Abstract
Recently, two new ligands of the Arp2/3 complex have been described that may shed light on the way cells organize complex networks of actin in response to signals. Abp1p, a yeast protein involved in endocytosis, and cortactin, a mammalian src substrate, both enhance the ability of the Arp2/3 complex to assemble branched actin filament networks.
Actin polymerization is required for many types of cell motility. Within the past few years it has become clear that the Arp2/3 complex is a key regulator of actin polymerization downstream of various receptors (Machesky and Insall, 1998; Svitkina and Borisy, 1999). Perhaps the best-characterized activators of the Arp2/3 complex are the Wiskott-Aldrich Syndrome protein (WASP)* family proteins (Mullins, 2000). In a recent issue of The Journal of Cell Biology, Goode et al. (2001) showed that Saccharomyces cerevisiae Abp1p, a protein that has been suggested to connect the actin cytoskeleton with endocytic trafficking, binds to and activates the Arp2/3 complex. Cortactin, a mammalian Src kinase substrate, has also recently been shown to activate Arp2/3 complex–mediated actin assembly (Uruno et al., 2001; Weaver et al., 2001). Unlike the WASP family, these proteins are thought to be mainly involved in enhancing the branching role of the Arp2/3 complex. These exciting findings suggest that new classes of Arp2/3 complex modulating proteins may account for some of the diversity of actin structures found in cells.
The Arp2/3 complex contains seven subunits, two of them related to actin (actin-related proteins). Arp2 and Arp3 may mimic the fast-growing end (barbed end) of an actin filament to allow the Arp2/3 complex to nucleate actin assembly, cap the slow-growing end (pointed end), and cross-link actin filaments (Mullins et al., 1998; Welch et al., 1998; for review see May, 2001). The Arp2/3 complex nucleates new filament branches from the side of existing filaments with a characteristic 70° angle between the branches. This process is known as dendritic nucleation, and may provide a rigid structure by which actin polymerization triggers membrane protrusion (Mullins, 2000).
Although purified Arp2/3 complex weakly activates actin nucleation, its activity can be dramatically enhanced by cellular activators, including: the WASP family proteins (Machesky and Insall, 1998; Higgs and Pollard, 1999); the Listeria protein ActA (Welch et al., 1998); the type I myosin Myo1p from Schizosaccharomyces pombe (Lee et al., 2000); and recently cortactin and Abp1p. Despite their diversity, each activator interacts with the Arp2/3 complex through a conserved sequence, the acidic “A” domain (Machesky and Insall, 1998). However, the mechanism of stimulation of the Arp2/3 complex differs from some of the activators. All members of the WASP family and ActA appear to require the monomeric actin-binding region (WASP homology [WH]2) (Higgs et al., 1999; Machesky et al., 1999; Rohatgi et al., 1999) (Fig. 1) . However, fungal myosin I's, lack this sequence, but may interact with actin monomer binding proteins such as verprolin or a yeast WASP homologue (Evangelista et al., 2000; Lechler et al., 2000; Lee et al., 2000). Abp1p and cortactin represent a novelty in the mechanism of activation of the Arp2/3 complex, in that they both require a filamentous actin-binding region that consists of an actin depolymerizing factor/cofilin homology domain, or a six to seven–tandem repeat fragment, respectively. Thus, actin monomer binding is not a strict requirement for Arp2/3 complex activation.
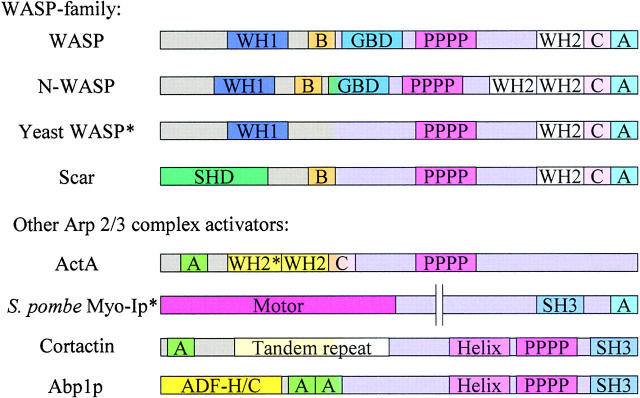
Figure 1.
