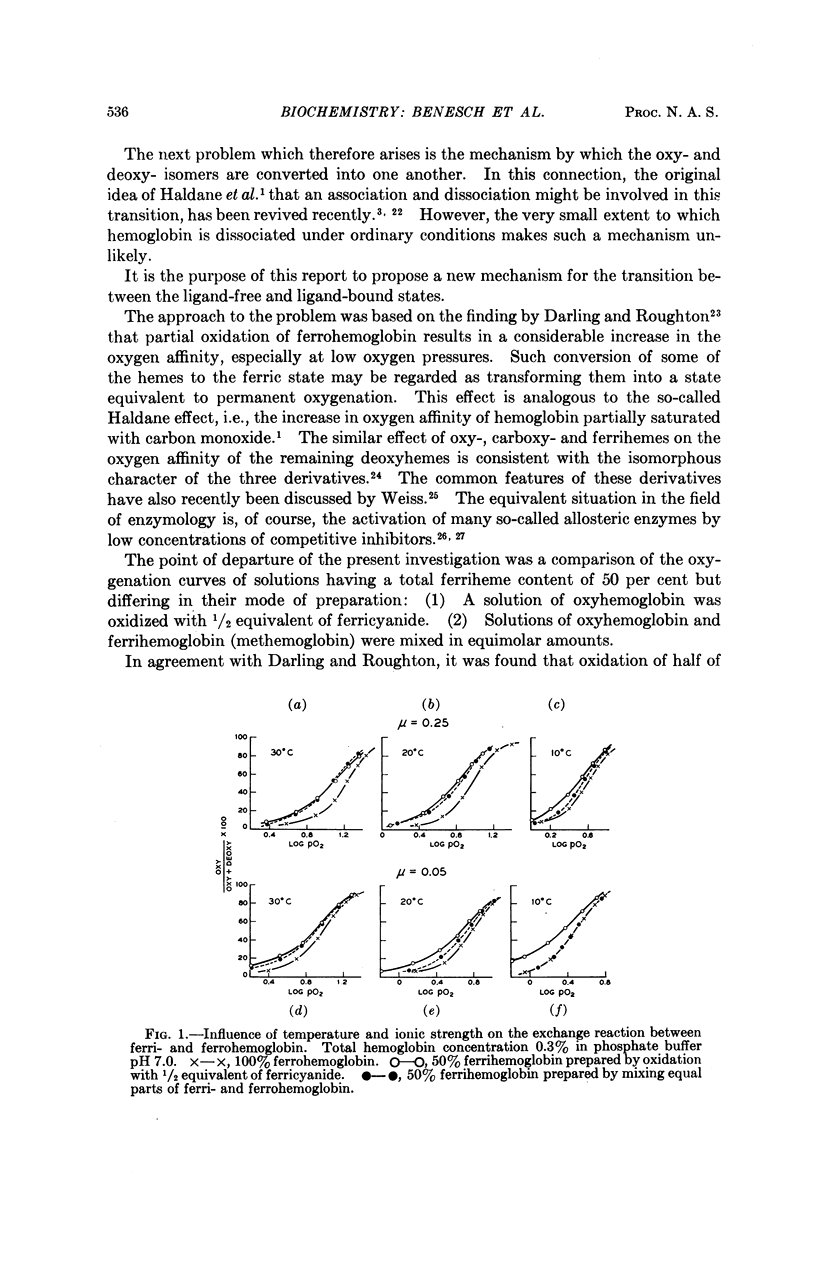
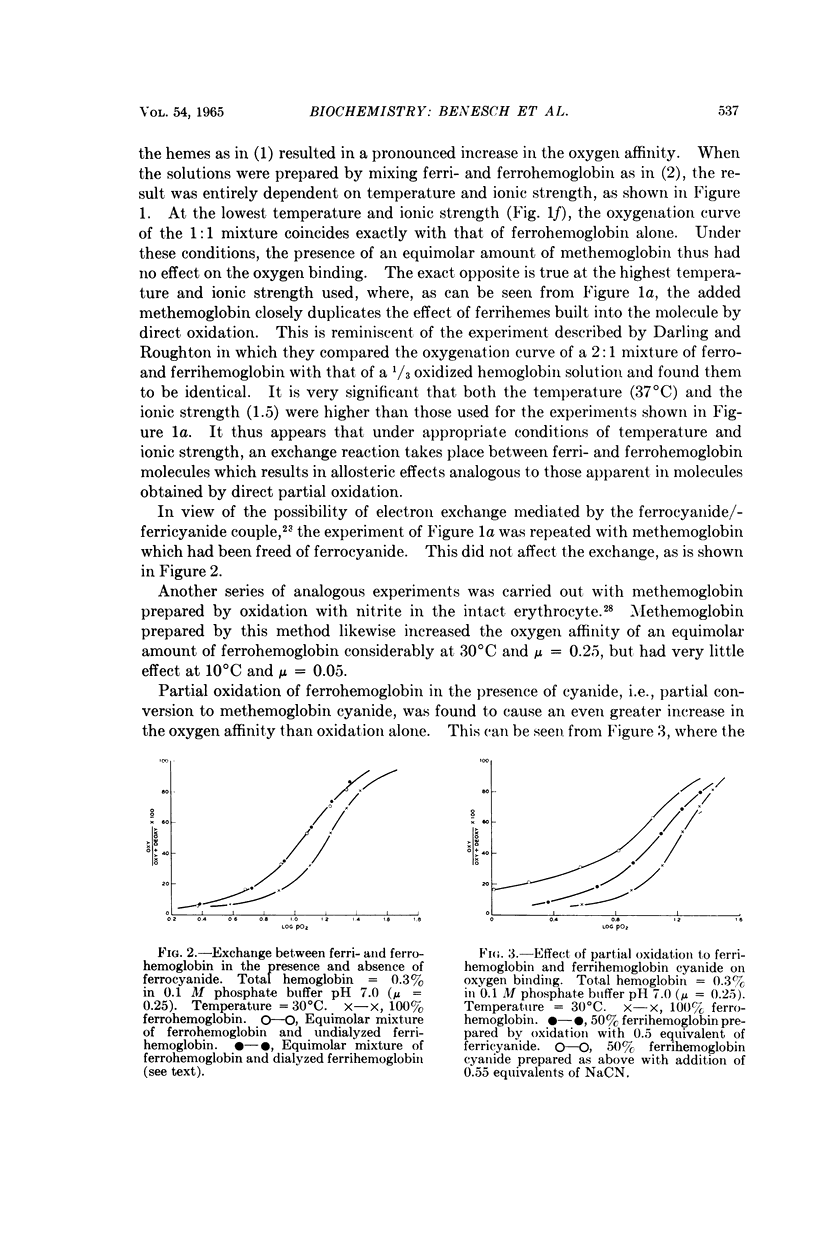
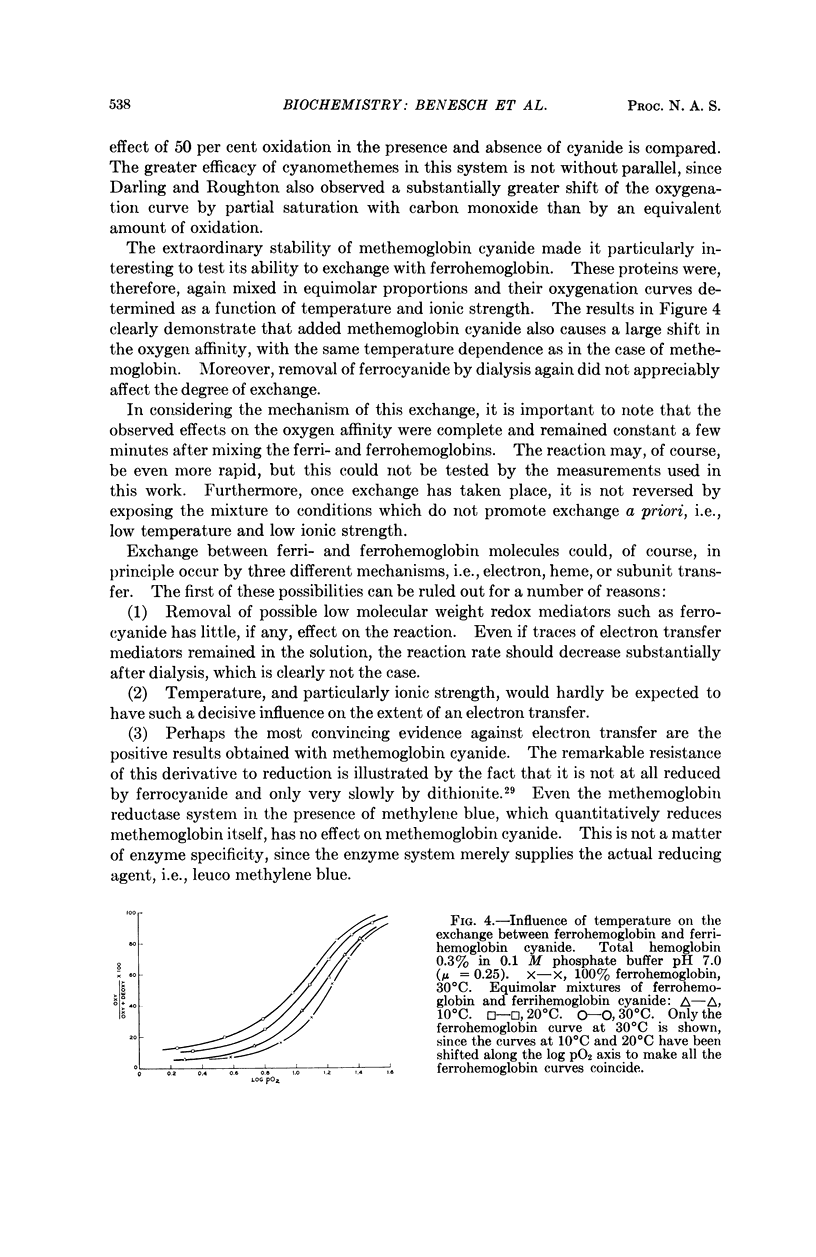
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., THOMPSON T. E. DETERMINATION OF STOICHIOMETRY AND EQUILIBRIUM CONSTANTS FOR REVERSIBLY ASSOCIATING SYSTEMS BY MOLECULAR SIEVE CHROMATOGRAPHY. Proc Natl Acad Sci U S A. 1965 Feb;53:342–349. doi: 10.1073/pnas.53.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANTONINI E., WYMAN J., BRUNORI M., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. STUDIES ON THE RELATIONS BETWEEN MOLECULAR AND FUNCTIONAL PROPERTIES OF HEMOGLOBIN. IV. THE BOHR EFFECT IN HUMAN HEMOGLOBIN MEASURED BY PROTON BINDING. J Biol Chem. 1963 Sep;238:2950–2957. [PubMed] [Google Scholar]
- ANTONINI E., WYMAN J., BRUNORI M., TAYLOR J. F., ROSSI-FANELLI A., CAPUTO A. STUDIES ON THE OXIDATION-REDUCTION POTENTIALS OF HEME PROTEINS. I. HUMAN HEMOGLOBIN. J Biol Chem. 1964 Mar;239:907–912. [PubMed] [Google Scholar]
