Abstract
Myogenesis in vitro involves myoblast cell cycle arrest, migration, and fusion to form multinucleated myotubes. Extracellular matrix (ECM) integrity during these processes is maintained by the opposing actions of matrix metalloproteinase (MMP) proteases and their inhibitors, the tissue inhibitor of metalloproteinases (TIMPs). Here, we report that TIMP-2, MMP-2, and MT1-MMP are differentially expressed during mouse myoblast differentiation in vitro. A specific role for TIMP-2 in myogenesis is demonstrated by altered TIMP-2−/− myotube formation. When differentiated in horse serum-containing medium, TIMP-2−/− myotubes are larger than wild-type myotubes. In contrast, when serum-free medium is used, TIMP-2−/− myotubes are smaller than wild-type myotubes. Regardless of culture condition, myotube size is directly correlated with MMP activity and inversely correlated with β1 integrin expression. Treatment with recombinant TIMP-2 rescues reduced TIMP-2−/− myotube size and induces increased MMP-9 activation and decreased β1 integrin expression. Treatment with either MMP-2 or MMP-9 similarly rescues reduced myotube size, but has no effect on β1 integrin expression. These data suggest a specific regulatory relationship between TIMP-2 and β1 integrin during myogenesis. Elucidating the role of TIMP-2 in myogenesis in vitro may lead to new therapeutic options for the use of TIMP-2 in myopathies and muscular dystrophies in vivo.
Keywords: fusion, hypertrophy, integrin, migration, MMP-2, MMP-9, myoblast, myogenesis, proteolysis
Introduction
Skeletal muscle development involves the migration of myogenic cells from somites, myoblast cell cycle arrest, myocyte fusion, and finally differentiation into multinucleated contractile myofibers. The molecular mechanisms that regulate these events have been extensively studied [1, 2]. Communication between cells and the surrounding microenvironment, including cell-cell and cell-matrix interactions, play a role in all phases of myogenesis [3].
Matrix metalloproteinases (MMPs) contribute to morphogenesis by degrading extracellular matrix (ECM) [4] as well as non-matrix [5] constituents. MMPs, which are comprised of secreted and membrane-associated (membrane type, MT) members, belong to a larger family of zinc-dependent neutral endopeptidases, which includes ADAM (A Disintegrin And Metalloproteinase) and ADAM-TS (ADAM proteases with thrombospondin domains) proteases [6, 7]. These proteases are produced as inactive zymogens. Most MMPs are activated extracellularly by other active MMPs and serine proteases. However, some MMPs, in particular MT-MMPs, are activated intracellularly via a furin-like convertase. A role for MMPs in myogenesis has long been proposed [8] and their participation in myoblast migration [9, 10] and fusion [11] has been investigated.
To ensure appropriate morphogenetic remodeling, ECM degradation must be precisely controlled. MMP activity is regulated by interactions with tissue inhibitor of metalloproteinases (TIMPs) [12]. The 4 known TIMPs are responsible for the inhibition of 25 MMPs, the 29 ADAMs with protease activity (of 42 total members), and 19 ADAM-TSs. In addition to MMP inhibition, TIMP-2 also plays a role in MMP activation. The activation of proMMP-2 by MT1-MMP requires the formation of a trimolecular complex between proMMP-2, MT1-MMP, and TIMP-2 [13]. In this complex, the N-terminal TIMP-2 domain binds to the active site of MT1-MMP while the C-terminal domain binds to proMMP-2’s hemopexin domain. This complex brings the zymogen close to the active site of a second MT1-MMP; thus, allowing for proMMP-2 activation. In the absence of TIMP-2, proMMP-2 activation is impaired [14, 15]. In addition to their MMP-dependent activities, it is now well accepted that TIMPs alter cell growth and survival in an MMP-independent manner [16, 17]. These MMP-independent functions are mediated via integrins, most specifically α3β1 integrin [17, 18].
Integrins form a major family of cell surface adhesion receptors, which are non-covalently associated dimeric transmembrane glycoproteins [19]. The 18 α and 8 β chains combine to form 24 different dimers which regulate diverse functions [20], including myoblast proliferation [21, 22], migration [23–26], fusion [27], and muscle contraction [28, 29]. The observations that myogenesis is largely normal in the absence of α7 [30], α4 [31], α6 [32], and α5 [33] integrin indicates the redundancy in integrin function. In contrast, disruption of β1 integrin in vitro or in vivo has profound effects on myogenesis [22, 27, 34–36].
Previously, we reported that TIMP-2−/− mice exhibit a motor phenotype that is associated with histological neuromuscular junction (NMJ) alterations [37]. Further investigation revealed decreased MMP activity and β1 integrin expression in muscle, particularly within fast-twitch muscle [38]. Because both MMP activity and β1 integrin are required for proper myogenesis and, to the best of our knowledge, there are no reports on the role of TIMP-2 in myogenesis, we investigated the role of TIMP-2 on myogenesis in vitro. Here, we report that TIMP-2−/− myotubes form in vitro, but that myotube size is dependent upon culture condition (i.e., horse serum or serum-free differentiation medium). Most strikingly, treatment of TIMP-2−/− myotubes with recombinant TIMP-2, MMP-2, or MMP-9 rescues reduced TIMP-2−/− myotube size, but only TIMP-2 treatment alters β1 integrin expression, suggesting a regulatory relationship between TIMP-2 and β1 integrin.
Materials and Methods
Animals
TIMP-2−/− mice, which have been described elsewhere [14], and wild-type controls were used for myoblast collection. Hindlimb muscles from entire litters of homozygous matings were pooled to obtain sufficient numbers of myoblasts. Procedures that involved animals were in accordance with the institutional guidelines of the University of Vermont Animal Care and Use Committee.
Antibodies
Primary antibodies: sheep anti-human TIMP-2 for immunocytochemistry (200X; Biogenesis Inc., Kingston, NH), rabbit anti-human TIMP-2 for western blot (1500X; AB801; Chemicon, Temecula, CA), rabbit anti-human MT1-MMP (500X for immunocytochemistry, 2500X for western blot; ab38971; Abcam, Cambridge, MA), rabbit anti-human MMP-2 for immunocytochemistry (500X; AB809, Chemicon), mouse anti-human MMP-2 for western blot (2500X; MAB3308; Chemicon), rabbit anti-human MMP-9 (500X for immunocytochemistry, 2500X for western blot; ab38906; Abcam), rabbit anti-human β1 integrin for immunocytochemistry (500X; AB1952; Chemicon), rat anti-mouse β1 integrin for western blot (2000X; MAB1997; Chemicon), and goat anti-human actin (1000X; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies: Cy3-conjugated donkey anti-sheep (500X), Cy3-conjugated goat anti-rabbit (500X), horseradish peroxidase (HRP)-conjugated donkey anti-sheep or anti-rabbit (3000X), and HRP-conjugated goat anti-mouse (3000X) were from Jackson ImmunoResearch (West Grove, PA). HRP-conjugated mouse anti-rat IgG2a (3000X) was from Southern Biotech (Birmingham, AL).
Primary Myoblast Culture
Myoblast cultures were established as previously described [39]. Hindlimb muscles from neonatal mice (3–5 days old) were collected into Dulbecco’s Phosphate Buffered Saline (DPBS; Invitrogen, Carlsbad, CA), minced with a razor blade, and briefly centrifuged to sediment the tissue. Cells were enzymatically digested in 2 ml digestion solution (DPBS supplemented with 2.5 mM CaCl2, 2.4 U/ml grade II dispase [Roche; Indianapolis, IN], and 100 μg/ml Liberase Blendzyme III [Roche]) by agitation on a rotary shaker for 45 minutes at 37°C. Cells were dispersed with a 5 ml plastic pipette every 15 minutes and finally passed through 80 μm nylon mesh (Nitex; Dynamic Aqua Supply; Surrey, British Columbia, Canada). The filtrate was spun at 800 rpm for 5 minutes to sediment the dissociated cells, the cell pellet was resuspended in growth medium, and the suspension was plated on collagen-coated dishes (40 μg/ml type I rat tail collagen size fractionated with 0.2 N glacial acetic acid; Upstate Biotechnology, Lake Placid, NY). Cells were plated at a density of 400 cells/mm2 in 35-mm dishes or 24-well plates. Cells were maintained in growth media consisting of Ham’s F-10 nutrient mixture (Invitrogen) supplemented with 20% fetal bovine serum (FBS, HyClone Laboratories, Inc., Logan, UT) and 2.5 ng/ml basic fibroblast growth factor (bFGF, kindly provided by Dr. Felix Eckenstein, UVM Department of Neurology). After 24 hours, cells were switched to differentiation medium, which consisted of Dulbecco’s Modified Eagles Medium (DMEM, Mediatech, Herndon, VA) supplemented with 2% heat-inactivated horse serum (HyClone Laboratories) or N2 supplement (Invitrogen). All media contained 50U/ml penicillin and 50 μg/ml streptomycin (Invitrogen). After 24 hours, cultures were treated with 10 μM cytosine-D-arabinofuranoside (AraC). Cultures were maintained at 37°C in a humidified air/5% CO2 incubator with media replenished daily. Conditioned media was collected and cells were harvested after 1, 2, 3, and 5 days in differentiation medium.
