A lectin from C. roseum seeds (CRL) has been purified, characterized and crystallized.
Keywords: Cymbosema roseum, Diocleinae, lectins
Abstract
A lectin from Cymbosema roseum seeds (CRL) was purified, characterized and crystallized. The best crystals grew in a month and were obtained by the vapour-diffusion method using a precipitant solution consisting of 0.1 M Tris–HCl pH 7.8, 8%(w/v) PEG 3350 and 0.2 M proline at a constant temperature of 293 K. A data set was collected to 1.77 Å resolution at a synchrotron-radiation source. CRL crystals are orthorhombic, belonging to space group P212121. Crystallographic refinement and full amino-acid sequence determination are in progress.
1. Introduction
Plant lectins are a structurally heterogeneous group of carbohydrate-binding proteins of non-immune origin that exhibit a variety of interactions in cellular processes such as cell communication, host defence, fertilization, parasitic infection and tumour metastasis. These lectins are present in various organisms, animal and vegetal, including leguminous plants. Despite the high level of conservation exhibited in their sequences, leguminous lectins show considerable diversity in their carbohydrate-binding properties and biological effects (Wah et al., 2001 ▶).
The Diocleinae lectins, a well studied group of closely related leguminous lectins, exhibit biological effects such as histamine release from rat peritoneal mast cells (Ferreira et al., 1996 ▶) and anti- (Assreuy et al., 1997 ▶) and pro-oedematogenic effects (Alencar et al., 1999 ▶). Minor differences in the ratios of dimeric and tetrameric forms in the lectins, together with differences in the relative orientations of the carbohydrate-binding sites in the quaternary structures, have been hypothesized to contribute to the differences in biological activities exhibited by Diocleinae lectins (Sanz-Aparicio et al., 1997 ▶).
The species Cymbosema roseum belongs to the Diocleinae subtribe of the Leguminosae family and is widespread throughout the Amazonian forest (Anavilhanas archipelago, Amazonas, Brazil). This work reports the purification, partial characterization, crystallization and preliminary X-ray diffraction analysis of a lectin from C. roseum (CRL).
2. Materials and methods
2.1. Purification and crystallization of C. roseum lectin (CRL)
C. roseum seeds were ground to a fine powder in a coffee mill. The powder was stirred with 0.15 M NaCl [1:10(w:v)] at room temperature for 4 h and then centrifuged at 10 000g for 20 min at 278 K. The resultant supernatant was applied onto a Sepharose-4B-mannose column (0.5 × 10 cm) equilibrated with 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2. After removing unbound material, the lectin was eluted with 0.1 M glycine, 0.15 M NaCl pH 2.6. Purified CRL was monitored by SDS–PAGE as described by Laemmli (1970 ▶) and was used to perform further characterization. N-terminal sequence analysis was performed using an Applied Biosystems pulsed-liquid phase 477A protein sequencer with a 120A PTH amino-acid analyzer, following the method described by the manufacturer.
Haemagglutinating activity and haemagglutination-inhibition studies were carried out in micro-titration plates using a standard procedure (Faria et al., 2004 ▶). The haemagglutinating activity of the lectin was determined using native and enzyme-treated rabbit erythrocytes. Native cells were prepared by washing the erythrocytes three times with 0.1 M NaCl and then resuspending them to a final concentration of 2%(v/v) in 0.15 M NaCl. To prepare enzyme-treated cells, washed packed erythrocytes were incubated with an equal volume of papain or trypsin for 30 min at 310 K. Treated cells were washed three times in 0.15 M NaCl and resuspended to a final concentration of 2%(v/v) in 0.15 M NaCl. Haemagglutination tests were performed on serial twofold dilutions of lectin solutions in PBS, each dilution having a final volume of 0.2 ml. A 0.2 ml aliquot of the 2% erythrocyte suspension was added to each dilution. The plates were gently shaken and left for 90 min at room temperature, after which time the degree of macroscopic agglutination was recorded.
