Abstract
Another giant protein has been detected in cross-striated muscle cells. Given the name obscurin, it was discovered in a yeast two-hybrid screen in which the bait was a small region of titin that is localized near the Z-band. Obscurin is about 720 kD, similar in molecular weight to nebulin, but present at about one tenth the level (Young et al., 2001). Like titin, obscurin contains multiple immunoglobulin-like domains linked in tandem, but in contrast to titin it contains just two fibronectin-like domains. It also contains sequences that suggest obscurin may have roles in signal transduction. During embryonic development, its localization changes from the Z-band to the M-band. With these intriguing properties, obscurin may not remain obscure for long.
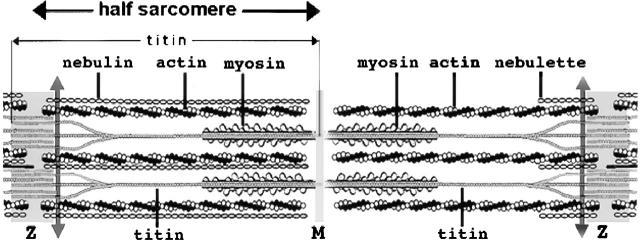
The basic unit that provides the structural framework for contraction of cross-striated muscles is the sarcomere (Fig. 1) . In order for the myosin thick filaments and the interdigitating actin filaments to interact optimally and generate force, many other proteins of the sarcomere are required. The two largest proteins in the sarcomere, nebulin and titin, help link the actin and myosin filaments in the sarcomere. The thin actin filaments are tethered in the Z-band, a dense substructure that is home to several known and unknown proteins and the site where the ends of titin filaments and nebulin molecules are also embedded. In vertebrate skeletal muscles (Fig. 1, left), 800 kD nebulin has its COOH terminus embedded in the Z-band. Two nebulin molecules extend along the length of each 1-μm long actin filament to form a thin filament (Labeit and Kolmerer, 1995a). Titin, the largest known protein at 3–3.7 million D has its NH2 terminus embedded in the Z-band and extends for 1 μm with its COOH terminus localized in the middle (M-band) of the aligned myosin filaments (A-band). Thus, each Z-band has two sets of overlapping NH2-terminal titin ends, and the M-band has two sets of overlapping COOH-terminal titin ends. The titin filaments are elastic and attach the thick filaments to the Z-bands (for review see Gregorio et al., 1999). Recent work by Liversage et al. (2001) using scanning transmission electron microscopy indicates that there may be just six titin filaments per half myosin filament, that is, each myosin filament binding to two sets of six overlapping oppositely polarized titin filaments.
Figure 1.
Diagram of a sarcomere bounded by the Z-bands. The left side of the sarcomere represents a half sarcomere found in vertebrate skeletal myofibrils. Note that the nebulin molecules are part of and extend the entire length of the thin filaments. The right side of the sarcomere reflects a half sarcomere in cardiac muscle cells. The smaller nebulin isoform, nebulette, begins within the Z-band and extends only a short distance along the thin filament. Titin is shown with its NH2 termini from adjacent sarcomeres overlapping in the Z-band. Groups of three titin filaments are shown aligned together in the half sarcomere and overlapping in the M-band with groups of three from the other half sarcomere. The scale of the drawing does not allow the ratio of six titins per half-thick filament or the two nebulin isoforms per actin thin filament to be illustrated. The double-headed arrows indicate the position of the region of titin used as a bait to pull out obscurin. The M-band is the mid-point of the group of aligned thick myosin filaments (A-band) where obscurin binds in cultured neonatal cardiomyocytes and in adult muscles.
