Abstract
Objective
To examine whether solid versus liquid meal-replacement products differentially affect appetite and appetite-regulating hormones in older adults.
Methods
On two occasions, 9 subjects (age: 61 ± 3 years; BMI: 25.6 ± 1.3 kg/m2) consumed 25 % of daily energy needs as solid or liquid meal-replacements of similar energy contents. Blood and appetite ratings were collected over 4 hours.
Results
The post-prandial hunger composite (area under the curve) was lower following the solid versus liquid meal-replacement (p < 0.005) and remained below baseline over 4 hours (p < 0.05). Similar responses were observed with the desire to eat. The insulin and ghrelin composites were lower following the solid trial compared to the liquid [insulin: 5825 (range: 4676–11639) vs. 7170 (4472–14169) uIU/l · 240 min, p < 0.01; ghrelin: − 92798 (range: − 269130–47528) vs. − 56152 (range: − 390855–30840) pg/ml · 240 min, p < 0.05]. Ghrelin also remained below baseline over 4 hours (p < 0.05). No differences in cholecystokinin and leptin were observed between products.
Conclusion
The consumption of comparable meal-replacement products in solid versus liquid versions with similar energy contents led to differential appetitive responses and should not be viewed as dietary equivalents in older adults.
Keywords: food rheology, ghrelin, energy balance, elderly
Introduction
Aging has been associated with compromised regulation of energy balance leading to obesity, anorexia, and frailty [1, 2]. Specifically, in 2003–2004, 71 % of adults of 60 years age and above were considered overweight or obese [3]. For many other older adults (30–50 %), the imbalance of energy intake and expenditure results in weight loss and an increased risk of frailty [2, 4]. Thus, it is critical to examine potential dietary strategies that may aid in the management of energy balance and body weight.
Commercial meal replacement products, in the form of snack bars and shakes (i.e., solid and liquid versions), have been readily available since the early 1990s and have become popular among individuals trying to lose weight [5]. Several studies have found excellent compliance along with sustained weight loss when meal replacements were incorporated into a weight loss program in overweight and obese individuals [5–7]. Meal-replacement products may also be useful in the prevention of weight loss in individuals with poor energy balance regulation, which is a common occurrence in older adults [8–10]. Specifically, meal-replacements have been shown to increase body weight in lean, older adults through increased 24-hour energy intake [9]. However, the manufacturers of these meal-replacement products promote comparable results for their respective solid and liquid versions and in fact equally incorporate them into their diet programs. Further, many of the scientific studies previously mentioned do not examine the potential differences between the solid and liquid meal-replacements utilized in their study [5–10]. There is strong evidence that liquid foods elicit weaker appetitive and dietary responses than solid foods [11, 12]. Furthermore, the sensation of satiety occurs more rapidly following solid food consumption compared to liquid consumption and exists for a longer time period, delaying the return of hunger [12]. Thus, the primary aim of this study was to examine whether comparable solid (S) and liquid (L) meal-replacement products (MRPs) with similar energy content differentially affect appetite (hunger, fullness, and desire to eat) in older adults. Numerous hormones have also been shown to influence appetite and the regulation of energy balance [13, 14]. Specifically, insulin, leptin, ghrelin, and cholecystokinin (CCK) are involved with acute and/or chronic regulation of food intake and/or body weight [13]. While knowledge is rapidly increasing concerning the role that these hormones play in appetite control, little is known about how solids and liquids affect these hormones. Thus, the secondary aim of this study was to examine whether the previously mentioned MRPs will differentially affect specific appetite-regulating hormones in older adults.
Materials and Methods
Subjects
Potential participants were recruited by newspaper advertisements and flyers posted in the greater Lafayette, Indiana region. Study inclusion was based on the following criteria: men and women between the ages of 50 and 80 years; not dieting; no weight loss or gain (> 5 lbs) within the last six months; females: post-menopausal (>1 year); body mass index between 22–35 kg/m2; normal liver and kidney functions; and, not diabetic. Nine subjects completed the study (Table 1). All study procedures were approved by the Purdue University Biomedical Institutional Review Board, and all subjects were informed of the purpose, procedures, and potential risks of the study prior to signing the informed consent document. Monetary compensation was provided to the participants.
