Abstract
Birds use photoperiod to control the time of breeding and moult. However, it is unclear whether responses are dependent on absolute photoperiod, the direction and rate of change in photoperiod, or if photoperiod entrains a circannual clock. If starlings (Sturnus vulgaris) are kept on a constant photoperiod of 12 h light : 12 h darkness per day (12 L : 12 D), then they can show repeated cycles of gonadal maturation, regression and moult, which is evidence for a circannual clock. In this study, starlings kept on constant 11.5 L : 12.5 D for 4 years or 12.5 L : 11.5 D for 3 years showed no circannual cycles in gonadal maturation or moult. So, if there is a circannual clock, it is overridden by a modest deviation in photoperiod from 12 L : 12 D. The responses to 11.5 L : 12.5 D and 12.5 L : 11.5 D were very different, the former perceived as a short photoperiod (birds were photosensitive for most of the time) and the latter as a long photoperiod (birds remained permanently photorefractory). Starlings were then kept on a schedule which ranged from 11.5 L : 12.5 D in mid-winter to 12.5 L : 11.5 D in mid-summer (simulating the annual cycle at 9 °N) for 3 years. These birds entrained precisely to calendar time and changes in testicular size and moult were similar to those of birds under a simulated cycle at 52 °N. These data show that birds are very sensitive to changes in photoperiod but that they do not simply respond to absolute photoperiod nor can they rely on a circannual clock. Instead, birds appear to respond to the shape of the annual change in photoperiod. This proximate control could operate from near equatorial latitudes and would account for similar seasonal timing in individuals of a species over a wide range of latitudes.
Keywords: bird, starling, photoperiodism, circannual rhythms, latitude
1. Introduction
Since the work of Rowan (1929), it has been clear that the primary environmental factor used by birds outside the tropics to time reproduction is the annual change in photoperiod. Birds have extra-retinal photoreceptors which they use, in conjunction with a circadian clock, to measure photoperiod (Dawson et al. 2001). Experimental transfer of birds from a short to a long photoperiod induces neuroendocrine changes within hours (Meddle & Follett 1997; Yoshimura et al. 2003) and these lead to gonadal maturation during subsequent days and weeks. The longer the photoperiod is, the greater the rate of gonadal maturation (Farner & Wilson 1957; Dawson & Goldsmith 1983). The timing of gonadal regression at the end of the breeding season and the associated moult are also controlled by photoperiod. Many temperate zone species undergo spontaneous gonadal regression (photorefractoriness) during prolonged exposure to long photoperiods, and the longer the photoperiod the sooner the regression and moult occurs (Dawson et al. 2001). However, it is unclear whether responses are dependent on the absolute duration of photoperiod, the direction and rate of change in photoperiod, or if photoperiod serves to entrain a circannual clock.
Many species of birds have breeding ranges that span a wide range of latitudes. Several species even have transequatorial breeding ranges, extending from high northern latitudes to well south of the equator (Lofts & Murton 1968). Individuals at different latitudes will experience very different changes in photoperiod during the year. Yet, breeding seasons do not differ greatly; at lower latitudes, breeding seasons tend to start slightly earlier but last longer, so that timing of the end of the breeding season and the postnuptial moult may be similar. For example, blackbirds, Turdus merula, start to breed two weeks earlier in southern England than in Scotland (Myres 1955), but postnuptial moult occurs at the same time (personal analysis of British Trust for Ornithology moult data). Similarly, great tits, Parus major, in northern Europe start to lay later, but also have a lower frequency of second clutches (Sanz 1998). If photoperiod exerts direct control on physiology and responses are proportional to absolute photoperiod, it is difficult to explain how breeding seasons could be similar over a wide range of latitudes unless there are marked genetic differences allowing different populations to respond differently to photoperiod (Silverin et al. 1993).
At the equator, there is no annual change in photoperiod, yet breeding in many species of birds remains seasonal (Lofts & Murton 1968). This may be brought about by an endogenous circannual clock. If tropical birds are kept on a constant equatorial photoperiod (12 h light and 12 h darkness per day (12 L : 12 D)), then they can undergo repeated cycles of gonadal maturation, regression and moult (Gwinner & Dittami 1990; Gwinner et al. 1995; Gwinner 1996). These cycles must be driven endogenously because cycle length varies between individuals and they become desynchronized from calendar time. The environmental factor that normally acts as a Zeitgeber to synchronize the endogenous cycles to true calendar time is unclear.
