Abstract
Myosin light-chain kinase (MLCK) of smooth muscle is multifunctional, being composed of N-terminal actin-binding domain, central kinase domain, and C-terminal myosin-binding domain. The kinase domain is the best characterized; this domain activates the interaction of smooth-muscle myosin with actin by phosphorylating the myosin light chain. We have recently shown that the Met-1–Pro-41 sequence of MLCK binds to actin to inhibit this interaction. However, it is not known whether the myosin-binding domain modifies the actin–myosin interaction. We designed MLCK⋅cDNA to overexpress the Asp-777–Glu-972 sequence in Escherichia coli. The purified Asp-777–Glu-972 fragment, although devoid of the kinase activity, exerted a stimulatory effect on the ATPase activity of dephosphorylated myosin (Vmax = 7.36 ± 0.44-fold, Km = 1.06 ± 0.20 μM, n = 4). When the N-terminal 39 residues of the fragment were deleted from the fragment, the resultant fragment, Met-816–Glu-972, lost the stimulatory activity. We synthesized the Ala-777–Ser-815 peptide that was deleted from the fragment and confirmed its stimulatory effect of the peptide (Vmax = 3.03 ± 0.22-fold, Km = 6.93 ± 1.61 μM, n = 3). When this peptide was further divided into Asp-777–Met-795 and Ala-796–Ser-815 peptides, the stimulatory activity was found in the latter. We confirmed that the myosin phosphorylation did not occur during the experiments with the above fragments and peptides. Therefore, we suggest that phosphorylation is not obligatory for smooth-muscle myosin not to be active.
Myosin light-chain kinase (MLCK) is an enzyme that phosphorylates the light chain of myosin in the presence of Ca2+ and calmodulin (Ca/CaM) (see refs. 1 and 2 for reviews). This catalytic domain is in the central part of MLCK neighbored by the regulatory domain that binds Ca/CaM. The N-terminal portion consists of actin-binding domains. At the Met-1–Pro-41 sequence, MLCK binds actin filaments (3) in a Ca/CaM-dependent manner (4). The Ca/CaM-independent actin-binding site is located somewhere between the Ca/CaM-dependent site and the catalytic domain (4). These actin-binding sites are expected to bundle actin filaments (5). The C terminus of MLCK is present in smooth-muscle cells as a gene product called telokin, which is independent of MLCK expression (6). Because telokin binds myosin to assemble it into thick filaments (7), the C terminus of MLCK is considered to be a myosin-binding domain.
The myosin phosphorylated by the catalytic domain of MLCK is in an active form and interacts with actin filaments to contract smooth muscle. This mode of phosphorylation is widely accepted as the regulatory path for actin–myosin interaction (see refs. 1 and 2 for reviews). However, there are a number of observations that are not explained by the mode of phosphorylation (see ref. 8 for review). To solve this problem, Ebashi and colleagues have proposed that MLCK can activate the actin–myosin interaction without phosphorylating myosin (9–11).
To search for the domain that is responsible for the stimulation, we overexpressed and purified various actin-binding fragments of MLCK in Escherichia coli, but we found inhibitory effects (see ref. 12 for review).
In this report, we focused on the C-terminal domain of MLCK and found the stimulatory effects of the domain on the actin–myosin interaction. Using recombinant proteins and synthetic peptides, we identified the amino acid sequences responsible for the stimulation.
MATERIALS AND METHODS
Native Proteins.
MLCKs were purified from the smooth muscles of chicken gizzard (13) and bovine stomach (10). Unless specified, MLCK was from chicken gizzard because we used this in most of our experiments. Telokin was purified from chicken gizzard as described by Ito et al. (14). Actin was purified from the acetone powder of chicken skeletal muscle (15) and used for experiments after polymerization. Myosin was purified from chicken-gizzard smooth muscle as described by Ebashi (16) and was mainly used in its dephosphorylated state. Part of myosin was phosphorylated by MLCK in the presence of Ca/CaM as described previously (17). Bovine-brain CaM was purchased from Sigma.
Recombinant Proteins.
The fragments of MLCK were obtained as described previously (4). In short, the pET21 vectors (Novagen) carrying cDNAs for the fragments were transfected to E. Coli BL 21 (DE 3). The fragments were overexpressed and then purified by the ammonium sulfate fractionation followed by DEAE- and CM-column chromatographies. The fragments were 777D/972E, 816M/972E, and 1M/515V, being composed of 777–972, 816–972, and 1–515 sequences, respectively, according to the primary structure of chicken-gizzard MLCK (18). Because the cDNA used for the construction of the vector encodes bovine-stomach MLCK (19), the figures were adjusted for chicken-gizzard MLCK by using the dnasis computer program.
Synthetic Peptides.