Co-culture of TIMP-2−/− and wild-type myotubes was performed as previously described [40]. This co-culture method assures that cells are in close, but not direct contact with each other. Therefore, it is used to determine whether a phenotype is driven by soluble or cell surface associated molecules. TIMP-2−/− myoblasts were plated directly onto the collagen-coated surface of 24-well plates as described above. Wild-type myoblasts were plated onto specially prepared collagen-coated cover slips (i.e., 12-mm diameter round cover slips containing 3 small 1-mm high feet with the feet facing upwards) in adjacent wells of the 24-well plate. After 24 hours in vitro (i.e., the day cells are switched to differentiation media), the cover slips containing wild-type cells were gently picked up with tweezers and placed, with feet facing downwards, into the wells containing TIMP-2−/− cells. Media was changed every 24 hours. After 3 days in differentiation media, the cover slips were removed, TIMP-2−/− myotubes were fixed, and phase contrast images were acquired (20X) to determine whether TIMP-2−/− myotube size was rescued in the presence of overlying wild-type myotubes. To verify that wild-type cells simply did not detach from the overlying coverslip, wild-type cells were transfected with GFP. No GFP-positive wild-type cells were observed among the TIMP-2−/− myotubes on the bottom of the well (data not shown).
For the treatment of TIMP-2−/− cells with recombinant protein, myoblasts were plated onto collagen-coated 24-well plates. After 1 day, cells were gently rinsed twice with serum-free medium to remove any residual FBS and serum-free differentiation media containing recombinant active mouse TIMP-2 (#39315, Abcam), MMP-2 (#39303, Abcam), or MMP-9 (#39309, Abcam) was added. Medium with fresh recombinant protein was replenished daily.
Western Blot Analysis
Conditioned medium was collected, briefly centrifuged to pellet any cells, and medium rapidly frozen at −80°C without the addition of protease inhibitors (to detect MMP activity). The cells were gently rinsed twice with DPBS, then lysed with Triton Lysis Buffer (20 mM Tris pH 7.4, 137 mM NaCl, 25 mM β-glycerolphosphate pH 7.4, 2 mM sodium pyrophosphate, 2 mM EDTA pH 7.4, 1% Triton X-100, 10% glycerol) containing a cocktail of protease inhibitors (phenyl methyl sulfonylfluoride [1 mM] to inhibit serine protease, N-ethylmaleimide and ε-amino-n-caproic acid [5 mM each] to inhibit cysteine proteases, pepstatin A [5 μg/ml] to inhibit acid proteases, and leupeptin [5 μg/ml] to inhibit serine and cysteine proteases). Lysates were spun at 14,000 rpm for 10 minutes at 4°C, and the supernatant used for western blot analysis. Protein concentrations were determined by the method of [41] using the Bio-Rad reagent (Hercules, CA) and bovine serum albumin (BSA) as a standard. Cell lysates (25 μg per lane) or conditioned medium (25 μl for treated cells or 50 μl for developmental time course, representing 1/10th of the total medium volume) were combined with sample buffer (20 mM Tris pH 6.8, 3% SDS, 10% glycerol, 0.01% bromophenol blue) and 0.1% β-mercaptoethanol (BME), boiled for 5 min, and electrophoresed by SDS-PAGE minigel (12%) in 50 mM Tris, 0.38 M glycine, 0.2% SDS [42]. Samples probed for β integrin and MMP-2 expression were run under native, non-reducing conditions (i.e., in the absence of BME). The proteins were electrophoretically transferred to nitrocellulose (Immobilon-P membranes; Schleicher & Schuell; Keene, NH) overnight at 50 mA in 25 mM Tris, 0.192 M glycine, 0.01% SDS and 20% methanol [43]. Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.4% Tween-20 (TBST; 10 mM Tris, pH 7.5, 150 mM NaCl), followed by overnight incubation at 4°C with primary antibody diluted in TBST and 3% BSA. After three 15 minute washes with TBST, membranes were incubated for 2 hours at room temperature with the appropriate HRP-conjugated secondary antibody diluted in TBST with 5% nonfat dry milk. After washing as before, immunocomplexes were visualized by enhanced chemiluminescence according to manufacturer’s instructions (PerkinElmer Life Sciences, Boston, MA). Densitometry was performed using Quantity One software (Bio-Rad).
Zymography
Conditioned medium (25 μl for treated cells or 50 μl for developmental time course) was combined with sample buffer (50 mM Tris pH 6.8, 0.1% SDS, 10% glycerol, 0.025% bromphenol blue) and electrophoresed by SDS-PAGE (12% non-reducing gel containing 1% gelatin and 0.1% SDS) in 25 mM Tris, 0.25 M glycine, 0.1% SDS. After electrophoresis, the gel was washed once for 15 min and then overnight in wash buffer (2.5% Triton X-100, 50 mM Tris pH 7.5, and 5 mM CaCl2) to remove SDS. The gel was then rinsed three times (30 minutes each) in water, followed by an 18 hour incubation at 37°C in incubation buffer (50 mM Tris pH 7.5 and 5 mM CaCl2). The gel was stained for 4 hours in Coomassie brilliant blue (0.05% Coomassie blue, 10% acetic acid, 30% isopropanol) and destained (10% isopropanol, 10% acetic acid). Recombinant human MMP-2/9 (Abcam) was used as a positive control.
Gelatinase Assay
Net gelatinolytic activity in 100 μl of conditioned media was determined using the EnzCheck Gelatinase Assay according to manufacturer’s instructions (Molecular Probes; Eugene, OR). MMP activity is reported as the rate of fluorescence increase over 15 hours normalized to assay reagent containing DQ gelatin and differentiation medium alone. The specificity of the assay was determined by the inclusion of the MMP inhibitor 1,10 phenanthroline (1mM) or a cocktail of protease inhibitors (not shown).
Immunocytochemistry
Immunocytochemistry was performed exactly as previously described [44]. Cells were fixed with 2% paraformaldehyde (by adding an equal volume of 4% paraformaldehyde directly to the cultured cells) for 20 minutes at room temperature and then washed three times for 10 minutes with DPBS. Cells were incubated in blocking buffer (DMEM, 5% FBS, 0.1% glycine, 0.1% lysine) plus 0.2% Triton X-100 for 1 hour at room temperature. Cell were incubated in primary antibody, diluted in blocking buffer, at 4°C overnight. After washing three times for 10 minutes with DPBS, the cells were incubated in secondary antibody, diluted in blocking buffer, for 1 hour at room temperature. After washing as before, cells were examined using a Nikon inverted epifluorescence microscope (MicroVideo Instruments, Avon, MA) and digital images captured with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI). Figures were prepared using Adobe Photoshop (Adobe Systems Inc., San Jose, CA) with minor adjustment to contrast.
Myotube area of wild-type and TIMP-2−/− cells was measured on images (20X) captured from fixed cells harvested either after 1 day or 3 days in differentiation media. In contrast, myotube size of treated cells was measured on images captured from live cells at 1 day and then cells within the same well 2 days later. At least two randomly selected, non-overlapping fields were photographed with a SPOT RT camera. Within each image at least 10 randomly selected myotubes were encircled and total myotube area (μm2) measured using software provided with the SPOT camera. Thus, mean myotube area was determined from at least 20 myotubes from at least 3 independent cultures.
Statistics
Data, presented as mean ± s.e.m, represents at least three independent cultures. Significant interactions were identified by unpaired t-test using Prism software (GraphPad; San Diego, CA). Criterion for statistical significance is p < 0.05.
Results
Previously, we reported the regulation of TIMP-2, MMP-2, and MT1-MMP expression during C2C12 differentiation [44]. Although this mouse myoblastic cell line is a well-accepted model to study myogenesis in vitro, the use of a cell line may not accurately mimic myogenesis in vivo. Therefore, this study was undertaken to further characterize the role of TIMP-2, MMP-2, and MT1-MMP during wild-type primary cultured murine myoblast differentiation. More importantly, the specific role of TIMP-2 in myogenesis was examined by comparing myotube formation derived from TIMP-2−/− mice.
TIMP-2, MMP-2, and MT1-MMP expression is altered during TIMP-2−/− myogenesis
Myogenesis in vitro can be investigated using a well-established myoblast culture technique [39]. Myoblasts are isolated from lower extremity muscles of postnatal day 3 (P3) – P5 mice. After allowing myoblasts to proliferate in the presence of trophic factors (e.g., 20% fetal bovine serum and basic fibroblast growth factor, bFGF), differentiation is induced upon serum reduction (e.g., 2% heat-inactivated horse serum).
The gradual increase in TIMP-2 expression during C2C12 differentiation [44] suggested it participates in myogenesis. Therefore, we first determined whether TIMP-2 deletion altered the number of myotubes formed after 3 days in differentiation media (i.e., the peak of myotube elongation in vitro). Myotube number (per 20X field) in wild-type (WT = 4.06 ± 0.32, n = 4) and TIMP-2−/− (KO = 4.22 ± 0.24, n = 3) cultures was comparable (p = 0.72). However, TIMP-2−/− myotube area (4.00 ± 0.17 μm2 × 1000) was larger than wild-type myotubes (3.23 ± 0.01 μm2 × 1000; p = 0.0008) (Fig. 1A).
Figure 1. TIMP-2−/− myotubes are larger than wild-type myotubes when cultured in the presence of horse serum.