Temperate zone species kept on a constant photoperiod of 12 L : 12 D can also undergo repeated cycles of gonadal maturation, regression and moult (Schwab 1971; Gwinner 1981, 1996; Dawson 1997). It has therefore been postulated that a circannual clock may also underpin seasonal cycles in species outside the tropics, and that the annual cycle in photoperiod entrains this to calendar time in an analogous way to that in which circadian clocks control daily cycles and are entrained by dawn (Gwinner 1996). In theory, this could explain the similarity in breeding seasons at different latitudes. However, there are also problems with this explanation, one of which is that circannual cycles are only apparent if birds are kept on, or close to, 12 L : 12 D. If starlings (Sturnus vulgaris), for example, are held on a constant 13 L : 11 D photoperiod, the gonads remain fully regressed indefinitely, whereas on constant 11 L : 13 D they remain mature indefinitely (Hamner 1971; Schwab & Rutledge 1973; Gwinner & Wozniak 1982). Thus, under seasonal changes in photoperiod outside the tropics, the circannual clock would only tick for two short periods each year, at the equinoxes.
The initial aim of this study was to determine the range of photoperiod either side of 12 L : 12 D within which circannual rhythms are still expressed in starlings. Circannual rhythms were not expressed under constant 11.5 L : 12.5 D or 12.5 L : 11.5 D. (Under 11.5 L : 12.5 D birds showed fluctuations in testicular size, but there were no significant periods of photorefractoriness, cycle length varied widely and mean cycle length exceeded 12 months.) However, the responses to constant 11.5 L : 12.5 D were very different from those to 12.5 L : 11.5 D. The former was generally perceived as a short photoperiod and the latter as a long photoperiod. This led to the question; what would happen to birds held under a natural sinusoidal annual cycle in photoperiod, in which the photoperiod varied between a minimum of 11.5 L : 12.5 D and a maximum of 12.5 L : 11.5 D, i.e. an amplitude of 1 h? This is the range over which photoperiod varies annually at a latitude of just 9 °N, i.e. well within the tropics (less than 23°). The responses to this treatment were compared to control birds held under a regime that simulated annual photoperiodic changes at 52 °N.
2. Material and methods
Starlings were caught from the wild at 52 °N as juveniles and held initially in an outdoor aviary. During the experiments, the birds were kept in indoor aviaries (3.1×3.1×2.6 m), in which they were allowed to fly freely. Temperature was maintained at approximately 18°C. Lighting was provided by three coolwhite fluorescent tubes and one grolux tube to provide UV, resulting in approximately 1500 lux at perch height. Testis size was assessed frequently after unilateral laparotomy under isoflurane anaesthesia (Home Office Licence 80/1521). The dimensions of the left testis were recorded to the nearest 0.1 mm using a binocular microscope and volume was calculated as 4/3πa2b, where a is half the diameter and b half the length. The progress of moult was scored as a proportion of final primary feather mass (Dawson & Newton 2004). In experiment 1, eight male starlings were moved from the outdoor aviary in January 2000 to a constant photoperiod of 11.5 L : 12.5 D for 4 years. In experiment 2, 10 starlings were moved from the outdoor aviary in January 2002 to a constant photoperiod of 12.5 L : 11.5 D for 3 years. In experiment 3, 12 starlings were moved from the outdoor aviary in December 2002 to a photoperiodic regime that followed the natural annual sinusoidal pattern in photoperiod, but with an amplitude of 1 h, i.e. a mid-winter photoperiod of 11.5 L : 12.5 D and a mid-summer photoperiod of 12.5 L : 11.5 D, for 3 years. Photoperiod (sunrise to sunset) for each day of the year was calculated using the Astronomical Applications Department of the US Naval Observatory (http://aa.usno.navy.mil/) for latitude 9 °N. Twelve control birds were held on a photoperiodic regime simulating 52 °N (an amplitude of 9 h, i.e. a mid-winter photoperiod of 7.5 L : 16.5 D and a mid-summer photoperiod of 16.5 L : 7.5 D). The time that lights were switched on and off was changed daily to match the calculated photoperiod.
