Abstract
The long-term history of Zeiraphera diniana Gn. (the larch budmoth, LBM) outbreaks was reconstructed from tree rings of host subalpine larch in the European Alps. This record was derived from 47 513 maximum latewood density measurements, and highlights the impact of contemporary climate change on ecological disturbance regimes. With over 1000 generations represented, this is the longest annually resolved record of herbivore population dynamics, and our analysis demonstrates that remarkably regular LBM fluctuations persisted over the past 1173 years with population peaks averaging every 9.3 years. These regular abundance oscillations recurred until 1981, with the absence of peak events during recent decades. Comparison with an annually resolved, millennium-long temperature reconstruction representative for the European Alps (r=0.72, correlation with instrumental data) demonstrates that regular insect population cycles continued despite major climatic changes related to warming during medieval times and cooling during the Little Ice Age. The late twentieth century absence of LBM outbreaks, however, corresponds to a period of regional warmth that is exceptional with respect to the last 1000+ years, suggesting vulnerability of an otherwise stable ecological system in a warming environment.
Keywords: climate change, population dynamics, Zeiraphera diniana, tree rings, European Alps
1. Introduction
Periodic oscillations in abundance, termed ‘population cycles’, are one of the most remarkable characteristics of animal population dynamics. While these cycles are not present in all species, they are common in many forest insect populations (Elton 1924; Kendall et al. 1998; Stenseth 1999; Bjørnstad & Grenfell 2001; Berryman 2002). During peak activity, populations may reach very high densities over large areas, and the resultant episodes of massive defoliation and/or tree mortality are known to serve as important forest disturbances that fundamentally affect ecosystem processes (Mattson & Addy 1975; Lovett et al. 2002).
While there is considerable debate about the precise mechanisms causing these oscillations, there is a general consensus that most cycles are the result of trophic interactions or maternal effects that contribute delayed negative feedback (Berryman 1996; Hunter et al. 1997; Kendall et al. 1999; Hanski et al. 2001). A multitude of negative feedbacks from lower (e.g. host plants, prey) and/or higher trophic levels (e.g. predators, diseases) are capable of producing periodic oscillations if there are time lags in their effects (Haukioja 1990; Berryman 1996). It, however, remains less clear how these cycles are modulated or regulated by climatic influences. The complexity of ecological interactions creates difficulty in measuring or predicting changes in ecosystem processes related to climate change (Walther et al. 2002; Ims & Fuglei 2005) and specifically predicting impacts on forest insect outbreaks (Volney & Fleming 2000, Bale et al. 2002).
Geographically disjunct populations of the same species may exhibit variation in the strength, period or amplitude of abundance oscillations. One recurring pattern of such geographical variation is the existence of latitudinal gradients in periodicity (Hansson & Henttonen 1985). For example, northern populations of the autumnal moth, Epirrita autumnata, in Fennoscandia display periodic oscillations causing widespread defoliation of host trees during population peaks (Ruohomaeki et al. 1997). However, more southerly populations exhibit little periodicity, and this gradient has been hypothesized to result from the dominance of specialist and generalist predators in northern and southern latitudes, respectively (Klemola et al. 2002).
Much less information exists about temporal variation in periodicity. Theoretical exploration of ‘transient’ population dynamics indicates that nonlinear trophic interactions may lead to populations transitioning between periodic, chaotic and random behaviour (Hastings 2004). While such transient dynamics have been observed in microcosm experiments (Henson et al. 1998), less evidence exists in natural populations. Unfortunately, the detection of temporal changes in periodicity necessitates longer time-series, which are not common for most natural systems. Some of the best examples of temporal variability in periodicity come from the foliage-feeding Lepidoptera, where relatively short time-series have been compiled from multi-annual surveys (Baltensweiler & Rubli 1999; Yamamura et al. 2006), but longer series are only available via reconstruction from tree-ring data (Weber 1997; Rolland et al. 2001; Boulanger & Arseneault 2004).
Dendrochronological methods can be used to analyse annual growth rings for decreases in increment and changes in morphology that are indicative of defoliation to reconstruct the abundance of forest Lepidoptera species (Schweingruber 1979; Swetnam & Lynch 1993). For example, the application of dendrochronological methods to reconstruct spruce budworm, Choristoneura fumiferana, populations over 450 years in eastern Canada indicates regular oscillations with little evidence for changes in either cycle period or amplitude (Krause 1997; Boulanger & Arseneault 2004). Similar analyses of western spruce budworm, Choristoneura occidentalis, and pandora moth, Coloradia pandora, dynamics in western USA suggest no evidence for long-term changes in periodicity (Swetnam & Lynch 1993; Ryerson et al. 2003) and unsystematic variations in the period of oscillations (Speer et al. 2001), respectively. In Europe, LBM gained notoriety for the regularity of population cycles that oscillate with a median periodicity of 8–9 years (Baltensweiler et al. 1977; Schweingruber 1979; Baltensweiler & Rubli 1999). Though several competing theories have been advanced to explain the mechanism behind these oscillations, there is little question that outbreaks have recurred in a periodic fashion over the last century.