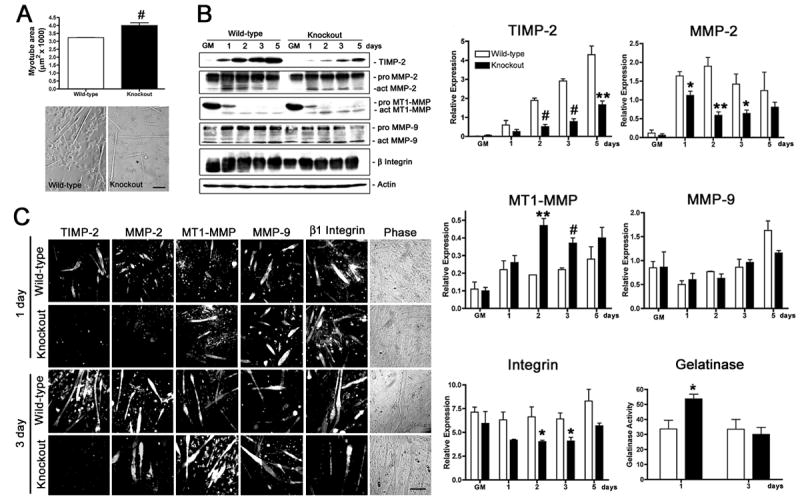
A) After 3 days in differentiation media containing 2% heat-inactivated horse serum, cells were fixed, and myotube area was measured (at least 20 randomly selected myotubes were measured from four wild-type and three TIMP-2−/− cultures). Data are presented as mean ± s.e.m. TIMP-2−/− myotubes are larger than wild-type myotubes. Myonuclei number is also increased in TIMP-2−/− myotubes, suggesting increased fusion (not shown). B) Western blot analysis with whole cell lysates (25 μg protein) of rapidly proliferating cells maintained in growth media (GM) for 24 hours after plating and after 1, 2, 3, and 5 days in differentiation media containing 2% heat-inactivated horse serum. Densitometric analysis (normalized to actin at each time point) reveals a dramatic reduction in TIMP-2 and active MMP-2 expression, increased active MT1-MMP expression, and no change in active MMP-9 expression in TIMP-2−/− myotubes. Similar to TIMP-2−/− muscle in vivo, total cellular β1 integrin expression, as well as cell surface expression (not shown), is decreased in TIMP-2−/− myotubes at 3 days, when myotube size is increased. Using a fluorescently caged gelatin substrate (i.e., DQ gelatin), protease activity was measured in 100 μl conditioned media (normalized to unconditioned medium containing serum). No difference in net gelatinolytic activity is detected at 3 days; in contrast, gelatinolytic activity is significantly increased at 1 day, the period of myoblast migration and fusion. Data are presented as mean ± s.e.m. C) Immunocytochemistry of Triton permeabilized cells confirms the western blot data of decreased TIMP-2 and MMP-2 expression and increased MT1-MMP expression in TIMP-2−/− myotubes. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bar = 100 μm.
To determine the mechanism underlying increased TIMP-2−/− myotube size, TIMP-2, MMP-2, and MT1-MMP expression was investigated. TIMP-2 expression in wild-type myotubes was undetectable in rapidly proliferating myoblasts (collected 24 hours after plating), but steadily increased coincident with myotube formation (Figs. 1B, C). In TIMP-2−/− myotubes, TIMP-2 expression was significantly reduced, but not absent. This supports our recent report of TIMP-2 mRNA and protein in the adult mouse brain in vivo [45]. While TIMP-2 was expressed within TIMP-2−/− cells, no secreted TIMP-2 was detected [45], (Fig. 2B), indicating a functional knockout for extracellular TIMP-2 activity. Given that TIMP-2 is required for MT1-MMP-mediated proMMP-2 activation [13], it was not surprising that active MMP-2 expression was significantly reduced in TIMP-2−/− myotubes (Figs. 1B, C). This supports previous reports of impaired proMMP-2 activation in TIMP-2 deficient cells [14, 15, 45]. Nonetheless, the developmental up-regulation of MMP-2 expression during myogenesis was similar in wild-type and TIMP-2−/− myotubes. ProMT1-MMP expression was markedly decreased upon trophic withdrawal in both wild-type and TIMP-2−/− myotubes (Figs. 1B, C). Active MT1-MMP expression in wild-type myotubes was up-regulated after 1 day in differentiation media, but then largely remained unchanged throughout differentiation. In contrast, active MT1-MMP expression was considerably increased in TIMP-2−/− myotubes. This up-regulation may serve as a mechanism to increase proMMP-2 activation, similar to that observed in mouse lung [14]. To determine whether MMP-9 (i.e., gelatinase B) expression is up-regulated in TIMP-2−/− myotubes to compensate for decreased MMP-2 (i.e., gelatinase A) activation, its expression also was examined (Figs. 1B, C). In contrast to the developmental regulation of MMP-2, MMP-9 was moderately expressed in rapidly proliferating myoblasts, its expression then declined upon serum withdrawal, but then steadily increased coincident with myotube formation. No differences in active MMP-9 expression were detected in TIMP-2−/− myotubes relative to wild-type myotubes. The differential regulation of TIMP-2, MMP-2, and MT1-MMP expression suggests that each subserves distinct functions during myogenesis.
Figure 2. TIMP-2−/− myotubes are smaller than wild-type myotubes when cultured in serum-free media.
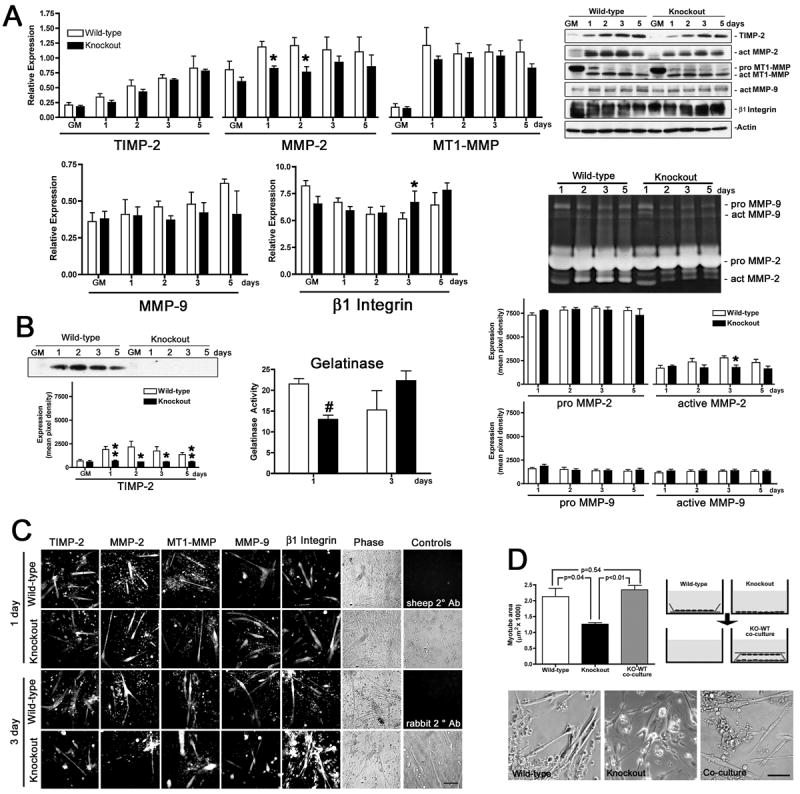
A) Western blot analysis (25 μg whole cell lysates) of myotubes differentiated in serum-free medium reveals that the protein expression profile and developmental regulation differs little between wild-type and TIMP-2−/− myotubes. Notable exceptions include decreased active MMP-2 expression at 1 and 2 days and increased β1 integrin expression at 3 days. B) Analysis of conditioned media. Western blot analysis (50 μl conditioned medium) shows that although TIMP-2−/− myotubes possess TIMP-2 protein (Fig. 1B) it is not secreted, indicating a biological knockout for extracellular TIMP-2 activity (i.e., MMP inhibition and proMMP-2 activation). Gelatinase activity (in 100 μl conditioned differentiation medium normalized to unconditioned media containing N2 supplement) is decreased at 1 day, but unchanged at 3days. By zymography (50 μl conditioned medium), decreased active MMP-2 expression is detected at 3 days, but not at 1 day, indicating that MMPs other than MMP-2 and MMP-9 contribute to the decreased net gelatinolytic activity. C) Immunocytochemistry of Triton permeabilized cells confirms the western blot data and reveals that both wild-type and TIMP-2−/− cultures contain a greater number of undifferentiated cells relative to cultures differentiated with horse serum. Secondary antibody alone controls (sheep and rabbit 2° Ab) demonstrate the specificity of immunolabeling. D) After 3 days in serum-free differentiation medium, cells were fixed and myotube area was measured. In contrast to myotubes differentiated with 2% horse serum (Fig. 1), TIMP-2−/− myotubes in serum-free media are smaller than wild-type myotubes. Co-culture of TIMP-2−/− (KO) with wild-type (WT) myotubes in close, but not direct, contact increases myotube size comparable to wild-type levels, suggesting soluble factor(s) are responsible for the rescue. Photomicrographs representative of the three culture conditions are shown. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bars = 100 μm.