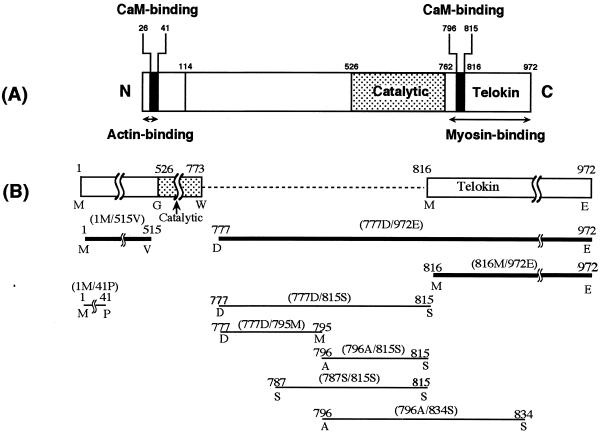
The gizzard MLCK sequences (18) of 1–41, 777–815, 777–795, 796–815, 787–815, and 796–834 were synthesized by using MilliGen 9050 peptide synthesizer and then purified according to the manufacturer’s instructions for N-(9-fluorenyl)methyloxy carbonyl (Fmoc) chemistry. They were used as 1M/41P peptide, 777D/815S peptide, 777D/795M peptide, 796A/815S peptide, 787S/815S peptide, and 796A/834S peptide, respectively. The topology of these peptides within MLCK is shown in Fig. 1 together with that of the recombinant fragments.
Figure 1.
Location of the recombinant fragments and synthetic peptides in MLCK. The number corresponds to the amino acid primary sequence number based on translation of the full length cDNA of chicken-gizzard MLCK (18). (A) Domain structure of MLCK. (B) Enlarged view of the part between catalytic and telokin domains to illustrate the various constructs. The thick and thin lines denote recombinant fragments and synthetic peptides, respectively.
ATPase Activity Measurements.
The effects of recombinant proteins and synthetic peptides were examined by measuring the ATPase activity of myosin (0.5 μM dephosphorylated myosin or 0.05 μM phosphorylated myosin) in the presence or absence of actin (10 μM for dephosphorylated myosin or 2 μM for phosphorylated myosin) under the conditions of 60 mM KCl/0.5 mM ATP/5 mM MgCl2/0.2 mM EGTA/1 mM DTT/20 mM Tris⋅HCl (pH 7.5) and various concentrations of MLCK or peptides. The phosphate liberated for 10 min at 25°C was quantified in duplicate or triplicate by the malachite green method (20). We ensured that the rate of ATP hydrolysis was steady and that the specific activity of the ATPase did not differ from our previously reported values. Thus, we were able to express the activity as a normalized ATPase activity (21). Vmax and Km were obtained by double reciprocal plots.
The effect of 777D/815S peptide on myosin ATPase activity was confirmed by single-turnover assays with 2′(3′)-O-N-methylanthraniloyl-ATP (mant-ATP) as the fluorescent analogue of ATP. This N-methylanthraniloyl derivative of ATP was prepared by reaction with N-methylisatoic anhydride (Molecular Probes) as described by Hiratsuka (22), except that after reaction the analogue was purified on a DEAE-cellulose column (23).
All fluorescence measurements were performed at 25°C in a RF-5000 spectrofluorometer (Shimadzu). One micromolar mant-ATP was added to a cuvette containing 0.5 μM dephosphorylated myosin, 60 mM KCl, 5 mM MgCl2, 20 mM Tris⋅HCl, pH 7.5, and 0.2 mM EGTA, so that the fluorescence enhancement and decay accompanying binding of mant-ATP to myosin and dissociation from myosin could be monitored. Mant-ATP fluorescence was excited at 356 nm, and the emission was monitored at 445 nm.
Interaction of Synthetic Peptides with Myosin and Actin.
The binding interaction was measured by surface plasmon resonance with the IAsys Cuvette System (Fisons, Cambridge, U.K.). The 777D/815S or 777D/795M peptide was immobilized to the cuvette of carboxy methylated dextran matrix via N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide according to the manufacturer’s protocol. Actin and either dephosphorylated or phosphorylated myosin were added to the cuvette and the resultant resonance at 25°C and pH 7.5 was recorded.
Other Methods.
SDS/PAGE was carried out by using the method of Laemmli (24) with a slight modification (25) so that we could check the purity (>95%) of every protein used in this study. The extent of phosphorylation of the 20-kDa light chain of smooth muscle myosin was examined by urea-PAGE, as previously described (26). Protein concentrations were determined by using the methods of Bradford (27) and/or Lowry et al. (28) with BSA as the standard.
RESULTS
Distinct Effects of MLCK on Phosphorylated and Dephosphorylated Myosins.
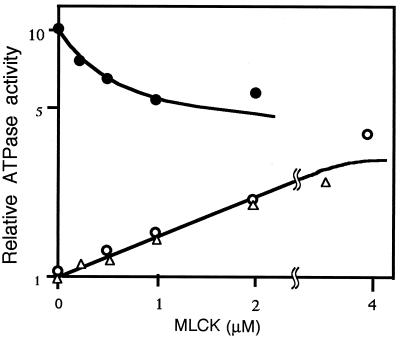
The actin-activated ATPase activities of myosin were measured in the presence of various concentrations of MLCK (Fig. 2). When myosin was phosphorylated beforehand (filled circles), the activity was inhibited by MLCK in a dose-dependent manner, confirming our previous reports (4, 29, 30, 31). With dephosphorylated myosin (open circles), the effect of MLCK is distinct; the activity was low in the absence of MLCK (ordinate), but it increased with the increase in MLCK concentrations. The increase was the same for MLCK from both chicken gizzard (open circles) and bovine stomach (open triangles). Double reciprocal plots of five independent experiments showed that the Vmax and Km were 4.31 ± 0.89-fold and 1.14 ± 0.26 μM (mean ± SEM, n = 6), respectively (Table 1).