Here, we present a dendrochronological reconstruction of historical larch budmoth (LBM; Zeiraphera diniana Gr.) outbreaks over a 1173-year time span (AD 832–2004). The analysis used radiodensitometric techniques to characterize tree-ring density profiles from 180 larch, Larix decidua Mill., samples spanning the past millennium. LBM outbreaks were identified based upon characteristic maximum latewood density (MXD) patterns in wood samples, and verified using more traditional techniques of comparison with tree-ring chronologies from non-host tree species. It is demonstrated that during the vast majority of the past millennium, outbreaks have reliably recurred with a remarkably constant return time. This represents the longest continuous time period over which any population cycle has ever been documented. We, however, also address a change in population dynamics since the 1980s, in which high-amplitude LBM oscillations have stopped. It is shown how this transition in LBM dynamics coincides with a period of unprecedented climatic warming. This change in outbreak oscillations is discussed with respect to previous studies, suggesting a causal relationship between recent warming and LBM cyclicity.
2. The larch budmoth system
Zeiraphera diniana is a foliage-feeding Lepidoptera that is widespread throughout the European Alps (Baltensweiler et al. 1977). In subalpine larch forests, LBM exhibits considerable temporal variability in density ranging from 1 to 30 000 larvae per host tree (Baltensweiler & Rubli 1999). In the years of high population densities, larvae may completely defoliate larch trees, but defoliation rarely results in tree mortality. Historical records of defoliation compiled by Baltensweiler & Rubli (1999) documented that outbreaks have recurred with a high degree of regularity every 8–9 years since the 1850s. More detailed records of annual budmoth densities document a series of five population cycles between 1950 and 1992; however, the population peak occurring in 1988 did not reach sufficient levels to cause noticeable defoliation (Baltensweiler 1993a). In addition to its subalpine distribution, LBM is also known to occur on larch at lower elevations in the Alps, but these populations apparently never reach outbreak levels.
Several different mechanisms have been proposed to explain oscillations seen in LBM populations. These theories have invoked behavioural changes in population quality (Baltensweiler 1993b), budmoth/disease interactions (Anderson & May 1980), induced host defences (Fischlin & Baltensweiler 1979) and host/parasitoid interactions (Turchin et al. 2003) as causes of periodic outbreaks. Virtually all of these mechanisms have made some assumption of delayed density-dependent effects. LBM females are good fliers and dispersal is considered to play an important role in the spatial distribution of outbreaks, including the existence of outbreak waves emanating from distinct regional foci (Bjørnstad et al. 2002; Johnson et al. 2004).
3. Material and methods
(a) Density measurements
Larix decidua tree-ring density variations were measured at 10 μm resolution using a WALESCH-2003 X-ray densitometer (Eschbach et al. 1995). Brightness variations on X-ray films were transferred into g cm−3 wood density using a calibration wedge of defined optical depths. Sub-annual profiles (figure 1) show a characteristic seasonal pattern of earlywood densities typically below 0.4 g cm−3 obtained from large and thin-walled cells formed at the beginning of a vegetation period, followed by latewood densities up to approximately 1.2 g cm−3 obtained from small, tangentially compressed and thick-walled cells formed during summer until the end of a vegetation period (Schweingruber et al. 1979).
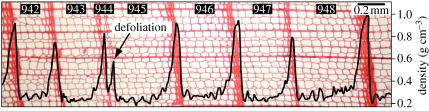
Figure 1.
LBM fingerprint in larch wood. Photomicrograph of larch tree rings (AD 942–948) and their density profile (black curve). During outbreak years, such as AD 944, LBM larvae feeding hinder ‘normal’ growth and cause narrow rings and an exceptionally low (frequently below 0.6 g cm−3) maximum latewood density.
The peak value in density (MXD) occurring near the annual ring boundary is severely reduced by LBM defoliation, as seen in AD 944 in figure 1. The basic mechanism of this signal consists of the (i) consumption of portions of larch needles by budmoth larvae, (ii) necrosis/discoloration of needles and reduction in photosynthetic activity, (iii) regrowth of a second needle generation using stored reserves, and (iv) reduced growth, division and wall thickening of tracheid cells (Schweingruber 1979; Baltensweiler & Rubli 1999). The critical mass for visible needle discoloration stimulating these characteristic tree-ring patterns is of the order of 100 LBM larvae per kilogram foliated branches (Auer 1977).
(b) Composite chronology and LBM detection procedure
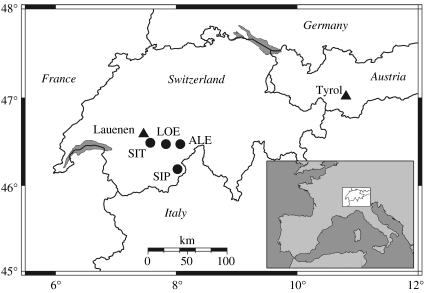
A total of 47 513 density profiles were measured from 180 larch wood samples from four locations in the Swiss Alps (figure 2). Samples were collected from living trees and historical buildings, all from elevations above 1600 m.a.s.l., and combined to a composite (living and relict) chronology using a well-established dendrochronological technique, cross-dating (Douglass 1929), whereby all annual rings were precisely dated to their calendar year of formation. After truncation at a minimum replication of eight samples, the chronology spans from AD 832 to 2004 (see figure S1 of the electronic supplementary material for more details).
Figure 2.