Although both of these giant proteins, nebulin and titin, are 1-μm long, their molecular structures are quite different. Nebulin is composed of four domains (Labeit and Kolmerer, 1995a; Wang et al., 1996). A COOH-terminal Src homology (SH)*3 domain and a short linker domain are embedded in the Z-band. The third domain begins at the Z-band margin and is composed of >200 repeats (each ∼35 amino acids) that stretch along the actin filament. The fourth domain is a short acidic NH2-terminal region (84 amino acids) that binds near the pointed end of the actin filament. A smaller isoform of nebulin, nebulette (107 kD), replaces nebulin in the myofibrils in cardiac muscles (Fig. 1, right; Moncman and Wang, 1995, 1999; Millevoi et al., 1998). This isoform has only 22 of nebulin's 35 amino acid repeats, but there is extensive homology of the COOH- and NH2-terminal domains of nebulette and nebulin (Millevoi et al., 1998; Moncman and Wang, 1999). In contrast to nebulin isoforms, both titin (Labeit and Kolmerer, 1995b) and the new protein obscurin reported in this issue (Young et al., 2001) are composed of repeating domains of the Ig- and fibronectin (FN)3–like types. Titin has ∼166 copies of the Ig domain and 132 FN3 repeats (Labeit and Kolmerer, 1995b; Gregorio et al., 1999). Obscurin, with a calculated molecular weight of 720 kD, has 55 Ig domains and only 2 FN3 domains (Young et al., 2001). Each of these Ig and FN3 domains are ∼4-nm long (Liversage et al., 2001), giving obscurin an estimated length of ≥200 nm.
Binding partners of titin have been discovered previously with the yeast two-hybrid technique. The center of the Z-band region of titin, that is, the z-repeats, was found to bind the Z-band protein α-actinin (Young et al., 1998). Even one of these z-repeats, ∼40–50 amino acids each, can target a partial titin construct to the Z-band (Ayoob et al., 2000). In another hunt, bait constructed from a small part of the NH2-terminal region of titin was found to bind to a protein that was named T-CAP (19 kD) (Gregorio et al., 1998). Subsequently, sequencing of the Z-band protein telethonin showed that T-CAP and telethonin were the same protein (Mues et al., 1998).
The kinase domain of titin, located in the M-band, can phosphorylate telethonin in muscle extracts (Mayans et al., 1998), but it is not known if this phosphorylation occurs in vivo before telethonin is incorporated into the Z-band. Overexpression of either the kinase domain of titin (Mayans et al., 1998) or the NH2-terminal region of titin that binds telethonin (Gregorio et al., 1998) leads to the loss of myofibrils in transfected muscle cells. Telethonin/T-CAP has taken on added importance due to its causal role in limb-girdle muscular dystrophy type 2G (Moreira et al., 2000). Another titin-binding protein, the p94 skeletal muscle-specific calpain (a protease), when mutated, causes limb-girdle muscular dystrophy type 2A (for review see Sorimachi et al., 2000).
In this issue, Young et al. (2001) chose a small region of titin that localizes just outside the Z-band as a yeast two-hybrid bait and found a novel binding partner. The detected partner, named obscurin, is an intriguing molecule. It has a calculated molecular weight of 720 kD. The protein is present at <10% of the level of nebulin. Since nebulin is ∼2–3% of the protein content of the myofibrils, obscurin would account for <0.3% of the myofibrillar proteins. In each half sarcomere of vertebrate skeletal muscles, there are two actin filaments, four nebulin molecules, and six titin filaments for each myosin half filament (for review see Sanger et al., 2000). Thus, for every one obscurin molecule in a half sarcomere, there could be at most 5 actin filaments, 10 nebulin molecules, 15 titins and 2.5 myosin half filaments. Like titin, obscurin has an impressive length of tandem Ig domains extending from its NH2 terminus. The COOH terminus has a more varied and interesting composition with several signaling domains: one IQ domain (binding motif for calmodulin-like proteins), one SH3 domain (mediating protein–protein interactions), one Dbl homology/RhoGTPase nucleotide exchange factor domain followed by the usually associated pleckstrin homology domain. It is not known yet if these domains in the COOH terminus of obscurin are involved in any signal transduction pathways. Obscurin, with a calculated length of ≥200 nm (Young et al., 2001), could cross-link myosin filaments and/or mediate signaling.