Inhibition tests were carried out using stock solutions of sugars and glycoproteins in 0.15 M NaCl. A twofold dilution series was prepared for each potentially inhibitory substance in 0.15 M NaCl containing 5 mM CaCl2 and 5 mM MnCl2. Each dilution had a final volume of 0.2 ml. The lectin samples were diluted in 0.15 M NaCl to give a solution containing four units of haemagglutinating activity per millilitre (the greatest dilution of the lectin that can agglutinate erythrocytes, i.e. the titre, was defined as containing one haemagglutinating unit per millilitre). 0.2 ml aliquots of the diluted lectin (four units) were added to each well of the diluted inhibitor series. The plates were then left at room temperature for 1 h before 0.2 ml of 2% native or enzyme-treated rabbit erythrocytes was added to each well. The plates were then allowed to stand at room temperature for a further 1 h before being examined for haemagglutination. The haemagglutination-inhibition titre was recorded as the the highest dilution of inhibitor which inhibited the agglutination produced by four haemagglutination units of lectin sample.
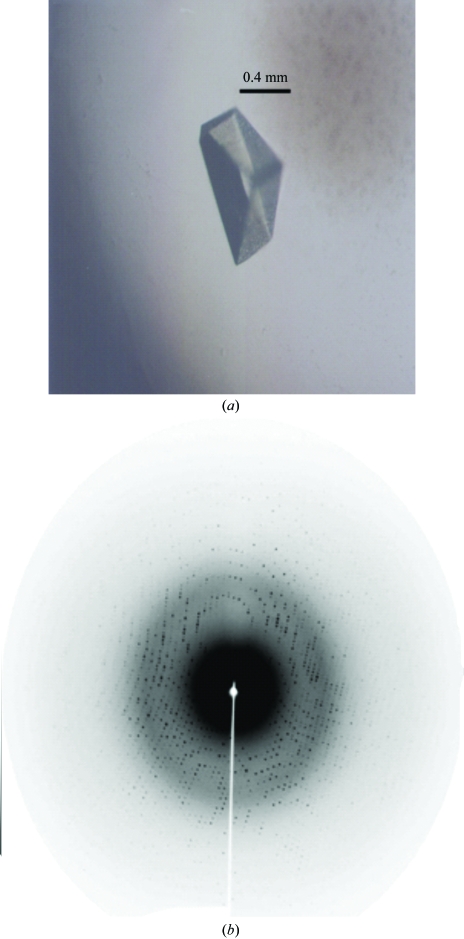
The lyophilized purified CRL was dissolved to a concentration of 12 mg ml−1 in 20 mM Tris–HCl pH 8.0 containing 0.5 mM CaCl2 and MnCl2 and used for crystallization trials. Crystallization screening by the hanging-drop vapour-diffusion method was performed in Linbro plates at 293 K using Hampton Research Crystal Screens I and II, SaltRx, Index and PEG/Ion Screens (Hampton Research, Aliso Viejo, CA, USA). The drops were composed of equal volumes (2 µl) of protein solution and reservoir solution and were equilibrated against 500 µl reservoir solution. An example of a crystal of CRL is shown in Fig. 1 ▶(a).
Figure 1.
(a) Crystal and (b) diffraction pattern of C. roseum lectin.
2.2. X-ray data collection
A crystal was transferred to a cryoprotectant solution consisting of 30% glycerol in the crystallization reservoir solution. Data were collected at 1.42 Å wavelength at a synchrotron-radiation source (beamline MX1, CPr station, Laboratório Nacional de Luz Síncrotron–LNLS, Campinas, Brazil) using a MAR Research CCD imaging plate at a crystal-to-detector distance of 70 mm. A set of 100 1° oscillation images was recorded (an image is shown in Fig. 1 ▶ b). Diffraction data were indexed, integrated and scaled using MOSFLM and SCALA (Collaborative Computational Project, Number 4, 1994 ▶).
An alignment analysis was performed with ClustalW (Thompson et al., 1994 ▶) that compared the N-terminal sequence of CRL (which was evidence of the success of the purification) with those of all non-redundant proteins deposited in the National Center of Biotechnology Information (NCBI). Determinations of the full amino-acid sequence and the three-dimensional structure are in progress.