Larch sampling sites are Aletsch (ALE), Lötschental (LOE), Simmental (SIT) and Simplon (SIP) in the Swiss Alps. The majority of tree-ring series originates from LOE (110 series, 1258–2004 period), followed by SIP (39, 735–1510 period), ALE (21, 1792–1974 period) and SIT (10, 1681–1986 period). Locations of the non-host (fir and spruce) Lauenen and Tyrol chronologies used for comparison are indicated with triangles.
Following the dendrochronological skeleton-plot technique (Schweingruber et al. 1990), the density data and X-ray images were visually screened for characteristically narrow tree-ring width (TRW) and low MXD values. This assessment resulted in 4649 tree rings that were identified as being affected by LBM outbreaks. Affected rings were then removed from the dataset, and the gaps were statistically re-estimated with the information obtained from remaining un- or less-affected rings in the same year of the chronology. To detect the LBM signal, a difference series between the gap-filled and original larch chronologies was computed. For details on chronology development, gap-filling procedure, age-effect correction of the LBM signal and validation of the LBM reconstruction, see the electronic supplementary material.
(c) Alpine temperature reconstruction
For a comparison of the LBM reconstruction with regional climate fluctuations, a millennium-long temperature record representative for high-elevation environments in the European Alps was composed (figure 3). This record combines a TRW-based reconstruction from AD 951 to 2002 integrating 1527 pine and larch samples (Büntgen et al. 2005) and a MXD-based reconstruction from AD 755 to 2004 based upon the same 180 larch samples used in the current study for LBM signal detection (Büntgen et al. 2006). Both datasets have been standardized using a technique (Esper et al. 2002, 2003) to remove the age trend, while preserving long-term temperature variations. The temperature signal in these records is weighted towards the summer season.
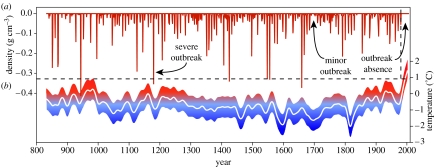
Figure 3.
Long-term LBM and temperature reconstructions for the European Alps. (a) MXD-based LBM outbreak reconstruction since AD 832. Time-series is the age-corrected difference series between gap-filled and original MXD data (for details on gap-filling and age-correction procedures, see the electronic supplementary material). Values less than −0.005 g cm−3 are shown. (b) The temperature model (white curve) is shown together with the standard error (coloured band) derived from the fit with instrumental data. Dashed lines indicate the last LBM mass outbreak in 1981 (vertical) and the upper standard error limit recorded in the late ninth century (horizontal).
We combined these reconstructions by calculating the arithmetic mean of the normalized records over the AD 951–2002 common period. Climate fluctuations before this period, i.e. between AD 832 and 950, are represented only by the density-based reconstruction. The combined proxy record was regressed over the 1864–2002 period (r=0.72) against high-elevation instrumental June–August mean temperatures provided by the Central Institute for Meteorology and Geodynamics (ZAMG) in Vienna (Böhm et al. 2001). We calculated the standard error (s.e.) of the long-term temperature estimate by multiplying the square root of the unexplained variance by the standard deviation of the target instrumental data during the 1864–2002 calibration period, and smoothed the reconstruction and s.e. using a 30-year spline filter (Cook & Peters 1981).
4. Results
(a) Long-term LBM reconstruction
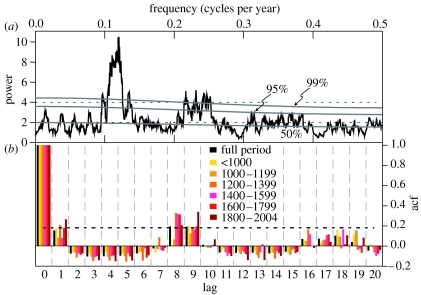
The long-term LBM outbreak reconstruction indicated that cyclic populations have persisted for more than a millennium in the European Alps (figure 3a). The longest interruption of these oscillations was recorded in the late twentieth century, the period since 1981. Analysis of the frequency spectrum of the reconstruction indicated a significant periodicity between 8.1 and 9.3 years and some resonance variability in the higher-frequency domain (figure 4a). The dominance of the 8–9-year cycle was confirmed using estimated autocorrelation functions (acf) applied to the full time-series and various shorter segments (figure 4b). All approximately 200-year segments reached 95% significance at either 8 or 9 years, suggesting this signal was stationary in the long-term LBM record.
Figure 4.
Temporal characteristics of the long-term LBM reconstruction shown in figure 3a. (a) MTM spectrum (Mann & Lees 1996) of the reconstruction. Grey curves are estimated significance levels. (b) Estimated autocorrelation functions (acf) for lags 0–20 of the reconstruction. Colours indicate different periods (full period and segments) used for acf calculation. Dashed curve is the 95% significance level.
The intensity of events, however, did exhibit some variation over the past 1000+ years. Low-intensity periods were, for example, reconstructed during the early fourteenth and nineteenth centuries, with more intense outbreaks during the mid-fourteenth and mid-sixteenth centuries (figure 3a). Two consecutive peak intensity episodes were, for example, reconstructed for 1548 and 1556. While the corrections for age effects in LBM detection (see the electronic supplementary material for details) increased the likelihood that this reconstruction accurately resolved intensity variations, additional data are needed for a more conclusive interpretation.