Inasmuch as proteases participate in myoblast migration and fusion, we next examined whether MMP proteolytic activity was altered in the conditioned medium of TIMP-2−/− cultures (Fig. 1B). The presence of MMPs and TIMPs in serum precluded western blot and zymographic analysis. Rather, net gelatinase activity was measured using a fluorescently caged gelatin (denatured collagen) substrate. Cleavage of this substrate by MMPs in the conditioned media yields peptides whose fluorescence is proportional to proteolytic activity. Gelatinolytic activity in conditioned media was normalized to unconditioned differentiation medium. While MMP activity is reduced in TIMP-2−/− muscles in vivo [38], no change in MMP activity was observed at 3 days when myotube size was increased (WT = 33.5 ± 6.51, n = 4; KO = 30.0 ± 4.74, n = 3; p = 0.68). However, gelatinase activity was increased in media collected from TIMP-2−/− cultures (53.67 ± 3.18) when compared to wild-type cultures (33.67 ± 5.78, p = 0.039) at 1 day. The protease(s) responsible for the increased gelatinolytic activity is, at present, not known. However, it suggests that altered extracellular matrix integrity may form the foundation of the increased myotube size.
Since β1 integrin expression is altered during muscle development in TIMP-2−/− mice [38] and β1 integrins play a role in myoblast migration and fusion, β1 integrin expression was examined (Figs. 1B, C). No change in β1 integrin expression was detected at 1 day, the time point with increased MMP activity. In contrast, at 3 days, TIMP-2−/− myotubes expressed less β1 integrin than wild-type myotubes. This is similar to our in vivo observation of decreased β1 integrin expression in P14 TIMP-2−/− extensor digitorum longus (EDL) muscle relative to wild-type EDL [38]. Thus, when differentiated in the presence of horse serum, TIMP-2−/− myotube size at 3 days is directly correlated with increased gelatinolytic activity at 1 day and inversely correlated with β1 integrin expression at 3 days. The decreased integrin expression at 3 days may simply be a compensation for increased MMP activity at 1 day.
TIMP-2−/− myotubes are smaller than wild-type myotubes when differentiated in serum-free defined media
The observed changes in TIMP-2−/− myotubes are intriguing; however, the presence of MMPs and TIMPs in serum confounds the data. Therefore, TIMP-2−/− myoblast differentiation was examined in serum-free media using a N2 supplement that contains insulin, transferrin, selenium, putrescine, and progesterone necessary for myogenesis (Fig. 2). We previously used this supplement to investigate MMP activity during C2C12 cell differentiation and observed no major differences in myogenesis relative to cells differentiated in horse serum [44].
The protein expression profile of myotubes differentiated in serum-free media differs from myotubes differentiated in the presence of horse serum (Fig. 2). Similar to serum containing media, TIMP-2 expression increased coincident with myotube formation in both wild-type and TIMP-2−/− myotubes (Fig. 2A). However, unlike serum-containing media, TIMP-2 expression was not reduced in TIMP-2−/− myotubes (Figs. 2A, C), suggesting that TIMP-2 expression by wild-type myotubes is dependent upon serum-derived factors. Similar to serum-containing media, active MMP-2 expression was reduced in TIMP-2−/− myotubes at 1 and 2 days (Figs. 2A, C). However, there was no increased active MT1-MMP expression in TIMP-2−/− myotubes (Figs. 2A, C), supporting our hypothesis that the increased MT1-MMP expression in TIMP-2−/− myotubes differentiated in serum is a response to reduced TIMP-2 expression. In contrast to TIMP-2, whose expression was up-regulated coincident with myotube formation, the developmental up-regulation of MT1-MMP and MMP-9 expression was lost in serum-free media (Figs. 2A, C). Similar to serum-containing media, β1 integrin expression in serum-free media was altered at 3 days; however, its expression was increased rather than decreased (Figs. 2A, C). Finally, proteolytic activity in conditioned medium was examined (Fig. 2B). In contrast to serum-containing conditioned media (Fig. 1B), the only source of MMPs and TIMPs in N2 conditioned media is that secreted by the cells. Western blot analysis (Fig. 2B) and reverse zymography [45] demonstrated that although TIMP-2−/− cells possess TIMP-2 protein, it is not secreted into the media. Similar to cells differentiated with horse serum, no change in gelatinolytic activity was observed in N2 differentiated cells at 3 days (WT = 15.25 ± 4.68, n = 4; KO = 22.33 ± 2.33, n = 3; p = 0.28); however, a significant reduction in net gelatinase activity was detected in TIMP-2−/− myotubes at 1 day (WT = 22.5 ± 1.32, n = 4; KO = 13.0 ± 1.0, n = 3; p = 0.003). While the gelatinase assay detects decreased MMP activity at 1 day, no decrease in either active MMP-2 or MMP-9 was detected by zymography, suggesting other MMPs contribute to the gelatinolytic activity in the condition media. Regardless of the culture condition, TIMP-2−/− myotube size at 3 days is directly correlated with MMP activity at 1 day and inversely correlated with β1 integrin expression at 3 days.
To determine the effect of these changes in protein expression, myotube size was measured (Fig. 2D). In contrast to C2C12 cells where no differences were observed in myotubes differentiated in the presence or absence of serum, both wild-type and TIMP-2−/− myotube size was reduced in serum-free media. This is likely due to the fact that myogenesis of primary myoblasts is more tightly regulated than C2C12 cells. Reduced myotube size was not associated with decreased myotube numbers (WT = 7.2 ± 1.27, n = 4; KO = 6.86 ± 0.23, n = 3; p = 0.83), suggesting that all components necessary for differentiation are present. Although both wild-type and TIMP-2−/− myotubes were reduced in size, there was a two-fold greater reduction in TIMP-2−/− myotube size relative to wild-type (WT = 34%, KO = 69%). Even when normalized to the fact that TIMP-2−/− myotubes were larger in horse serum-containing media, there was a 61% size reduction (WT = 2.13 ± 0.26, KO = 1.26 ± 0.05 μm2 × 1000, p = 0.04). Because myotubes are multinucleated, myotube size is dictated by the number of myonuclei capable of supporting a restricted amount of cytoplasm [46]. To determine whether the reduced myotube size was due to impaired cytoplasmic growth or cell fusion, the number of nuclei per myotube was determined at 3 days. TIMP-2−/− myotubes contained significantly fewer nuclei than wild-type myotubes (WT = 3.21 ± 0.09, KO = 2.40 ± 0.17, p = 0.005). Therefore, the initial formation of a multinucleated myotube proceeds normally, but cell fusion with myotubes is impaired. These observations not only demonstrate a role for TIMP-2 in myogenesis in vitro they also suggest that TIMP-2 acts in concert with serum-derived factors.
To address whether soluble or membrane bound signals are responsible for reduced TIMP- 2−/− myotube size, TIMP-2−/− myoblasts were co-culture with wild-type myoblasts in close apposition, but not direct contact [40] (Fig. 2D). This co-culture paradigm completely rescued TIMP-2−/− myoblast size comparable to wild-type levels (2.34 ± 0.14 μm2 × 1000, n = 3, p = 0.002 relative to KO, p = 0.54 relative to WT). To rule out the possibility that even TIMP-2−/− myoblasts secrete myogenic factors that are simply concentrated by the presence of the overlying cover slip, TIMP-2−/− myoblasts were co-cultured with each other. No change in myotube size was observed (data not shown). This clearly demonstrates that soluble signals released from wild-type myotubes into the extracellular milieu activated a myogenic program in TIMP-2−/− myoblasts.
TIMP-2 treatment rescues reduced TIMP-2−/− myotube size and down-regulates β1 integrin expression
The ability of wild-type myotubes to rescue the reduced TIMP-2−/− myotube size when co-cultured in close proximity suggests that soluble factors regulate myogenesis, but does not identify the responsible factor(s). First, whether recombinant TIMP-2 could rescue reduced TIMP-2−/− myotube size was tested (Fig. 3A). TIMP-2 was added when cultures were switched from growth to differentiation media and replenished daily. Myotube size was not altered by 12.5 nM TIMP-2 treatment after 1 day (untreated = 1.05 ± 0.13 μm2 × 1000, treated = 2.51 ± 0.70 μm2 × 1000, n = 4 p = 0.09) or 3 day (untreated = 2.24 ± 0.22 μm2 × 1000, treated = 3.48 ± 0.58 μm2 × 1000, p = 0.09). In contrast, 25 nM TIMP-2 produced a significant increase in myotube size both at 1 day (2.69 ± 0.18 μm2 × 1000, p = 0.003) and 3 days (4.68 ± 0.59 μm2 × 1000, p = 0.008). Interestingly, myotubes treated with 50 nM TIMP-2 were smaller than those treated with 25 nM TIMP-2 treatment at both 1 day (2.15 ± 0.27 μm2 × 1000) and 3 days (3.71 ± 0.51 μm2 × 1000). This is likely due to the fact that at low concentrations, TIMP-2 promotes proMMP-2 activation; hence, promoting MMP-mediated ECM remodeling required for myoblast migration and fusion. In contrast, at higher concentrations TIMP-2 serves its traditional role of MMP inhibition; thus, reducing myoblast migration, fusion, and myotube size.
Figure 3. TIMP-2 rescues reduced TIMP-2−/− myotube size, increases active MMP-9, and decreases β1 integrin expression.