Figure 2.
Effect of MLCK on the actin-activated ATPase activity of myosin. ATPase activities of dephosphorylated (filled circles) and phosphorylated (open circles and triangles) myosin were measured in the presence of actin, the various concentrations of chicken-gizzard MLCK (circles), and bovine-stomach MLCK (triangles). They were plotted on a logarithmic scale (ordinate) against MLCK concentrations (abscissa). Because CaM was not used in the assay, the effects were not mediated by the kinase activity of MLCK.
Table 1.
Effect on ATPase activities of dephosphorylated and phosphorylated myosins
| Actin-activated ATPase
|
Myosin ATPase
|
|||||
|---|---|---|---|---|---|---|
| Dephosphorylated myosin
|
Phosphorylated myosin
|
Dephosphorylated myosin
|
||||
| Vmax (-fold) | Km (μM) | Vmax (-fold) | Km (μM) | Vmax (-fold) | Km (μM) | |
| Native MLCK | 4.31 ± 0.89 (6) | 1.14 ± 0.26 (6) | Inhibition (refs. 31, 40) | (∗) | 3.85 (1) | 0.59 (1) |
| 777D/972E fragment | 7.36 ± 0.44 (4) | 1.06 ± 0.20 (4) | No stimulation (2) | No stimulation (2) | 3.70 (1) | 0.67 (1) |
| 816M/972E fragment | No stimulation (2) | No stimulation (2) | NT | NT | NT | NT |
| native Telokin | No stimulation (2) | No stimulation (2) | NT | NT | NT | NT |
| 777D/815S peptide | 3.03 ± 0.22 (3) | 6.93 ± 1.61 (3) | No stimulation (1) | No stimulation (1) | 3.45, 2.70 (2) | 9.70, 3.17 (2) |
| 777D/795M peptide | No stimulation (6) | No stimulation (6) | No stimulation (2) | No stimulation (2) | No stimulation (1) | No stimulation (1) |
| 796A/815S peptide | 3.24 ± 0.48 (5) | 20.0 ± 0.96 (5) | NT | NT | NT | NT |
| 787S/815S peptide | 2.68 ± 0.68 (3) | 15.4 ± 4.57 (3) | NT | NT | NT | NT |
| 7776A/834S peptide | 1.47, 2.94 | 14.7, 37.0 | NT | NT | NT | NT |
| 1M/41P peptide | No stimulation (2) | No stimulation (2) | NT | NT | NT | NT |
The numbers in parentheses refer to the number of experiments. NT, not tested; No stimulation, relative ATPase activities were plotted against the protein (0–5 μM) and peptide (0–0.3 mM) concentrations. When they were within 0.95–1.19-fold for the proteins and 0.90–1.50-fold for the peptides, it was considered no stimulation. ∗, half-maximal inhibition was observed at 0.15–0.20 μM, as described in refs. 31 and 40.
Although the measurement was carried out in the absence of Ca/CaM, it could be argued that the stimulation is caused by the residual kinase activity of MLCK. To address this argument, we repeated this experiment in the presence of wortmannin, a MLCK inhibitor (32) and found no effect on stimulation (data not shown). After the ATPase measurement, we also subjected the actomyosin to urea-PAGE and detected no evidence of myosin phosphorylation (data not shown).
Effects of Recombinant Fragments Devoid of the Kinase Domain of MLCK.
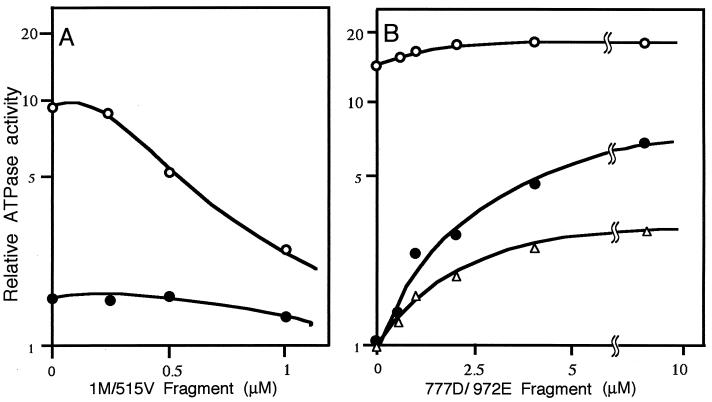
The N-terminal 1M/515V fragment exerted an inhibitory effect on the actin-activated ATPase activity of phosphorylated myosin but little effect on the activity of dephosphorylated myosin (Fig. 3A). Because the 1M/515V fragment contains the 1–41 sequence, where MLCK binds to actin to exert an inhibitory effect (3, 4), these data indicate that inhibitory effect observed with parent MLCK is specific for the phosphorylated myosin.