Schematic representation of the sequences of the different Arp2/3 complex activators: WASP, N-WASP, yeast WASP homologues (S. pombe Wsp1 and S. cerevisiae Las17p/Bee1p), Scar/WAVE, ActA, yeast Myosin-I homologue S. pombe Myo1p, Abp1p, and cortactin. All activators contain the A (acidic) sequence to bind to the Arp2/3 complex. The WASP family proteins and ActA all contain one or two WH2 motifs that bind to actin monomer. Myosin I may recruit another molecule that binds actin monomers (such as a WASP homologue). Abp1p and cortactin bind to actin filaments through the actin depolymerizing factor (ADF)-H/C–cofilin homology region and the tandem repeat region, respectively. B, basic; GBD, GTPase binding domain; PPPP, proline-rich region; C, central basic region; SHD, Scar homology domain; WH2*, weaker homology to WASP. Although we have not drawn a basic sequence in yeast WASP, Las17p/Bee1p contains a cluster of basic residues in this region, whereas Wsp1p does not appear to.
Abp1p has been implicated in endocytic processes, as it has a redundant function with Sla2p in endocytic trafficking and it interacts with other proteins that function in endocytosis, such as Rvs167p/amphiphysin. Abp1p binds to Rvs167p via the proline-rich region of Abp1p binding to the Src homology (SH)3 domain of Rvs167p (Fig. 1). Abp1p also has an SH3 domain at its COOH terminus (Fig. 1). Goode et al. (2001) show that the ability of Abp1p to bind to the Arp2/3 complex and to actin filaments is also relevant in vivo. A point mutation in Abp1p that makes it unable to activate the Arp2/3 complex acts synergistically with a mutation in fimbrin, an actin filament cross-linking protein. Fimbrin has been connected with endocytosis (Kubler and Riezman, 1993) and although current evidence is quite preliminary, fimbrin and Abp1p may both have roles in linking actin and endocytosis. It is not clear yet whether the mammalian homologue of Abp1p, Abp1, has a similar role. In mammalian cells, Abp1 localizes to puncta in the perinuclear region, and upon activation of the GTPase Rac it translocates to the leading edge of the cell with the Arp2/3 complex (Kessels et al., 2000). Although the mammalian Abp1 lacks the Arp2/3 complex binding domain A, it may use a partner that could bind to the Arp2/3 complex. For example, Abp1 binds to dynamin, a GTPase implicated in endocytic trafficking (Kessels et al., 2000), which in turn could form a complex with WASP (Qualmann et al., 1999).
Cortactin has no direct homologue in yeast. In mammals it is overexpressed in several cancers (Wu et al., 1991; Schuuring et al., 1993), and it is localized in invasive structures that can degrade the extracellular matrix (Bowden et al., 1999). Cortactin has been linked to cell–matrix contacts through the Hax-1 and the polycystic kidney disease protein PDK2 (Gallagher et al., 2000). It is a substrate of nonreceptor tyrosine kinases, such as Src, Syk, and Fer (Wu et al., 1991; Maruyama et al., 1996; Gallet et al., 1999), and when it is tyrosine phosphorylated by Src it exhibits reduced actin filament cross-linking ability (Huang et al., 1997). Like Abp1, cortactin localizes to the leading edge of the cell in lamellipodia, and in other punctate structures rich in actin (Wu et al., 1991; Huang et al., 1998). It is also involved in endocytic processes, colocalizing with actin and the Arp2/3 complex on endosomal vesicles (Kaksonen et al., 2000). This colocalization may be by direct binding to the Arp2/3 complex, and/or through its COOH-terminal SH3 domain which can bind to dynamin (McNiven et al., 2000) (Fig. 1). Thus, cortactin may serve as a scaffold protein in various large protein complexes used to regulate adhesion to the extracellular matrix, actin assembly, and/or endocytosis.
Both Abp1p and cortactin increase the affinity of the Arp2/3 complex for actin filaments. For Abp1p, the increase is as much as 30-fold. Although it has not yet been determined whether cortactin increases the affinity of the Arp2/3 complex for actin filaments, cortactin binds to filaments with high affinity, giving it the potential to recruit the Arp2/3 complex and promote branching.
