Abstract
Polished pebbles occasionally found within skeletons of giant herbivorous sauropod dinosaurs are very likely to be gastroliths (stomach stones). Here, we show that based on feeding experiments with ostriches and comparative data for relative gastrolith mass in birds, sauropod gastroliths do not represent the remains of an avian-style gastric mill. Feeding experiments with farm ostriches showed that bird gastroliths experience fast abrasion in the gizzard and do not develop a polish. Relative gastrolith mass in sauropods (gastrolith mass much less than 0.1% of body mass) is at least an order of magnitude less than that in ostriches and other herbivorous birds (gastrolith mass approximates 1% of body mass), also arguing against the presence of a gastric mill in sauropods. Sauropod dinosaurs possibly compensated for their limited oral processing and gastric trituration capabilities by greatly increasing food retention time in the digestive system. Gastrolith clusters of some derived theropod dinosaurs (oviraptorosaurs and ornithomimosaurs) compare well with those of birds, suggesting that the gastric mill evolved in the avian stem lineage.
Keywords: gizzard, digestion, sauropod dinosaurs, extant birds, gastroliths
1. Introduction
Sauropod dinosaurs of the Jurassic and Cretaceous periods were the largest herbivores ever to have evolved, commonly exceeding body masses of 30 tons or more (Seebacher 2001). In many forms, the small head and weak dentition appear ill suited for oral processing of the enormous quantity of plant matter required to support their high growth rates (Sander 2000; Erickson et al. 2001), if not endothermic metabolism (Padian & Horner 2004).
Polished pebbles occasionally found with sauropod skeletons are generally interpreted as gastroliths or stomach stones, i.e. stones ingested voluntarily by the animal and retained in its digestive tract (Janensch 1929; Christiansen 1996; Ratkevich 1998; Bonaparte & Mateus 1999; Sanders et al. 2001). Polish of stones found in association with sauropods ranges from dull to fatty or waxy to a silky or even a highly reflective surface (figure 1a,b; Gillette 1994; Wings 2004). The pebbles are also believed to have comprised a gastric mill of the kind employed by modern birds (Galton 1986; Christiansen 1996; Upchurch & Barrett 2000; Barrett & Upchurch 2005; Fastovsky & Weishampel 2005). Although our taphonomic and sedimentological research (Wings 2004) supports the view that these pebbles are gastroliths and not just a sedimentological phenomenon as proposed by some authors (Calvo 1994; Lucas 2000), we here provide evidence that sauropods lacked a gastric mill.
Figure 1.
Genuine and alleged gastroliths from sauropods and ostriches. All images have the same scale. (a) Sauropod gastroliths from Cedarosaurus (Denver Museum of Natural History 39045). Note that some pebbles are composed of relatively soft sandstone and that even the quartz pebbles exhibit a dull surface, except where covered by diagenetic hematite coating. (b) Typical alleged sauropod gastroliths, composed of quartz and exhibiting a high polish (Yale Peabody Museum 1782). (c) Natural ostrich gastrolith composed of white vein quartz (Institute of Palaeontology, University Bonn (IPB) R563). (d) Black chert pebbles before and after abrasion experiment (IPB R564). (e) Abrasion sequence of experimental granite samples (IPB R565) in the ostrich gizzards. Samples are from experiment illustrated in figure 2. Note that the general shape of the stones remained intact during the experiment and that the experimental gastroliths did not develop any polish. Numbers below specimens in (c), (d) and (e) indicate residence time in gizzard in days.
Herbivorous and granivorous birds, lacking oral processing capabilities, must rely on their gastric mill in the gizzard to efficiently triturate food stuff for hind gut fermentation (Gionfriddo & Best 1999). Other components of the avian gastric mill are strong, paired stomach muscles and a tough lining of the stomach consisting of a keratin-like substance forming a specialized tissue, koilin (Duke 1986). The number and mass of rocks in a gastric mill are important variables in the system. None of the components of the gastric mill except for gastroliths have the potential to fossilize, however.
To test the hypotheses that sauropod dinosaurs possessed a bird-like gastric mill and that the polished pebbles found in association with some sauropod skeletons are its remains, we first investigated gastrolith properties and function in the largest herbivorous bird, the ostrich (Struthio camelus). In specifically designed feeding experiments, we assessed whether gastroliths develop a polish and how long they last in the gizzard. Second, we compared relative gastrolith mass data for ostriches, other birds and sauropod dinosaurs.
