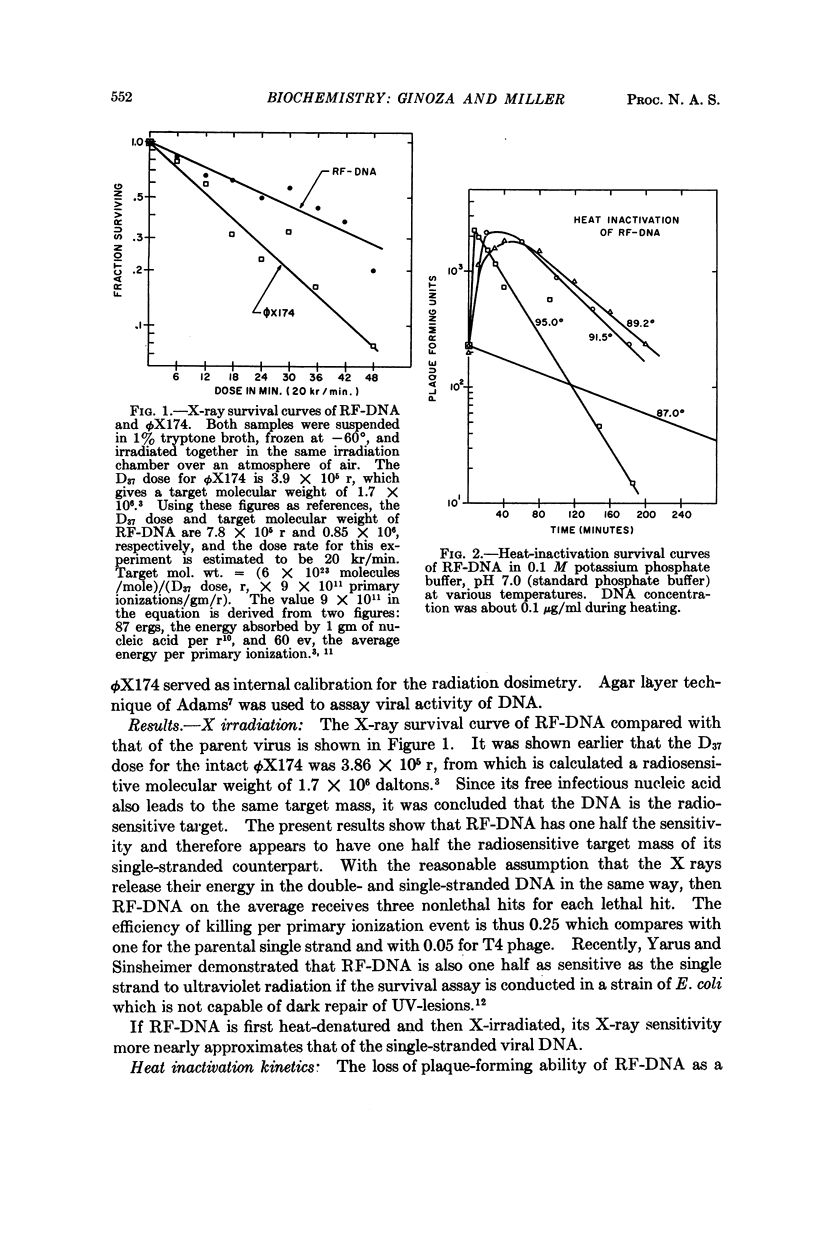
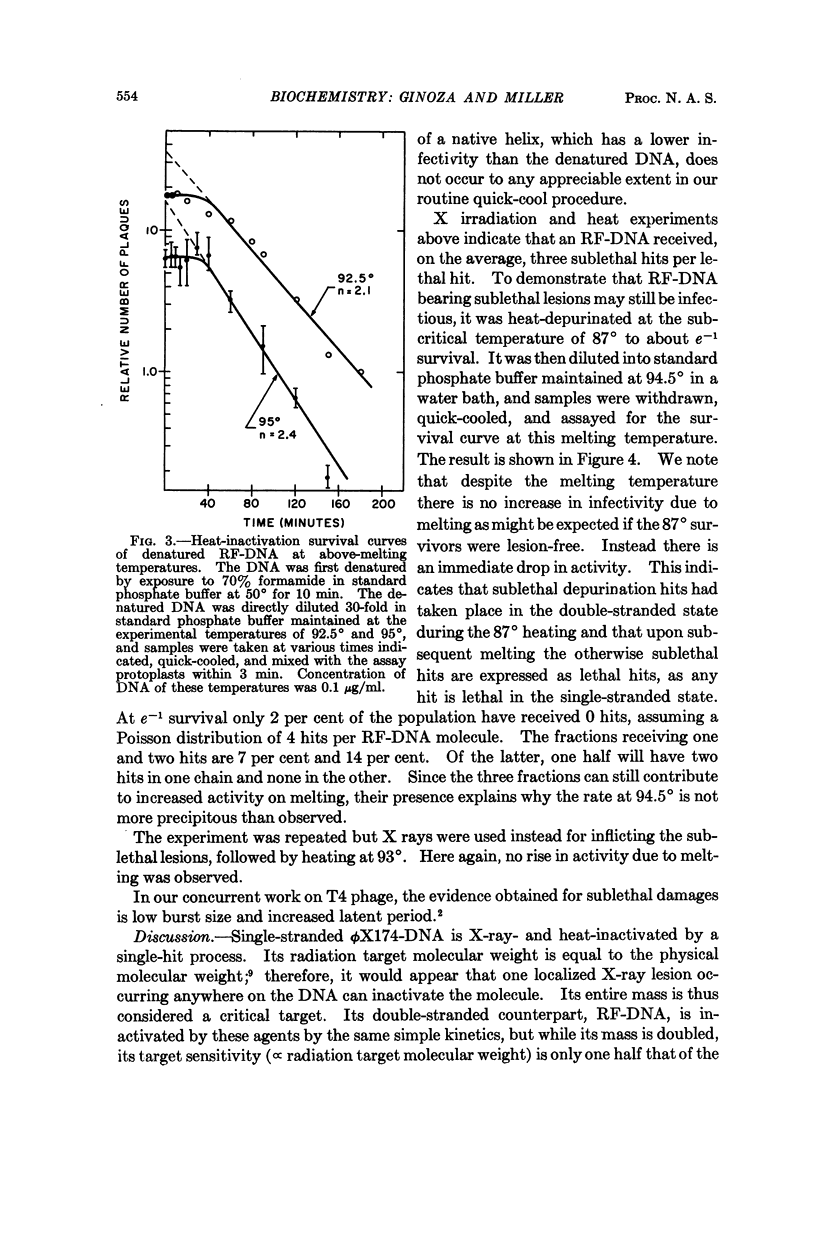
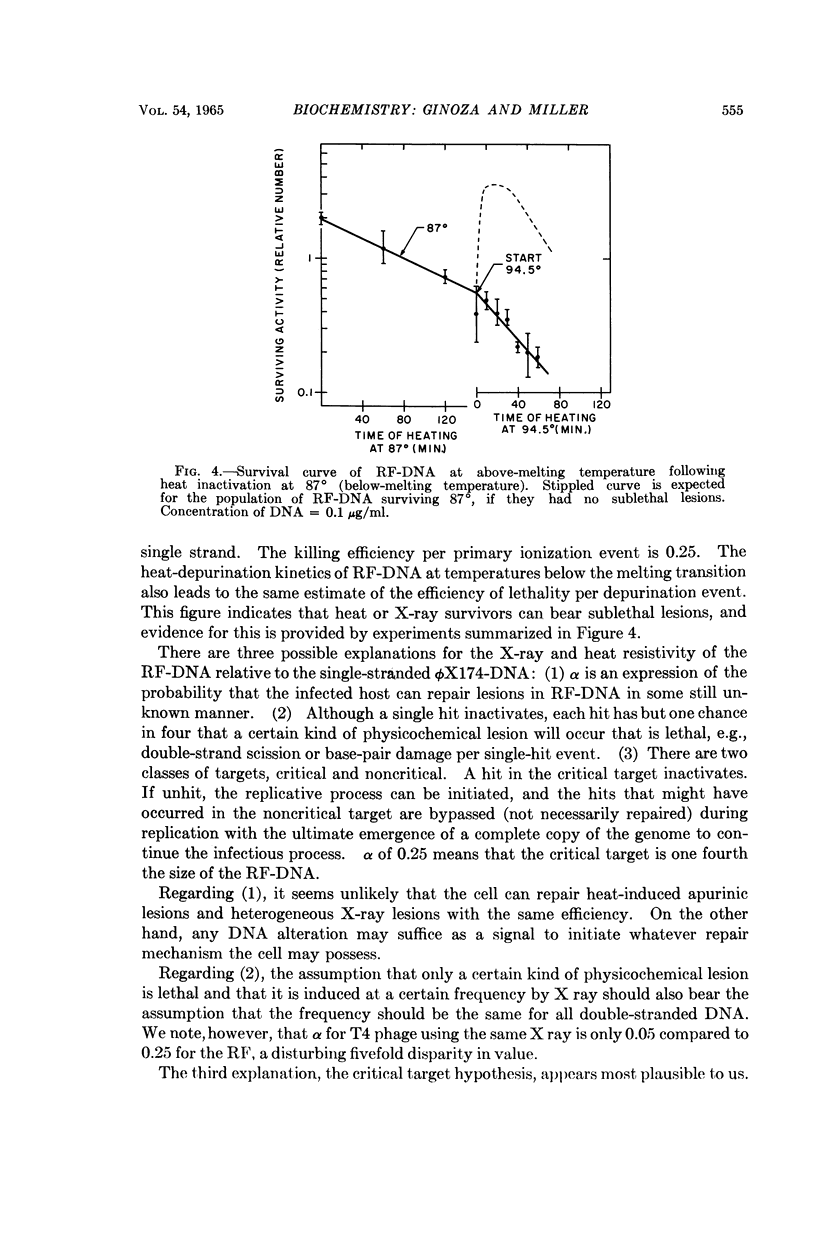
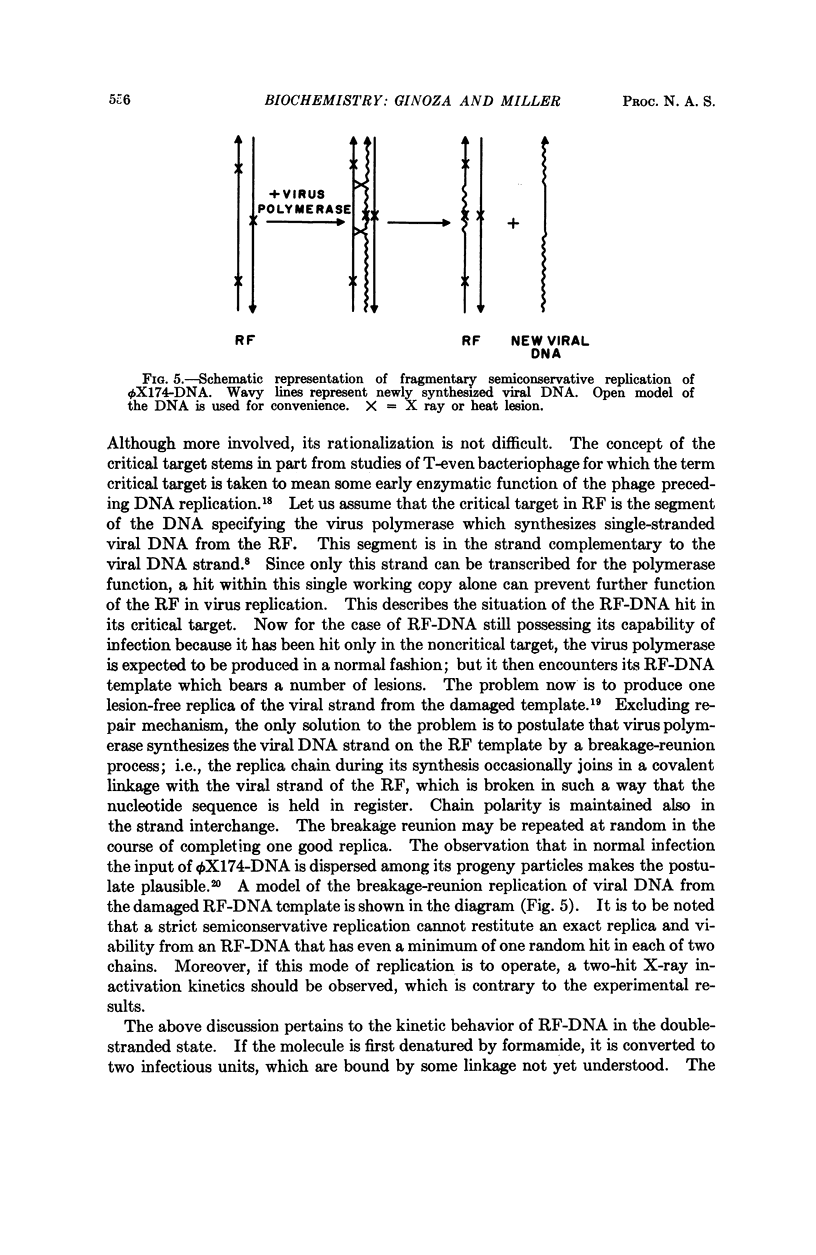
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GINOZA W., HOELLE C. J., VESSEY K. B., CARMACK C. MECHANISMS OF INACTIVATION OF SINGLE-STRANDED VIRUS NUCLEIC ACIDS BY HEAT. Nature. 1964 Aug 8;203:606–609. doi: 10.1038/203606a0. [DOI] [PubMed] [Google Scholar]
- GINOZA W. RADIOSENSITIVE MOLECULAR WEIGHT OF SINGLE-STRANDED VIRUS NUCLEIC ACIDS. Nature. 1963 Aug 3;199:453–456. doi: 10.1038/199453a0. [DOI] [PubMed] [Google Scholar]