- ANTONINI E., WYMAN J., BUCCI E., FRONTICELLI C., ROSSI-FANELLI A. The dissociation and recombination of subunits of human, horse, and canine hemoglobin. J Mol Biol. 1962 May;4:368–375. doi: 10.1016/s0022-2836(62)80017-5. [DOI] [PubMed] [Google Scholar]
- ATKINSON D. E., HATHAWAY J. A., SMITH E. C. KINETICS OF REGULATORY ENZYMES. KINETIC ORDER OF THE YEAST DIPHOSPHOPYRIDINE NUCLEOTIDE ISOCITRATE DEHYDROGENASE REACTION AND A MODEL FOR THE REACTION. J Biol Chem. 1965 Jun;240:2682–2690. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R., MACDUFF G. THE DISSOCIATION OF HEMOGLOBINS A AND H IN CONCENTRATED SODIUM CHLORIDE. Biochemistry. 1964 Aug;3:1132–1135. doi: 10.1021/bi00896a021. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R. The influence of oxygenation on the reactivity of the--SH groups of hemoglobin. Biochemistry. 1962 Sep;1:735–738. doi: 10.1021/bi00911a002. [DOI] [PubMed] [Google Scholar]
- BENESCH R. E., BENESCH R., WILLIAMSON M. E. The influence of reversible oxygen binding on the interaction between hemoglobin submits. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2071–2075. doi: 10.1073/pnas.48.12.