There are some puzzling and intriguing immunofluorescent localization results for obscurin (Young et al., 2001). Antibodies generated against four different regions of the molecule stained the M-bands of cultured neonatal rat cardiomyocytes. One of the antibodies (directed against the titin-binding region of obscurin, designated as α-Ob19/20) also yielded weak staining of Z-bands of these cardiomyocytes. The M-band localization is a surprise, since obscurin was identified when it bound a region of titin that localizes within the sarcomere at a site far from the M-band. Transfection of the neonatal cardiomyocytes with just the titin-binding region of obscurin revealed the expected Z-band localization. Staining of cross-striated muscle tissues in different stages of development with the α-Ob19/20 antibody indicated that in early embryonic stages obscurin was localized in the Z-band. However, in older embryos the staining with this antibody became concentrated in the M-band. Antibodies directed against the region containing the IQ domain and one against the Dbl homology/RhoGTPase nucleotide exchange factor domain did not stain the myofibrils until late in development when they bound to the M-band. The staining results in the cultured rat neonatal cardiomyocytes reported by Young et al. (2001) correspond to the staining in later stages of development and in adult animals where all of the antibodies stain the M-bands. It would be interesting to see these antibodies used on cultured embryonic chick cardiomyocytes where new myofibrils are assembled within hours (Dabiri et al., 1997), especially after cell divisions (Sanger and Sanger, 2001). It will also be interesting to determine which M-band proteins bind obscurin and how the binding is regulated during myofibril maturation.
Earlier this year, Centner et al. (2001) used the titin M-band serine-threonine kinase domain (the domain that phosphorylated telethonin in muscle extracts [Mayans et al., 1998]) as bait in a two-hybrid screen. The surprise catch was a muscle isoform of a ring finger protein, MURF-1 (38 kD). Ring finger proteins have a two zinc binding cysteine-histidine motif and are involved in several cellular processes including signal transduction, ubiquitination, and morphogenesis (Jackson et al., 2000; Spencer et al., 2000). In turn, MURF-1 was used as a bait and a MURF-2 was found. When MURF-2 was used as a bait, a third isoform was discovered, MURF-3. MURFs 1 and 3 are muscle specific isoforms. MURF-3 (41 kD) is identical to the MURF that was first discovered by Spencer et al. (2000) and had been shown to localize to the surprising combination of Z-bands and microtubules (Spencer et al., 2000). Thus, the MURFs may integrate signal pathways that are mediated by proteins in the sarcomere and on microtubules.
Centner et al. (2001) demonstrated that MURF-2 does not localize to the Z-band but is soluble in the cytoplasm of fetal hearts. Antibodies to MURF-1 reveal results that are puzzling. MURF-1 staining was concentrated at the M-band only in isolated adult rat heart myofibrils: a region expected of a protein that was selected using a bait encoding a region of titin's M-band domain. However, in sectioned adult rat heart and skeletal muscles (psoas) MURF-1 antibodies stained either the Z-bands alone or a combination of Z- and M-bands. Do MURF-1 proteins also move from one area of the sarcomere as obscurin may? What proteins bind MURF-1 and MURF-3 to Z-bands? We now have two muscle proteins that have been identified by binding to defined regions of titin in yeast two-hybrid screens but which localize to an unexpected region of the sarcomere: obscurin and MURF-1.