We chose ostriches for our comparative research on the putative sauropod gastric mills for three reasons: (i) being birds, ostriches are the closest living relatives of sauropod dinosaurs, (ii) the diet of ostriches, fibrous plant matter, is a comparable diet to that assumed for sauropods (Upchurch & Barrett 2000; Barrett & Upchurch 2005; Fastovsky & Weishampel 2005), and (iii) ostriches possess a well-developed gastric mill in their gizzard with a known gastrolith function: triturating and mixing ingested plant foodstuff. Although sample material is abundant and easily accessible at ostrich farms, thus permitting statistical tests, no comprehensive study has been conducted on ostrich gastroliths before.
Ostriches, like all ratites, lack a crop. Instead, foodstuff is stored and mixed with stomach juices in the sac-like proventriculus. The gastro-intestinal tract leads into the second part of the stomach, the ventriculus or gizzard, which contains the food and the majority of the gastroliths. The remaining gastroliths are found in the proventriculus. The massive muscle packages that flank the gizzard contract two to three times per minute in ostriches, generating a continuous (and audible) movement of the stones grinding up foodstuff.
2. Material and methods
(a) Feeding experiments with ostriches
Different rock types were offered to German farm ostriches and recovered after residence times from 1 to 60 days (figures 1 and 2). In November 2002, cubes of 2 cm side length of three rock types (limestone, granite and rose quartz) were presented to three groups of 1-year-old ostriches on the farm ‘Gemarkenhof’ near Remagen (western Germany), with each group being offered one rock type only. Since stones are a normal part of their dietary uptake, the birds swallowed the stones deliberately. Each of the ostriches swallowed at least three stones of its designated rock type. Rose quartz was chosen because it has the same physical properties as the white vein quartz (of which most of the naturally acquired gastroliths consist), but can be separated from these by colour. In addition, polished black chert pebbles were offered to a fourth group of ostriches. Since all birds were later slaughtered during normal farm operations, the stones were retrieved at irregular intervals. Once the stomachs (proventriculus and gizzard) were removed, the stomach contents were collected, washed and screened, and the experimental rocks were separated and examined for mass loss, surface modifications and shape change.
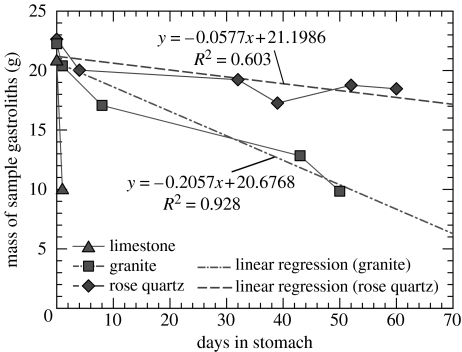
Figure 2.
Results of the gastrolith-feeding experiments with German farm ostriches (Struthio camelus) using three different rock types: limestone; granite; and rose quartz. The mass of the fed stones is plotted against the number of days of residence in the stomach. Each data point represents one slaughtered animal that contained a sample of known residence time. Rose quartz was by far the most resistant rock type, followed by granite, limestone being destroyed almost immediately. Number of days between intake of experimentally prepared gastroliths and eventual slaughter was decided independently by the farmer. Hence, duration of the interval between samples is not of equal length.
(b) Amounts of gastroliths in ostriches, other birds and sauropod dinosaurs
More than 300 stomachs of slaughtered ostriches of known body mass from two different groups of free-ranging farm animals in Germany and South Africa were sampled for a statistical analysis of absolute and relative gastrolith mass (see electronic supplementary material for details). To augment the ostrich data and to detect scaling effects, we added reliable data for 26 species of birds compiled from ornithological literature. The data came from insectivorous, omnivorous and herbivorous bird species belonging to several families (see electronic supplementary material for details).
Despite the hundreds of sauropod skeletons found worldwide, data for gastrolith mass in these dinosaurs is scarce, in particular because only very few specimens preserve any. We analysed the three sauropod finds with the highest gastrolith masses, i.e. Seismosaurus hallorum (Gillette 1994), Cedarosaurus weiskopfae (Sanders et al. 2001) and Dinheirosaurus lourinhanensis (Bonaparte & Mateus 1999). Data for the first two sauropods were collected from the literature and calibrated during museum visits, the mass for the latter was kindly provided by O. Mateus (2004, personal communication; see electronic supplementary material for details).