This ability to link the Arp2/3 complex and actin filaments enables Abp1p and cortactin to activate the Arp2/3 complex. Abp1p induces actin nucleation through the Arp2/3 complex with the same efficiency as the Listeria monocytogenes protein, ActA, and uses similar sequences to WASP family proteins (A and C domains, Fig. 1) to bind to and activate the Arp2/3 complex. Cortactin modestly activates the Arp2/3 complex–mediated actin nucleation, ∼2–3-fold, whereas ActA or WASP can activate it >10-fold. Together, cortactin and WASP show an ∼20-fold increase in activation of the Arp2/3 complex, thus having a slight synergistic effect. Both the actin filament and the Arp2/3 complex binding domains of cortactin are required for the Arp2/3 complex activation.
Goode et al. (2001), Uruno et al. (2001), and Weaver et al. (2001) indicate that Abp1p and cortactin activate the Arp2/3 complex by stabilizing its interaction with actin filaments, acting as “hand-holds” for branching. One could imagine that Abp1p or cortactin could displace WASP family proteins by binding to the Arp2/3 complex, thus promoting stability of filament branches. Alternatively, WASP family proteins, Abp1p, or cortactin could be used in separate situations in which different types of actin networks are required (Fig. 2) . It is likely that WASP family proteins activate the Arp2/3 complex by a “hit and run” mechanism (Higgs and Pollard, 1999), requiring only transient interactions, whereas Abp1p binds more tightly to the complex. However, it is unclear whether Abp1p is localized purely via binding to the Arp2/3 complex, or whether other signals such as activated small GTPases could also direct it to the plasma membrane or to sites of actin assembly. The mechanism of cortactin localization to the membrane is also unknown. Notably, cortactin can only bind to actin filaments every 15 actin monomer subunits, thus inducing branch formation separated by a characteristic distance. With or without WASP, cortactin stabilizes actin branches and protects them from debranching. The importance of branching near the plasma membrane seems clear, but the role in endocytic trafficking is less obvious.
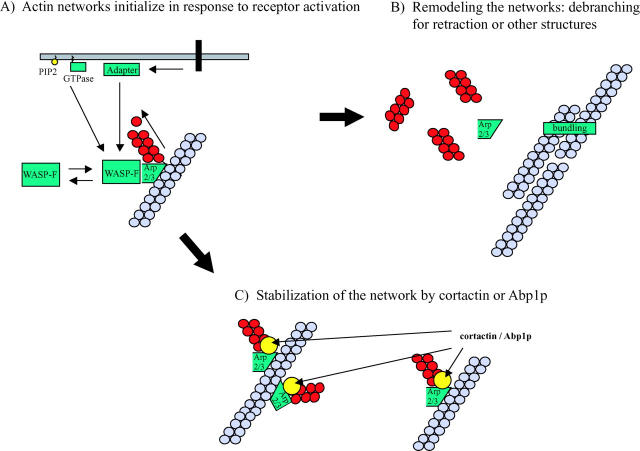
Figure 2.
Model depicting possible role of cortactin and Abp1p in the stabilization of actin networks during cell motility or membrane trafficking. (A) Events at the plasma membrane including receptor activation, small GTPase activation, and interaction with acidic phospholipids such as phosphatidylinositol 4,5-bisphosphate (PIP2) are thought to bring about the activation of WASP family proteins. Once active, a WASP family protein transiently binds to and activates the Arp2/3 complex. The Arp2/3 complex becomes stably incorporated into an actin filament branch, which elongates until capping protein blocks the polymerization at the barbed end. (B) For networks that will be remodelled, debranching may facilitate the formation of parallel filament bundles (by α-actinin, fascin, or another bundling protein) or depolymerization of filaments during filopodia formation or retraction of new pseudopodia. (C) If a branched network is to be maintained longer term, the activities of cortactin and Abp1p may be to stabilize branches and provide additional rigidity to the network.
As cortactin is overexpressed in many cancers (Schuuring et al., 1993), it is striking that the overexpression of WASP, Scar, Abp1p, and cortactin can all cause defects in actin organization that lead to malfunction of the cells (Drubin et al., 1988; Machesky and Insall, 1998; Uruno et al., 2001). It is unclear whether this is a general characteristic of all Arp2/3 complex activators, but it is possible that overexpression of these proteins may cause delocalization of the Arp2/3 complex, resulting in disruption of actin morphology. It would be interesting to determine whether the delocalization of the Arp2/3 complex is involved in the progression of tumors or in the formation of the invasive structures where cortactin localizes.