Table 1.
Subject characteristics of the 9 subjects
| Subject Characteristics | Mean ± SEM |
|---|---|
| Gender | |
| Male (n) | 2 |
| Female (n) | 7 |
| Age (years) | 61 ± 3 |
| Height (cm) | 170 ± 2 |
| Weight (kg) | 74.7 ± 4.5 |
| BMI (kg/m2) | 25.7 ± 1.3 |
| Fasting glucose (mg/dl) | 94 ± 2 |
| Fasting insulin (uIU/l) | 8.2 ± 1.1 |
Experimental design
This was a randomized, repeated-measures study including two trials for each subject. Subjects reported to the laboratory at 7 AM following an overnight fast on two separate days with one week between testing days. A venous catheter was inserted for blood draws. After the baseline blood sample was taken, the subject was provided with a meal consisting of 25 % of estimated daily energy needs as a commercially available MRP bar or beverage. Subjects were also required to consume 237 mL of water during the S-MRP to minimize differences in thirst and oral wetting. An equal amount of water (237 mL) was also consumed during the L-MRP. The subject consumed the meal within a 15-minute period. Blood sampling was performed at 15, 60, 120, 180, and 240 minutes after meal consumption. Plasma glucose, insulin, ghrelin, CCK, and leptin concentrations were assessed from blood samples collected in ethylenediaminetetraacetic acid (EDTA) tubes that were centrifuged, aliquoted, and stored at − 80 ° C for analysis. Appetitive sensations (i.e., hunger, fullness, desire to eat) were rated using visual analog scales [15] following each blood draw.
Meal-replacement products
The foods consumed in this study were Slim · fast® Original Meal-Replacement Bars and Shakes (Slim · fast® Foods Company, Englewood, NJ). Table 2 lists the energy and macronutrient composition of the S and L-MRPs. The subjects were asked to consume 25 % of their estimated daily energy needs, determined as 1.5 times their estimated resting energy expenditure [16]. Thus, each subject consumed an average of 2.5 bars or shakes.
Table 2.
Total energy and macronutrient composition of solid and liquid meal-replacements
| Solid meal- replacement | Liquid meal- replacement | |
|---|---|---|
| Energy Content | ||
| Single Serving (kcal/serving) | 220 | 220 |
| (gram weight/serving) | 54 | 217* |
| Meal (kcal/meal) | 540 ± 28 | 540 ± 28 |
| (gram weight) | 138 ± 7 | 553 ± 29* |
| Macronutrient Composition | ||
| (% of energy intake) | ||
| Carbohydrate | 64 | 73 |
| Protein | 15 | 18 |
| Fat | 25 | 10 |
| (g/meal) | ||
| Carbohydrate | 86 ± 4 | 98 ± 5* |
| Protein | 20 ± 1 | 24 ± 1* |
| Fat | 15 ± 0.8 | 6 ± 1* |
| Fiber | 2 ± 1 | 12 ± 1* |
Data expressed as Mean ± SEM
Solid vs. Liquid Meal-replacement; Two-tailed Wilcoxon Test (p<0.05)
Measurement of glucose and hormones
Plasma glucose was measured by an oxidase method on a COBAS analyzer (Mira Plus, Roche Diagnostic Systems, Serial ♯ 31–1718, Indianapolis, IN, USA). The limit of detection was 0–500 mg/dl with a sensitivity of 1 mg/dl. Plasma insulin was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). The range of the kit was 3–300–2084 uIU/l. The assay sensitivity was < 1 uIU/ml; the intra-assay and inter-assay coefficients of variation for the assay controls were 2.6 and 1.3 % for the low controls and 5.2 and 6.2 % for the high controls, respectively. Plasma total ghrelin was measured with an EIA (enzyme immunoassay) kit (Phoenix Pharmaceuticals, Belmont, CA, USA). The range of the kit was 0–100 ng/ml. The assay sensitivity was 0.08 ng/ml; the intra-assay and inter-assay coefficients of variation for the assay control were 4 and < 15 %, respectively. Plasma CCK was measured with an EIA (enzyme immunoassay) kit (Phoenix Pharmaceuticals, Belmont, CA, USA). The range of the kit was 0–100 ng/ml. The assay sensitivity was 0.08 ng/ml; the intra-assay and inter-assay coefficients of variation for the assay control were 4 and < 15 %, respectively. Plasma leptin was measured with an enzyme-linked immunosorbent assay (ELISA) kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA). The range of the kit was 0–100 ng/ml. The assay sensitivity was 0.47 ng/ml; the intra-assay and inter-assay coefficients of variation for the assay controls were 4.4 and 6.2 % for the low controls and 4.9 and 5.3 % for the high controls, respectively. All samples from a given subject were tested in duplicate and analyzed in the same assay.