3. Results and discussion
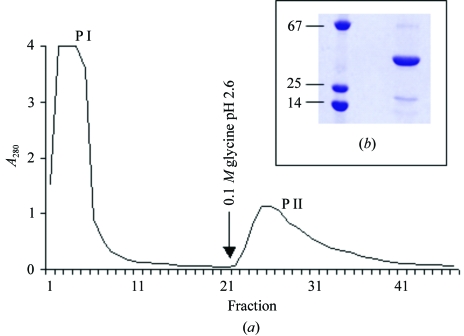
C. roseum lectin (CRL) was purified by a single step using a Sepharose-4B-mannose affinity chromatography column (Fig. 2 ▶ a). All purification steps were monitored by haemagglutinating activity and SDS–PAGE.
Figure 2.
Affinity chromatography and SDS–PAGE of the purified C. roseum lectin (CRL). (a) Sepharose-4B-mannose chromatography. (b) SDS–PAGE showing protein markers (left) and a purified CRL band (right). The molecular-weight markers are bovine serum albumin (67 kDa), chymotrypsinogen (25 kDa) and lysozyme (14 kDa). The gel was Coomassie blue stained.
CRL showed haemagglutinating activity towards papain-treated, trypsin-treated and untreated rabbit erythrocytes. The minimal concentration of purified protein that agglutinated a 2% rabbit erythrocyte suspension was <2 µg ml−1. This haemagglutinating property is similar to those of other Diocleinae lectins. From the inhibitory substances tested, mannose was the most potent, with a minimum concentration of 19.5 mM. The CRL N-terminal sequence was found to be ADTIVAVELDSYPNTDIGDPSYPH. This sequence is very similar to those of other lectins (Table 1 ▶) from the subtribe Diocleinae [100% identity with Dioclea lehmanni I (Perez et al., 1990 ▶) and Canavalia brasiliensis (Moreira & Cavada, 1984 ▶); 92% identity with D. grandiflora (Moreira et al., 1983 ▶), ConA (Hague, 1975 ▶) and Cratylia floribunda (Oliveira et al., 1991 ▶)]. The apparent molecular weight of CRL was determined by SDS–PAGE after heating in the presence of SDS (Fig. 2 ▶ b). The lectin appears to be composed of three polypeptide chains of approximate molecular weights of 30, 18 and 12 kDa. This variety of molecular weights is similar to that found for other lectins from Diocleineae species such as Canavalia ensiformis (Hague, 1975 ▶). This latter lectin, commonly known as concanavalin A, has been shown to initially be expressed with an initial set of termini, which led to the supposition that the gene product is then cleaved post-translationally to form two chains and a second set of termini and then fused into a single chain again by peptide-bond formation that joins the initial set of termini, a kind of circular permutation of the sequence. In the absence of other evidence, we suppose that the smaller chains are analogous to the two chains of the cleaved gene product and the largest chain is analogous to the fused final product (Cunningham et al., 1979 ▶).
Table 1. N-terminal sequence alignment of Diocleinae lectins.
| Lectin | Sequence |
|---|---|
| Cymbosema roseum | ADTIVAVELDSYPNTDIGDPSYPH |
| Dioclea lehmanni I | ADTIVAVELDSYPNTDIGDPSYPH |
| Dioclea grandiflora | ADTIVAVELNSYPNTDIGDPNYPH |
| Canavalia ensiformis | ADTIVAVELDTYPNTDIGDPSYPH |
| Canavalia brasiliensis | ADTIVAVELDSYPNTDIGDPSYPH |
| Cratylia floribunda | ADTIVAVELDSYPNTDIGDPNYQH |
Microcrystals were obtained using 0.1 M HEPES pH 7.5 containing 10%(w/v) PEG 3350 and 0.2 M proline. Improvement of this crystallization condition was obtaining by increasing the pH and PEG concentration. Suitable crystals were obtained from drops containing 0.1 M Tris–HCl pH 7.8, 8%(w/v) PEG 3350 and 0.2 M proline. CRL crystals grew within a month to maximum dimensions of approximately 0.8 × 0.4 × 0.4 mm (Fig. 1 ▶ a). The diffraction data showed the CRL crystals to be orthorhombic, belonging to space group P212121, with unit-cell parameters a = 67.8, b = 103.1, c = 122.1 Å. CRL crystal data were scaled in the resolution range 34.92–1.77 Å. Statistics of the data collection can be found in Table 2 ▶. Assuming the presence of four molecules of 25 kDa (as is standard for Diocleinae lectins that present an apparent weight of 30 kDa in SDS–PAGE, such as ConA and D. lehmanni lectin I) in each monomer in the asymmetric unit, the calculated V M was 2.1 Å3 Da−1 (Matthews, 1968 ▶), indicating a solvent content of 41.9%. Elucidation of the complete amino-acid sequence of CRL, now in progress, will permit the refinement of a three-dimensional CRL structure.