Because dendritic nucleation in lamellipodia is an active process in which actin filaments must be assembling and disassembling quickly, branch stabilization by cortactin must be regulated. Perhaps tyrosine phosphorylation by Src could downregulate cortactin's branch stabilizing activity in order to promote rapid turnover of actin networks and rapid motility. On the other hand, tyrosine kinase receptor activation leading to cell motility or phagocytosis by the Fc receptor is generally thought to promote the formation of branched networks in lamellipodia; thus it is unclear how cortactin may be regulated in this situation.
Future experiments might also address whether Abp1p and cortactin function to activate the Arp2/3 complex by themselves or always in concert with a WASP family protein, and whether these activities lead to the assembly of specific actin structures. Clearly, cortactin and Abp could stabilize actin branches and alter the structure, rigidity, or persistence of filament networks (Fig. 2). This could be extremely useful when the cell is forming specialized actin structures. For example, filopodia do not contain visibly branched actin filament networks, yet Cdc42 activation of N-WASP is thought to be connected to filopodia formation (for review see Mullins, 2000). Either this idea is wrong, or actin structures formed by N-WASP and the Arp2/3 complex are modulated to minimize branching and favor tightly bundled parallel filaments. For example, downregulation of cortactin in filopodia could lead to destabilization of branches, and perhaps allow other cross-linking proteins to dominate.
It seems clear that the Arp2/3 complex functions in large multimolecular complexes. New activating and modulatory proteins will no doubt continue to be found at a considerable rate. At present, our understanding of the Arp2/3 complex is quite simplistic and depends almost completely on reconstituted systems with at most a few purified proteins present. Filling in the gaps in our understanding of different actin assembly processes and actin structures in cells promises to keep the field busy for a while. Cortactin and Abp1p are examples of the exciting connections between processes such as membrane trafficking, adhesion, and actin assembly that we are just beginning to understand.
Footnotes
Abbreviations used in this paper: SH, Src homology; WASP, Wiskott-Aldrich Syndrome protein; WH, WASP homology.
References
- Bowden, E.T., M. Barth, D. Thomas, R.I. Glazer, and S.C. Mueller. 1999. An invasion-related complex of cortactin, paxillin and PKCmu associates with invadopodia at sites of extracellular matrix degradation. Oncogene. 18:4440–4449. [DOI] [PubMed] [Google Scholar]
- Drubin, D.G., K.G. Miller, and D. Botstein. 1988. Yeast actin-binding proteins: evidence for a role in morphogenesis. J. Cell Biol. 107:2551–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista, M., B.M. Klebl, A.H. Tong, B.A. Webb, T. Leeuw, E. Leberer, M. Whiteway, D.Y. Thomas, and C. Boone. 2000. A role for myosin-I in actin assembly through interactions with Vrp1p, Bee1p, and the Arp2/3 complex. J. Cell Biol. 148:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher, A.R., A. Cedzich, N. Gretz, S. Somlo, and R. Witzgall. 2000. The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc. Natl. Acad. Sci. USA. 97:4017–4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet, C., M.P. Rosa, A. Habib, M. Lebret, S. Levy-Toledano, and J. Maclouf. 1999. Tyrosine phosphorylation of cortactin associated with Syk accompanies thromboxane analogue-induced platelet shape change. J. Biol. Chem. 274:23610–23616. [DOI] [PubMed] [Google Scholar]
- Goode, B.L., A.A. Rodal, G. Barnes, and D.G. Drubin. 2001. Activation of the Arp2/3 complex by the actin filament binding protein Abp1p. J. Cell Biol. 153:627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgs, H.N., and T.D. Pollard. 1999. Regulation of actin polymerization by Arp2/3 complex and WASP/Scar proteins. J. Biol. Chem. 274:32531–32534. [DOI] [PubMed] [Google Scholar]
- Higgs, H.N., L. Blanchoin, and T.D. Pollard. 1999. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry. 38:15212–15222. [DOI] [PubMed] [Google Scholar]
- Huang, C., Y. Ni, T. Wang, Y. Gao, C.C. Haudenschild, and X. Zhan. 1997. Down-regulation of the filamentous actin cross-linking activity of cortactin by Src-mediated tyrosine phosphorylation. J. Biol. Chem. 272:13911–13915. [DOI] [PubMed] [Google Scholar]