Statistical analyses
Due to the small sample size, non-parametric two-tailed Wilcoxon Signed Rank tests were performed on the meal characteristics and appetite and hormonal area under or over the curve (AUC or AOC) data between the S and L-MRP trials. The subject and dietary characteristics are expressed as mean ± SEM. The appetite and hormonal measurements are expressed as median (range). If any differences in baseline hormonal data were observed between MRPs, the data was examined and expressed according to change from baseline (time point value minus baseline). p-Value < 0.05, 2-tailed is considered statistically significant. Statistical analyses were performed using SPSS (Version 12.0; Chicago, IL).
Results
Appetite
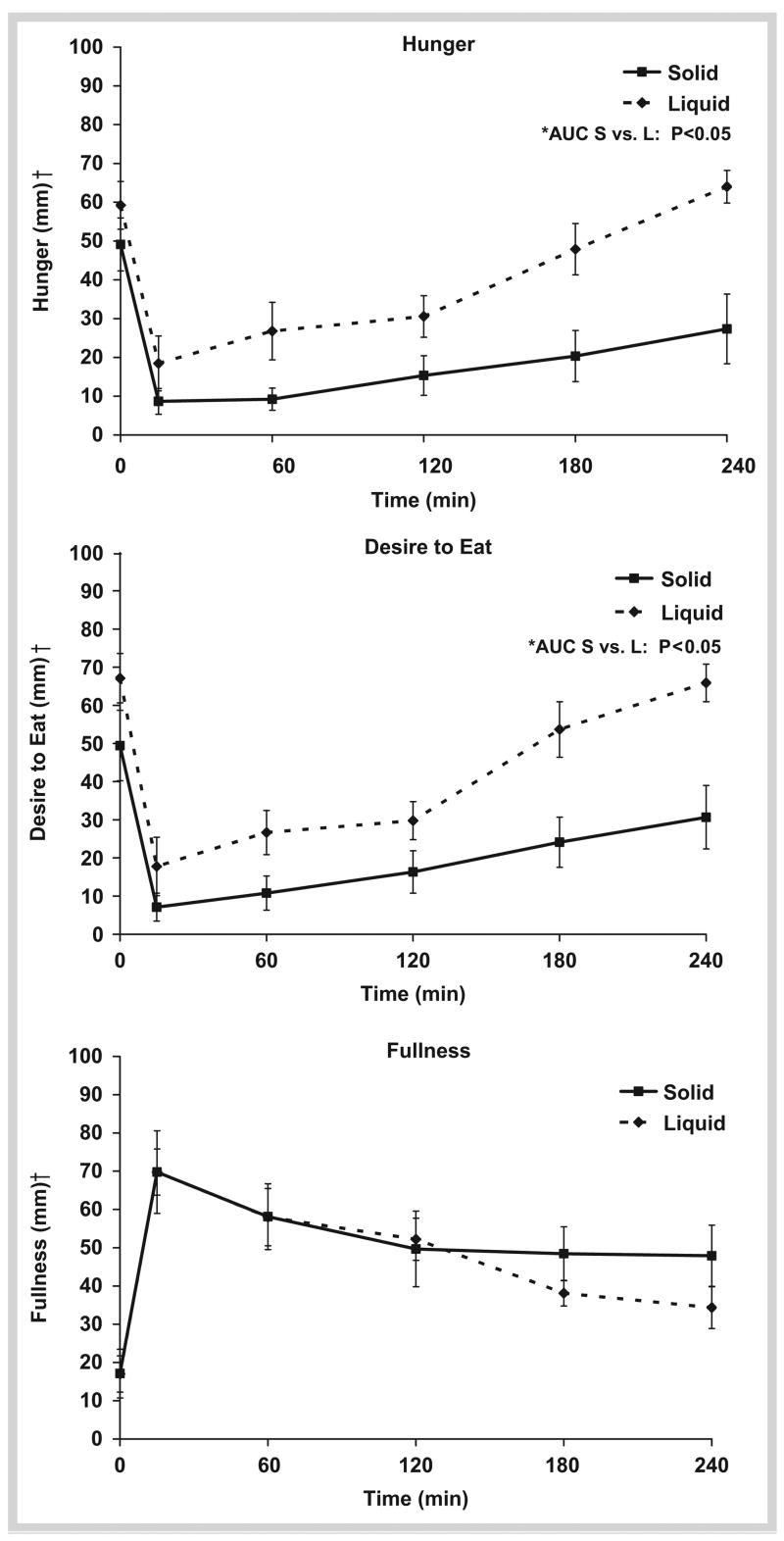
Hunger immediately declined following meal consumption, reaching nadir levels 15 minutes post-prandially regardless of meal type. However, within 60 minutes after S and L ingestion, hunger began to progressively increase but remained lower at every time point in the S-MRP compared to the L (p < 0.05; Fig. 1). At 4-hour post-meal