The Z-band appears to be a home to an increasing number of molecules involved in focal adhesions and signal transduction (Brugge, 1998; Honda et al., 1998). Three recently described Z-band proteins owe their localization to the Z-band by virtue of binding to α-actinin, a titin-binding protein. The first two proteins are (1) ALP (actinin-associated LIM protein; 39 kD; Xia et al., 1997) and (2) cypher1 (77 kD; mouse isoforms; Zhou et al., 1999) or ZASP (Z-band alternatively spliced PDZ motif protein; 78 kD human isoforms of cypher1; Faulkner et al., 1999). ALP has one LIM domain, whereas cypher1/ZASP has three. LIM domain proteins can be in the nucleus and in the cytoplasm where they are thought to be involved in actin filament organization (Zhou et al., 1999). These two Z-band LIM proteins also each contain one PDZ domain, a module involved in protein–protein interactions (Faulkner et al., 1999). The third new protein, myotilin (57 kD), interacts via residues near its NH2 terminus with the spectrin repeats of α-actinin (Salmikangas et al., 1999; Hauser et al., 2000). The COOH-terminal part of myotilin contains two Ig-like domains that have the highest homology to titin's Z7-Z8 (Ig) domains. A missense mutation in the myotilin gene is found in an autosomal dominant form of limb-girdle muscular dystrophy 1A (Hauser et al., 2000). Nevertheless, the mutated myotilin protein (T57I) binds α-actinin and localizes to the Z-bands of the affected muscles. The cause of the Z-band abnormalities characteristic of this disorder may be a function of altered interactions of the mutant myotilin with other Z-band proteins.
Two other interesting proteins reported to be associated with the Z-bands of muscle are neuroendocrine-specific protein–like (Nspl)1 (22.2 kD; Geisler et al., 1999) and Arg-binding protein (ArgBP)2 (71 kD; Wang et al., 1997). Nspl1 binds to the intermediate filaments in myoblasts and becomes localized to the Z-bands of mature myofibrils (Geisler et al., 1999). ArgBP2 contains three SH3 domains, has a region rich with serines and threonines (a possible target for protein kinases), and associates with and is phosphorylated by Arg and c-Abl, members of the Abelson family of tyrosine kinases. ArgBP2 is an excellent candidate for involvement in signal transduction cascades. The transcript for ArgBP2 is 50–200-fold higher in the heart than in other tissues including skeletal muscle cells.
It will be important to determine how the Z-band constituents of cardiac and skeletal muscle cells differ from each other and what effect these differences have on myofibrillar functions. For example, p130cas, an adaptor molecule that binds several focal adhesion signaling proteins directly and indirectly (Brugge, 1998), is preferentially expressed in mouse cardiac muscle Z-bands (Honda et al., 1998). Embryonic mice lacking p130cas die in utero at a stage when the normal developing heart is beating (11.5–12.5 d post coitum). In the p130cas mutants, the cardiac myofibrils but not the skeletal myofibrils are disorganized and their Z-bands disrupted (Honda et al., 1998). These observations indicate an important role for the p130cas in cardiac myofibrillogenesis.
Muscle proteins have been studied for more than a century. The contribution by Young et al. (2001) indicates that we are in an exciting and challenging era, as we continue the search to sort out the molecules and signaling pathways that cardiac and skeletal muscle cells use to assemble and maintain their myofibrils.
( of ) Fishing out proteins that bind to titin | Sanger and Sanger
Acknowledgments
The muscle work in our laboratory is supported by grants from the National Institutes of Health, Muscular Dystrophy Association, and the American Heart Association.
Footnotes
Abbreviations used in this paper: ArgBP, argose-binding protein; FN, fibronectin; MURF, muscle isoform of a ring finger; Nspl, neuorendocrine-specific protein–like; SH, Src homology.