3. Results
(a) Experiment 1
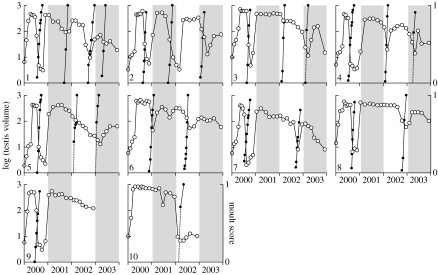
All the birds showed initial testicular maturation, followed shortly afterwards by regression and moult (apart from bird no. 10). Thus, responses during the first nine months were similar to a normal annual cycle. The end of moult was followed by rapid testicular recrudescence and, thereafter, the testes tended to remain large with brief periods of partial testicular regression followed by moult. The number of these cycles varied between 1 and 4 over the 4 years. Mean cycle length (inter-moult period for the eight birds that moulted at least twice) was 17.6±5.4 (s.d.) months (figure 1).
Figure 1.
Changes in testis volume (open circles, left axis) and the occurrence of moult (solid circles, right axis) under constant 11.5 L : 12.5 D. Ten starlings were moved from an outdoor aviary in January 2000 to a constant photoperiod of 11.5 h light : 12.5 h darkness per day for 4 years. The birds are shown in the order of the number of moults undertaken during the 4 years, from 4 for bird no. 1 to 1 for bird nos. 9 and 10.
(b) Experiment 2
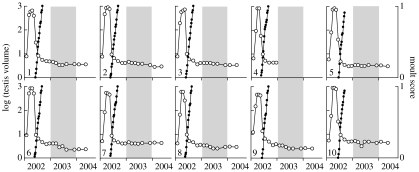
All the birds showed initial testicular maturation, followed by complete regression and moult. Thereafter, the testes remained fully regressed for 2 years. There was no evidence of gonadal cycles and no further moult (figure 2).
Figure 2.
Changes in testis volume (open circles, left axis) and the occurrence of moult (solid circles, right axis) under constant 12.5 L : 11.5 D. Ten starlings were moved from an outdoor aviary in January 2002 to a constant photoperiod of 12.5 h light per day for 3 years.
(c) Experiment 3
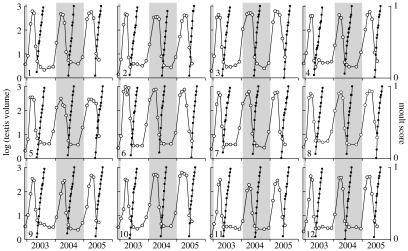
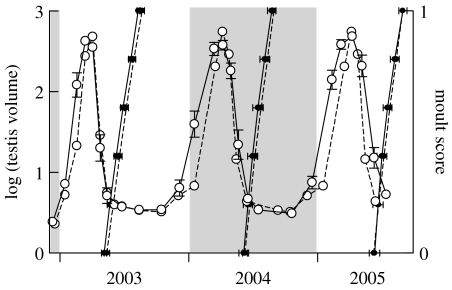
All the birds showed three repeated cycles of testicular maturation, followed by complete regression and moult during the 3 years (figure 3). Mean cycle length (inter-moult period) was 12.1±0.6 (s.d.) months. Maximum and minimum testis sizes matched those of control birds (figure 4).
Figure 3.
Changes in testis volume (open circles, left axis) and the occurrence of moult (solid circles, right axis) in photoperiodic cycles simulating 9 °N. Twelve starlings were moved from an outdoor aviary in December 2002 to a photoperiodic regime that followed the natural annual pattern in photoperiod, but with an amplitude of 1 h, i.e. a mid-winter photoperiod of 11.5 L : 12.5 D and a mid-summer photoperiod of 12.5 L : 11.5 D, for 3 years. All the birds showed repeated cycles of testicular maturation, followed by complete regression and moult. Mean cycle length (inter-moult period) was 12.1±0.6 (s.d.) months.
Figure 4.