ingestion, hunger was 14 ± 10 % higher than fasting with the L-MRP; whereas hunger remained below fasting with the S-MRP by 45 ± 19 %. The hunger AUC was lower with the S-MRP [2408 mm · 240 min (range: 945–8805 mm · 240 min)] compared to the L-MRP [8235 mm · 240 min (range: 2572–12892 mm · 240 min);p < 0.005]. Similar responses were observed with the desire to eat ( Fig. 1). Specifically, the desire to eat at each time point and AUC over the 4-hour period were lower with the S-MRP [AUC: 3315 mm · 240 min (range: 225–11025 mm · 240 min)] than with the L-MRP [AUC: 7958 mm · 240 min (range: 2348–13455 mm · 240 min; p < 0.05)]. While there was an increase in fullness after both MRPs, no significant differences were observed at individual time points ( Fig. 1) or AUC [S: 11475 mm · 240 min (range: 4800–21270 mm · 240 min); L: 12442 mm · 240 min (range: 8978–15592 mm · 240 min); p = 0.60].
Fig. 1.
Appetite ratings over 4 hours for liquid vs. solid meal-replacements. Meal was consumed at 0 minute. *AUC Solid vs. Liquid Meal-replacement; Two-tailed Wilcoxon Test (p<0.05). †Visual Analog Scale (1–100 mm).