Estimates for sauropod body mass were derived from Seebacher (2001) who used graphic reconstructions to produce volume estimates that were transformed into mass estimates.
3. Results
(a) Feeding experiments
More than half of the mass of the limestone cubes was lost from the ostrich gizzard after 24 h, a result of the acidic environment. Consequently, no later sample contained any trace of the limestone cubes. Granite, although very resistant to weathering, was rather quickly eroded in the highly destructive environment of the gizzard (figure 1). The lowest abrasion rate was observed in rose quartz: after 60 days, about 15% of the mass of the stones was eroded (figure 2).
Shape change resulting from the abrasion experiments was limited: even after strong weight loss of 55.7% (granite sample after 50 days), the cube shape of the sample remained recognizable (figure 1e). Owing to the high abrasion rate, initially smooth cut surfaces became rough, and the samples maintained this rough surface throughout the abrasion experiments. Abrasion rate was obviously much too high for the samples to develop a polish. Similarly, the polished black chert pebbles became dull within a day (figure 1d).
From the 15% weight loss within 60 days residence time in the gizzard, the total survival time of quartz pebbles with a typical diameter of 2 cm can be extrapolated to ca 1 year in the ostrich stomach. As ostriches live for 30–40 years (Folch 1992), our experiments explain why the birds need a constant supply of rocks.
The dull surface of sample gastroliths is in accordance with natural ostrich gastroliths from the same stomachs that never showed a polish (figure 1c). The implication of our experiments is that natural stones taken up by a bird will not develop any polish in its gastric mill. This result is consistent with observations for many other herbivorous birds.
(b) Amounts of gastroliths
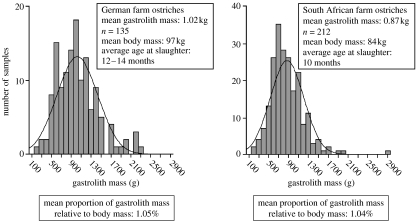
In both sample groups of ostriches, gastrolith mass correlated closely with body mass, and relative gastrolith mass showed a tight normal distribution around a mean gastrolith mass of 1.05% of body mass (figure 3). In the data from other bird taxa, there is also a clear relationship between body mass and gastrolith mass (figure 4). Herbivorous species have the highest proportion of gastroliths in the stomach, repeatedly reaching 1% of body mass, independently of size which spans four orders of magnitude (figure 4).
Figure 3.
Gastrolith masses of South African and German free-ranging farm ostriches. Both groups exhibit a normal distribution of data. The Kolmogorov–Smirnov test gave an asymptotic significance of 0.414 for German ostrich gastrolith mass and an asymptotic significance of 0.084 for South African ostrich gastrolith mass. While the South African birds were two to four months younger at slaughter, and therefore had a lower mean body mass, the proportions of gastrolith and body mass is almost identical in both groups.
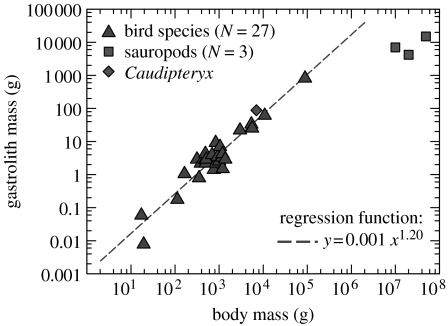
Figure 4.
Relationship of body mass to gastrolith mass in birds and dinosaurs. Data were compiled for 27 bird species of several families (ostriches, wild birds and domesticated; see electronic supplementary material) as well as for the theropod Caudipteryx and the three sauropod finds with the highest mass of associated gastroliths (type specimens of Seismosaurus, Cedarosaurus and Dinheirosaurus). Note that values of relative gastrolith mass in sauropods lie well below the regression line for birds and the Caudipteryx data.
Allometric comparison of sauropod gastrolith data with that of living birds indicates that sauropod relative gastrolith mass was at least one order of magnitude lower than in herbivorous birds (figure 4). The highest gastrolith mass recorded for any sauropod is ca 15 kg of gastroliths found associated with the skeleton of Seismosaurus. These compare with a body mass estimate of approximately 50 000 kg (Seebacher 2001) and thus account for only 0.03% of body mass (figure 4). Based on the ratio derived from extant ostriches, a gastrolith mass in excess of 500 kg would be predicted for Seismosaurus. Even if a very conservative mass estimate of 30 000 kg is used, a prediction of 300 kg of gastroliths would result.