Changes in testis volume (open circles, left axis) and the occurrence of moult (solid circles, right axis) in photoperiodic cycles simulating 9 °N and 52 °N. Mean±s.e. values for the 12 individual birds shown in figure 3 that were held on a simulated 9 °N photoperiodic regime (solid lines) superimposed on values for 12 control birds (broken lines) held on a photoperiodic regime simulating 52 °N (an amplitude of 9 h, i.e. a mid-winter photoperiod of 7.5 L : 16.5 D and a mid-summer photoperiod of 16.5 L : 7.5 D). Mean and s.e. for moult were calculated by regressing day on moult score (Dawson & Newton 2004). The timing of testicular maturation was slightly advanced in the second two cycles in the 9 °N birds, but the time of regression and moult was exactly the same as in the 52 °N birds.
The timing of testicular maturation was slightly advanced in the second two cycles in the 9 °N birds compared with the 52 °N birds, but the time of regression and moult was exactly the same as in the 52 °N birds.
4. Discussion
The first part of this study demonstrated that circannual rhythmicity was not sustained under constant 11.5 L : 12.5 D or 12.5 D : 11.5 L. If starlings are held under constant 11 L : 13 D, the testes remain large indefinitely and birds do not moult (Hamner 1971). In starlings under 11.5 L : 12.5 D, there was some evidence for irregular periods of testicular regression and moult, but generally the testes remained large and the mean inter-moult interval was well in excess of 12 months (figure 1). Under 12 L : 12 D, a proportion of starlings do undergo apparent circannual cycles, but cycle length varies widely, between 9 and 15 months (Gwinner 1981; Dawson 1997). Under 12.5 L : 11.5 D or 13 L : 11 D, testes remain regressed indefinitely (figure 2; Hamner 1971; Gwinner & Wozniak 1982). Thus, there is a graded response, but only under a constant photoperiod close to 12 L : 12 D is there evidence for sustained rhythmicity. Similarly, stonechats (Saxicola torquata) held under 12.8 L : 11.2 D did not show rhythmicity but did under 12.25 L : 11.75 D (Gwinner 1996). Moreover, the lack of apparent rhythmicity under 13 L : 11 D is not because this photoperiod masks the circannual clock. The response of starlings to a change in photoperiod from 13 L : 11 D is the same irrespective of how long birds had been held under 13 L : 11 D (Gwinner & Wozniak 1982). Thus, the ‘circannual clock’ must stop when photoperiod is not almost exactly 12 L : 12 D, which means that for birds more than 9° latitude away from the equator, any circannual clock can only tick twice each year, at the equinoxes.
As with birds, it has been argued for mammals that seasonality may result from a circannual rhythm which is entrained by annual change in photoperiod. The evidence, however, is mixed (see Goldman 2001). There is evidence for circannual periodicity in reproductive function in sheep and ground squirrels held under a constant photoperiod. Conversely, several species of photoperiodic rodents remain reproductively active for most of their lives when held under a constant long photoperiod, yet show rapid gonadal regression if transferred to a short photoperiod.
In marked contrast to starlings held under constant 11.5 L : 12.5 D or 12.5 L : 11.5 D, birds held under simulated annual cycle at 9 °N, ranging from 11.5 L : 12.5 D to 12.5 L : 11.5 D, did entrain precisely under this schedule, in that cycles were exactly 12 months apart (figure 3) and that minimum and maximum testicular sizes attained were the same as in natural temperate zone cycles (figure 4). The spotted antbird (Hylophylax naevioides), a tropical species living at 9 °N, is sensitive to small changes in photoperiod. Birds showed gonadal maturation in response to an increase in photoperiod from 12 L : 12 D to 13 L : 11 D and even an increase to 12.28 L appeared to have an effect (Hau et al. 1998). Clearly, such photoperiodic sensitivity is not restricted to tropical species since starlings, a temperate zone species, entrained precisely to a 9 °N simulated photoperiodic cycle.