Glucose
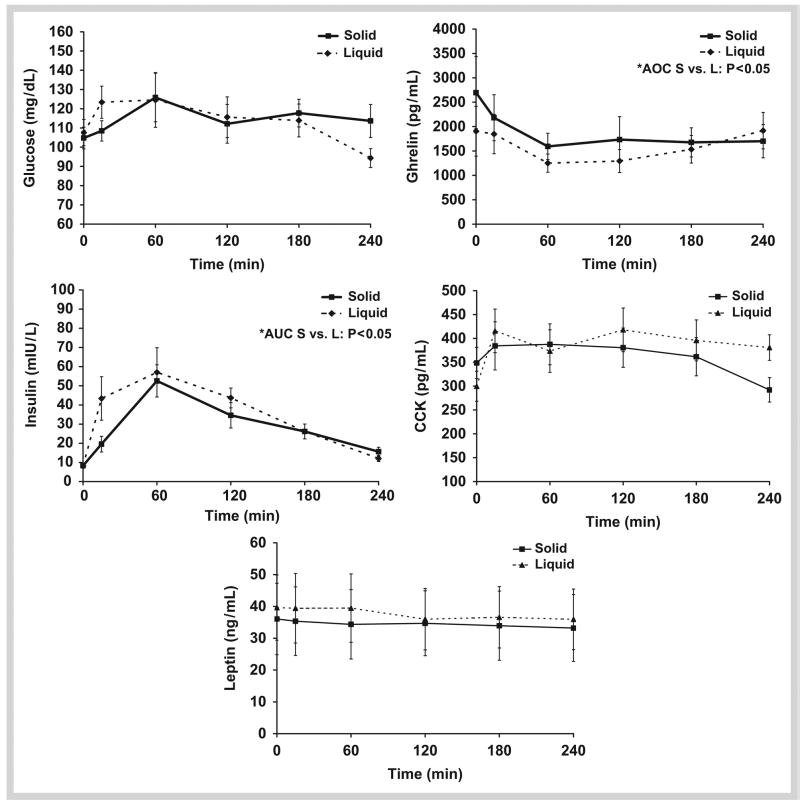
Within 15 minutes of meal consumption, plasma glucose concentrations significantly increased with the L-MRP (p < 0.01) but did not reach significance until 60 minutes with the S-MRP (p < 0.05). No significant differences existed between plasma glucose AUC in the S [23861 mg/dl · 240 min (range: 21304–39161 mg/dl · 240 min)] vs. L-MRP [28185 mg/dl · 240 min (range: 21364–40271 mg/dl · 240 min; p = 0.68] ( Fig. 2).
Fig. 2.
Appetite-regulating hormone concentrations over 4 hours for liquid vs. solid meal-replacements. Meal was consumed at 0 minute. *AUC; AOC Solid vs. Liquid Meal-replacement; Two-tailed Wilcoxon Test (p<0.05).
Appetite-regulating Hormones
After meal consumption, plasma insulin concentrations increased and peaked at 60 minutes with both MRPs ( Fig. 2). Insulin concentrations were lower in the S-MRP at 15 minutes (p < 0.05) and 120 minutes (p < 0.05) compared to the L-MRP ( Fig. 2). The insulin AUC response was lower following the S-MRP (5825 uIU/l · 240 min (range: 4676–11639 uIU/l · 240 min) vs. the L-MRP (7170 uIU/l · 240 min (range: 4472–14169 uIU/l · 240 min); p < 0.01).
Plasma ghrelin concentrations declined following the consumption of both meals and reached nadir levels at 60 minutes (p < 0.02). At the end of the testing period, ghrelin tended to be lower in the S-MRP compared to the L-MRP (p = 0.086). Specifically, with the S-MRP, plasma ghrelin remained below baseline 4 hours after meal consumption (p < 0.05), whereas the plasma ghrelin following the L-MRP returned to baseline at 4 hours after the meal ( Fig. 2). Because the baseline ghrelin concentrations were significantly different between meals [S: 2721 pg/ml (range: 468–6367 pg/mL) vs. L: 1676 pg/ml (range: 792–5869 pg/mL); p < 0.05], the AOC was examined according to change from baseline. Thus, ghrelin AOC (expressed as change from baseline) was lower following the S-MRP [−92798 pg/ml · 240 min (range: −269130–47528 pg/ml · 240 min)] compared to the L-MRP [−56152 pg/ml · 240 min (range: −390855–30840 pg/ml · 240 min)]; p < 0.05).
Because the baseline CCK concentrations were significantly different between meals [S: 330 pg/ml (range: 205–469 pg/ml) vs. L: 266 pg/ml (range: 174–411 pg/ml); p < 0.05], the AUC was examined according to change from baseline. The AUC was not different between meals [S: − 1290 pg/ml · 240 min (range: −35078–6555 pg/ml · 240 min) vs. L: −5528 pg/ml · 240 min (range: −14122–6540 pg/ml · 240 min); p = 0.14].