Comparison of the long-term LBM record with the newly composed Alpine climate reconstruction (figure 3b) showed that regular population cycles continued despite major fluctuations in regional temperature including the transition from warm conditions during medieval times into the Little Ice Age, with the coldest episodes in the late sixteenth and early nineteenth centuries. This finding, together with various calibration tests using (non-smoothed) 500-year seasonal temperature reconstructions averaged over the respective grid cells in the European Alps (Luterbacher et al. 2004), demonstrated that there was no systematic relationship between LBM activity and climatic variations.
While these tests suggested that there is no link between LBM and climate in the high-frequency domain, the long-term reconstruction, however, underscores the exceptional character of the late twentieth century absence of LBM mass outbreaks. This absence falls in a period of extreme regional warmth—outside the climatic envelope seen over the past 1200 years (figure 3b)—suggesting that some threshold in temperature variation has recently been passed, disturbing an otherwise stable ecological system of population cycles.
(b) Detailed budmoth history
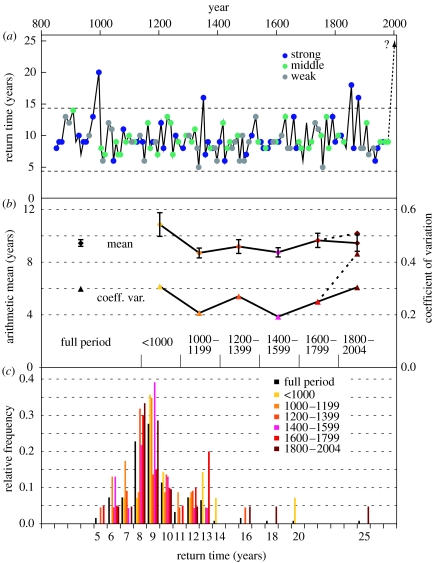
Analysis of the long-term LBM reconstruction and validation using alternative signal detection approaches—including consideration of lag-1 TRW data and comparison with non-host tree species (see the electronic supplementary material)—enabled the identification of 123 defoliation events since AD 832 (table 1). The return time record of these events demonstrated that, except for the recent period since 1981, no discontinuity in LBM mass outbreaks longer than two decades has occurred over the past 1173 years (figure 5a). Overall, there are four other intervals in the tenth, fourteenth and nineteenth centuries exceeding the two standard deviation thresholds, but none reached the length—currently 25 years—of the late twentieth century absence period. While the four longest (14, 16, 18 and 20 years) and the two shortest (2×5 years) intervals coincided with very strong and weak MXD signals (see the colours in figure 5a), additional tests using the recurrence time and MXD reduction data revealed no further relationship between these variables.
Table 1.
One hundred and twenty-three defoliation events derived from the long-term LBM reconstruction. (All years listed exceeded the −0.005 g cm−3 threshold in this record. In cases where two or more ‘competing years’—i.e. consecutive years of similar magnitude—were recorded, only the first year was denoted. Consequently, the years listed here do not represent the full time-series as shown in figure 3. Bold years are outbreaks confirmed in the historical literature (Baltensweiler & Rubli 1999) back to 1850 (horizontal line 1). Letters indicate synchronous negative deviations found using other detection methods. The Lauenen and Tyrol data span the 982–1976 and 1324–1975 periods, respectively (horizontal lines 2 and 3).)
| twentieth–nineteenth century | eighteenth–seventeenth century | sixteenth–fifteenth century | fourteenth–thirteenth century | twelfth–eleventh century | tenth–ninth century |
|---|---|---|---|---|---|
| 1981 | 1792a–e | 1599b–e | 1398 | 1196 | 996a–c 3 |
| 1972a | 1779b,c,e | 1590b,d | 1392 | 1189 | 976a |
| 1963a,b,d,e | 1771c,d | 1581a–e | 1384 | 1180a–c | 963 |
| 1954a | 1758a,c,d | 1572a–e | 1376a–e | 1171 | 953a |
| 1945a–e | 1753b,e | 1564e | 1368a 2 | 1163 | 944 |
| 1937a,c,d,e | 1743 | 1556a–e | 1359a | 1151a,b | 935 |
| 1931 | 1732d | 1548a–e | 1352a–c | 1145b,c | 928 |
| 1923c–e | 1720a | 1539a,d | 1336b | 1136b,c | 918 |
| 1915a | 1711a,c | 1530c,e | 1331a–c | 1126a–c | 909 |
| 1908a,b,c,e | 1703d | 1517b,d | 1323 | 1117a–c | |
| 1507 | 1312b | 1108a–c | |||
| 1303 | |||||
| 1896 | 1691 | 1498a–e | 1293a | 1099a–c | 895a |
| 1888a,b,d | 1685a,b,d | 1491a | 1283e | 1089a,b | 883a |
| 1880a,b,c,e | 1677a–e | 1485 | 1275 | 1080a–c | 870a |
| 1864 1 | 1668b–e | 1475 | 1267a,c | 1069b,c | 961a |
| 1856a,b,d,e | 1660b–e | 1469b,d | 1258a,c | 1062a–c | 852a |
| 1838 | 1647c | 1459a,b,d,e | 1248b,c | 1055b | 844a |
| 1830b–e | 1639b–e | 1450a–e | 1241 | 1046a–c | |
| 1821b–e | 1631a,b | 1442b,c,e | 1229a,b | 1040c | |
| 1811a,b,d | 1618 | 1433a,b | 1216a,c | 1029b | |
| 1801b–e | 1608a–e | 1425a–e | 1208a,c | 1017b,c | |
| 1419 | 1010b,c | ||||
| 1407a–e | 1004b,c |
Combined larch MXD plus TRW (lag-1).