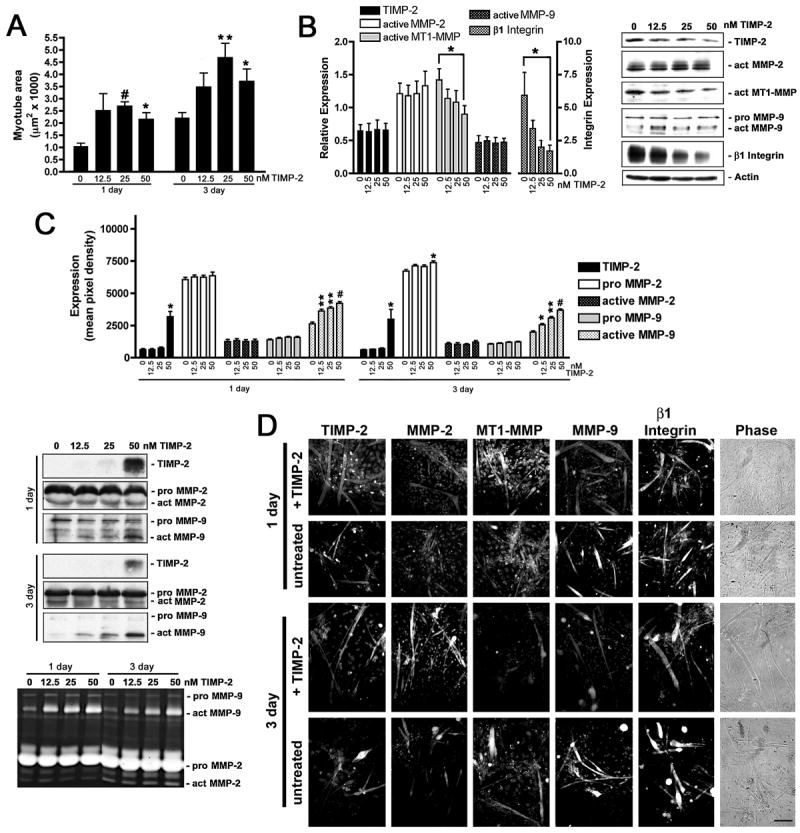
A) TIMP-2−/− myotube area was measured on live cells after 1 and 3 days in differentiation media in the absence or presence of recombinant active mouse TIMP-2 (12.5, 25, or 50 nM). Both at 1 and 3 days, 25 nM TIMP-2 is most effective at rescuing the reduced TIMP-2−/− myotube size. B) Western blot analysis (25 μg whole cell lysates) shows decreased active MT1-MMP and β1 integrin expression in TIMP-2 treated cultures at 3 days. C) Western blot and zymographic analysis (25 μl conditioned medium) reveals a dose-dependent increase in active MMP-9 secretion in response to TIMP-2 treatment both at 1 and 3 days. D) Immunocytochemistry of Triton permeabilized untreated and TIMP-2 treated (25 nM) cells. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bar = 100 μm.
To determine the effect of increasing TIMP-2−/− myotube size, alterations in protein expression were determined at 3 days (Figs. 3B, C). TIMP-2 treatment had no effect of cellular TIMP-2, active MMP-2, or active MMP-9 expression (Figs. 3B–D). In sharp contrast, active MT1-MMP and β1 integrin expression dramatically declined in a dose-dependent manner. Although TIMP-2 treatment had no effect on cellular MMP-9 expression, both western blot and zymographic analyses of conditioned media demonstrate significantly increased MMP-9 activity at 1 and 3 days (Fig. 3C). Somewhat surprisingly, TIMP-2 treatment did not increase detectable levels of active MMP-2 in the conditioned media (Fig. 3C). This may be due to decreased MT1-MMP expression in response to TIMP-2 treatment (Fig. 3B).
MMP-2 and MMP-9 treatment rescues reduced TIMP-2−/− myotube size without affecting β1 integrin expression
ProMMP-2 activation is impaired in TIMP-2−/− cells [14, 15, 45], MMP-9 activation is increased coincident with rescued TIMP-2−/− myotube size (Fig. 3), and both molecules have been shown to play a role in myogenesis [10, 47]. Therefore, we tested whether MMP-2 (Fig. 4) or MMP-9 (Fig. 5) could rescue the reduced TIMP-2−/− myotube size.
Figure 4. MMP-2 rescues reduced TIMP-2−/− myotube size and increases active MMP-9 without altering β1 integrin expression.
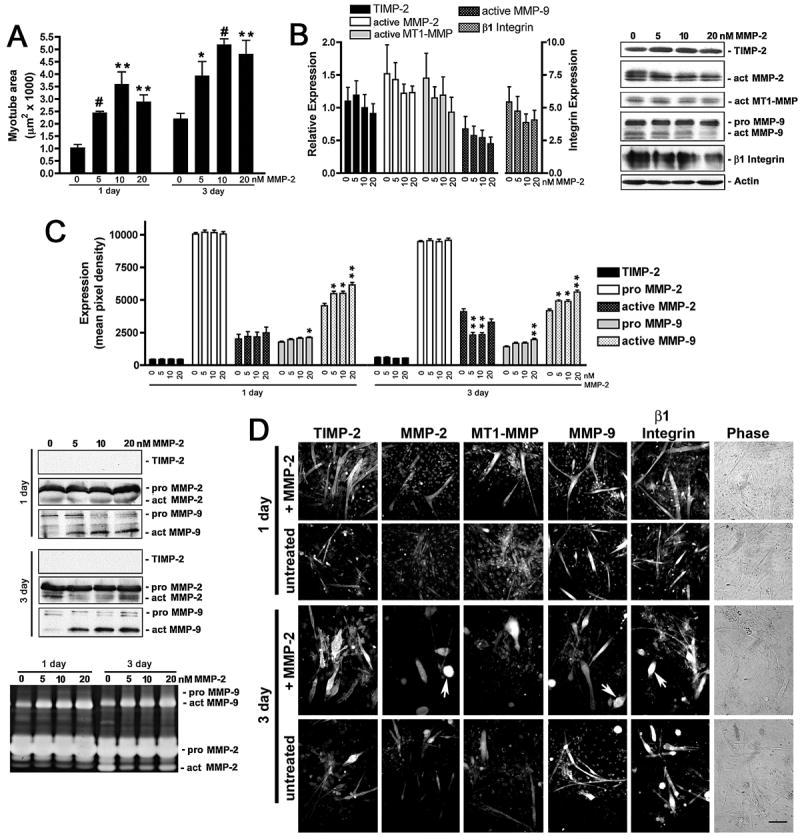
A) TIMP-2−/− myotube area was measured on live cells after 1 and 3 days in differentiation media in the absence or presence of recombinant active mouse MMP-2 (5, 10, 20 nM). Both at 1 and 3 days, 10 nM MMP-2 is most effective at rescuing the reduced TIMP-2−/− myotube size. B) Western blot analysis (25 μg whole cell lysates) shows that MMP-2 treatment has no effect on the proteins analyzed. C) Western blot and zymographic analysis (25 μl conditioned medium) reveals a dose-dependent increase in active MMP-9 secretion in response to MMP-2 treatment both at 1 and 3 days. In addition, MMP-2 treatment (5 and 10 nM) decreases active MMP-2 secretion at 3 days. D) Immunocytochemistry of Triton permeabilized untreated and MMP-2 treated (10 nM) cells. Note that the number of myoballs (arrows) is increased in MMP-2 treated cultures at 3 days. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bar = 100 μm.
Figure 5. MMP-9 rescues reduced TIMP-2−/− myotube size without altering β1 integrin expression.
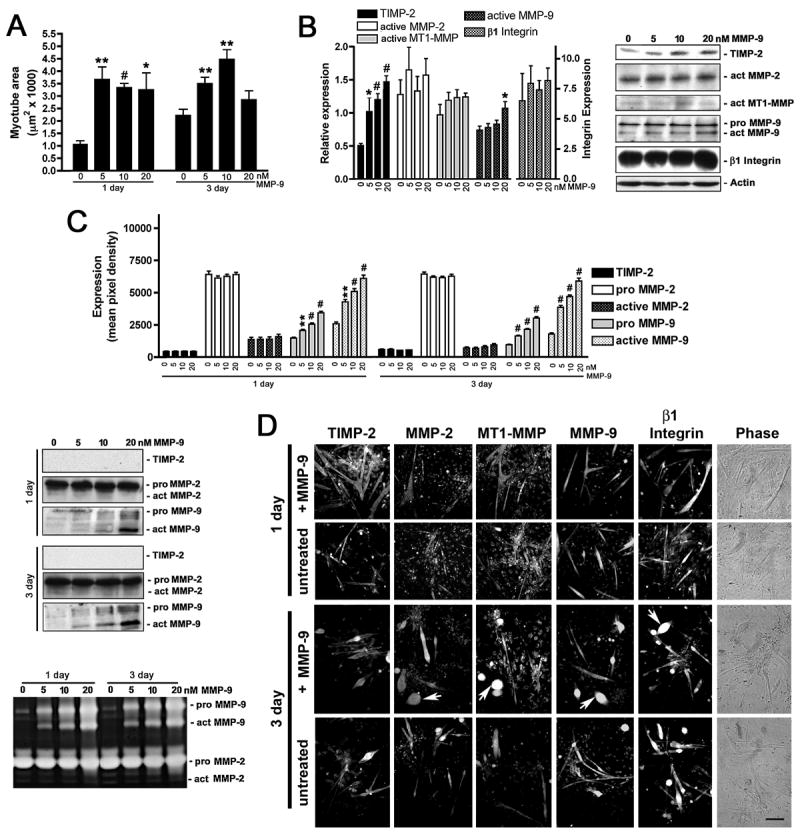
A) TIMP-2−/− myotube area was measured on live cells after 1 and 3 days in differentiation media in the absence or presence of recombinant active mouse MMP-9 (5, 10, 20 nM). MMP-9 rescues TIMP-2−/− myotube size at a lower concentration (5 nM) than MMP-2 (10 nM) at both 1 and 3 days. B) Western blot analysis (25 μg whole cell lysates) shows that MMP-9 treatment increases cellular TIMP-2 expression, but has no effect on β1 integrin expression. C) Western blot and zymographic analysis (25 μl conditioned medium) detects increased active MMP-9 in the medium of MMP-9 treated cells. However, no change in TIMP-2 secretion is detected. D) Immunocytochemistry of Triton permeabilized untreated and MMP-9 treated (10 nM) cells. Note that, like MMP-2 treatment, the number of myoballs (arrows) is increased in MMP-9 treated cultures at 3 days. *p < 0.05, **p ≤ 0.01, #p ≤ 0.001. Scale bar = 100 μm.