Plasma leptin remained relatively unchanged throughout the 4-hour period with no differences between meals [AUC: S-MPR: 5503 ng/ml · 240 min (range: 693–22370 ng/ml · 240 min) vs. L-MRP: 7543 ng/ml · 240 min (range: 945–19911 ng/ml · 240 min); p=0.17] ( Fig. 2).
Discussion
Previous research documents that solid foods lead to greater and more prolonged reductions of hunger were compared to low viscosity foods such as liquids [11, 12, 17, 18]. Specifically, Mattes et al. and Rothacker et al, [11, 18] compared meal-replacement bar and shake effects on hunger, fullness, and desire to eat and found that hunger and desire to eat remained below baseline for 3 hours following consumption of the liquid MRP. The bar led to a reduced hunger and desire to eat for 5 hours [11, 18]. Almiron-Roig et al. examined the satiety response following solid and liquid study foods (i.e., cola and cookies) and found no differences in satiety [19]. However, the study foods were consumed during a low-hunger state, thus limiting the power to observe differential appetitive responses. Our research findings are consistent with the findings of weaker responses to liquids in that the S-MRP elicited lower hunger and desire to eat compared to the L-MRP. While these differential appetite data provide further insight surrounding the physiological responses to solids vs. liquids, future studies are needed to identify whether these responses would lead to differential food intake.
Consistent with the appetite data, we observed a greater and more prolonged reduction in plasma total ghrelin concentration following the S-MRP compared to the L-MRP. The S-MRP also led to lower plasma insulin concentration throughout the 4-hour period compared to the L-MRP. Lastly, our study showed that despite the higher hunger and desire to eat following the L-MRP, plasma leptin and CCK concentrations remained fairly stable for both S and L-MRPs.
While the S and L-MRPs had similar energy content, the macro-nutrient compositions were not matched. Specifically, the L-MRP contained more carbohydrate, protein, and fiber, and less fat than the S-MRP. These differences may have affected the appetite and hormonal responses, making it difficult to draw quantitative conclusions about an independent effect of solids vs. liquids. Due to the higher protein, higher fiber, and lower fat content in the L-MRP compared to the S-MRP, a greater reduction in hunger and desire to eat would be expected. However, the S-MRP led to reduced hunger and desire to eat indicating that the properties of solids and liquids were stronger appetite regulators than macronutrient composition. Concerning the hormonal responses to macro-nutrients, insulin responds to glucose and protein and remains virtually unaffected by dietary fat and fiber intake [20]. Thus, it is possible that the lower insulin response observed during the solid meal may have been due to the lower glucose and protein concentrations. The effect of macronutrient composition on the ghrelin response is limited and controversial. However, recent studies show significant declines in ghrelin following carbohydrate [20], protein [21, 22], and fiber consumption [23]. Thus, we likely biased the trial to yield a greater reduction in post-prandial ghrelin concentration with the liquid meal. The fact that reduced post-prandial ghrelin was observed lends support for a strong role for the properties of solid foods. CCK responds to dietary protein [13] and fiber [24]. Specifically, Bourdon et al. found that CCK was twice as high following the consumption of a high fiber meal compared to a low-fiber meal [24]. In the present study, the liquid meal-replacement had higher fiber compared to the solid meal replacement yet no differences in CCK were found. Several studies suggest leptin is responsive to dietary fat and/or protein [25]. However, energy density, which is a characteristic difference between solids and liquids of the same energy content, appears to have a greater influence on appetite and the related hormones compared to the macronutrient content [26]. Our results would also support this statement as the S-MRP was more energy dense than the L-MRP with similar energy content. Further studies comparing liquid vs. solid meals with identical macronutrient composition are needed to quantitatively and independently identify the effects of solids vs. liquids on these appetite-regulating hormones.