Lauenen versus gap-filled data (MXD).
Lauenen versus gap-filled data (TRW).
Tyrol versus gap-filled data (MXD).
Tyrol versus gap-filled data (TRW).
Figure 5.
Characteristics of LBM outbreak events reconstructed over the past millennium (see table 1 for complete list). (a) Return time record calculated from the 123 LBM defoliation events. Colours indicate a classification of the intensity of outbreaks, i.e. the reduction of wood density (in g cm−3) caused by LBM. Dashed curve in the late twentieth century refers to the recent absence in mass outbreaks since 1981. Horizontal dashed curves are the two standard deviations. (b) Arithmetic mean and standard error (bars), and coefficient of variation computed for the full return time record (AD 832–2004) and six sub-periods. Dashed curves in recent centuries result from including a 25-year return time interval since 1981, i.e. an LBM mass outbreak in 2006 was assumed. (c) Relative frequency distribution of the return time data. Colours indicate different periods. Note that a single event transforms to bars of different relative heights (e.g. all spikes in classes above 14 years correspond to single events).
Calculation of the mean recurrence times of various approximately 200-year segments indicated an increased interval length in the early period before AD 1000, significantly greater than the 9.3 years obtained for the full record (figure 5b). This deviation was influenced by the inclusion of a 20-year interval—the second longest interval on record (see the yellow spike in the tail of the relative distribution shown in figure 5c)—that occurred in the first millennium AD from 976 to 996. Yet, because replication and spatial distribution in these early larch data were significantly reduced in comparison with the second millennium AD, we cannot exclude the possibility that a single LBM outbreak was missed in this earliest period. Similarly, it is also possible that an undetected LBM outbreak occurred during the 18-year interval 1838–1856 (brown spike in the distribution). We recorded several deviations between the original and the gap-filled MXD records during this period (e.g. 1843), but none of these reached the 0.005 g cm−3 threshold necessary for classification as an LBM mass outbreak. However, the exclusion of only one of these longer intervals would substantially affect the conclusion on the exceptional character of the recent outbreak absence.
Considering these uncertainties and the circumstance that the right margin of the frequency distributions contains very few data, it is not feasible to robustly derive extreme-value test statistics. Observations supporting the conclusion that the recent outbreak absence period is truly exceptional include (i) the similarity of the sub-period frequency distributions indicating that the LBM recurrence time data were stationary over the past millennium (figure 5c), (ii) the deviation of the coefficient of variation after including a 25-year interval to account for the recent absence (figure 5b, dashed curve), and (iii) the sheer length of the outbreak absence reached by now, making this an unprecedented episode with respect to the last 1173 years.
5. Discussion
Understanding the cyclic dynamics of animal populations, and evaluating the vulnerability of such ecological systems to exogenous forcing such as climate change, requires long time-series of natural population variation. We report here on such a time-series of Z. diniana outbreaks from the European Alps, derived from a dataset of MXD measurements from living and historical L. decidua wood samples. The MXD parameter is a particularly sensitive indicator for LBM outbreaks, since defoliation events severely reduce cell wall thickening and result in abnormally low MXD values. The dataset of 47 513 high-resolution density profiles used in this study is unique in terms of its length, replication and diagnostic capabilities for detection of insect outbreaks.
The LBM reconstruction was achieved by identifying larch tree rings—sample-by-sample, year-by-year—that were affected by defoliation events, removing these rings, and statistically re-estimating values using data from trees unaffected during the same year. This procedure allowed the calculation of a difference series between MXD data that were ‘contaminated’ by LBM fingerprints and MXD data that were ‘cleaned’ from this contamination. Potential uncertainty in the LBM reconstruction could, however, result from the combination of living and relict tree-ring material. Whereas in the collection of samples from living trees we had control over the origin of material, the origin of historical samples varied between 1600 and 2100 m.a.s.l. within the investigated valleys, i.e. site control and sample homogeneity decline from living towards historic data.
The striking persistence and regularity of budmoth reoccurrence over the past 1000+ years reflects positively on the reconstruction method presented here, since it demonstrated that LBM detection succeeded without major restriction throughout the past millennium. Conversely, if we had reconstructed prolonged periods without any LBM peak signals, there would have been some ambiguity in interpreting these periods, since missing signals could have been due to the origin of wood material from portions of the Alps where LBM outbreaks did not occur or due to the insensitivity or inadequateness of our detection methods.
Reconstructed LBM defoliation events were also supported by various tests with comparable non-host time-series and a combined MXD plus lag-1 TRW record. Results from comparisons with non-host data, however, tended to be less complete than the time-series based on the gap-filling method (see the electronic supplementary material). This was probably due to the chance occurrence of LBM defoliation in years that were exceptionally cold. In these cases, both the host and the non-host chronologies would be expected to show negative outliers, making LBM detection more difficult.