4. Discussion
We reject the hypothesis that sauropod dinosaurs had an avian-style gastric mill, and that polished pebbles occasionally found with sauropod skeletons are its remains, for a number of reasons: the high abrasion rate and the lack of polish in bird gastroliths (figures 1 and 2); the rare occurrence of gastroliths in sauropod fossils; and, where present, the extremely low values of relative gastrolith mass in sauropods compared with birds (figure 4).
We are aware of the dangers of extrapolating over four orders of magnitude from a 89 kg ostrich to a 50 000 kg sauropod, but note that the ratio of body mass to gastrolith mass observed in birds also includes a body mass range of four orders of magnitude, from a 17 g robin to a 89 kg ostrich. Several other methods for estimating sauropod mass are reviewed in Seebacher (2001). Although there is considerable variation between these estimates, they are all of the same order of magnitude, not affecting the extremely low ratios of gastrolith mass to body mass in sauropods calculated in this study.
Compared to the estimated dimensions of an average sauropod stomach, the mass and number of the discovered gastroliths would have been negligible for stomach function as a gastric mill. Other characteristics also argue against such a function, such as the size of the pebbles (very small compared with the dimensions of the throat and the total body size of the sauropods) and the rock type of the gastroliths. For example, 31% of the Cedarosaurus gastroliths are composed of soft rocks such as sandstones and siltstones (Sanders et al. 2001), which could not have served efficiently as grinding agents.
If sauropods did indeed employ a gastric mill, many reasonably complete skeletons should be accompanied by very noticeable amounts of pebbles or even cobbles. Hypothetically, strong under-representation of gastroliths could be, in part, attributable to taphonomic processes such as scavenging by theropod dinosaurs, which preferentially could have removed the digestive tract (as seen in modern scavengers) or the dropping of gastroliths out of floating carcasses (as observed in an experiment on ostriches; Wings 2003). However, some sauropod fossil deposits are autochthonous (e.g. Howe Quarry; Michelis 2004), and our extensive fieldwork revealed that sauropod gastroliths are an extremely rare occurrence (see electronic supplementary material). Also, there are other groups of dinosaurs (see below) and further fossil vertebrates (primitive diapsids, plesiosaurs, crocodilians and birds) that regularly preserve gastroliths (Whittle & Everhart 2000; Wings 2004; Wings in press) despite having been subject to similar taphonomic processes as sauropods. The presence of isolated polished clasts in sauropod-bearing formations is not necessarily a result of the deposition of former gastroliths but has also been attributed to transport in hyperconcentrated flows (Zaleha & Wiesemann 2005). Consequently, such isolated clasts should not be called ‘gastroliths’ but rather ‘exoliths’ (i.e. exotic rocks in fine-grained sediments which may show a high polish and which potentially—but not necessarily—were former gastroliths; Wings in press).
Since there is no evidence for gastric mills in sauropod dinosaurs, another explanation regarding occurrence and function of sauropod gastroliths should be sought. Possible alternatives include accidental or pathologic ingestion, or intentional ingestion for mineral uptake, especially calcium supply (Wings in press). Similar to what has been observed in several extant bird taxa (Gionfriddo & Best 1999; Wings 2004), stones might have been swallowed indiscriminately. While the calcium-rich limestone would have been quickly dissolved, acid-resistant rock types, such as quartz, could have remained in the stomach and may have accumulated as gastroliths, thereby developing polish through prolonged exposure to mild abrasion.
The lack of a gastric mill also raises the question of how sauropods processed their plant food. Like all strictly herbivorous tetrapods, sauropod dinosaurs needed bacterial fermentation to access the nutrients in the plant cell walls. Fermentation, which probably occurred in specialized parts of the hindgut (Farlow 1987; Dunham et al. 1989; Marshall & Stevens 2000), would have been greatly speeded up by foodstuff having been reduced to small particles. While the robust, broad-crowned teeth of some basal sauropods and early macronarians (e.g. Camarasaurus and Brachiosaurus) indicate some oral processing capability (Upchurch & Barrett 2000; Barrett & Upchurch 2005; Chatterjee & Zheng 2005), the narrow-crowned, pencil-shaped teeth of Jurassic diplodocids and derived titanosauromorphs are inconsistent with oral processing (Upchurch & Barrett 2000; Barrett & Upchurch 2005). Sauropods possibly secured sufficient nutrient uptake by extremely prolonged food retention times permitted by their large body size (Farlow 1987; Clauss & Hummel 2005).