Figure 3.
Effect of 1M/515V and 777D/972E fragments on the actin-activated ATPase activity of myosin. Recombinant fragments of the N-terminal 1M/515V and C-terminal 777D/972E were designed to be devoid of the catalytic domain of MLCK. Their effects were examined by measuring actin-activated ATPase activity. (A) Effect of 1M/515V fragment. Open circles, effect on phosphorylated myosin; filled circles, effect on dephosphorylated myosin. (B) Effect of 777D/972E fragment. Open circles, effect on phosphorylated myosin; filled circles, effect on dephosphorylated myosin; triangles, effect on dephosphorylated myosin where 0.2 mM EGTA was replaced with CaM at equal molarity to 777D/972E fragment and 0.2 mM Ca2+. The stimulatory effect on the ATPase activity of dephosphorylated myosin was observed only with 777D/972E fragment.
Fig. 3B shows the effect of the C-terminal 777D/972E fragment. When myosin was phosphorylated, the stimulatory effect was slight. However, it exerted a stimulatory effect on the ATPase activity of dephosphorylated myosin with Vmax = 7.36 ± 0.04-fold and Km = 1.06 ± 0.20 μM (mean ± SEM, n = 4, Table 1). From this result by using a recombinant fragment devoid of the kinase activity of MLCK, we conclude that the stimulatory effect of parent MLCK on the ATPase activity is brought about by a mode that is not mediated by myosin phosphorylation.
Because the 777D/972E fragment contains the CaM-binding site of the 796–813 regulatory domain sequence (see ref. 33 for review), we examined the effect of Ca/CaM and found that Ca/CaM reduced the stimulatory effect to some extent (see Discussion).
Analysis of the Primary Sequence Responsible for the Stimulation.
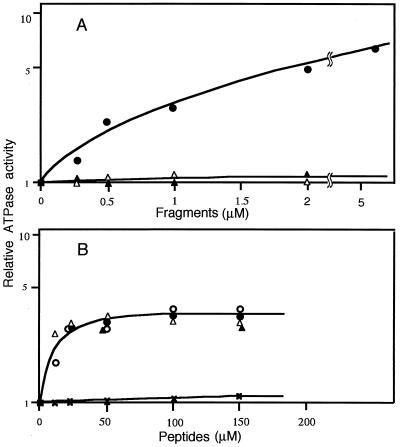
We designed another recombinant fragment, the 816M/972E fragment, which is devoid of the N-terminal 39 residues of the 777D/972E fragment. As shown in Fig. 4A, the stimulatory effect observed in the 777D/972E fragment (filled circles) was lost in the 816M/972E fragment (open triangles). Because the 816M/972E fragment is the same as telokin that was expressed in smooth-muscle cells as an independent gene product of MLCK (6, 7), we examined the effect of native telokin (filled triangles in Fig. 4A) and confirmed the absence of the stimulation. These observations indicate that the deleted 777–815 sequence is responsible for the stimulation.
Figure 4.
Effects of MLCK fragments and synthetic peptides on the actin-activated ATPase activity of dephosphorylated myosin. The actin-activated ATPase activity of myosin was examined in the presence of various concentrations of MLCK fragments and synthetic peptides under the conditions described in the legend to Fig. 2. (A) Effect of MLCK fragments. Filled circles, 777D/972E fragment; open triangles, 816M/972M fragment; filled triangles, telokin. (B) Effect of synthetic peptides. Filled circles, 777D/815S peptide; open circles, 796A/815S peptide; open triangles, 787S/815S peptide; filled triangles, 796A/834S peptide; crosses, 777D/795M.
We synthesized the above sequence as the 777D/815S peptide and examined its stimulatory effect. As shown by the filled circles in Fig. 4B, it stimulated with Vmax = 3.03 ± 0.22-fold and Km = 6.93 ± 1.61 μM (mean ± SEM, n = 3, Table 1).
We further deleted the C-terminal 20 residues from 777D/815S peptide to construct 777D/795M peptide. The peptide did not show any stimulation (crosses in Fig. 4B; Table 1), suggesting the 20 residues of 796–815 sequence play a key role in the stimulation. To confirm this suggestion, we synthesized three peptides of different length that shared the 20 residues, i.e., 796A/815S, and 796A/834S, 787S-815S (see Fig. 1) and then examined their effects. As shown in Fig. 4B, they all exerted stimulatory effects to a similar extent (Table 1), confirming the importance of the 796–815 sequence.
Binding of Myosin with Synthetic Peptides.
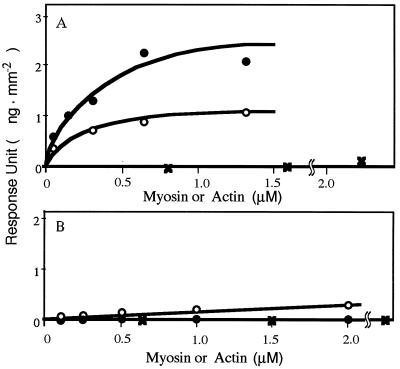
To investigate the interaction of myosin with the stimulatory peptides, we chose to immobilize the 777D/815S peptide in the IAsys cuvette, as this had the lowest Km of the synthetic peptides (Table 1). As shown in Fig. 5A, both phosphorylated and dephosphorylated myosins interacted with the peptide but not with actin. The higher signal for dephosphorylated myosin is compatible with the stimulatory effect on it by the peptide (see 777D/815S peptide in Table 1).