MMP-2 treatment produced a greater than 3-fold increase in myotube size at all concentrations tested (Fig. 4A). Treatment with MMP-2 had the most pronounced effect at 1 day (untreated = 1.05 ± 0.13 μm2 × 1000, 5 nM MMP-2 = 2.46 ± 0.04 μm2 × 1000, n = 4, p <0.0001) and least effect at 3 days (untreated = 2.24 ± 0.22 μm2 × 1000, 5 nM MMP-2 = 3.95 ± 0.56 μm2 × 1000, p = 0.03). Similar to TIMP-2 treatment, increasing MMP-2 concentrations (to 10 nM) initially increased myotube size (1 day = 3.61 ± 0.48 μm2 × 1000, p = 0.002; 3 day = 5.20 ± 0.22 μm2 × 1000, p <0.0001), but myotube size began to decrease with further increased MMP-2 (to 20 nM) (1 day = 2.90 ± 0.27 μm2 × 1000, p = 0.0008; 3 day = 4.82 ± 0.54 μm2 × 1000, p = 0.004). This suggests that MMP-mediated ECM remodeling is required for myoblast migration and fusion, but excessive proteolysis decreases myogenesis. In contrast to TIMP-2 mediated myotube rescue, MMP-2 treatment had no effect on the cellular expression of MT1-MMP or β1 integrin (Figs. 4B, D). However, similar to TIMP-2 treatment, the levels of active MMP-9 detected in the conditioned media increased in a dose-dependent manner (Fig. 4C).
Like MMP-2, MMP-9 treatment (Fig. 5A) was most effective at rescuing reduced TIMP-2−/− myotube size at 1 day (untreated = 1.05 ± 0.13 μm2 × 1000, 10 nM MMP-9 = 3.34 ± 0.17 μm2 × 1000, n = 4, p <0.0001) and less effective at 3 days (untreated = 2.24 ± 0.22 μm2 × 1000, 10 nM MMP-9 = 4.47 ± 0.39 μm2 × 1000, p = 0.003). The greatest fold increase in myotube size was achieved at lower MMP-9 concentrations than that achieved by MMP-2 at 1 day (5 nM, 3.67 ± 0.50 μm2 × 1000, p = 0.002); however, 5 nM MMP-9 was less effective at rescuing reduced TIMP-2−/− myotube size at 3 days (3.50 ± 0.25 μm2 × 1000, p = 0.01), suggesting that MMP-9 plays a critical role in myoblast migration and initial fusion than secondary fusion with myotubes. Unlike MMP-2, increasing concentrations of MMP-9 (to 20 nM) did not reduce myotube size at 1 day (3.25 ± 0.68 μm2 × 1000, p = 0.21 relative to untreated, p = 0.64 relative to 5 nM), but 20 nM MMP-9 significantly reduced myotube size at 3 days (2.85 ± 0.36 μm2 × 1000, p = 0.20 relative to untreated, p = 0.02 relative to 10 nM). The significant size reduction in MMP-2 (Fig. 4D) and MMP-9 (Fig. 5D) treated myotubes at 3 days stems from the increased number of myoballs (i.e., desmin-positive round cells) in these cultures, indicating the importance of ECM-mediated cell adhesion in differentiated myotubes. While MMP-9 was as effective as MMP-2 and TIMP-2 at rescuing reduced TIMP-2−/− myotube size, MMP-9, like MMP-2, had no effect on active MT1-MMP or β1 integrin expression (Figs. 5B, D). Surprisingly, MMP-9 treatment induced a dose-dependent increase in cellular TIMP-2 expression. Increased cellular TIMP-2 expression did not, however, result in TIMP-2 secretion into the conditioned media (Fig. 5C), even when concentrated 20-fold (data not shown). Taken together, these data suggest a strong role for TIMP-2 in myogenesis in vitro and suggests a regulatory relationship between TIMP-2 and β1 integrin expression in myotube formation.
Discussion
Recently, we reported that TIMP-2−/− mice exhibit a movement phenotype suggestive of muscle weakness [37] that is associated with reduced β1 integrin expression in fast-twitch muscle [38]. The present study was undertaken to characterize myogenesis of TIMP-2−/− myoblasts in vitro. Here, we showed that TIMP-2−/− myotubes are larger than wild-type myotubes when differentiated in media containing 2% horse serum, but significantly smaller than wild-type myotubes when serum-free media is used. Regardless of culture condition, the size changes are directly correlated with MMP activity and inversely correlated with β1 integrin expression in TIMP-2−/− myotubes. More strikingly, treatment with TIMP-2 or MMP-2 rescued the reduced TIMP-2−/− myotube size and increased active MMP-9 secretion, but only TIMP-2 treatment decreased β1 integrin expression. This suggests a regulatory relationship between TIMP-2 and β1 integrin expression during myogenesis.
Because myofiber diameter influences its contractile strength, myofiber size must be tightly regulated. The formation of a syncytial multinucleated myofiber from mononucleated myoblasts involves an initial fusion between myoblasts, and subsequent fusion of myoblasts with nascent myotubes resulting in increased myofiber size. Many molecules regulating initial myoblast fusion have been identified [48], including α3 [49], α4 [50], and β1 [27, 51] integrins, as well as proteases [8, 11, 47, 51] and their inhibitors [52, 53]. The second phase of myoblast fusion/elongation is regulated by a smaller cadre of molecules, including rolling pebbles in Drosophila [54, 55], and the nuclear factor of activated T cell (NFAT) family member NFATc2 [56] and interleukin-4 [57, 58] in mammals. Based on the data presented here, we propose that TIMP-2 regulates myofiber size during the initial phase of myoblast fusion.
A role for metalloproteases in myogenesis has long been proposed [8] and some of the responsible proteases have been identified. Recent studies have demonstrated a role for MMP-2 [9, 47], MT1-MMP [47, 59], and MMP-9 [10] in myoblast migration and fusion. In the absence of TIMP-2-mediated MMP inhibition, one would expect increased MMP activity and enhanced myogenesis. Indeed, increased net gelatinolytic activity was detected in TIMP-2−/− myotubes in horse serum-containing media at 1 days, the period of myoblast migration and fusion; thus, resulting in increased myotube size at 3 days. The exact opposite occurred in serum-free media, net gelatinase activity at 1 day, and myotube size at 3 days, was decreased. Although net gelatinolytic activity in TIMP-2−/− serum-free conditioned media was decreased, no decrease in MMP-2 or MMP-9 activity could be detected by western blot or zymographic analyses. This suggests that other MMPs contribute to the overall reduced gelatinase activity. While most MMPs possess gelatinase activity, MMP-3 is of particular relevance to this study. MMP-3 is expressed in muscle [60], removes agrin from the synaptic basal lamina in an activity-dependent manner [61], MMP-3−/− mice [62] show NMJ alterations similar to TIMP-2−/− mice [38], and proMMP-3 is activated via MMP-2/TIMP-2. Whether MMP-3 activity is reduced in TIMP-2−/− muscle in vitro or in vivo needs to be determined.
The role of TIMPs during myogenesis in vitro has not been investigated. Of the four known TIMPs, TIMP-2 is the most abundantly expressed in muscle in vivo and its expression is maintained throughout adulthood [38, 60]. TIMP-2 is a complex molecule in that it is involved in MMP activation as well as inhibition. Extracellular TIMP-2 is required for proMMP-2 activation via MT1-MMP [13]. Thus, even though MT1-MMP expression was increased in TIMP-2−/− myotubes cultured in horse serum, proMMP-2 activation was reduced. MT2-MMP can activate proMMP-2 in the absence of TIMP-2 [63] and MT2-MMP is expressed in muscle [60]. Nonetheless, proMMP-2 activation was impaired; thus, demonstrating the importance of the MT1-MMP/TIMP-2 complex in proMMP-2 activation. We demonstrated here, and elsewhere [45], that TIMP-2−/− mice possess a TIMP-2 protein variant that is not secreted. The expression of the TIMP-2 variant was significantly reduced in TIMP-2−/− myotubes, relative to wild-type myotubes, in the presence of horse serum, but not reduced under serum-free conditions, suggesting that the TIMP-2 promoter contains serum response element(s). Regardless of culture condition, TIMP-2 expression significantly increased coincident with wild-type and TIMP-2−/− myotube formation. The presence of multiple E-box consensus sequences in the TIMP-2 promoter (unpublished observation) suggests it may be a target of myogenic regulatory factors similar to the membrane-anchored MMP-regulator RECK [53]. TIMP-2 also alters cell growth and survival in an MMP-independent manner via integrins [17, 18]. The increased β1 integrin expression at 3 days in TIMP-2−/− myotubes differentiated in serum-free media may simply be a compensatory up-regulation to promote myoblast migration/fusion due to decreased MMP expression at 1 day. However, the treatment of TIMP-2−/− myotubes with TIMP-2, MMP-2, and MMP-9 points to a more specific regulatory relationship between TIMP-2 and β1 integrin.
