boyle et al., 1991; Warn-Cramer et al., 2000), oxidized with performic acid, lyophilized to dryness, and digested overnight at 37°C with N-tosyl-l-phenylalanine chloromethylketone–treated trypsin (Worthington). The resulting phosphotryptic peptides were resolved on TLC plates by electrophoresis in pH 1.9 buffer for 1 h at 1000 V followed by ascending chromatography in the second dimension in an isobutyric acid buffer. After air drying, the plates were exposed to Eastman Kodak Co. X-Omat XAR-5 film with the aid of an intensifying screen at −70°C.
In vitro analysis of the protein kinase activity of v-Src
Cells were lysed in Ripa buffer, clarified, and immunoprecipitated using tumor-bearing rabbit (TBR) serum 6-1-4 obtained from rabbits bearing Rous sarcoma virus–induced tumors (Brugge and Erikson, 1977). The IgG heavy chain of the TBR serum served as a substrate for active v-Src in an immune complex kinase assay (Collett et al., 1980). Immunoprecipitated v-Src was assayed in vitro in a 20-μl reaction volume-containing kinase buffer (Loo et al., 1995) with 10 μCi of [γ-32P]ATP for 10 min at room temperature. The reaction was stopped by the addition of 20 μl of 2× SDS sample buffer and boiling. Proteins were resolved by SDS-PAGE, and the gels were dried and autoradiographed using Eastman Kodak Co. X-Omat XAR-5 films.
Immunoblotting analysis of Cx43 and activated MAP kinase
Cx43 expression was measured by immunoblotting cell lysates obtained from confluent monolayers. Cells were harvested, lysed in lysis buffer (Loo et al., 1999), and protein concentrations were determined by the Bradford method (Bio-Rad Laboratories). Samples containing equal amounts of protein were resolved by SDS-PAGE. Cx43 was detected with Cx43CT368 antiserum using the ECL immunoblotting kit (Amersham Pharmacia Biotech).
To quantify the relative levels of pTyr, Cx43 was immunoprecipitated from lysates of cells grown to confluence on 60-mm culture dishes using equal amounts of Cx43CT368 antiserum under conditions of antigen excess. Samples were separated by SDS-PAGE and transferred to Immobilon-P membranes (Millipore). pTyr was detected with an anti-pTyr monoclonal antibody (PY99) (Santa Cruz Biotechnology, Inc.) using the ECL-plus immunoblotting kit (Amersham Pharmacia Biotech) and Eastman Kodak Co. X-Omat XAR-5 films. The films were scanned using a MultiImager, and the relative amounts of pTyr of Cx43 were quantified as described for the phosphoamino acid analysis.
To examine MAP kinase activation, v-Src–expressing cells were treated with either 100 μM MEK inhibitor, PD98059, in DMSO or DMSO alone for 1 h or 4 h at 37°C. Cx43 KO cells reexpressing wt Cx43 were treated with EGF at 100 ng/ml for 2 min as a positive control for activated MAP kinase (Warn-Cramer et al., 1998). Cells were harvested, lysed, and equal amounts of protein were resolved by SDS-PAGE and then electrotransferred to Immobilon-P membranes. Activated MAP kinase was detected with a rabbit antibody specific for the phosphorylated activated form of MAP kinase (Promega) using the ECL-plus immunoblotting kit and Eastman Kodak Co. X-Omat XAR-5 films. After the detection of activated MAP kinase, the membranes were stripped as described (ECL immunoblotting kit) and reblotted with an antibody recognizing all isoforms of p42 MAP kinase (Santa Cruz Biotechnology, Inc.). The relative amounts of MAP kinase were quantified as described for the pTyr immunoblotting. The ratio of the amounts of active MAP kinase to all isoforms of MAP kinase in the wtS2 cells without MEK inhibitor treatment was set to 100%. The percentages of the other samples were normalized accordingly.
Measurement of GJC
Cells were grown to confluence on 35-mm culture dishes. Single cells were microinjected with a glass micropipette (Eppendorf) containing the fluorescent dye Lucifer yellow (10%, wt/vol) using an Eppendorf Transjector 5246 and an Eppendorf micromanipulator that relies on pressure for dye injection. The fluorescent images were visualized with a ZEISS phase–contrast inverted microscope equipped with epifluorescence. The number of adjacent cells that became fluorescent was counted 1 min after microinjection. GJC values were determined by the microinjection of approximately six cells/plate from an average of six plates/sample in studies carried out on at least two different days. P values were determined by two-tailed Student's t tests. Fluorescent images from single cell microinjections were captured 4–6 min after microinjection using the Mocha software.