Figure 5.
Interaction of synthetic peptides with myosin and actin. Surface plasmon resonance responses of synthetic peptides [(A) 777D/815S; B, 777D/795M) immobilized in the IAsys cuvette was determined by adding actin (crosses), dephosphorylated myosin (filled circles), and phosphorylated myosin (open circles).
The 777D/795M peptide, which exhibited no effect on the activity (see 777D/795M peptide in Table 1), did not bind either phosphorylated or dephosphorylated myosin (Fig. 5B).
Single Turnovers.
The stimulation by 777D/815S peptide of the ATPase activity of dephosphorylated myosin was detectable in the absence of actin (see 777D/815S peptide in Table 1). This steady-state measurement may be influenced by a small population of high-activity molecules. To study this, we carried out single-turnover experiments with mant-ATP in the presence of various concentrations of the 777D/815S peptide.
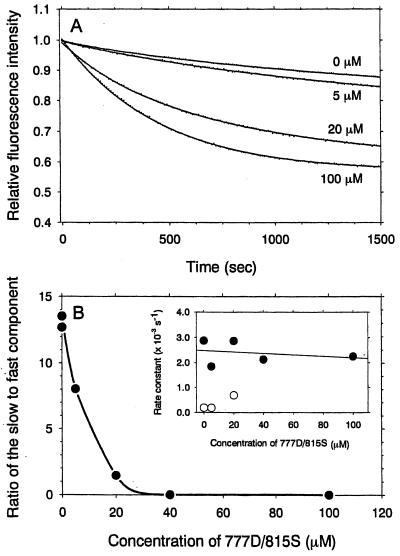
Addition of mant-ATP to the myosin solutions causes an abrupt increase in fluorescence because of mant-ATP binding. To prevent further mant-ATP binding, we added a 100-fold molar excess of ATP at the plateau of this transient binding. The subsequent decay in mant-nucleotide fluorescence corresponding to release of the hydrolysis products, mant-ADP and Pi, was measured (Fig. 6A). The time courses of this fluorescence decay were fitted to double exponential. The slow and fast components had rate constants of about 3 × 10−4 s−1 and about 2 × 10−3 s−1, respectively, which were independent of peptide concentration (Fig. 6B Inset). The slow component could be ascribed to the products released by dephosphorylated myosin (34). Increasing the peptide concentration to 40 μM resulted in the loss of the slow component and only the fast component was observed (Fig. 6B), supporting the idea of a single population of stimulated myosin in the presence of more than 40 μM of the peptide.
Figure 6.
Effects of 777D/815S peptide on the single turnover of Mg-ATPase of dephosphorylated myosin. (A) The decays in fluorescence intensity of mant-nucleotide were elicited by the addition of an excess of ATP. First, 0.5 μM dephosphorylated myosin was incubated with 1 μM mant-ATP and 0–100 μM 777D/815S peptide in 60 mM KCl/5 mM MgCl2/20 mM Tris⋅HCl, pH 7.5/0.2 mM EDTA. The mant fluorescence is enhanced as mant-ATP binds to myosin. At the plateau of this binding transient, a 100-fold molar excess of ATP was added to the myosin solution. The decay traces were fitted to double exponential plots. (B) The ratio of the fast and slow components of product release changed drastically in the presence of 777D/815S peptide. Increasing the concentration of 777D/815S peptide to 40 μM resulted in loss of the slow component and only the faster component with a rate constant of 2 × 10−3 s−1 was observed. (Inset) The rate constants of the mant fluorescence decay obtained by the double exponential fit were plotted against the peptide concentrations. Irrespective of the peptide concentrations, the rate constants of the fast (filled circles) and slow components (open circles) were about 2 × 10−3 s−1 and about 3 × 10−4 s−1, respectively, the latter being characteristic of the dephosphorylated myosin.
DISCUSSION
This report shows that MLCK is able to stimulate myosin ATPase activity without phosphorylating the light chain of myosin. The amino acid sequence responsible for the stimulation is the 796–815 sequence, which has been characterized as a regulatory domain for the kinase activity (see ref. 33 for review). Stimulation because of the mode of MLCK was about 3.5-fold (Table 1), which is much lower than the stimulation through the phosphorylating mode (Fig. 3B).