- Huang, C., J. Liu, C.C. Haudenschild, and X. Zhan. 1998. The role of tyrosine phosphorylation of cortactin in the locomotion of endothelial cells. J. Biol. Chem. 273:25770–25776. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., H.B. Peng, and H. Rauvala. 2000. Association of cortactin with dynamic actin in lamellipodia and on endosomal vesicles. J. Cell Sci. 24:4421–4426. [DOI] [PubMed] [Google Scholar]
- Kessels, M.M., A.E. Engqvist-Goldstein, and D.G. Drubin. 2000. Association of mouse actin-binding protein 1 (mAbp1/SH3P7), a Src kinase target, with dynamic regions of the cortical actin cytoskeleton in response to Rac1 activation. Mol. Biol. Cell. 11:393–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler, E., and H. Riezman. 1993. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 12:2855–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler, T., A. Shevchenko, and R. Li. 2000. Direct involvement of yeast type I myosins in Cdc42-dependent actin polymerization. J. Cell Biol. 148:363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, W.L., M. Bezanilla, and T.D. Pollard. 2000. Fission yeast myosin-I, Myo1p, stimulates actin assembly by Arp2/3 complex and shares functions with WASp. J. Cell Biol. 151:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky, L.M., and R.H. Insall. 1998. Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex. Curr. Biol. 8:1347–1356. [DOI] [PubMed] [Google Scholar]
- Machesky, L.M., R.D. Mullins, H.N. Higgs, D.A. Kaiser, L. Blanchoin, R.C. May, M.E. Hall, and T.D. Pollard. 1999. Proc Natl. Acad. Sci. USA. 96:3739–3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, S., T. Kurosaki, K. Sada, Y. Yamanashi, T. Yamamoto, and H. Yamamura. 1996. Physical and functional association of cortactin with Syk in human leukemic cell line K562. J. Biol. Chem. 271:6631–6635. [DOI] [PubMed] [Google Scholar]
- May, R.C. 2001. The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell. Mol. Life. Sci. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven, M.A., L. Kim, E.W. Krueger, J.D. Orth, H. Cao, and T.W. Wong. 2000. Regulated interactions between dynamin and the actin-binding protein cortactin modulate cell shape. J. Cell Biol. 151:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins, R.D. 2000. How WASp family proteins and the Arp2/3 complex convert intracellular signals into cytoskeletal structures. Curr. Opin. Cell Biol. 12:91–96. [DOI] [PubMed] [Google Scholar]
- Mullins, R.D., J.A. Heuser, and T.D. Pollard. 1998. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. USA. 95:6181–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann, B., J. Roos, P.J. DiGregorio, and R.B. Kelly. 1999. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol. Biol. Cell. 10:501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, and M.W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell. 97:221–231. [DOI] [PubMed] [Google Scholar]
- Schuuring, E., E. Verhoeven, S. Litvinov, and R.J. Michalides. 1993. The product of the EMS1 gene, amplified and overexpressed in human carcinomas, is homologous to a v-src substrate and is located in cell-substratum contact sites. Mol. Cell. Biol. 13:2891–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T.M., and G.G. Borisy. 1999. Progress in protrusion: the tell-tale scar. Trends Biochem. Sci. 24:432–436. [DOI] [PubMed] [Google Scholar]
- Uruno, T., J. Liu, P. Zhang, Yx. Fan, C. Egile, R. Li, S.C. Mueller, and X. Zhan. 2001. Activation of Arp2/3 complex-mediated actin polymerization by cortactin. Nat. Cell Biol. 3:259–266. [DOI] [PubMed] [Google Scholar]
- Weaver, A.M., A.V. Karginov, A.W. Kinley, S.A. Weed, Y. Li, J.T. Parsons, and J.A. Cooper. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr. Biol. 11:370–374. [DOI] [PubMed] [Google Scholar]
- Welch, M.D., J. Rosenblatt, J. Skoble, D.A. Portnoy, and T.J. Mitchison. 1998. Interaction of human Arp2/3 complex and the Listeria monocytogenes ActA protein in actin filament nucleation. Science. 281:105–108. [DOI] [PubMed] [Google Scholar]
- Wu, H., A.B. Reynolds, S.B. Kanner, R.R. Vines, and J.T. Parsons. 1991. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol. Cell. Biol. 11:5113–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]