Acknowledgments
We thank Dr. Martha Kanemitsu for providing the GSTCx43CT fusion protein with the P253-P256 deletion, Dr. Tona Gilmer for the pMvsrc plasmid, Dr. David Paul for the Y265F mutant cx43 gene, and Dr. Kendra Martyn for reviewing the manuscript.
This research was supported by grants from the National Cancer Institute, National Institutes of Health (CA52098) to A.F. Lau, and the American Heart Association (HIFW-17-98) to R. Lin.
Footnotes
Abbreviations used in this paper: CT, COOH-terminal tail; Cx, connexin; GJC, gap junctional communication; GST, glutathione S-transferase, KO, knockout; MAP, mitogen-activated protein; MEK, MAP kinase kinase; NRK, normal rat kidney; pSer, phosphoserine; pThr, phosphothreonine; pTyr, phosphotyrosine; wt, wild-type.
References
- Alexandropoulos, K., and D. Baltimore. 1996. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 10:1341–1355. [DOI] [PubMed] [Google Scholar]
- Atkinson, M.M., and J.D. Sheridan. 1985. Reduced junctional permeability in cells transformed by different viral oncogenes. In Gap Junctions. M.V.L. Bennett and D.C. Spray, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 205–213.
- Atkinson, M.M., A.S. Menko, R.G. Johnson, J.R. Sheppard, and J.D. Sheridan. 1981. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J. Cell Biol. 91:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer, E.C., D.L. Paul, and D.A. Goodenough. 1987. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J. Cell Biol. 105:2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, W.J., P. Van der Geer, and T. Hunter. 1991. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin layer cellulose plates. Meth. Enzymol. 201:110–149. [DOI] [PubMed] [Google Scholar]
- Brown, M.T., and J.A. Cooper. 1996. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1287:121–149. [DOI] [PubMed] [Google Scholar]
- Brugge, J.S., and R.L. Erikson. 1977. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 269:346–348. [DOI] [PubMed] [Google Scholar]
- Collett, M.S., A.F. Purchio, and R.L. Erikson. 1980. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 285:167–169. [DOI] [PubMed] [Google Scholar]
- Crow, D.S., E.C. Beyer, D.L. Paul, S.S. Kobe, and A.F. Lau. 1990. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol. Cell. Biol. 10:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow, D.S., W.E. Kurata, and A.F. Lau. 1992. Phosphorylation of connexin43 in cells containing mutant src oncogenes. Oncogene. 7:999–1003. [PubMed] [Google Scholar]
- Domann, R., and J. Martinez. 1995. Alternative to cloning cylinders for isolation of adherent cell clones. Biotechniques. 18:594–595. [PubMed] [Google Scholar]
- Filson, A.J., R. Azarnia, E.C. Beyer, W.R. Loewenstein, and J.S. Brugge. 1990. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ. 1:661–668. [PubMed] [Google Scholar]
- Giepmans, B.N.G., T. Hengeveld, F.R. Postma, and W.G. Moolenaar. 2001. Interaction of c-Src with gap junction protein connexin-43. J. Biol. Chem. 276:8544–8549. [DOI] [PubMed] [Google Scholar]
- Goldberg, G.S., and A.F. Lau. 1993. Dynamics of connexin43 phosphorylation in pp60v-src-transformed cells. Biochem. J. 295:735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough, D.A., J.A. Goliger, and D.L. Paul. 1996. Connexins, connexins, and intercellular communication. Annu. Rev. Biochem. 65:475–502. [DOI] [PubMed] [Google Scholar]
- Higuchi, R. 1989. Using PCR to engineer DNA. In PCR Technology. H.A. Erlich, editor. Stockton Press, New York. 61–70.