There are three other dinosaur groups that are regularly found with gastrolith clusters: the ornithischian dinosaur Psittacosaurus (Xu 1997; You & Dodson 2004), ornithomimids (Kobayashi et al. 1999; Ji et al. 2003; Barrett 2005), and basal oviraptorosaurs (Ji et al. 1998), raising the question whether these may represent the remains of a gastric mill. All described specimens of the feathered Lower Cretaceous oviraptorosaur Caudipteryx have well-defined clusters of gastroliths in the abdominal region of the skeletons (Ji et al. 1998). These clusters are composed of pebbles which closely resemble bird gastroliths in size and composition (O. Wings 2003, personal observation on two National Geological Museum of China specimens: NGMC-97-4-A and NGMC-97-9-A). For these specimens, we estimate a gastrolith mass/body mass ratio of 1.25% (see electronic supplementary material), which is in remarkable agreement with the ratio in living birds, suggesting strongly that Caudipteryx had an avian-style gastric mill. Gastrolith-bearing specimens of the ornithomimid theropod Sinornithomimus (Kobayashi & Lü 2003) appear to have a similarly high relative gastrolith mass, which has been interpreted as evidence for a gastric mill (Kobayashi & Lü 2003) and a herbivorous diet (Barrett 2005).
The gastrolith clusters in Caudipteryx and other derived non-avian theropods suggest that the gizzard is not an autapomorphy of crown-group birds, but rather evolved much earlier along the avian stem lineage, repeating a now well-known pattern of supposed ‘bird’ characteristics appearing phylogenetically earlier than in Archaeopteryx. None of the specimens of the Urvogel preserve gastroliths, but some other Mesozoic bird skeletons do (Zhou & Zhang 2003; Zhou et al. 2004), although the record is patchy and influenced by taphonomic loss of gastrolith and possible seasonal dietary shifts (Zhou et al. 2004). Since Psittacosaurus is a derived ornithischian, its gastrolith clusters are most parsimoniously interpreted as homoplastic to those of derived non-avian theropods.
Acknowledgments
We thank R. Schumacher and A. Olivier for their help during the ostrich research. K. Carpenter, S. Lucas and O. Mateus provided access to the sauropod gastroliths and made data available. M. Clauss, D. Fowler, R. v. Geldern, W. Joyce and G. Oleschinski helped in various ways during the preparation of this manuscript. Financial support was provided by the ‘Graduiertenförderung Nordrhein-Westfalen’, the DFG Graduiertenkolleg ‘Evolution und Biodiversität in Raum und Zeit’ and ‘The Jurassic Foundation’. This is contribution no. 13 by the DFG Research Unit ‘Biology of the Sauropod Dinosaurs: The Evolution of Gigantism’.
Supplementary Material
Detailed supplementary methods of data gathering from ostriches, body mass and gastrolith mass data for all 27 species of birds (supplementary table 1), including references, additional information about the Seismosaurus gastroliths, body mass and gastrolith mass data for Caudipteryx, methods of taphonomic field work in Upper Jurassic sauropod quarries (visited sites, collections, etc.), detailed results of taphonomic field work, including summary of relevant information in supplementary table 2
References
- Barrett P.M. The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria) Palaeontology. 2005;48:347–358. doi:10.1111/j.1475-4983.2005.00448.x [Google Scholar]
- Barrett P.M, Upchurch P. Sauropod diversity through time: macroevolutionary and paleoecological implications. In: Curry Rogers K.A, Wilson J.A, editors. The Sauropods. Evolution and paleobiology. University of California Press; Berkeley, CA: 2005. pp. 125–156. [Google Scholar]
- Bonaparte J.F, Mateus O. A new diplodocid, Dinheirosaurus lourinhanensis gen. et sp. nov., from the Late Jurassic beds of Portugal. Revista del Museo Argentino de Ciencias Naturales. 1999;5:13–29. [Google Scholar]
- Calvo J.O. Gastroliths in sauropod dinosaurs. Gaia. 1994;10:205–208. [Google Scholar]
- Chatterjee S, Zheng Z. Neuroanatomy and dentition of Camarasaurus lentus. In: Tidwell V, Carpenter K, editors. Thunder-Lizards. The sauropodomorph dinosaurs. Indiana University Press; Bloomington, IN: 2005. pp. 199–211. [Google Scholar]
- Christiansen P. The evidence for and implications of gastroliths in sauropods (Dinosauria, Sauropoda) Gaia. 1996;12:1–7. [Google Scholar]
- Clauss M, Hummel J. The digestive performance of mammalian herbivores: why big may not be that much better. Mamm. Rev. 2005;35:174–187. doi:10.1111/j.1365-2907.2005.00062.x [Google Scholar]
- Duke G.E. Alimentary canal: anatomy, regulation of feeding, and mobility. In: Sturkie P.D, editor. Avian physiology. Springer; New York, NY: 1986. pp. 269–288. [Google Scholar]
- Dunham, A. E., Overall, K. L., Porter, W. P. & Forster, C. A. 1989 Implications of ecological energetics and biophysical and developmental constraints for life-history variation in dinosaurs. In Paleobiology of the dinosaurs (ed. J. O. Farlow). GSA special paper 238, pp. 1–21. Boulder, CO: The Geological Society of America, Inc.