- GINOZA W., ZIMM B. H. Mechanisms of inactivation of deoxyribonucleic acids by heat. Proc Natl Acad Sci U S A. 1961 May 15;47:639–652. doi: 10.1073/pnas.47.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., BURTON A., SINSHEIMER R. L. ELECTRON MICROSCOPY OF THE REPLICATIVE FORM OF THE DNA OF THE BACTERIOPHAGE PHI-X174. Science. 1963 Nov 15;142(3594):961–961. doi: 10.1126/science.142.3594.961. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- ROGER M., HOTCHKISS R. D. Selective heat inactivation of pneumococcal transforming deoxyribonucleate. Proc Natl Acad Sci U S A. 1961 May 15;47:653–669. doi: 10.1073/pnas.47.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINSHEIMER R. L., STARMAN B., NAGLER C., GUTHRIE S. The process of infection with bacteriophage phi-XI74. I. Evidence for a "replicative form". J Mol Biol. 1962 Mar;4:142–160. doi: 10.1016/s0022-2836(62)80047-3. [DOI] [PubMed] [Google Scholar]
- TESSMAN I. Some unusual properties of the nucleic acid in bacteriophages S13 and phi X174. Virology. 1959 Mar;7(3):263–275. doi: 10.1016/0042-6822(59)90197-7. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YARUS M., SINSHEIMER R. L. THE U.V.-RESISTANCE OF DOUBLE-STRANDED PHIX174 DNA. J Mol Biol. 1964 Apr;8:614–615. doi: 10.1016/s0022-2836(64)80018-8. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN F., KROEGER H., HAGEN U., KECK K. THE EFFECT OF X-IRRADIATION ON THE PRIMING ABILITY OF DNA IN THE RNA POLYMERASE SYSTEM. Biochim Biophys Acta. 1964 May 18;87:160–162. doi: 10.1016/0926-6550(64)90058-1. [DOI] [PubMed] [Google Scholar]