- Kamps, M.P., and B.M. Sefton. 1989. Acid and base hydrolysis of phosphoproteins bound to Immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal. Biochem. 176:22–27. [DOI] [PubMed] [Google Scholar]
- Kanemitsu, M.Y., L.W. Loo, S. Simon, A.F. Lau, and W. Eckhart. 1997. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J. Biol. Chem. 272:22824–22831. [DOI] [PubMed] [Google Scholar]
- Kanner, S.B., A.B. Reynolds, H.C. Wang, R.R. Vines, and J.T. Parsons. 1991. The SH2 and SH3 domains of pp60src direct stable association with tyrosine phosphorylated proteins p130 and p110. EMBO J. 10:1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata, W.E., and A.F. Lau. 1994. p130gag-fps disrupts gap junctional communication and induces phosphorylation of connexin43 in a manner similar to that of pp60v-src. Oncogene. 9:329–335. [PubMed] [Google Scholar]
- Laird, D.L., M. Castillo, and L. Kasprzak. 1995. Gap junction turnover, intracellular trafficking, and phosphorylation of connexin43 in Brefeldin A–treated rat mammary tumor cells. J. Cell Biol. 131:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein, W.R. 1981. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol. Rev. 61:829–913. [DOI] [PubMed] [Google Scholar]
- Loo, L.W., M.Y. Kanemitsu, and A.F. Lau. 1999. In vivo association of pp60v-src and the gap-junction protein connexin 43 in v-src-transformed fibroblasts. Mol. Carcinog. 25:187–195. [PubMed] [Google Scholar]
- Loo, L.W.M., J.M. Berestecky, M.Y. Kanemitsu, and A.F. Lau. 1995. pp60src-mediated phosphorylation of connexin 43, a gap junction protein. J. Biol. Chem. 270:12751–12761. [DOI] [PubMed] [Google Scholar]
- Martyn, K.D., W.E. Kurata, B.J. Warn-Cramer, J.M. Burt, E. TenBroek, and A.F. Lau. 1997. Immortalized connexin43 knockout cell lines display a subset of biological properties associated with the transformed phenotype. Cell Growth Differ. 8:1015–1027. [PubMed] [Google Scholar]
- Miller, A.D., D.G. Miller, J.V. Garcia, and C.M. Lynch. 1993. Use of retroviral vectors for gene transfer and expression. Meth. Enzymol. 217:581–599. [DOI] [PubMed] [Google Scholar]
- Morgenstern, J., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucl. Acid Res. 18:3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley, G.E., S.M. Taffet, and M. Delmar. 1996. Intramolecular interactions mediate pH regulation of connexin43 channels. Biophys. J. 70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, D.L., L. Ebihara, L.J. Takemoto, K.I. Swenson, and D.A. Goodenough. 1991. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 115:1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, M.P., and W.T. Miller. 2000. A peptide model system for processive phosphorylation by Src family kinases. Biochemistry. 39:14531–14537. [DOI] [PubMed] [Google Scholar]
- Songyang, Z., K.L. Carraway, M. Eck, S.C. Harrison, R.A. Feldman, M. Mohammadl, J. Schlessinger, S.R. Hubbard, D.P. Smith, C. Eng, et al. 1995. Catalytic specificity of protein-tyrosine kinase is critical for selective signaling. Nature. 373:536–539. [DOI] [PubMed] [Google Scholar]
- Swenson, K.I., H. Piwnica-Worms, H. McNamee, and D.L. Paul. 1990. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, S.J., and D. Shalloway. 1994. An RNA-binding protein associated with Src through its SH2 and SH3 domains in mitosis. Nature. 368:867–871. [DOI] [PubMed] [Google Scholar]
- Toyofuku, T., Y. Akamatsu, H. Zhang, T. Kuzuya, M. Tada, and M. Hori. 2001. c-Src regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J. Biol. Chem. 276:1780–1788. [DOI] [PubMed] [Google Scholar]
- Trosko, J.E., and R.J. Ruch. 1998. Cell-cell communication in carcinogenesis. Front Biosci. 3:D208–D236. [DOI] [PubMed] [Google Scholar]
- Unger, V.M., N.M. Kumar, N.B. Gilula, and M. Yeager. 1999. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 283:1176–1180. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer, B.J., G.T. Cottrell, J.M. Burt, and A.F. Lau. 1998. Regulation of connexin-43 gap junctional intercellular communication by mitogen-activated protein kinase. J. Biol. Chem. 273:9188–9196. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer, B.J., W.E. Kurata, and A.F. Lau. 2000. Biochemical analysis of connexin phosphorylation. Meth. Mol. Biol. 154:431–446. [DOI] [PubMed] [Google Scholar]
- Willecke, K., and S. Haubrich. 1996. Connexin expression systems: to what extent do they reflect the situation in the animal? J. Bioenerg. Biomembr. 28:319–326. [DOI] [PubMed] [Google Scholar]
- Zhou, L., E.M. Kasperek, and B.J. Nicholson. 1999. Dissection of the molecular basis of pp60v-src induced gating of connexin 43 gap junction channels. J. Cell Biol. 144:1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]