Both TIMP-2 and MMP-2 treatment was associated with increased MMP-9 activity in the conditioned media, suggesting that the rescued TIMP-2−/− myotube size was mediated by MMP-9. That the rescue was more pronounced at 1 day, than 3 days, supports our hypothesis that initial myoblast fusion is affected. The TIMP-2 dose response was also consistent with an MMP-dependent mechanism. At low doses, TIMP-2 serves to activate proMMP-2; thus, MMP activity and myotube size increase. At higher doses, TIMP-2 inhibits MMP activity and myotube size began to decrease. MMP-9 treatment rescued the reduced TIMP-2−/− myotube size at a lower concentration than MMP-2. This suggests that MMP-9, although expressed at lower levels than MMP-2, may play a more important role in myogenesis than MMP-2. It was particularly striking that MMP-9 treatment up-regulated the cellular expression of TIMP-2, even though its proposed promoter region was deleted [14]. This up-regulation cannot be due to increased myotube size since both TIMP-2 and MMP-2 treatment increased myotube size, but had no effect on TIMP-2 expression. Rather, increased TIMP-2 expression is likely due to increased cellular MMP-9 expression, which did not occur in TIMP-2 and MMP-2 treated cultures. The contribution of the intracellular TIMP-2 to myogenesis warrants further investigation.
While reduced TIMP-2−/− myotube size in serum-free media was rescued by treatment with recombinant TIMP-2, MMP-2, or MMP-9, only TIMP-2 treatment was associated with decreased β1 integrin expression. The mechanism of how TIMP-2 alters β1 integrin expression is presently not known. What is clear it that β1 integrin expression is not decreased simply due to increased myotube size since both MMP-2 and MMP-9 treatment increased myotube size, but did not alter β1 integrin expression. It is interesting to note that TIMP-2, but not MMP-2 or MMP-9, treatment also decreased MT1-MMP expression. The interaction of MMPs and TIMPs with integrins has been demonstrated in a number of systems. Integrins have been shown to serve as a docking system for the proMMP-2, TIMP-2, MT1-MMP complex [64, 65] and promote the activation of proMMP-2 and proMT1-MMP [66–68]. Furthermore, ADAM-12 regulates myogenic migration and fusion by binding α3β1 integrin [49]. ADAM-12 has also been shown to regulate myogenic cell differentiation via α9β1 integrin [51] via an RGD-independent mechanism [69]. Whether TIMP-2 interacts with β1 integrins in an RGD-dependent or RGD-independent manner needs to be addressed. In addition, whether the regulation of integrin expression occurs at the transcriptional level needs to be determined.
The co-localization of TIMP-2 with β1 integrins at costameres in wild-type fast-twitch muscle and decreased β1 expression in TIMP-2−/− fast-twitch muscle [38], combined with the regulation of β1 integrin expression in response to TIMP-2 treatment suggests a regulatory relationship between these two molecules. Further studies are needed to enhance our understanding of the role of MMPs and TIMPs during myogenesis. The data garnered from these studies potentially will open new therapeutic avenues for neuromuscular disorders and myopathies.
Acknowledgments
We thank Dr. Felix Eckenstein for kindly providing bFGF, Dr. Rae Nishi for providing cover slips for the co-culture experiments and use of the microplate reader, and Dr. Carson Cornbrooks for critical review of the manuscript. This work was supported by Grant NS045225 co-funded by NINDS and NCRR (DMJ). Genotyping was performed using equipment provided by the University of Vermont Neuroscience Center of Biomedical Research Excellence Molecular Core facility (NIH NCRR IP20 RR16435). Densitometric analysis was performed in the VT Cancer Center DNA Analysis Facility and was supported, in part, by grant P30CA22435 from the NCI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parker MH, Seale P, Rudnicki MA. Looking back to the embryo: defining transcriptional networks in adult myogenesis. Nat Rev Genet. 2003;4:497–507. doi: 10.1038/nrg1109. [DOI] [PubMed] [Google Scholar]
- 2.Brand-Saberi B. Genetic and epigenetic control of skeletal muscle development. Ann Anat. 2005;187:199–207. doi: 10.1016/j.aanat.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Krauss RS, Cole F, Gaio U, Takaesu G, Zhang W, Kang JS. Close encounters: regulation of vertebrate skeletal myogenesis by cell-cell contact. J Cell Sci. 2005;118:2355–2362. doi: 10.1242/jcs.02397. [DOI] [PubMed] [Google Scholar]
- 4.Nagase H, Woessner JF. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 5.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 6.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386:15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huovila AP, Turner AJ, Pelto-Huikko M, Karkkainen I, Ortiz RM. Shedding light on ADAM metalloproteinases. Trends Biochem Sci. 2005;30:413–422. doi: 10.1016/j.tibs.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Couch CB, Strittmatter WJ. Rat myoblast fusion requires metalloendoprotease activity. Cell. 1983;32:257–265. doi: 10.1016/0092-8674(83)90516-0. [DOI] [PubMed] [Google Scholar]
- 9.El Fahime E, Torrente Y, Caron NJ, Bresolin MD, Tremblay JP. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258:279–287. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- 10.Lewis MP, Tippett HL, Sinanan AC, Morgan MJ, Hunt NP. Gelatinase-B (matrix metalloproteinase-9; MMP-9) secretion is involved in the migratory phase of human and murine muscle cell cultures. J Muscle Res Cell Motil. 2000;21:223–233. doi: 10.1023/a:1005670507906. [DOI] [PubMed] [Google Scholar]
- 11.Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- 12.Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Butler GS, Butler MJ, Atkinson SJ, Will H, Tamura T, van Westrum SS, Crabbe T, Clements J, d’Ortho M-P, Murphy G. The TIMP2 membrane type 1 metalloproteinase “receptor” regulates the concentration and efficient activation of progelatinase A. J Biol Chem. 1998;273:871–880. doi: 10.1074/jbc.273.2.871. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caterina JJ, Yamada S, Caterina NCM, Longenecker G, Holmbäck K, Shi J, Yermovsky AE, Engler JA, Birkedal-Hansen H. Inactivating mutation of the mouse Tissue Inhibitor of Metalloproteinases-2 (Timp-2) gene alters proMMP-2 activation. J Biol Chem. 2000;275:26416–26422. doi: 10.1074/jbc.M001271200. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez CA, Butterfield C, Jackson G, Moses MA. Structural and functional uncoupling of the enzymatic and angiogenic inhibitory activities of tissue inhibitor of metalloproteinase-2 (TIMP-2): loop 6 is a novel angiogenesis inhibitor. J Biol Chem. 2003;278:40989–40995. doi: 10.1074/jbc.M306176200. [DOI] [PubMed] [Google Scholar]
- 17.Seo DW, Li H, Guedez L, Wingfield PT, Diaz T, Salloum R, Wei BY, Stetler-Stevenson WG. TIMP-2 mediated inhibition of angiogenesis: an MMP-independent mechanism. Cell. 2003;114:171–180. doi: 10.1016/s0092-8674(03)00551-8. [DOI] [PubMed] [Google Scholar]
- 18.Pérez-Martínez L, Jaworski DM. Tissue inhibitor of metalloproteinase-2 promotes neuronal differentiation by acting as an anti-mitogenic signal. J Neurosci. 2005;25:4917–4929. doi: 10.1523/JNEUROSCI.5066-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 20.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 21.Belkin AM, Retta SF. β1D integrin inhibits cell cycle progression in normal myoblasts and fibroblasts. J Biol Chem. 1998;273:15234–15240. doi: 10.1074/jbc.273.24.15234. [DOI] [PubMed] [Google Scholar]
- 22.Rohwedel J, Guan K, Zuschratter W, Jin S, Ahnert-Hilger G, Furst D, Fassler R, Wobus AM. Loss of β1 integrin function results in a retardation of myogenic, but an acceleration of neuronal, differentiation of embryonic stem cells in vitro. Dev Biol. 1998;201:167–184. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]
- 23.Jaffredo T, Horwitz AF, Buck CA, Rong PM, Dieterlen-Lievre F. Myoblast migration specifically inhibited in the chick embryo by grafted CSAT hybridoma cells secreting an anti-integrin antibody. Development. 1988;103:431–446. doi: 10.1242/dev.103.3.431. [DOI] [PubMed] [Google Scholar]
- 24.Yao CC, Ziober BL, Squillace RM, Kramer RH. α7 integrin mediates cell adhesion and migration on specific laminin isoforms. J Biol Chem. 1996;271:25598–25603. doi: 10.1074/jbc.271.41.25598. [DOI] [PubMed] [Google Scholar]
- 25.Echtermeyer F, Schober S, Poschl E, von der Mark H, von der Mark K. Specific induction of cell motility on laminin by α7 integrin. J Biol Chem. 1996;271:2071–2075. doi: 10.1074/jbc.271.4.2071. [DOI] [PubMed] [Google Scholar]
- 26.Crawley S, Farrell EM, Gu WWM, Huang HY, Huynh V, Hodges BL, Cooper DN, Kaufman SJ. The α7β1 integrin mediates adhesion and migration of skeletal myoblasts on laminin. Exp Cell Res. 1997;235:274–286. doi: 10.1006/excr.1997.3671. [DOI] [PubMed] [Google Scholar]