Numerous studies have examined the effects of meal-replacements on body weight regulation. Heymsfield et al. performed a meta-analysis of 30 weight loss studies utilizing meal-replacement products and found that meal-replacement products are as effective as conventional meal plans for prolonged weight loss and are more convenient [5]. Recently, Noakes et al. incorporated both solid and liquid Slim · fast® meal-replacements, similar to the products in our study, into a six-month weight loss program and found that these meal-replacements led to a 9.4 % reduction in body weight [27]. While meal-replacements lead to successful weight loss, our study shows that the consumption of comparable solid and liquid MRPs leads to differential appetitive responses. While speculative, this would suggest greater compliance and therefore greater and more prolonged weight loss if solid MRPs are incorporated into weight loss programs in overweight and obese older adults. Alternately, Wouters-Wesseling et al. evaluated the effects of consuming a liquid supplement on body weight over a 6-month period in healthy elderly individuals [9]. The liquid supplement led to greater weight gain (+ 1.6 kg) compared to those individuals not consuming the liquid [9]. Similar results were also observed in frail, undernourished elderly people over a 16-week period [10]. Taken together, these studies support the application of L-MRPs in promoting weight gain and the prevention of body weight reduction in lean older adults through reduced suppression of hunger and desire to eat. While our study was not designed to examine whether solid meal-replacements lead to better energy balance or weight control, the differential responses in appetite between solids and liquids may aid in management of energy balance and body weight.
Conclusion
Lower hunger, desire to eat, insulin, and ghrelin responses observed with the solid meal-replacement product compared to the liquid version underscore that they should not be used interchangeably for energy balance and weight control in overweight or underweight older individuals.
Acknowledgments
This study was supported by NIH grant R01 AG021911-0102 (WWC) which included a diversity assistantship to AJS, a Purdue graduate assistantship (AJS), Phyllis Izant gift funds, and summer Howard Hughes Internships (SMT and RAS).
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.MacIntosh C, Morley JE, Chapman IM. The anorexia of aging. Nutrition. 2000;16:983–995. doi: 10.1016/s0899-9007(00)00405-6. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 4.Yeh SS, Schuster MW. Geriatric cachexia: the role of cytokines. Am J Clin Nutr. 1999;70:183–197. doi: 10.1093/ajcn.70.2.183. [DOI] [PubMed] [Google Scholar]
- 5.Heymsfield SB, van Mierlo CA, van der Knaap HC, Heo M, Frier HI. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes Relat Metab Disord. 2003;27:537–549. doi: 10.1038/sj.ijo.0802258. [DOI] [PubMed] [Google Scholar]
- 6.Flechtner-Mors M, Ditschuneit HH, Johnson TD, Suchard MA, Adler G. Metabolic and weight loss effects of long-term dietary intervention in obese patients: four-year results. Obes Res. 2000;8:399–402. doi: 10.1038/oby.2000.48. [DOI] [PubMed] [Google Scholar]
- 7.Ditschuneit HH, Flechtner-Mors M, Johnson TD, Adler G. Metabolic and weight-loss effects of a long-term dietary intervention in obese patients. Am J Clin Nutr. 1999;69:198–204. doi: 10.1093/ajcn/69.2.198. [DOI] [PubMed] [Google Scholar]
- 8.Rolls BJ, Dimeo KA, Shide DJ. Age-related impairments in the regulation of food intake. Am J Clin Nutr. 1995;62:923–931. doi: 10.1093/ajcn/62.5.923. [DOI] [PubMed] [Google Scholar]
- 9.Wouters-Wesseling W, Van Hooijdonk C, Wagenaar L, Bindels J, de Groot L, Van Staveren W. The effect of a liquid nutrition supplement on body composition and physical functioning in elderly people. Clin Nutr. 2003;22:371–377. doi: 10.1016/s0261-5614(03)00034-7. [DOI] [PubMed] [Google Scholar]