Table 2. Summary of data-collection statistics for CRL.
Values in parentheses are for the highest resolution shell (1.87–1.77 Å).
| X-ray wavelength (Å) | 1.427 |
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 67.82, b = 103.14, c = 122.09 |
| Resolution limits (Å) | 34.92–1.77 |
| Asymmetric unit content | 4 molecules |
| Total reflections measured | 286361 |
| Unique reflections measured | 80568 |
| Completeness (%) | 97.00 (97.0) |
| Rmerge (%) | 5.4 (32.5) |
| 〈I/σ(I)〉 | 7.1 (2.2) |
Acknowledgments
We thank Dr Juan J. Calvete of Instituto de Biomedicina de Valencia for his technical support. This work was partly supported by Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), FAPESP (SMOLBNet, 01/07532-0), Universidade Regional do Cariri (URCA), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Laboratório Nacional de Luz Síncrotron (LNLS) in Brazil. BSC, AHS and WFA are senior investigators of CNPq.
References
- Alencar, N. M. N., Teixeira, E. H., Assreuy, A. M. S., Cavada, B. S., Flores, C. A. & Ribeiro, R. A. (1999). Mediators Inflamm.8, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assreuy, A. M. S., Shibuya, M. D., Martins, G. J., Souza, M. L. P., Cavada, B. S., Moreira, R. A., Oliveira, J. T. A., Ribeiro, R. A. & Flores, C. A. (1997). Mediators Inflamm.6, 201–210. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763. [Google Scholar]
- Cunningham, B. A., Hemperly, J. J., Hopp, T. P. & Gerald, M. (1979). Proc. Natl Acad. Sci. USA, 76, 3218–3222. [DOI] [PMC free article] [PubMed]
- Faria, R. A., Andrade-Neto, M., Pinto, L. S., Castellon, R. R., Calvete, J. J. & Cavada, B. S. (2004). Arch. Latinoam. Nutr.54, 349–353. [PubMed] [Google Scholar]
- Ferreira, R. R., Cavada, B. S., Moreira, R. A., Oliveira, J. T. A. & Gomes, J. G. (1996). Inflamm. Res.45, 442–447. [DOI] [PubMed] [Google Scholar]
- Hague, D. R. (1975). Plant Physiol.55, 636–642. [DOI] [PMC free article] [PubMed]
- Laemmli, U. K. (1970). Nature (London), 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed] [Google Scholar]
- Moreira, R. A., Barros, A. C. H., Stewart, J. C. & Pusztai, A. (1983). Planta, 158, 63–69. [DOI] [PubMed]
- Moreira, R. A. & Cavada, B. S. (1984). Biologia Plant.26, 113–120.
- Oliveira, J. T. A., Cavada, B. S. & Moreira, R. A. (1991). Rev. Bras. Bot.14, 63–68.
- Perez, G., Hernandez, M. & Mora, E. (1990). Phytochemistry, 29, 1745–1749. [DOI] [PubMed] [Google Scholar]
- Sanz-Aparicio, J., Hermoso, J., Grangeiro, T. B., Calvete, J. J. & Cavada, B. S. (1997). FEBS Lett.405, 114–118. [DOI] [PubMed] [Google Scholar]
- Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1994). Nucleic Acids Res.22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wah, D. A., Romero, A., Gallego del Sol, F., Cavada, B. S., Ramos, M. V., Grangeiro, T. B., Sampaio, A. H. & Calvete, J. J. (2001). J. Mol. Biol.310, 885–894. [DOI] [PubMed] [Google Scholar]