References
- Ayoob, J.C., K.K. Turnacioglu, B. Mittal, J.M. Sanger, and J.W. Sanger. 2000. Targeting of cardiac muscle titin fragments to the Z-bands and dense bodies of living muscle and non-muscle cells. Cell Motil. Cytoskel. 45:67–82. [DOI] [PubMed] [Google Scholar]
- Brugge, J.S. 1998. Casting light on focal adhesions. Nat. Genet. 19:309–311. [DOI] [PubMed] [Google Scholar]
- Centner, T., J. Yano, E. Kimura, A.S. McElhinny, K. Pelin, C.C. Witt, M.-L. Bang, K. Trombitas, H. Granzier, C.C. Gregorio, et al. 2001. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domains. J. Mol. Biol. 306:717–726. [DOI] [PubMed] [Google Scholar]
- Dabiri, G.A., K.K. Turnacioglu, J.M. Sanger, and J.W. Sanger. 1997. Myofibrillogenesis in living embryonic cardiac cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 94:9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner, G., A. Palavicini, E. Formentin, A. Comelli, C. Ievolella, S. Trevisan, G. Bortoletto, P. Scannapieco, M. Salamon, V. Mouly, et al. 1999. ZASP; a new Z-band alternatively spliced PDZ-motif protein. J. Cell Biol. 146:465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, J.G., R.J. Palmer, L.J. Stubbs, and M.L. Mucenski. 1999. Nspl1, a new Z-band-associated protein. J. Mus. Res. Cell Motil. 20:661–668. [DOI] [PubMed] [Google Scholar]
- Gregorio, C.C., H. Granzier, H. Sorimachi, and S. Labeit. 1999. Muscle assembly: a titanic achievement? Curr. Opin. Cell Biol. 11:18–25. [DOI] [PubMed] [Google Scholar]
- Gregorio, C.C., K. Trombitas, T. Centner, B. Kolmerer, G. Stier, K. Kunke, K. Suzuki, F. Obermayr, H. Granzier, H. Sorimachi, et al. 1998. The NH2 terminus of titin spans the Z-disc: its interaction with a novel 19-kD ligand (T-cap) is required for sarcomeric integrity. J. Cell Biol. 143:1013–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser, M.A., S.K. Horrigan, P. Salmikangas, U.M. Torian, K.D. Viles, R. Dancel, R.W. Tim, A. Taivainen, L. Bartoloni, J.M. Gilchrist, et al. 2000. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum. Mol. Genet. 9:2141–2147. [DOI] [PubMed] [Google Scholar]
- Honda, H., H. Oda, T. Nakamoto, Z.-i. Honda, R. Sakai, T. Suzuki, T. Saito, K. Nakamura, K. Nakao, T. Ishikawa, et al. 1998. Cardiovascular anomaly, impaired actin bundling and resistance to src-induced transformation in mice lacking p130Cas. Nat. Genet. 19:361–366. [DOI] [PubMed] [Google Scholar]
- Jackson, P.K., A.G. Eldridge, E. Freed, L. Furstenthal, J.Y. Hsu, B.K. Kaiser, and J.D.R. Reiman. 2000. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10:429–439. [DOI] [PubMed] [Google Scholar]
- Labeit, S., and B. Kolmerer. 1995. a. The complete primary structure of human nebulin and its correlation to muscle structure. J. Mol. Biol. 248:308–315. [DOI] [PubMed] [Google Scholar]
- Labeit, S., and B. Kolmerer. 1995. b. Titins, giant proteins in charge of muscle ultrastructure and elasticity. Science. 270:293–296. [DOI] [PubMed] [Google Scholar]
- Liversage, A.D., D. Holmes, P.J. Knight, L. Tskhovrebova, and J. Trinick. 2001. Titin and the sarcomere symmetry paradox. J. Mol. Biol. 305:401–409. [DOI] [PubMed] [Google Scholar]
- Mayans, O., P.F.M. van der Ven, M. Wilm, A. Mues, P. Young, D.O. Fuerst, M. Wilmanns, and M. Gautel. 1998. Structural basis for activation of the titin kinase domain during myofibrillogenesis. Nature. 395:863–869. [DOI] [PubMed] [Google Scholar]
- Millevoi, S., K. Trombitas, B. Kolmerer, S. Kostin, J. Schaper, K. Pelin, H. Granzier, and S. Labeit. 1998. Characterization of nebulette and nebulin and emerging concepts of their roles for vertebrate Z-discs. J. Mol. Biol. 282:111–123. [DOI] [PubMed] [Google Scholar]