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENESCH R., MACDUFF G., BENESCH R. E. DETERMINATION OF OXYGEN EQUILIBRIA WITH A VERSATILE NEW TONOMETER. Anal Biochem. 1965 Apr;11:81–87. doi: 10.1016/0003-2697(65)90045-x. [DOI] [PubMed] [Google Scholar]
- CHANGEUX J. P. ALLOSTERIC INTERACTIONS INTERPRETED IN TERMS OF QUATERNARY STRUCTURE. Brookhaven Symp Biol. 1964 Dec;17:232–249. [PubMed] [Google Scholar]
- Douglas C. G., Haldane J. S., Haldane J. B. The laws of combination of haemoglobin with carbon monoxide and oxygen. J Physiol. 1912 Jun 12;44(4):275–304. doi: 10.1113/jphysiol.1912.sp001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H. The photochemical formation of a quickly reacting form of haemoglobin. Biochem J. 1959 Feb;71(2):293–303. doi: 10.1042/bj0710293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDOTTI G., CRAIG L. C. Dialysis studies. VIII. The behavior of solutes which associate. Proc Natl Acad Sci U S A. 1963 Jul;50:46–54. doi: 10.1073/pnas.50.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUISMAN T. H., DOZY A. M. Studies on the heterogeneity of hemoglobin. V. Binding of hemoglobin with oxidized glutathione. J Lab Clin Med. 1962 Aug;60:302–319. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MUIRHEAD H., PERUTZ M. F. STRUCTURE OF HAEMOGLOBIN. A THREE-DIMENSIONAL FOURIER SYNTHESIS OF REDUCED HUMAN HAEMOGLOBIN AT 5-5 A RESOLUTION. Nature. 1963 Aug 17;199:633–638. doi: 10.1038/199633a0. [DOI] [PubMed] [Google Scholar]
- PERUTZ M. F., BOLTON W., DIAMOND R., MUIRHEAD H., WATSON H. C. STRUCTURE OF HAEMOGLOBIN. AN X-RAY EXAMINATION OF REDUCED HORSE HAEMOGLOBIN. Nature. 1964 Aug 15;203:687–690. doi: 10.1038/203687a0. [DOI] [PubMed] [Google Scholar]
- RIGGS A. The binding of N-ethylmaleimide by human hemoglobin and its effect upon the oxygen equilibrium. J Biol Chem. 1961 Jul;236:1948–1954. [PubMed] [Google Scholar]
- SCHEJTER A., ADLER A. D., GLAUSER S. C. STOICHIOMETRY OF HEMOGLOBIN REACTIONS. Science. 1963 Aug 30;141(3583):784–788. doi: 10.1126/science.141.3583.784. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]