Ebashi and his colleagues induced superprecipitation of actin and dephosphorylated myosin by adding MLCK and found that MLCK is able to activate the actin–myosin interaction while keeping myosin phosphorylation in the basal level (9, 10). The present study confirmed their study by detecting a stimulatory effect of MLCK on the actin-activated ATPase activity of dephosphorylated myosin (Fig. 1). The failure of our previous studies (4, 29–31) to detect the stimulation can be explained by the use of phosphorylated myosin for the assay. However, we could not confirm the effect of Ca/CaM; for the induction of superprecipitation by MLCK, Ca/CaM is required (9, 10), but our stimulation of the ATPase activity was detectable in the absence of Ca/CaM. Indeed, Ca/CaM worked so as to obscure the stimulation (Fig. 3B, open triangles). The recombinant proteins used in the present study are not subject to post-transcriptional modification, which may be one of the causes for the discrepancy.
Contraction of smooth muscle is typically composed of phasic followed by tonic contraction. The latter, which is weaker than the former, is observed until agonists are washed out (see ref. 35 for review). This prolonged contraction is not associated with the elevation of either cytosolic Ca2+ concentration or myosin phosphorylation (see ref. 36 for review). The new regulatory mode reported in the present study is characterized by the weak stimulation of myosin ATPase activity under low Ca2+ concentrations with no sign of myosin phosphorylation. The obscuring of stimulation by Ca/CaM (Fig. 3B) is in accordance with the reports by Nishimura and van Breemen (37) that the tonic contraction of skinned fibers of smooth muscle relax much faster when Ca2+ is present than absent. We speculate that MLCK may support such a contraction through the new mode.
As shown in Fig. 2, maximum stimulation requires high concentration of MLCK, i.e., several-fold in excess of myosin. This aspect suggests that the MLCK effect is not enzymic and that MLCK remains bound to myosin during stimulation. According to the estimation by Dabrowska et al., MLCK in gizzard was only 1/17 of myosin on a molar basis (38). Thus the moiety of myosin that is associated with MLCK in vivo is too low to conclude the physiological relevance in terms of concentration. However, MLCK has been suggested to bind to actin/myosin with much higher affinity in vivo than in vitro (39). We need additional experiments to establish the physiological role of the present stimulatory effect.
It is an interesting observation that this mode of stimulation is more obvious in the 777D/972E fragment than in parent MLCK (Table 1). As reported previously (5), MLCK binds actin to assemble actin filaments into bundles. The 777D/972E fragment is devoid of these actin-binding sites, however (Fig. 1). This bundling activity may interfere with the access of myosin to actin, causing weaker activation by actin of myosin ATPase activity. Another expectation for the lower stimulatory activity of parent MLCK is that the 796–815 sequence of 777D/972E fragment is exposed so that it may interact with myosin more freely. In parent MLCK, however, this sequence is located at the position that does not allow myosin easy access to the sequence, resulting in weaker stimulation of myosin ATPase activity. The crystal structure of whole MLCK, which has not yet been resolved (see ref. 2 for review), will answer this speculation.
In relation to the above discussion, we also noted that the stimulatory effect of the 777D/972E fragment exceeded that of the 796A/815S peptide (Table 1). The fragment contains telokin in addition to the 796–815 sequence (see Fig. 1). The preliminary characterization of the MLCK fragment with the N-terminal 39 residues deleted from the 777D/972E fragment showed a weaker stimulation in myosin ATPase activity (data not shown). Therefore, the telokin domain may also play some unessential role in the stimulation. We speculate that the domain may increase the affinity of the 39 residues to myosin.
The stimulatory effect of MLCK because of this mode is brought about by the binding of MLCK to the myosin (Fig. 5A). A similar example is the stimulation of myosin ATPase activity by caldesmon and calponin (40–42). Although these are actin-binding proteins that inhibit the activity (43, 44), they stimulate the activity when actin is absent (see refs. 45 and 46 for review). Stimulation unassociated with myosin phosphorylation has been also reported by Ito et al., who found that monoclonal antibodies to subfragment 2 of myosin stimulate myosin ATPase activity (47). They explained the stimulation by the shape-activity hypothesis (48), where the conformational change of myosin from its 6S form to the 10S form on phosphorylation is an important factor in determining its enzymatic activity. It will be of interest to see whether the 39 residues of MLCK responsible for this mode can induce such a change.
The single-turnover experiments showed that there are two populations of dephosphorylated myosin under the present experimental conditions and that 777D/815S peptide shifted the equilibrium from slow to fast states. The rate constants for displacement of mant-ATP by an excess of ATP as determined by double exponential plots were 3 × 10−4 s−1 and 2 × 10−3 s−1 (Fig. 6). These rate constants did not change over the range of the 777D/815S peptide concentrations (Fig. 6B Inset). Nevertheless, the proportion of the fast and slow components was changed by the concentration of the peptide (Fig. 6B). The slow component of the mant-ATP fluorescence decay would represent a product release by a myosin population that was not stimulated by the 777D/815S peptide. The fast component is stimulated and capable of releasing product at about 2 × 10−3 s−1. The 777D/815S peptide thus increased this population of the molecules by the mechanism independent of the phosphorylation state of the myosin light chain. As discussed above, the shape change of myosin may be involved in such a mode of stimulation.