- 27.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. β1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4:673–685. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 28.Belkin AM, Retta SF, Pletjushkina OY, Balzac F, Silengo L, Fassler R, Koteliansky VE, Burridge K, Tarone G. Muscle β1D integrin reinforces the cytoskeleton-matrix link: modulation of integrin adhesive function by alternative splicing. J Cell Biol. 1997;139:1583–1595. doi: 10.1083/jcb.139.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boppart MD, Burkin DJ, Kaufman SJ. α7β1 integrin regulates mechanotransduction and prevents skeletal muscle injury. Am J Physiol Cell Physiol. 2006;290:C1660–1666. doi: 10.1152/ajpcell.00317.2005. [DOI] [PubMed] [Google Scholar]
- 30.Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin α7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17:318–323. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 31.Yang JT, Rando TA, Mohler WA, Rayburn H, Blau HM, Hynes RO. Genetic analysis of α4 integrin functions in the development of mouse skeletal muscle. J Cell Biol. 1996;135:829–835. doi: 10.1083/jcb.135.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 33.Taverna D, Disatnik MH, Rayburn H, Bronson RT, Yang J, Rando TA, Hynes RO. Dystrophic muscle in mice chimeric for expression of α5 integrin. J Cell Biol. 1998;143:849–859. doi: 10.1083/jcb.143.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menko AS, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 35.Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 1995;9:1883–1895. doi: 10.1101/gad.9.15.1883. [DOI] [PubMed] [Google Scholar]
- 36.Schwander M, Shirasaki R, Pfaff SL, Muller U. β1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J Neurosci. 2004;24:8181–8191. doi: 10.1523/JNEUROSCI.1345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaworski DM, Soloway P, Caterina J, Falls WA. Tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice display motor deficits. J Neurobiol. 2006;66:82–94. doi: 10.1002/neu.20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lluri G, Langlois GD, McClellan B, Soloway PD, Jaworski DM. Tissue inhibitor of metalloproteinase-2 (TIMP-2) regulates neuromuscular junction development via a β1 integrin-mediated mechanism. J Neurobiol. 2006;66:1365–1377. doi: 10.1002/neu.20315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banker GA. Trophic interactions between astroglial cells and hippocampal neurons in culture. Science. 1980;209:809–810. doi: 10.1126/science.7403847. [DOI] [PubMed] [Google Scholar]
- 41.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lluri G, Jaworski DM. Regulation of TIMP-2, MT1-MMP, and MMP-2 expression during C2C12 differentiation. Muscle Nerve. 2005;32:492–499. doi: 10.1002/mus.20383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaworski DM, Beem-Miller M, Lluri G, Barrantes-Reynolds R. A potential regulatory relationship between the nested gene DDC8 and its host gene Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) Physiol Genomics. 2006;28:168–178. doi: 10.1152/physiolgenomics.00160.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen DL, Roy RR, Edgerton VR. Myonuclear domains in muscle adaptation and disease. Muscle Nerve. 1999;22:1350–1360. doi: 10.1002/(sici)1097-4598(199910)22:10<1350::aid-mus3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 47.Oh J, Takahashi R, Adachi E, Kondo S, Kuratomi S, Noma A, Alexander DB, Motoda H, Okada A, Seiki M, Itoh T, Itohara S, Takahashi C, Noda M. Mutations in two matrix metalloproteinase genes, MMP-2 and MT1-MMP, are synthetic lethal in mice. Oncogene. 2004;23:5041–5048. doi: 10.1038/sj.onc.1207688. [DOI] [PubMed] [Google Scholar]
- 48.Horsley V, Pavlath GK. Forming a multinucleated cell: molecules that regulate myoblast fusion. Cells Tissues Organs. 2004;176:67–78. doi: 10.1159/000075028. [DOI] [PubMed] [Google Scholar]
- 49.Brzóska E, Bello V, Darribere T, Moraczewski J. Integrin α3 subunit participates in myoblast adhesion and fusion in vitro. Differentiation. 2006;74:105–118. doi: 10.1111/j.1432-0436.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 50.Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- 51.Lafuste P, Sonnet C, Chazaud B, Dreyfus PA, Gherardi RK, Wewer UM, Authier FJ. ADAM12 and α9β1 integrin are instrumental in human myogenic cell differentiation. Mol Biol Cell. 2005;16:861–870. doi: 10.1091/mbc.E04-03-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barnoy S, Glasner T, Kosower NS. The role of calpastatin (the specific calpain inhibitor) in myoblast differentiation and fusion. Biochem Biophys Res Commun. 1996;220:933–938. doi: 10.1006/bbrc.1996.0509. [DOI] [PubMed] [Google Scholar]
- 53.Echizenya M, Kondo S, Takahashi R, Oh J, Kawashima S, Kitayama H, Takahashi NM C. The membrane-anchored MMP-regulator RECK is a target of myogenic regulatory factors. Oncogene. 2005;24:5850–5857. doi: 10.1038/sj.onc.1208733. [DOI] [PubMed] [Google Scholar]
- 54.Rau A, Buttgereit D, Holz A, Fetter R, Doberstein SK, Paululat A, Staudt N, Skeath J, Michelson AM, Renkawitz-Pohl R. rolling pebbles (rols) is required in Drosophila muscle precursors for recruitment of myoblasts for fusion. Development. 2001;128:5061–5073. doi: 10.1242/dev.128.24.5061. [DOI] [PubMed] [Google Scholar]
- 55.Menon SD, Osman Z, Chenchill K, Chia W. A positive feedback loop between Dumbfounded and Rolling pebbles leads to myotube enlargement in Drosophila. J Cell Biol. 2005;169:909–920. doi: 10.1083/jcb.200501126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Horsley V, Friday BB, Matteson S, Kegley KM, Gephart J, Pavlath GK. Regulation of the growth of multinucleated muscle cells by an NFATC2-dependent pathway. J Cell Biol. 2001;153:329–338. doi: 10.1083/jcb.153.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horsley V, Jansen KM, Mills ST, Pavlath GK. IL-4 acts as a myoblast recruitment factor during mammalian muscle growth. Cell. 2003;113:483–494. doi: 10.1016/s0092-8674(03)00319-2. [DOI] [PubMed] [Google Scholar]
- 58.Lafreniere JF, Mills P, Bouchentouf M, Tremblay JP. Interleukin-4 improves the migration of human myogenic precursor cells in vitro and in vivo. Exp Cell Res. 2006;312:1127–1141. doi: 10.1016/j.yexcr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 59.Ohtake Y, Tojo H, Seiki M. Multifunctional roles of MT1-MMP in myofiber formation and morphostatic maintenance of skeletal muscle. J Cell Sci. 2006;119:3822–3832. doi: 10.1242/jcs.03158. [DOI] [PubMed] [Google Scholar]
- 60.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563:129–134. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- 61.Werle MJ, VanSaun M. Activity dependent removal of agrin from synaptic basal lamina by matrix metalloproteinase 3. J Neurocytol. 2003;32:905–913. doi: 10.1023/B:NEUR.0000020631.69804.f5. [DOI] [PubMed] [Google Scholar]
- 62.VanSaun M, Herrera AA, Werle MJ. Structural alterations at the neuromuscular junctions of matrix metalloproteinase 3 null mutant mice. J Neurocytol. 2003;32:1129–1142. doi: 10.1023/B:NEUR.0000021907.68461.9c. [DOI] [PubMed] [Google Scholar]
- 63.Morrison CJ, Butler GS, Bigg HF, Roberts CR, Soloway PD, Overall CM. Cellular activation of MMP-2 (gelatinase A) by MT2-MMP occurs via a TIMP-2-independent pathway. J Biol Chem. 2001;276:47402–47410. doi: 10.1074/jbc.M108643200. [DOI] [PubMed] [Google Scholar]
- 64.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 65.Hornebeck W, Emonard H, Monboisse JC, Bellon G. Matrix-directed regulation of pericellular proteolysis and tumor progression. Semin Cancer Biol. 2002;12:231–241. doi: 10.1016/s1044-579x(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 66.Stanton H, Gavrilovic J, Atkinson S, d’Ortho MP, Yamada KM, Zardi L, Murphy G. The activation of proMMP-2 (gelatinase A) by HT1080 fibrosarcoma cells is promoted by culture on a fibronectin substrate and is concomitant with an increase in processing of MT1-MMP (MMP-14) to a 45 kDa form. J Cell Sci. 1998;111:2789–2798. doi: 10.1242/jcs.111.18.2789. [DOI] [PubMed] [Google Scholar]
- 67.Hofmann UB, Westphal JR, Van Kraats AA, Rutter DJ, Van Muijen GNP. Expression of integrin αVβ3 correlates with activation of membrane-type matrix metalloproteinase-1 (MT1-MMP) and matrix metalloproteinase-2 (MMP-2) in human melanoma cells in vitro and in vivo. Int J Cancer. 2000;87:12–19. doi: 10.1002/1097-0215(20000701)87:1<12::aid-ijc3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 68.Deryugina EI, Ratnikov BI, Yu Q, Baciu PC, Rozanov DV, Strongin AY. Prointegrin maturation follows rapid trafficking and processing of MT1-MMP in furin-negative colon carcinoma LoVo cells. Traffic. 2004;5:627–641. doi: 10.1111/j.1600-0854.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 69.Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin α9β1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. doi: 10.1074/jbc.M001953200. [DOI] [PubMed] [Google Scholar]