The timing of maximal testicular size, regression and moult were identical in starlings under the 9 °N schedule to those under the 52 °N schedule, but testicular maturation started somewhat earlier. This reflects what happens naturally; the breeding seasons of birds, e.g. great tits (Sanz 1998), start somewhat earlier but last longer at lower latitudes than at higher latitudes. One problem in photoperiodism is to explain how birds of the same species but at different latitudes have similar breeding seasons even though they experience very different changes in absolute photoperiod. One possible explanation is genetic differences such that different populations respond differently to the same absolute photoperiod (Silverin et al. 1993). The data presented here not only demonstrate that birds are highly sensitive to changes in photoperiod, but also that they do not simply respond to absolute photoperiod, since similar responses were elicited by very different photoperiods. For example, moult started at about the summer solstice in both the 9 °N birds and the 52 °N birds, even though for one group was 12.5 L : 11.5 D and for the other 16.5 L : 7.5 D. Birds can retain a memory of the previous day's photoperiod (Dawson & King 1994; Brandstätter et al. 2000; Brandstätter 2003) and so can assess change and, presumably, rate of change in photoperiod. In this study, not only was the absolute photoperiod very different between the two groups, but so too was the rate of change. This suggests that birds must be even more subtle and be able to assess the change in the rate of change; in other words, to use the shape of the annual cycle of photoperiod to time seasonal events. Another possible explanation is that the intensity of photorefractoriness induced by a marginally long photoperiod such as 12.5 L : 11.5 D is less than induced by a longer photoperiod and, consequently, that this is dissipated more rapidly as photoperiod decreases towards 11.5 L : 12.5 D. This could account for the earlier recrudescence in the 9 °N birds. Either mechanism could account for the similarity of breeding seasons at different latitudes.
Exactly at the equator, there is no change in photoperiod during the year, but light intensity does change (Gwinner & Scheuerlein 1998) in regions with wet and dry seasons. In starlings, light intensity alters perception of photoperiod—a photoperiod is perceived as slightly shorter than it is if light intensity is low (Bentley et al. 1998). Thus, at the equator, perception of, and responses to, a 12 L : 12 D photoperiod may change during the year as light intensity changes. A change in perception of just 30 min either side of 12 L : 12 D would, in theory, be sufficient for complete entrainment. Gwinner & Scheuerlein (1998) kept African stonechats (S. torquata) on constant 12.25 L : 11.75 D. Birds under constant light intensity showed almost random testicular cycles, whereas those given alternating six-month period of high- and low-light intensity did entrain to calendar time.
In conclusion, these data show that birds do require changes in photoperiod to time annual events. Birds do not rely on a circannual clock which is entrained by photoperiod. Although starlings are a temperate zone species, they are sensitive to small changes in photoperiod. However, they do not simply respond to absolute photoperiod. Instead, birds appear to respond in some way to the shape of the annual change in photoperiod. This proximate control could operate from near equatorial latitudes and would account for similar seasonal timing in individuals of a species over a wide range of latitudes.
References
- Bentley G.E, Goldsmith A.R, Dawson A, Briggs C, Pemberton M. Decreased light intensity alters the perception of day length by male European starlings (Sturnus vulgaris) J. Biol. Rhythms. 1998;13:148–158. doi: 10.1177/074873098128999998. doi:10.1177/074873098128999998 [DOI] [PubMed] [Google Scholar]
- Brandstätter R. Encoding time of day and time of year by the avian circadian system. J. Neuroendocrinol. 2003;15:398–404. doi: 10.1046/j.1365-2826.2003.01003.x. doi:10.1046/j.1365-2826.2003.01003.x [DOI] [PubMed] [Google Scholar]
- Brandstätter R, Kumar V, Abraham U, Gwinner E. Photoperiodic information acquired and stored in vivo is retained in vitro by a circadian oscillator, the avian pineal gland. Proc. Natl Acad. Sci. USA. 2000;97:12 324–12 328. doi: 10.1073/pnas.200354997. doi:10.1073/pnas.200354997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. Plasma luteinizing hormone and prolactin during circannual rhythms of gonadal maturation and molt in male and female European starlings. J. Biol. Rhythms. 1997;12:371–377. doi: 10.1177/074873049701200409. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith A.R. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J. Endocrinol. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, King V. Thyroidectomy does not affect the daily or free-running rhythms of plasma melatonin in European starlings. J. Biol. Rhythms. 1994;9:137–144. doi: 10.1177/074873049400900204. [DOI] [PubMed] [Google Scholar]