The extent of stimulation of ATPase by the 777D/815S peptide, as measured in single-turnover experiments (Fig. 6), is 3-fold greater than that determined in the steady-state ATPase measurements (Fig. 4). This discrepancy probably arises partly because of a dominating contribution in the steady-state assays in the absence of the peptide from a relatively minor fraction of myosin in the fast state. As shown in the ordinate of Fig. 6B, the population of myosin with the higher ATPase activity (2 × 10−3 s−1) was 10% of the total amount of myosin in the absence of the peptide, indicating that the rate expected under steady-state assays would be 5 × 10−4 s−1. The extent of the full stimulation by the peptide measured in steady-state assays thus would be 4-fold or less.
Thus, we detected stimulation of myosin ATPase activity by MLCK by steady-state and single-turnover assays. The stimulation was ascribed to the binding at its 796–815 sequence. Because the sequences have no kinase activity, the stimulatory activatory suggests that myosin phosphorylation is not obligatory for smooth-muscle myosin to be active.
Acknowledgments
This study was supported by grants (to K.K.) from the Mitsubishi Foundation and the Smoking Research Foundation and by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (to K.O.K.K.).
ABBREVIATIONS
- MLCK
myosin light-chain kinase
- mant-ATP
2′(3′)-O-N-methylanthraniloyl-ATP
- CaM
calmodulin
References
- 1.Somlyo A P, Somlyo A V. Nature (London) 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- 2.Barany M, editor. Biochemistry of Smooth Muscle Contraction. 1996. pp. 1–418. [Google Scholar]
- 3.Gallagher P J, Stull J T. Mol Cell Biochem. 1997;173:51–57. doi: 10.1023/a:1006876318155. [DOI] [PubMed] [Google Scholar]
- 4.Ye L-H, Hayakawa K, Kishi H, Imamura M, Nakamura A, Okagaki T, Takagi T, Iwata A, Tanaka T, Kohama K. J Biol Chem. 1997;272:32182–32189. doi: 10.1074/jbc.272.51.32182. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa K, Okagaki T, Higashi-Fujime S, Kohama K. Biochem Biophys Res Commun. 1994;199:786–791. doi: 10.1006/bbrc.1994.1298. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher P J, Herring B P. J Biol Chem. 1991;266:23945–23952. [PMC free article] [PubMed] [Google Scholar]
- 7.Shrinsky V P, Vorotnikow A V, Birukov K G, Nanaev A K, Collinge M, Lukas T J, Sellers J R, Watterson D M. J Biol Chem. 1993;268:16578–16583. [PubMed] [Google Scholar]
- 8.Kohama K, Saida K, editors. Smooth Muscle Contraction, New Regulatory Modes. Tokyo/Karger, Basel: Japan Sci. Soc. Press; 1995. pp. 1–159. [Google Scholar]
- 9.Kuwayama H, Ebashi S. J Biochem (Tokyo) 1988;104:858–861. doi: 10.1093/oxfordjournals.jbchem.a122564. [DOI] [PubMed] [Google Scholar]
- 10.Kuwayama H, Suzuki H, Koga R, Ebashi S. J Biochem (Tokyo) 1988;104:862–866. doi: 10.1093/oxfordjournals.jbchem.a122565. [DOI] [PubMed] [Google Scholar]
- 11.Ebashi S, Mikawa T, Kuwayama H, Suzuki M, Ikemoto H, Ishizaki Y, Koga R. In: Regulation and Contraction of Smooth Muscle. Siegman M J, Somlyo A P, Stephens N L, editors. New York: Liss; 1987. pp. 109–117. [Google Scholar]
- 12.Kohama K, Ye L-H, Hayakawa K, Kohama K. Trends Pharmacol Sci. 1996;17:284–287. doi: 10.1016/0165-6147(96)10033-x. [DOI] [PubMed] [Google Scholar]
- 13.Adelstein R S, Klee C B. J Biol Chem. 1981;256:7501–7509. [PubMed] [Google Scholar]
- 14.Ito M, Dabrowska R, Guerriero V, Jr, Hartshorne D J. J Biol Chem. 1989;264:13971–13974. [PubMed] [Google Scholar]
- 15.Kohama K. J Biochem (Tokyo) 1980;87:997–999. doi: 10.1093/oxfordjournals.jbchem.a132832. [DOI] [PubMed] [Google Scholar]
- 16.Ebashi S. J Biochem (Tokyo) 1976;79:229–231. doi: 10.1093/oxfordjournals.jbchem.a131052. [DOI] [PubMed] [Google Scholar]
- 17.Okagaki T, Higashi-Fujime S, Ishikawa R, Takano-Ohmuro H, Kohama K. J Biochem (Tokyo) 1991;108:856–866. doi: 10.1093/oxfordjournals.jbchem.a123471. [DOI] [PubMed] [Google Scholar]