- Erickson G.M, Rogers K.C, Yerby S.A. Dinosaurian growth patterns and rapid avian growth rates. Nature. 2001;412:429–433. doi: 10.1038/35086558. doi:10.1038/35086558 [DOI] [PubMed] [Google Scholar]
- Farlow J.O. Speculations about the diet and digestive physiology of herbivorous dinosaurs. Paleobiology. 1987;13:60–72. [Google Scholar]
- Fastovsky D.E, Weishampel D.B. 2nd edn. Cambridge University Press; Cambridge, UK: 2005. The evolution and extinction of the dinosaurs. [Google Scholar]
- Folch A. Family Struthionidae (Ostrich) In: Hoyo J.D, Elliott A, Sargatal J, editors. Handbook of the birds of the world. Ostrich to ducks. vol. 1. Lynx Editions; Barcelona, Spain: 1992. pp. 76–83. [Google Scholar]
- Galton P.M. Herbivorous adaptations of Late Triassic and Early Jurassic dinosaurs. In: Padian K, editor. The beginning of the age of dinosaurs. Cambridge University Press; New York, NY: 1986. pp. 203–221. [Google Scholar]
- Gillette D.D. Columbia University Press; New York, NY: 1994. Seismosaurus, the earth shaker. [Google Scholar]
- Gionfriddo J.P, Best L.B. Grit use by birds—a review. Curr. Ornithol. 1999;15:89–148. [Google Scholar]
- Janensch W. Magensteine bei Sauropoden der Tendaguru-Schichten. Palaeontographica. 1929;VII(Suppl.):135–144. [Google Scholar]
- Ji Q, Currie P.J, Norell M.A, Ji S.-A. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–761. doi:10.1038/31635 [Google Scholar]
- Ji Q, Norell M.A, Makovicky P.J, Gao K.-Q, Ji S.A, Yuan C. An Early ostrich dinosaur and implications for ornithomimosaur phylogeny. Am. Museum Noviates. 2003;3420:1–19. doi:10.1206/0003-0082(2003)420<0001:AEODAI>2.0.CO;2 [Google Scholar]
- Kobayashi Y, Lü J.-C. A new ornithomimid dinosaur with gregarious habits from the Late Cretaceous of China. Acta Palaeontol. Polonica. 2003;48:235–259. [Google Scholar]
- Kobayashi Y, Lu J.-C, Dong Z.-M, Barsbold R, Azuma Y, Tomida Y. Herbivorous diet in an ornithomimid dinosaur. Nature. 1999;402:480–481. doi:10.1038/44999 [Google Scholar]
- Lucas, S. G. 2000 The gastromyths of “Seismosaurus,” a Late Jurassic dinosaur from New Mexico. In Dinosaurs of New Mexico (ed. S. G. Lucas & A. B. Heckert). New Mexico Museum of Natural History and Science Bulletin 17, pp. 61–68.
- Marshall C.L, Stevens C.E. Yes Virginia, there were foregut fermenting dinosaurs. Proc. Comp. Nutr. Soc. 2000;2000:138–142. [Google Scholar]
- Michelis, I. 2004 Vergleichende Taphonomie des Howe Quarry's (Morrison Formation, Oberer Jura), Bighorn County, Wyoming, USA. Ph.D. thesis, University of Bonn, Bonn. (Accessible online at http://nbn-resolving.de/urn:nbn:de:hbz:5N-04632).