- Dawson A, Newton I. Use and validation of a molt score index corrected for primary feather mass. Auk. 2004;121:372–379. doi:10.1642/0004-8038(2004)121[0372:UAVOAM]2.0.CO;2 [Google Scholar]
- Dawson A, King V.M, Bentley G.E, Ball G.F. Photoperiodic control of seasonality in birds. J. Biol. Rhythms. 2001;16:366–381. doi: 10.1177/074873001129002079. doi:10.1177/074873001129002079 [DOI] [PubMed] [Google Scholar]
- Farner D.S, Wilson A.C. A quantitative examination of testicular growth in the white-crowned sparrow. Biol. Bull. 1957;113:254–267. doi: 10.2307/1539953. [DOI] [PubMed] [Google Scholar]
- Goldman B.D. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J. Biol. Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. doi:10.1177/074873001129001980 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannuale Rhythmen bei Tieren und ihre photoperiodische Synchronisation. Naturwissenschaften. 1981;68:542–551. doi: 10.1007/BF00401662. doi:10.1007/BF00401662 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Circannual clocks in avian reproduction and migration. Ibis. 1996;138:47–63. [Google Scholar]
- Gwinner E, Dittami J.P. Endogenous reproductive rhythms in a tropical bird. Science. 1990;249:906–908. doi: 10.1126/science.249.4971.906. doi:10.1126/science.249.4971.906 [DOI] [PubMed] [Google Scholar]
- Gwinner E, Scheuerlein A. Seasonal changes in day-light intensity as a potential zeitgeber of circannual rhythms in equatorial stonechats. J. Ornithologie. 1998;139:407–412. doi:10.1007/BF01653467 [Google Scholar]
- Gwinner E, Wozniak J. Circannual rhythms in European starlings: why do they stop under long photoperiods? J. Comp. Physiol. A. 1982;146:419–421. doi:10.1007/BF00609438 [Google Scholar]
- Gwinner E, König S, Zeman M. Endogenous gonadal, LH and molt rhythms in tropical stonechats—effects of pair bond on period, amplitude, and pattern of circannual cycles. J. Comp. Physiol. A—Sens. Neural Behav. Physiol. 1995;177:73–79. doi: 10.1007/BF00243399. [DOI] [PubMed] [Google Scholar]
- Hamner W.M. On seeking an alternative to the endogenous reproductive rhythm hypothesis in birds. In: Menaker M, editor. Biochronometry. National Academy of Sciences; Washington, DC: 1971. pp. 448–462. [Google Scholar]
- Hau M, Wikelski M, Wingfield J.C. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc. R. Soc. B. 1998;1391:89–95. doi:10.1098/rspb.1998.0268 [Google Scholar]
- Lofts B, Murton R.K. Photoperiodic and physiological adaptations regulating avian breeding cycles and their ecological significance. J. Zool., Lond. 1968;155:327–394. [Google Scholar]
- Meddle S.L, Follett B.K. Photoperiodically driven changes in Fos expression within the basal tuberal hypothalamus and median eminence of Japanese quail. J. Neurosci. 1997;17:8909–8918. doi: 10.1523/JNEUROSCI.17-22-08909.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myres M.T. The breeding of the blackbird, song thrush and mistle thrush in Great Britain: part 1, breeding seasons. Bird Study. 1955;1:2–24. [Google Scholar]
- Rowan W. Experiments in bird migration, I. Manipulation of the reproductive cycle: seasonal histological changes in the gonads. Proc. Boston Soc. Nat. Hist. 1929;39:115–208. [Google Scholar]
- Sanz J.J. Effects of geographical location and habitat on breeding parameters of Great tits. Auk. 1998;115:1034–1051. [Google Scholar]
- Schwab R.G. Circannian testicular periodicity in the European starling in the absence of photoperiodic change. In: Menaker M, editor. Biochronometry. National Academy of Science; Washington, DC: 1971. pp. 428–447. [Google Scholar]
- Schwab, R. G. & Rutledge, J. T. 1973 Effects of natural and artificial illumination on testicular metamorphosis in the European starling (Sturnus vulgaris): maturation, involution, and photorefractory phases. In The sun in the service of mankind, pp. B.15-1–B.15-11. UNESCO Conference, Paris. Paris, France: UNESCO.
- Silverin B, Massa R, Stokkan K.-A. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, Iigo M, Yamamura T, Hirunagi K, Ebihara S. Light-induced hormone conversion of T4 to T3 regulates photoperiodic responses of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. doi:10.1038/nature02117 [DOI] [PubMed] [Google Scholar]