- 18.Olson N J, Pearson R B, Needleman D S, Hurwitz M Y, Kemp B E, Means A R. Proc Natl Acad Sci USA. 1990;87:2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi H, Inoue A, Mikawa T, Kuwayama H, Hotta Y, Masaki T, Ebashi S. J Biochem (Tokyo) 1992;112:786–791. doi: 10.1093/oxfordjournals.jbchem.a123976. [DOI] [PubMed] [Google Scholar]
- 20.Kodama T, Fukui K, Kometani K. J Biochem (Tokyo) 1986;99:1465–1472. doi: 10.1093/oxfordjournals.jbchem.a135616. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Ishikawa R, Okagaki T, Ye L-H, Kohama K. Cell Motil Cytoskeleton. 1994;29:250–258. doi: 10.1002/cm.970290308. [DOI] [PubMed] [Google Scholar]
- 22.Hiratsuka T. Biochim Biophys Acta. 1983;742:496–508. doi: 10.1016/0167-4838(83)90267-4. [DOI] [PubMed] [Google Scholar]
- 23.Woodward S, Eccleston J F, Geeves M A. Biochemistry. 1990;30:422–430. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa R, Yamashiro S, Matsumura F. J Biol Chem. 1989;214:7490–7497. [PubMed] [Google Scholar]
- 26.Perrie W, Perry S V. Biochem J. 1970;119:31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Lowry O H, Rosebrough N J, Farr A L, Randall R. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Kohama K, Okagaki T, Hayakawa K, Lin Y, Ishikawa R, shimmen T, Inoue A. Biochem Biophys Res Commun. 1992;184:1204–1211. doi: 10.1016/s0006-291x(05)80010-5. [DOI] [PubMed] [Google Scholar]
- 30.Ye L-H, Hayakawa K, Lin Y, Okagaki T, Fujita K, Kohama K. J Biochem (Tokyo) 1994;116:1377–1382. doi: 10.1093/oxfordjournals.jbchem.a124690. [DOI] [PubMed] [Google Scholar]
- 31.Sato M, Ye L-H, Kohama K. J Biochem (Tokyo) 1995;118:1–3. doi: 10.1093/oxfordjournals.jbchem.a124862. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi S, Kakita S, Takahashi I, Kawahara K, Tsukuda E, Sano T, Yamada K, Yoshida M, Kase Y, Matsuda Y, et al. J Biol Chem. 1992;267:2157–2163. [PubMed] [Google Scholar]
- 33.Allen B G, Walsh M P. Trends Biochem Sci. 1994;19:362–368. doi: 10.1016/0968-0004(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 34.Cross R A, Tackson A P, Citi S, Kendrick-Jones J, Bagshaw C R. J Mol Biol. 1988;203:173–181. doi: 10.1016/0022-2836(88)90100-3. [DOI] [PubMed] [Google Scholar]
- 35.Kitazawa T, Somlyo A. In: Regulation of Smooth Muscle Contraction. Moreland R S, editor. New York: Plenum; 1991. pp. 97–109. [Google Scholar]
- 36.Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won K-J, Sato K. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- 37.Nishimura J, van Breemen C. J Pharmacol Exp Ther. 1991;258:397–402. [PubMed] [Google Scholar]
- 38.Dabrowska R, Hinks S, Walsh M P, Hartshorne D J. Biochem Biophys Res Commun. 1982;107:1524–1531. doi: 10.1016/s0006-291x(82)80172-1. [DOI] [PubMed] [Google Scholar]
- 39.Lin P -J, Luby-Phelps K, Stull J T. J Biol Chem. 1997;272:7412–7420. doi: 10.1074/jbc.272.11.7412. [DOI] [PubMed] [Google Scholar]
- 40.Lin Y, Ye L-H, Ishikawa R, Fujita K, Kohama K. J Biochem (Tokyo) 1993;113:643–645. doi: 10.1093/oxfordjournals.jbchem.a124096. [DOI] [PubMed] [Google Scholar]
- 41.Lin Y, Ishikawa R, Kohama K. J Biochem (Tokyo) 1993;114:279–283. doi: 10.1093/oxfordjournals.jbchem.a124167. [DOI] [PubMed] [Google Scholar]
- 42.Ishikawa R, Okagaki T, Higashi-Fujime S, Kohama K. J Biol Chem. 1991;266:21784–21790. [PubMed] [Google Scholar]
- 43.Sobue K, Sellers J R. J Biol Chem. 1991;266:12115–12118. [PubMed] [Google Scholar]
- 44.Takahashi K, Nadal-Ginard B. J Biol Chem. 1991;266:13284–13288. [PubMed] [Google Scholar]
- 45.Okagaki T, Kohama K. In: Smooth Muscle Contraction. Kohama K, Saida K, editors. Tokyo/Karger, Basel: Japan Sci. Soc. Press; 1995. pp. 17–35. [Google Scholar]
- 46.Ye L-H, Hayakawa K, Okagaki T, Kohama K. In: Regulation of the Contractile Cycle in Smooth Muscle. Nakano T, Hartshorne D J, editors. Tokyo: Springer; 1995. pp. 152–173. [Google Scholar]
- 47.Ito M, Pierce P R, Allen R E, Hartshorne D J. Biochemistry. 1989;28:5567–5572. doi: 10.1021/bi00439a034. [DOI] [PubMed] [Google Scholar]
- 48.Onishi H, Wakabayashi T, Kamata T, Watanabe S. J Biochem (Tokyo) 1983;94:1147–1154. doi: 10.1093/oxfordjournals.jbchem.a134459. [DOI] [PubMed] [Google Scholar]