- Moncman, C.L., and K. Wang. 1995. Nebulette; a 107 kD nebulin-like protein in cardiac muscle. Cell Motil. Cytoskel. 32:205–225. [DOI] [PubMed] [Google Scholar]
- Moncman, C.L., and K. Wang. 1999. Functional dissection of nebulette demonstrates actin binding of nebulin-like repeats and Z-line targeting of SH3 and linker domains. Cell Motil. Cytoskel. 44:1–22. [DOI] [PubMed] [Google Scholar]
- Moreira, E.S., T.J. Wiltshire, G. Faulkner, G. Nilforoushan, M. Vainzof, O.T. Suzuki, G. Valle, R. Reeves, M. Zatz, M.R. Passos-Bueno, et al. 2000. Limb-girdle muscular dystrophy type 2G is caused by mutations in the gene encoding the sarcomeric protein telethonin. Nat. Genet. 24:163–166. [DOI] [PubMed] [Google Scholar]
- Mues, A., P.F.M. van der Ven, P. Young, D.O. Furst, and M. Gautel. 1998. Two immunoglobulin-like domains of the Z-disc portion of titin interact in a conformational-dependent way with telethonin. FEBS Lett. 428:111–114. [DOI] [PubMed] [Google Scholar]
- Salmikangas, P., O.M. Mykkanen, M. Gronholm, L. Heiska, and O. Carpen. 1999. Myotilin, a novel sarcomeric protein with two Ig-like domains, is encoded by a candidate gene for two limb-girdle muscular dystrophy. Hum. Mol. Genet. 8:1329–1336. [DOI] [PubMed] [Google Scholar]
- Sanger, J.W., and J.M. Sanger. 2001. Green fluorescent proteins improve myofibril research. Biophotonics Int. 8:44–46. [Google Scholar]
- Sanger, J.W., J.C. Ayoob, P. Chowrashi, D. Zurawski, and J.M. Sanger. 2000. Assembly of myofibrils in cardiac muscle cells. Adv. Exp. Med. Biol. 481:89–102. [DOI] [PubMed] [Google Scholar]
- Sorimachi, H., Y. Ono, and K. Suzuki. 2000. Skeletal muscle-specific calpain, p94, and connectin/titin: their physiological functions and relationship to Limb-girdle muscular dystrophy type 2A. Adv. Exp. Med. Biol. 481:383–397. [DOI] [PubMed] [Google Scholar]
- Spencer, J.A., S. Eliazer, R.L. Ilaria, J.A. Richardson, and E.N. Olson. 2000. Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. J. Cell Biol. 150:771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, H., S.T. Winokur, W.-L. Kuo, M.R. Altherr, and D.S. Bredt. 1997. Actinin-associated LIM protein: identification of a domain interaction between PDZ and spectrin-like repeat motifs. J. Cell Biol. 139:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P., C. Ferguson, S. Banuelos, and M. Gautel. 1998. Molecular structure of the sarcomeric Z-disk: two types of titin interactions lead to an asymmetrical sorting of alpha-actinin. EMBO J. 17:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, P., E. Ehler, and M. Gautel. 2001. Obscurin, a giant sarcomeric Rho-GEF protein involved in sarcomere assembly. J. Cell Biol. 154:123–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B., E.A. Golemis, and G.D. Kruh. 1997. ArgBP2, a multiple Src homology 3 domain-containing, Arg/Abl-interacting protein is phosphorylated in v-Abl-transformed cells and localized in stress fibers and cardiocyte Z-disks. J. Biol. Chem. 272:17542–17550. [DOI] [PubMed] [Google Scholar]
- Wang, K., M. Knipfer, Q.-Q. Huang, A. van Heerden, L.C.-L. Hsu, G. Guitierrez, X.-L. Quian, and H. Stedtman. 1996. Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. J. Biol. Chem. 271:4304–4314. [DOI] [PubMed] [Google Scholar]
- Zhou, Q., P. Ruiz-Lozano, M.E. Martone, and J. Chen. 1999. Cypher, a striated muscle-restricted PDZ and LIM domain-containing protein, binds to alpha-actinin-2 and protein kinase C. J. Biol. Chem. 274:19807–19813. [DOI] [PubMed] [Google Scholar]