LBM defoliation has long captured public attention, since the forests of entire subalpine valleys and regions turn brownish during outbreak years, a phenomenon that visually transforms the European Alps. Yet, periodic outbreaks by forest defoliators, such as LBM, are known to serve as important disturbances affecting forest nutrient cycling and other ecosystem processes (Schowalter et al. 1986; Lovett et al. 2002). The reconstruction presented here reveals that these periodic events prevailed over the past millennium, and we can presume that the regular recurrence of outbreaks has played an important role in stable ecosystem functioning. From AD 832 to 1981, there were 123 LBM outbreaks with a mean reoccurrence time of 9.3 years, and prior to 1980 there was never a gap that lasted longer than two decades. Our analysis, however, also suggests that the absence of mass outbreaks since the 1980s is truly exceptional. Given this unprecedented change in the disturbance regime, we expect that nutrient cycling and other ecosystem processes operating in the subalpine larch forests of the Alps may be undergoing a drastic alteration.
Among the plethora of animal species known to exhibit population cycles (Kendall et al. 1998), oscillations of LBM in the European Alps are considered to exhibit particularly regular cycles (Berryman 1996, 2002). Our report here that these regular cycles persisted without significant interruption for more than 1000 years portrays these LBM cycles as even more remarkable. Although theoretical models of nonlinear trophic interactions, such as are thought to exist in the LBM system, are known to sometimes exhibit transient dynamics in which populations transition between periodic, chaotic and random behaviour (Hastings 2004), the results presented here provide little evidence of such behaviour. Furthermore, the persistence of regular population cycles in the LBM system demonstrates that the stability of these oscillations are resilient to the range of climatic variations and other disturbances occurring in the Alps over the last 1200 years.
Although LBM populations have experienced some variation in climate over the last 1200 years, an annually resolved temperature reconstruction from the Alps demonstrates that conditions during the late twentieth century represent the warmest period of the past millennium (figure 3b). The fact that this period also corresponds with an unprecedented lack of LBM outbreaks since 1981 implicates the role of extraordinary climatic conditions as the cause of outbreak failure. While this association suggests that contemporary temperature increase has induced a fundamental alteration in LBM dynamics, the mechanism for this connection is not totally certain. A probable hypothesis (Baltensweiler 1993a) explaining the failure of LBM to reach high population levels involves the influence of late winter weather patterns on the viability of LBM in the egg stage, when the moth overwinters on the upper site of larch branches. Warm winters trigger early LBM egg development and increased egg mortality, as well as increased larval mortality when first instars that hatch prior to the development of larch needles starve. As a consequence, larval populations may not reach defoliating densities and the LBM cycles may prematurely collapse.
Like most forest insect species, LBM populations are affected by a multitude of interactions with lower (i.e. host trees) and higher (i.e. parasitoids, predators and diseases) trophic levels and the unprecedented change in climate could thereby affect LBM populations indirectly, in addition to the direct mechanisms outlined above. Although LBM populations have not reached defoliating levels in most parts of the Alps since the early 1980s, they have attained sub-defoliating peak densities during this period (Baltensweiler 1993b; Baltensweiler & Rubli 1999). This suggests that the change in LBM population dynamics has been a diminishing of oscillation amplitude, but not necessarily an alteration of the period. It remains to be seen how this recent disruption of a major disturbance regime will alter ecosystem processes in subalpine larch forests and what other changes might be triggered as a result.
Acknowledgments
This work was supported by the Swiss National Science Foundation (NCCR Climate and EURO-TRANS, no. 200021–105663) and the European Union (ALPIMP, no. EVK2-CT-2002-00148).
Supplementary Material
Includes five figures
References
- Anderson R.M, May R.M. Infection diseases and population cycles of forest insects. Science. 1980;210:658–661. doi: 10.1126/science.210.4470.658. doi:10.1126/science.210.4470.658 [DOI] [PubMed] [Google Scholar]
- Auer C. Dynamik von Lärchenwicklerpopulationen längs des Alpenbogens. Mitt. Eidg. Forschungsanstalt Wald Schnee Landschaft. 1977;53:70–105. [Google Scholar]
- Bale J.S, et al. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biol. 2002;8:1–16. doi:10.1046/j.1365-2486.2002.00451.x [Google Scholar]
- Baltensweiler W. Why the larch bud moth cycle collapsed in the subalpine larch-cembran pine forests in the year 1990 for the first time since 1850. Oecologia. 1993a;94:62–66. doi: 10.1007/BF00317302. doi:10.1007/BF00317302 [DOI] [PubMed] [Google Scholar]
- Baltensweiler W. A contribution to the explanation of the larch bud moth cycle, the polymorphic fitness hypothesis. Oecologia. 1993b;93:251–255. doi: 10.1007/BF00317678. doi:10.1007/BF00317678 [DOI] [PubMed] [Google Scholar]
- Baltensweiler W, Rubli D. Dispersal—an important driving force of the cyclic population dynamics of the larch bud moth. Forest Snow Landscape Res. 1999;74:3–153. [Google Scholar]
- Baltensweiler W, Benz G, Bovey P, Delucchi V. Dynamics of larch bud moth populations. Annu. Rev. Entomol. 1977;22:79–100. doi:10.1146/annurev.en.22.010177.000455 [Google Scholar]
- Berryman A.A. What causes population cycles of forest Lepidoptera? Trends Ecol. Evol. 1996;11:28–32. doi: 10.1016/0169-5347(96)81066-4. doi:10.1016/0169-5347(96)81066-4 [DOI] [PubMed] [Google Scholar]
- Berryman A. A. (ed.) 2002 Population cycles: causes and analysis. In Population cycles: the case for tropic interactions, pp. 3–28. Oxford, UK: Oxford University Press .