- 10.Payette H, Boutier V, Coulombe C, Gray-Donald K. Benefits of nutritional supplementation in free-living, frail, undernourished elderly people: a prospective randomized community trial. J Am Diet Assoc. 2002;102:1088–1095. [PubMed] [Google Scholar]
- 11.Mattes RD, Rothacker D. Beverage viscosity is inversely related to post-prandial hunger in humans. Physiol Behav. 2001;74:551–557. doi: 10.1016/s0031-9384(01)00597-2. [DOI] [PubMed] [Google Scholar]
- 12.Leathwood P, Pollet P. Effects of slow release carbohydrates in the form of bean flakes on the evolution of hunger and satiety in man. Appetite. 1988;10:1–11. doi: 10.1016/s0195-6663(88)80028-x. [DOI] [PubMed] [Google Scholar]
- 13.Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi: 10.1677/joe.1.05866. [DOI] [PubMed] [Google Scholar]
- 14.Pliquett RU, Fuhrer D, Falk S, Zysset S, von Cramon DY, Stumvoll M. The effects of insulin on the central nervous system – focus on appetite regulation. Horm Metab Res. 2006;38:442–446. doi: 10.1055/s-2006-947840. [DOI] [PubMed] [Google Scholar]
- 15.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the ‘Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–334. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 16.Harris JA, Benedict FG. A biometric study of basal metabolism in man. Washington, D.C: Carnegie Institute of Washington; 1919. [Google Scholar]
- 17.Sepple CP, Read NW. Gastrointestinal correlates of the development of hunger in man. Appetite. 1989;13:183–191. doi: 10.1016/0195-6663(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 18.Rothacker DQ, Watemberg S. Short-term hunger intensity changes following ingestion of a meal replacement bar for weight control. Int J Food Sci Nutr. 2004;55:223–226. doi: 10.1080/09637480410001734111. [DOI] [PubMed] [Google Scholar]
- 19.Almiron-Roig E, Flores SY, Drewnowski A. No difference in satiety or in subsequent energy intakes between a beverage and a solid food. Physiol Behav. 2004;82:671–677. doi: 10.1016/j.physbeh.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89:3048–3054. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 21.Bowen J, Noakes M, Clifton PM. Appetite regulatory hormone responses to various dietary proteins differ by body mass index status despite similar reductions in ad libitum energy intake. J Clin Endocrinol Metab. 2006;91:2913–2919. doi: 10.1210/jc.2006-0609. [DOI] [PubMed] [Google Scholar]
- 22.Tannous dit El Khoury D, Obeid O, Azar ST, Hwalla N. Variations in postprandial ghrelin status following ingestion of high-carbohydrate, high-fat, and high-protein meals in males. Ann Nutr Metab. 2006;50:260–269. doi: 10.1159/000091684. [DOI] [PubMed] [Google Scholar]
- 23.Nedvidkova J, Krykorkova I, Bartak V, Papezova H, Gold PW, Alesci S, et al. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2003;88:1678–1682. doi: 10.1210/jc.2002-021669. [DOI] [PubMed] [Google Scholar]
- 24.Bourdon I, Olson B, Backus R, Richter BD, Davis PA, Schneeman BO. Beans, as a source of dietary fisber, increase cholecystokinin and apol-ipoprotein b48 response to test meals in men. J Nutr. 2001;131:1485–1490. doi: 10.1093/jn/131.5.1485. [DOI] [PubMed] [Google Scholar]
- 25.Weigle DS, Breen PA, Matthys CC, Callahan HS, Meeuws KE, Burden VR, et al. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am J Clin Nutr. 2005;82:41–48. doi: 10.1093/ajcn.82.1.41. [DOI] [PubMed] [Google Scholar]
- 26.Bell EA, Castellanos VH, Pelkman CL, Thorwart ML, Rolls BJ. Energy density of foods affects energy intake in normal-weight women. Am J Clin Nutr. 1998;67:412–420. doi: 10.1093/ajcn/67.3.412. [DOI] [PubMed] [Google Scholar]
- 27.Noakes M, Foster PR, Keogh JB, Clifton PM. Meal replacements are as effective as structured weight-loss diets for treating obesity in adults with features of metabolic syndrome. J Nutr. 2004;134:1894–1899. doi: 10.1093/jn/134.8.1894. [DOI] [PubMed] [Google Scholar]