- Padian K, Horner J.R. Dinosaur physiology. In: Weishampel D.B, Dodson P, Osmolska H, editors. The Dinosauria. University of California Press; Berkeley, CA: 2004. pp. 660–671. [Google Scholar]
- Ratkevich R. A new Cretaceous brachiosaurid dinosaur from Southern Arizona. J. Arizona–Nevada Acad. Sci. 1998;31:71–82. [Google Scholar]
- Sander P.M. Longbone histology of the Tendaguru sauropods: implications for growth and biology. Paleobiology. 2000;26:466–488. doi:10.1666/0094-8373(2000)026<0466:LHOTTS>2.0.CO;2 [Google Scholar]
- Sanders F, Manley K, Carpenter K. Gastroliths from the Lower Cretaceous sauropod Cedarosaurus weiskopfae. In: Tanke D.H, Carpenter K, editors. Mesozoic vertebrate life: new research inspired by the paleontology of Philip J. Currie. Indiana University Press; Bloomington, IN: 2001. pp. 166–180. [Google Scholar]
- Seebacher F. A new method to calculate allometric length-mass relationships of dinosaurs. J. Vertebr. Paleontol. 2001;21:51–60. doi:10.1671/0272-4634(2001)021[0051:ANMTCA]2.0.CO;2 [Google Scholar]
- Upchurch P, Barrett P.M. The evolution of sauropod feeding mechanisms. In: Sues H.-D, editor. Evolution of herbivory in terrestrial vertebrates: perspectives from the fossil record. Cambridge University Press; Cambridge, UK: 2000. pp. 79–122. [Google Scholar]
- Whittle, C. H. & Everhart, M. J. 2000 Apparent and implied evolutionary trends in lithophagic vertebrates from New Mexico and elsewhere. In Dinosaurs of New Mexico (ed. S. G. Lucas & A. B. Heckert). New Mexico Museum of Natural History and Science Bulletin 17, pp. 75–82.
- Wings O. Observations on the release of gastroliths from ostrich chick carcasses in terrestrial and aquatic environments. J. Taphon. 2003;1:97–103. [Google Scholar]
- Wings, O. 2004 Identification, distribution, and function of gastroliths in dinosaurs and extant birds with emphasis on ostriches (Struthio camelus). Ph.D. thesis, University of Bonn, Bonn. (Accessible online at http://nbn-resolving.de/urn:nbn:de:hbz:5N-04626)
- Wings, O. In press. A review of gastrolith function with implications for fossil vertebrates and a revised classification. Acta Palaeontologica Polonica
- Xu X. A new psittacosaur (Psittacosaurus mazongshanensis, sp. nov.) from Mazongshan Area, Gansu Province, China. In: Dong Z, editor. Sino-Japanese Silk Road dinosaur expedition. China Ocean Press; Beijing, China: 1997. pp. 48–67. [Google Scholar]
- You H.-L, Dodson P. Basal Ceratopsia. In: Weishampel D.B, Dodson P, Osmolska H, editors. The Dinosauria. University of California Press; Berkeley, CA: 2004. pp. 478–493. [Google Scholar]
- Zaleha M.J, Wiesemann S.A. Hyperconcentrated flows and gastroliths: sedimentology of diamictites and wackes of the upper Cloverly Formation, Lower Cretaceous, Wyoming, U.S.A. J. Sediment. Res. 2005;75:43–54. doi:10.2110/jsr.2005.005 [Google Scholar]
- Zhou Z, Zhang F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Canad. J. Earth Sci. 2003;40:731–747. doi:10.1139/e03-011 [Google Scholar]
- Zhou Z, Clarke J.A, Zhang F, Wings O. Gastroliths in Yanornis: an indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften. 2004;91:571–574. doi: 10.1007/s00114-004-0567-z. doi:10.1007/s00114-004-0567-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed supplementary methods of data gathering from ostriches, body mass and gastrolith mass data for all 27 species of birds (supplementary table 1), including references, additional information about the Seismosaurus gastroliths, body mass and gastrolith mass data for Caudipteryx, methods of taphonomic field work in Upper Jurassic sauropod quarries (visited sites, collections, etc.), detailed results of taphonomic field work, including summary of relevant information in supplementary table 2