- Bjørnstad O.N, Grenfell B.T. Noisy clockwork: time series analysis of population fluctuations in animals. Science. 2001;293:638–643. doi: 10.1126/science.1062226. doi:10.1126/science.1062226 [DOI] [PubMed] [Google Scholar]
- Bjørnstad O.N, Peltonen M, Liebhold A.M, Baltensweiler W. Waves of larch budmoth outbreaks in the European Alps. Science. 2002;298:1020–1023. doi: 10.1126/science.1075182. doi:10.1126/science.1075182 [DOI] [PubMed] [Google Scholar]
- Böhm R, Auer I, Brunetti M, Maugeri M, Nanni T, Schoner W. Regional temperature variability in the European Alps 1760–1998 from homogenized instrumental time series. Int. J. Climatol. 2001;21:1779–1801. [Google Scholar]
- Boulanger Y, Arseneault D. Spruce budworm outbreaks in eastern Quebec over the last 450 years. Can. J. Forest Res. 2004;34:1035–1043. doi:10.1139/x03-269 [Google Scholar]
- Büntgen U, Esper J, Frank D.C, Nicolussi K, Schmidhalter M. A 1052-year tree-ring proxy for alpine summer temperatures. Clim. Dyn. 2005;25:141–153. doi:10.1007/s00382-005-0028-1 [Google Scholar]
- Büntgen U, Frank D.C, Nievergelt D, Esper J. Alpine summer temperature variations, AD 755–2004. J. Clim. 2006;19:5606–5623. doi:10.1175/JCLI3917.1 [Google Scholar]
- Cook E.R, Peters K. The smoothing spline: a new approach to standardizing forest interior tree-ring width series for dendroclimatic studies. Tree Ring Bull. 1981;41:45–53. [Google Scholar]
- Douglass A.E. The secret of the Southwest solved by talkative tree rings. Nat. Geogr. Mag. 1929;56:736–770. [Google Scholar]
- Elton C.S. Periodic fluctuations in the number of animals: their causes and effects. Br. J. Exp. Biol. 1924;2:119–163. [Google Scholar]
- Eschbach W, Nogler P, Schär E, Schweingruber F.H. Technical advances in the radiodensitometrical determination of wood density. Dendrochronologia. 1995;13:155–168. [Google Scholar]
- Esper J, Cook E.R, Schweingruber F.H. Low-frequency signals in long tree-ring chronologies and the reconstruction of past temperature variability. Science. 2002;295:2250–2253. doi: 10.1126/science.1066208. doi:10.1126/science.1066208 [DOI] [PubMed] [Google Scholar]
- Esper J, Cook E.R, Krusic P.J, Peters K, Schweingruber F.H. Tests of the RCS method for preserving low-frequency variability in long tree-ring chronologies. Tree Ring Res. 2003;59:81–98. [Google Scholar]
- Fischlin A, Baltensweiler W. Systems analysis of the larch bud moth system: the larch-larch bud moth relationship. Mitt. Schweiz. Ent. Ges. 1979;52:273–289. [Google Scholar]
- Hanski I, Henttonen H, Korpimaki E, Oksanen L, Turchin P. Small rodent dynamics and predation. Ecology. 2001;82:1505–1520. doi:10.2307/2679796 [Google Scholar]
- Hansson L, Henttonen H. Gradients in density variations of small rodents: the importance of latitude and snow cover. Oecologia. 1985;67:394–402. doi: 10.1007/BF00384946. doi:10.1007/BF00384946 [DOI] [PubMed] [Google Scholar]
- Hastings A. Transients: the key to long-term ecological understanding? Trends Ecol. Evol. 2004;19:39–45. doi: 10.1016/j.tree.2003.09.007. doi:10.1016/j.tree.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Haukioja E. Induction of defense in trees. Annu. Rev. Entomol. 1990;36:25–42. doi:10.1146/annurev.en.36.010191.000325 [Google Scholar]
- Henson S.M, Cushing J.M, Costantino R.F, Dennis B, Desharnais R.A. Phase switching in population cycles. Proc. R. Soc. B. 1998;265:2229–2234. doi:10.1098/rspb.1998.0564 [Google Scholar]
- Hunter M.D, Varley G.C, Gradwell G.R. Estimating the relative roles of top-down and bottom-up forces on insect herbivore populations: a classic study revisited. Proc. Natl Acad. Sci. USA. 1997;94:9176–9181. doi: 10.1073/pnas.94.17.9176. doi:10.1073/pnas.94.17.9176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ims R.A, Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. BioScience. 2005;55:311–322. doi:10.1641/0006-3568(2005)055[0311:TICITE]2.0.CO;2 [Google Scholar]
- Johnson D.M, Bjornstad O.N, Liebhold A.M. Landscape geometry and travelling waves in the larch budmoth. Ecol. Lett. 2004;7:967–974. doi:10.1111/j.1461-0248.2004.00659.x [Google Scholar]
- Kendall B.E, Prendergast J, Bjørnstad O.N. The macroecology of population dynamics: taxonomic and biogeographic patterns in population cycles. Ecol. Lett. 1998;1:160–164. doi:10.1046/j.1461-0248.1998.00037.x [Google Scholar]
- Kendall B.E, Briggs C.J, Murdoch W.W, Turchin P, Ellner S.P, McCauley E, Nisbet R.M, Wood S.N. Why do populations cycle? A synthesis of statistical and mechanistic modeling approaches. Ecology. 1999;80:1789–1805. doi:10.2307/176658 [Google Scholar]
- Klemola T, Tanhuanpaa M, Korpimaki E, Ruohomaki K. Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos. 2002;99:83–94. doi:10.1034/j.1600-0706.2002.990109.x [Google Scholar]
- Krause C. The use of dendrochronological material from buildings to get information about past spruce budworm outbreaks. Can. J. Forest Res. 1997;27:69–75. doi:10.1139/cjfr-27-1-69 [Google Scholar]
- Lovett G.M, Christenson L.M, Groffman P.M, Jones C.G, Hart J.E, Mitchell M.J. Insect defoliation and nitrogen cycling in forests. Bioscience. 2002;52:335–341. doi:10.1641/0006-3568(2002)052[0335:IDANCI]2.0.CO;2 [Google Scholar]
- Luterbacher J, Dietrich D, Xoplaki E, Grosjean M, Wanner H. European seasonal and annual temperature variability, trends, and extremes since 1500. Science. 2004;303:1499–1503. doi: 10.1126/science.1093877. doi:10.1126/science.1093877 [DOI] [PubMed] [Google Scholar]
- Mann M.E, Lees J.M. Robust estimation of background noise and signal detection in climatic time series. Clim. Change. 1996;33:409–445. doi:10.1007/BF00142586 [Google Scholar]
- Mattson W.J, Addy N.D. Phytophagous insects as regulators of forest primary production. Science. 1975;190:515–522. [Google Scholar]
- Rolland C, Baltensweiler W, Petitcolas V. The potential of using Larix decidua ring widths in reconstructions of larch budmoth (Zeiraphera diniana) outbreak history: dendrochronological estimates compared with insect surveys. Trees. 2001;15:414–426. doi:10.1007/s004680100116 [Google Scholar]
- Ruohomaeki K, Virtanen T, Kaitaniemi P, Tammaru T. Old mountain birches at high altitudes are prone to outbreaks of Epirrita autumnata (Lepidoptera: Geometridae) Environ. Entomol. 1997;26:1096–1104. [Google Scholar]
- Ryerson D.E, Swetnam T.W, Lynch A.M. A tree-ring reconstruction of western spruce budworm outbreaks in the San Juan Mountains, Colorado, U.S.A. Can. J. Forest Res. 2003;33:1010–1028. doi:10.1139/x03-026 [Google Scholar]
- Schowalter T.D, Hargrove W.W, Crossley D.A., Jr Herbivory in forested ecosystems. Annu. Rev. Entomol. 1986;31:177–196. doi:10.1146/annurev.en.31.010186.001141 [Google Scholar]
- Schweingruber F.H. Auswirkungen des Lärchenwicklerbefalls auf die Jahrringstruktur der Lärche. Schweiz. Z. Forstwes. 1979;130:1071–1093. [Google Scholar]
- Schweingruber F.H, Bräker O.U, Schär E. Dendroclimatic studies on conifers from central Europe and Great Britain. Boreas. 1979;8:427–452. [Google Scholar]
- Schweingruber F.H, Eckstein D, Serre-Bachet F, Bräker O.U. Identification, presentation and interpretation of event years and pointer years in dendrochronology. Dendrochronologia. 1990;8:9–38. [Google Scholar]
- Speer J.H, Swetnam T.W, Wickman B.E, Youngblood A. Changes in pandora moth outbreak dynamics during the past 622 years. Ecology. 2001;82:679–697. doi:10.2307/2680188 [Google Scholar]
- Stenseth N.C. Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos. 1999;87:427–461. [Google Scholar]
- Swetnam T.W, Lynch A.M. Multi-century, regional-scale patterns of western spruce budworm history. Ecol. Monogr. 1993;63:399–424. doi:10.2307/2937153 [Google Scholar]
- Turchin P, Wood S.N, Ellner S.P, Kendall B.E, Murdoch W.W, Fischlin A, Casas J, McCauley E, Briggs C.J. Dynamical effects of plant quality and parasitism on population cycles of larch budmoth. Ecology. 2003;84:1207–1214. [Google Scholar]
- Volney W.J.A, Fleming R.A. Climate change and impacts of boreal forest insects. Agr. Ecosyst. Environ. 2000;82:283–294. doi:10.1016/S0167-8809(00)00232-2 [Google Scholar]
- Walther G.-R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.-M, Guldberg O.H, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Weber U. Dendrochronological reconstruction and interpretation of larch budmoth (Zeiraphera diniana) outbreaks in two central alpine valleys of Switzerland from 1470–1990. Trees. 1997;11:277–290. [Google Scholar]
- Yamamura K, Yokozawa M, Nishimori M, Ueda Y, Yokosuka T. How to analyze long-term insect population dynamics under climate change: 50-year data of three insect pests in paddy fields. Popul. Ecol. 2006;48:31–48. doi:10.1007/s10144-005-0239-7 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes five figures